Abstract
Background
There is a growing interest in the role of gut bacteria in a number of diseases and an emerging hypothesis that inflammatory bowel disease (IBD) is triggered by microbial dysbiosis in genetically susceptible individuals. Currently, fecal microbiota transplantation (FMT) is utilized for the treatment of Clostridium difficile colitis. Data on the efficacy of FMT for IBD are mixed, but patients are interested in its use for the treatment of IBD. We sought to describe the use of FMT (self or medical professional administered) in individuals with IBD using IBD Partners, an Internet-based cohort.
Methods
Patients enrolled in the IBD Partners cohort were offered the opportunity to complete an optional survey on the use of FMT between January 2017 to September 2018 (n = 5430). A cross-sectional analysis was performed within patients who completed the survey and did not have a pouch or ostomy. Patients’ demographic characteristics, disease activity and phenotype, mode of FMT delivery, and patient-reported efficacy were compared.
Results
Among 3274 eligible patients, 51 (1.6%) responded that they had an FMT in the past. Of patients undergoing FMT, 22 patients had the FMT for C. difficile while 29 reported that the FMT was for another indication. Most patients receiving FMT for an indication other than C. difficile had ulcerative colitis/indeterminate colitis (25, 86.2%). Colonoscopy (68.2%) and nasogastric tube (18.2%) were the most common routes of administration for patients receiving FMT for C. difficile colitis. Self-administration (72.4%) and enemas (17.2%) were the most common routes of administration in patients receiving FMT for an alternate indication. Patients reporting FMT for an indication other than C. difficile were less likely to have a physician directing their FMT treatment (20.6%) as compared to patients receiving FMT for C. difficile (86.3%). Patient-reported efficacy was lower for FMT given for a non-C. difficile indication.
Conclusions
Patients undergoing FMT for an indication other than C. difficile infection were more likely to have ulcerative colitis, self-administer FMT, and were less likely to be receiving FMT under the guidance of a medical professional. FMT was not as effective for symptoms when given for a non-C. difficile indication. Patients should be counseled on potential harms and lack of proven benefit associated with FMT for IBD indications to try to discourage self-administered FMT without proper medical oversite.
Keywords: Crohn’s disease, ulcerative colitis, inflammatory bowel disease, fecal microbiota transplant
INTRODUCTION
There are over 1014 bacterial cells in the human body, most of which are located in the gut. Currently, there is a growing interest in the role of gut bacteria in a variety of diseases. Although the precise etiology of inflammatory bowel disease (IBD) remains unknown, there is an increasing belief that IBD is triggered by the inappropriate activation of the immune system by the intestinal microbiota in genetically susceptible individuals.1 Microbial dysbiosis is thought to lead to a dysregulation of the balance between beneficial and injurious commensals.2 Patients with IBD have previously been found to have an abundance of Enterobacteriaceae and a paucity of Faecalibacterium3 as well as a significant reduction in the biodiversity of their microbiome. IBD patients were more likely to have been prescribed antibiotics before their diagnosis.4 This evidence raises the possibility that restoring a balanced microbiome may be of benefit in IBD.
Fecal microbiota transplant (FMT) is the process of administering fecal bacteria from the donor or commercial stool sources to restore microbial diversity. Studies have used various routes of administration including nasoduodenal tube, colonoscopy, enema, and combination of colonoscopy plus enema at various frequencies. FMT was initially developed and studied in Clostridium difficile infection nonresponsive to antibiotics and has demonstrated efficacy rates of more than 90%.5 FMT is currently recommended in updated infectious disease and gastroenterology guidelines for refractory C. difficile.6, 7 Given the proposed role of microbial dysbiosis in the pathogenesis of IBD, FMT has also been investigated as a potential treatment for Crohn’s disease (CD) and ulcerative colitis (UC).
Despite a paucity of high-quality evidence to support FMT in IBD and a potential for harm, a survey of patients with UC showed that the majority were interested in or willing to consider FMT.8 Interest in FMT was thought to reflect the perception that FMT was a “natural” treatment for UC. Little is known, however, on the current state of FMT use (including both self and medical professional administered FMT) more broadly in IBD populations. There are unanswered questions regarding types of FMT delivery, stool dosage per infusion, preparation of inoculum, frequency of FMT, indication for FMT (whether for refractory C. difficile infection or other uses), FMT donor source, and whether FMT is being done as part of a research study or off-label.
To learn more about the current use of FMT, we used a large online cohort of IBD patients to investigate the use of FMT, mode of administration, and perceived response.
METHODS
Study Design
We performed a cross-sectional analysis of a subset of patients within the Crohn’s and Colitis Foundation IBD Partners cohort.
Study Population
IBD Partners is a longitudinal Internet-based cohort of adult patients with IBD established in 2011. Development of this cohort has been previously described in detail.9 Briefly, patients were recruited to participate in this online cohort registry via email, social media, and Crohn’s and Colitis Foundation educational events. More than 15,000 patients with self-reported IBD have enrolled in the cohort since 2011. Participants are given a baseline survey at the time of entry into the study and are invited to complete follow-up surveys every 6 months. Data on demographics, disease subtype, medication use, patient-reported disease activity, and other patient-reported outcomes are collected. In January 2017 an optional, 13-question survey of fecal microbiota transplant was added to baseline and follow-up surveys. Patients were included in the study if they completed the survey. Patients were excluded from the study if they were younger than 18 years of age at the time of the survey. Patients with an ileoanal pouch or ostomy were also excluded from the analysis due to the potential heterogeneity in this group. As dates of FMT were not collected it could not be determined if patients had an FMT before or after pouch or ostomy creation. These patients may have received FMT after surgery for treatment of pouchitis and not IBD, making the data less interpretable.
Study Variables
Demographic data including age, gender, race, and ethnicity were collected. Clinical data included BMI, smoking status, disease phenotype, medication use, and prior hospitalization. Disease activity was assessed using 2 validated patient-reported instruments including the short Crohn’s disease activity index (sCDAI) for CD10 and the simple clinical colitis activity index (sCCAI) for UC.11
Statistical Analysis
Bivariate analyses were used to compare demographics, disease activity, medication use, and quality of life between FMT recipients and non-FMT recipients. FMT characteristics and efficacy were compared between FMT recipients for C.difficile and FMT recipients for other indication. Categorical variables were compared using chi-square statistics or Fisher’s test as appropriate. Continuous variables were compared using t tests or Wilcoxon rank-sum test as appropriate. Statistical significance was defined as a P-value less than 0.05. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Ethical Considerations
The study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
RESULTS
Among 5430 participants offered the survey between January 2017 and September 2018, 3647 participants completed the survey (67.2% response rate). Excluding patients with an ileoanal pouch or ostomy, 3274 patients were eligible for the analysis. Clinical characteristics of patients completing the FMT survey are summarized in Table 1. There were 51 people who reported a prior FMT. The average age of FMT recipients was 41.7 years old and 74.5% were women; similar to the overall Partners cohort (72% women). There were 31 (60.8%) FMT recipients with UC or indeterminate colitis (IC) versus 36.6% UC/IC in the non-FMT cohort. The average duration of disease was 13.7 years in patients reporting prior FMT. Patient-reported disease activity, as measured by the sCCAI and the sCDAI, was similar between FMT and non-FMT patients. FMT recipients were more likely to use rectal steroids (7.4% vs 2.6%, P = 0.022), budesonide (11.8% vs 3.6%, P = 0.002), and systemic steroids (13.7% vs 5.4%) as compared to non-FMT patients. FMT recipients also reported more current use of probiotics (56.9% vs 29.0%) and lifetime use of probiotics (96.1% vs 65.8%) as compared to non-FMT patients. Use of 5-ASA, immunomodulators, and biologics was not significantly different in FMT recipients (Table 2).
TABLE 1.
Descriptive Statistics of CCFA Partners Who Completed FMT Survey
| Prior FMT (N = 51) n (%) | No FMT (N = 3223) n (%) | P | |
|---|---|---|---|
| Patient Characteristics | |||
| Age, mean (SD) | 41.7 (13.9) | 46.1 (15.0) | 0.036 |
| Female | 38 (74.5) | 2319 (72.0) | 0.686 |
| Race | |||
| White | 47 (92.2) | 2882 (89.4) | |
| Black | 0 (0.0) | 35 (1.1) | |
| Other/unknown | 4 (7.8) | 306 (9.5) | |
| Ethnicity | |||
| Hispanic | 2 (4.1) | 77 (2.5) | 0.478 |
| BMI kg/m2, mean (SD) | 23.0 (4.3) | 25.9 (6.2) | 0.001 |
| Current smoker | 0 (0) | 132 (4.5) | 0.149 |
| Clinical Characteristics | |||
| CD | 20 (39.2) | 2004 (63.4) | <0.001 |
| UC/IC | 31 (60.8) | 1179 (36.3) | <0.001 |
| Family history of IBD | 1 (2.0) | 236 (7.4) | 0.147 |
| Years of disease, mean (SD) | 13.7 (10.7) | 16.8 (12.4) | 0.068 |
| Prior bowel surgery | 36 (70.6) | 1901 (59.0) | 0.094 |
| IBD hospitalizations | 7 (13.7) | 1099 (34.1) | 0.002 |
| Disease activity, mean (SD) | |||
| sCDAI | 161.3 (120.1) | 135.5 (88.5) | 0.197 |
| SCCAI | 3.3 (2.3) | 3.0 (2.6) | 0.501 |
TABLE 2.
Use of IBD Medications
| IBD Medications Current | IBD Medications Ever | |||||
|---|---|---|---|---|---|---|
| Prior FMT (N = 51) n (%) | No FMT (N = 3223) n (%) | P | Prior FMT (N = 51) n (%) | No FMT (N = 3223) n (%) | P | |
| Rectal steroids | 4 (7.8) | 89 (2.6) | 0.022 | 28 (54.9) | 1194 (37.1) | 0.009 |
| Oral/IV steroids | 7 (13.7) | 173 (5.4) | 0.009 | 47 (92.2) | 2700 (83.9) | 0.109 |
| Budesonide | 6 (11.8) | 117 (3.6) | 0.002 | 26 (51.0) | 1415 (43.9) | 0.315 |
| Antibiotics | 1 (2.0) | 77 (2.4) | 0.841 | 40 (78.4) | 2069 (64.3) | 0.036 |
| 5-ASA | 22 (43.1) | 1119 (34.8) | 0.213 | 48 (94.1) | 2916 (90.6) | 0.390 |
| Immunomodulator | 16 (31.4) | 831 (25.8) | 0.372 | 37 (72.5) | 2093 (65.0) | 0.263 |
| Biologic | 23 (45.1) | 1580 | 0.568 | 32 (62.7) | 2067 (64.2) | 0.831 |
| Probiotic | 29 (56.9) | 932 (29.0) | <0.001 | 49 (96.1) | 2117 (65.8) | <0.001 |
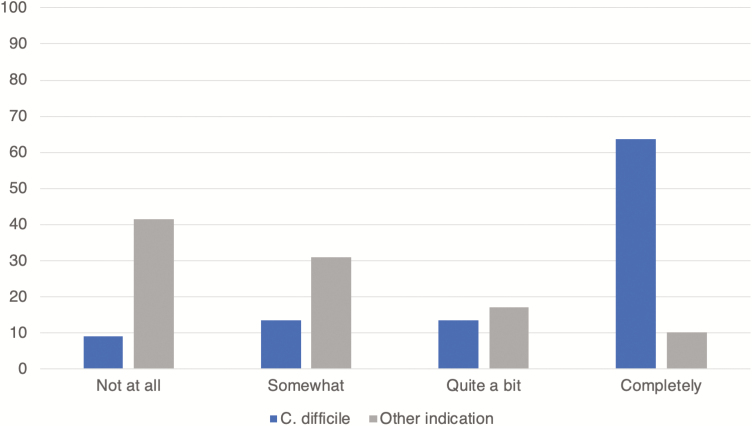
Of patients receiving FMT, 22 reported that the FMT was for C. difficile and 29 reported FMT for another indication (Table 3). Most patients receiving FMT for an indication other than C. difficile had UC/IC (25, 86.2%). The majority of patients (41, 80.4%) reported that their FMT was more than 1 year before completing the survey. Route of FMT delivery was significantly different in patients receiving FMT for C. difficile versus those receiving FMT for an alternate indication. Colonoscopy (68.2%) and nasogastric tube (18.2%) were the most common routes of administration for patients receiving FMT for C. difficile colitis. Self-administration (72.4%) and enemas (17.2%) were the most common routes of administration in patients receiving FMT for an alternate indication. Patients reporting FMT for an indication other than C. difficile were less likely to have a physician directing their FMT treatment (20.6%) as compared to patients receiving FMT for C. difficile (86.3%). Only 7 (13.7%) of all FMTs were done as part of a research study. FMT for an alternative indication was less effective in relieving symptoms as compared to FMT for C. difficile infection (Figure 1). Fourteen patients (63.6%) receiving FMT for C. difficile infection reported complete relief of symptoms versus 3 patients (10.3%) who reported complete relief of symptoms after FMT for another indication.
TABLE 3.
Characteristics of FMT Given in IBD Patients
| FMT for C. difficile (N = 22) n (%) | FMT for Other Indication (N = 29) n (%) | P | |
|---|---|---|---|
| Number of antibiotic courses for C. difficile before FMT 2 3–4 ≥5 | 7 (31.8) 9 (40.9) 6 (27.3) | — | 0.691 |
| Duration since last FMT Within 1 year More than 1 year ago | 5 (22.7) 17 (77.3) | 5 (17.2) 24 (82.8) | 0.685 |
| Route of FMT Nasogastric tube Colonoscopy Enema Pill/capsule Self-administration | 4 (18.2) 15 (68.2) 0 (0) 2 (9.1) 1 (4.5) | 0 (0) 2 (6.9) 5 (17.2) 1 (3.4) 21 (72.4) | <0.001 |
| Source of FMT Known donor/family member Commercial source Hospital or clinic source | 12 (54.5) 4 (18.2) 6 (27.3) | 25 (86.2) 0 (0) 4 (13.8) | 0.017 |
| FMT as part of a research study | 4 (18.2) | 3 (10.3) | 0.032 |
| Provider directing FMT treatment Primary care provider Gastroenterologist Other physician Other health care provider Self Other | 1 (4.5) 11 (50.0) 7 (31.8) 1 (4.5) 1 (4.5) 1 (4.5) | 1 (3.4) 2 (6.9) 3 (10.3) 3 (10.3) 20 (69.0) 0 (0.0) | <0.001 |
FIGURE 1.
Patient-reported effectiveness of FMT on IBD symptoms. Fourteen patients (63.6%) receiving FMT for C. difficile infection reported complete relief of symptoms versus 3 patients (10.3%) who reported complete relief of symptoms after FMT for another indication.
DISCUSSION
Here we present a large survey of FMT in a cohort of patients with UC and CD with a 67.2% response rate. Survery nonresponders were 6 years older, had the disease for about 5 more years, and had approximately 5% more hospitalizations and IBD surgery. These differences are likely of little clinical significance and represent a slightly older population with more time to have IBD and complications rather than a sicker population. Overall FMT was rare: only 51 patients (1.6%) reported having a prior FMT at the time of the survey. Patients receiving FMT were more likely to have UC as compared to the general IBD Partners cohort. While this may be explained by the higher rate of C. difficile in UC patients compared to CD patients,12 even the patients having an FMT for a non-C. difficile indication were much more likely to have UC (86.2%). This likely reflects the more promising outcomes of FMT for primary UC treatment as compared to CD. Despite similar disease activity indices between FMT recipients and the general cohort, FMT recipients were less likely to report prior IBD hospitalization (13.7% vs 34.1%, P = 0.002). FMT recipients were also more likely to use probiotics both currently and in the past. The lack of hospitalization and higher rates of probiotic use may reflect a greater interest in alternative or natural therapies which could be associated with a greater acceptance of fecal microbiota transplantation as a treatment in general.
There have been several prior studies of FMT for IBD, but data have been mixed and studies are often small. To date, there have been 4 randomized controlled trials (RCTs) investigating FMT in UC. Most recently Costello et al13 investigated steroid-free remission of UC in 73 patients with mild to moderate UC. Pooled donor FMT or autologous FMT was delivered via colonoscopy followed by 2 enemas over 7 days. At 8 weeks, overall steroid-free remission was achieved in 32% of the pooled donor FMT versus 9% in the autologous FMT (odds ratio 5.0, 95% confidence interval 1.2–20.1, P = 0.03) and 42% maintained remission at 12 months. However, prior RCTs showed mixed results in UC specifically with nasoduodenal administration.13–16 Furthermore, while Moayyedi et al14 showed remission in 24% of patients administered FMT, it was found that 7 of the 9 patients who achieved remission received fecal material from the same donor. Paramsothy et al17 found that among UC patients, an increased number of infusions (>10) was associated with a higher rate of clinical remission (49% vs 27% in patients receiving ≤10 infusions). Also, lower infusion (colonoscopy or enema) was more effective than upper administrations.
There are currently no RCTs in CD. A recent meta-analysis and systematic review17 identified 53 studies (41 in UC, 11 in CD, and 4 in pouchitis) of FMT in IBD, most were cohort studies with the exception of the 4 RCTs conducted in UC. Overall remission rates were 36% in UC, 50.5% in CD, and 21.5% in pouchitis patients. Posttransplant microbiota analysis was performed in 24 studies with many identifying increased diversity and a shift toward donor microbiota profile. In addition to low remission rates in IBD, FMT also carries the potential for harm. A recent study of patients receiving FMT for CD was halted early after 2 of the 10 patients had significant decompensations requiring an escalation of IBD therapy.18
Patients undergoing FMT for an indication other than C. difficile were mostly self-administering their treatment without the oversite of a medical practitioner. One prior study of patient perceptions around FMT for the treatment of UC showed that a majority of patients were willing to consider FMT despite good disease control on conventional therapy. This willingness existed in the absence of safety or efficacy data for FMT.8 Our study confirms that patients are not only willing to consider FMT for treatment but are also engaged in self-directed FMT without the supervision of a clinician. However, patient-perceived efficacy rates for FMT were significantly lower than in those undergoing FMT for C. difficile. These findings are particularly concerning in light of a recent FDA-issued safety alert for FMT after 2 episodes of invasive infections by extended-spectrum beta-lactamase-producing Escherichia coli and 1 death. These serious adverse events coupled with the potential for IBD flare after FMT should make physicians take pause before recommending FMT as a benign treatment. Furthermore, physicians should warn their patients of these potential harms and discourage patients from performing self-administered FMT without the guidance of a medical professional.
A strength of our study is the large sample size and diverse geographic enrollment in IBD Partners. Additionally, the majority of patients in IBD Partners are treated in a community setting. Therefore, we are able to describe practice patterns outside academic centers. Limitations of the study include the self-reported nature of our survey that may bias efficacy claims or recall of disease severity. However, a prior validation study revealed a 97% accuracy rate of self-reported IBD status and disease subtype within the CCFA Partners cohort.19 Another limitation of the study is that there were a larger number of women enrolled in the Partners cohort and responding to the FMT survey (74.5%). This may limit the external validity of the study and may not be generalizable due to the self-selection of survey respondents. Additionally, our validated measures of disease activity were not at the time of FMT, we therefore relied on patient perception of efficacy at that time as our endpoint.
CONCLUSIONS
In this cross-sectional study of patients with IBD, there were only 51 (1.6%) who reported having prior FMT. Twenty-two patients underwent FMT for C. difficile infection while the remaining underwent FMT of another indication. Patients undergoing FMT for an indication other than C. difficile infection were more likely to have UC, self-administer FMT, and were less likely to be receiving FMT under the guidance of a medical professional. FMT was not as effective for symptoms when given for a non-C. difficile indication. Although FMT in IBD patients is rare, there is increasing interest in the role of the microbiome and the use of FMT for several chronic diseases. FMT is also increasingly reported in the media. Patients should be warned of potential risks (including death) and lack of proven efficacy associated with FMT for IBD to try to discourage self-administered FMT without proper medical oversite.
Funding: This research was supported, in part, by grants from the National Institutes of Health (P30 DK034987) and the Crohn’s and Colitis Foundation.
Disclosures: C.M.B., R.S.S., and X.Z.: none; M.D.L.: consulting AbbVie, Pfizer, UCB, Salix, Valeant, Takeda, Prometheus, Janssen, and Target PharmaSolutions. Research support: Takeda and Pfizer.
REFERENCES
- 1. Wang ZK, Yang YS, Chen Y, et al. . Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol. 2014;20:14805–14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79–84. [DOI] [PubMed] [Google Scholar]
- 3. Sekirov I, Russell SL, Antunes LC, et al. . Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. [DOI] [PubMed] [Google Scholar]
- 4. Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2011;106:2133–2142. [DOI] [PubMed] [Google Scholar]
- 5. Quraishi MN, Widlak M, Bhala N, et al. . Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46:479–493. [DOI] [PubMed] [Google Scholar]
- 6. McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:987–994. [DOI] [PubMed] [Google Scholar]
- 7. Surawicz CM, Brandt LJ, Binion DG, et al. . Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498; quiz 499. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SA, Vachon A, Rodriquez D, et al. . Patient perceptions of fecal microbiota transplantation for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Long MD, Kappelman MD, Martin CF, et al. . Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis. 2012;18:2099–2106. [DOI] [PubMed] [Google Scholar]
- 10. Thia K, Faubion WA Jr, Loftus EV Jr, et al. . Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis. 2011;17:105–111. [DOI] [PubMed] [Google Scholar]
- 11. Walmsley RS, Ayres RC, Pounder RE, et al. . A simple clinical colitis activity index. Gut. 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. [DOI] [PubMed] [Google Scholar]
- 13. Costello SP, Hughes PA, Waters O, et al. . Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moayyedi P, Surette MG, Kim PT, et al. . Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109.e6. [DOI] [PubMed] [Google Scholar]
- 15. Rossen NG, Fuentes S, van der Spek MJ, et al. . Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118.e4. [DOI] [PubMed] [Google Scholar]
- 16. Paramsothy S, Kamm MA, Kaakoush NO, et al. . Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. [DOI] [PubMed] [Google Scholar]
- 17. Paramsothy S, Paramsothy R, Rubin DT, et al. . Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11:1180–1199. [DOI] [PubMed] [Google Scholar]
- 18. Gutin L, Piceno Y, Fadrosh D, et al. . Fecal microbiota transplant for Crohn disease: a study evaluating safety, efficacy, and microbiome profile. United European Gastroenterol J. 2019;7:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Randell RL, Long MD, Cook SF, et al. . Validation of an internet-based cohort of inflammatory bowel disease (CCFA partners). Inflamm Bowel Dis. 2014;20:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]