Abstract
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease globally. The primary initiating mechanism in DN is hyperglycemia-induced vascular dysfunction, but its progression is due to different pathological mechanisms, including oxidative stress, inflammatory cells infiltration, inflammation and fibrosis. Macrophages (Mφ) accumulation in kidneys correlates strongly with serum creatinine, interstitial myofibroblast accumulation and interstitial fibrosis scores. However, whether or not Mφ polarization is involved in the progression of DN has not been adequately defined. The prevalence of the different phenotypes during the course of DN, the existence of hybrid phenotypes and the plasticity of these cells depending of the environment have led to inconclusive results. In the same sense the role of the different macrophage phenotype in fibrosis associated or not to DN warrants additional investigation into Mφ polarization and its role in fibrosis. Due to the association between fibrosis and the progressive decline of renal function in DN, and the role of the different phenotypes of Mφ in fibrosis, in this review we examine the role of macrophage phenotype control in DN and highlight the potential factors contributing to phenotype change and injury or repair in DN.
Keywords: macrophages, diabetic nephropathy, fibrosis
1. Introduction
Diabetic nephropathy (DN) is currently the most prevalent chronic kidney disease and the leading cause of end-stage renal disease in adults [1]. A role for macrophages (Mφ) in both the development and progression of this disease is recognized.
Mφ and renal inflammation are associated with DN as an analysis of renal biopsies in diabetic patients has confirmed the presence of Mφ in both glomeruli and the interstitium at all stages of DN [2,3]. Mφ accumulate in the diabetic kidneys [4,5,6] has been related to renal disease progression [2]. This accumulation correlates strongly with serum creatinine, interstitial myofibroblast accumulation and interstitial fibrosis scores [7,8,9,10,11]. Additionally, in diabetic db/db mice, it has been shown that macrophage accumulation and activation provokes glomerular and tubular damage, albuminuria, elevated plasma creatinine, renal fibrosis and kidney expression of Mφ chemokines [12].
Mφ are considered an important source of tumor necrosis factor-alpha (TNF-α) and it is known that this cytokine does play a pivotal role in the development of DN. In this sense, TNF-α levels in kidneys are increased in experimental animal models of DN [13,14] and conditional knockout of TNF-α in Mφ revealed a complete block of TNF-α expression in diabetes-induced models. In addition, deletion of macrophage TNF-α provoked a reduction in hypertrophy, albuminuria and glomerular pathology [15]. Pharmacological inhibition of TNF synthesis reduced the loss of glomerular filtration rate in patients with DN [16] and high TNF receptors are indicative of disease progression in humans with DN [17,18].
The role of fibrosis in the progression of DN has also been recognized appearing to be critical for final progression of DN to kidney failure in diabetic Type 1 and 2 [19]. There is a positive correlation between the grade of fibrosis of the renal cortical interstitium and the serum creatinine concentration at the time of biopsy in patients with DN. This fibrosis appears to be largely due to increase cellular components and Mφ presence, which is followed by an increase in interstitial fibrillary collagen.
Mφ recruitment generates inflammatory cytokines that may stimulate cells to enhance its production or reduce the degradation of matrix proteins [20]. Targeted deletion of the macrophage scavenger receptor-A ameliorated many of the glomerular changes of experimental DN in mice. In these experimental conditions, Mφ infiltration was decreased, proinflammatory genes were suppressed and attachment of monocytes to type IV collagen was reduced [21]. In addition, glomerular and tubulointerstitial cells produce a multitude of inflammatory mediators in the diabetic milieu, especially as injury proceeds, which can augment inflammatory damage and modify Mφ behavior in fibrosis.
Given the strong associations between fibrosis and the progressive decline of renal function in DN, and the recognized role of Mφ as inductors of fibrosis, in this review, we discuss the role of Mφ in both the development and progression of fibrosis in DN. We examine the role of Mφ phenotype in fibrosis development and highlight its implications for new therapeutic strategies.
2. Macrophage Phenotype and Fibrosis
Fibrosis is a process characterized by excessive deposits of extracellular matrix that leads to the replacement of functional parenchyma by fibrotic tissue [22]. Renal fibrosis is the common pathological process in chronic kidney disease, despite the underlying cause, in which kidney gradually lost its ability to repair as a result of ongoing tissue injury and inflammation [23]. However, renal fibrosis is a multifactorial and dynamic process that carries many cellular events in response to the injurious stimuli. Within the several cells types that are implicated in the pathogenesis of renal fibrosis, Mφ gains attention due to the potential therapeutic approaches mediated by cell therapy transfer. These highly heterogeneous cells belong to the mononuclear phagocyte system and are virtually present in all tissues as monocyte-derived Mφ from bone marrow and/or as tissue-resident Mφ that arise from embryonic precursors; the latter self-renew in situ independent of circulating monocytes [24,25]. Mφ has the ability to eliminate pathogens, apoptotic cells or any other foreign body through phagocytosis or T cells activation, which can either contribute to tissue repair or promote further damage. These contrasting functions are the result of macrophage functional plasticity, since they change their phenotype in response to local microenvironment cues [26]. Thus, macrophage activation involves a complex interplay between infiltrated immune cells, resident damage cells and apoptotic cells orchestrated by a number of cytokines/chemokines and growth factors.
Traditionally, in vitro studies have classified Mφ as classically activated Mφ (M1) and alternatively activated Mφ (M2) based on the activation mechanism and cell function [27]. The M1 phenotype is triggered by microbial molecules or inflammatory cytokines, such as lipopolysaccharide (LPS) and interferon gamma (IFN-γ) and releases proinflammatory cytokines and cytotoxic mediators. Accordingly, M1 is involved in the initiation phase of inflammation and is related to tissue damage and proinflammatory functions. In contrast the M2 phenotype produces anti-inflammatory cytokines, growth factor and proangiogenic cytokines involved in the wound healing process (repair phase). Therefore, after the injury, if the interaction between inflammation and repair is well structured, the normal state of the tissue is restored. However, if the initial insult remains perpetuating the inflammation or if the wound healing process is deregulated, it can cause fibrosis [28]. This dichotomous M1/M2 classification of macrophage remains widely used, although represents a simple overview of the macrophage phenotype and function, since the tissue microenvironment has a rather complex combinations of stimuli. For instance, kidney resident Mφ characterized as F4/80+ CD64+ CD11b/cint were able to limit kidney injury through angiogenesis and wound healing while expressing both pro- and anti-inflammatory/fibrotic genes in a murine model of renal artery stenosis [29]. In another model of chronic kidney damage, known as unilateral ureteral obstruction (UUO), Sogawa et al. revealed that an impaired M1 infiltration suppressed fibrosis and inflammation after UUO in Nrf2 deficiency mice, implying that inflammasome activation can cause chronic inflammation and renal fibrosis through the M1 phenotype [30]. Recently, UUO-induced renal fibrosis was found ameliorated in a myeloid-specific RBP-J deficient macrophage model were both phenotypes (M1 and M2) were compromised by the Notch blockade, suggesting that Notch regulates macrophage phenotype outside the conventional M1/M2 classification [31].
In addition to the Mφ response to cytokine cues, other mechanisms have been determined. Zhang et al. exposed that a triggering receptor expressed on myeloid cells 1 (TREM-1) modulates the Mφ phenotype towards M1 under high-glucose in vitro conditions; in conjunction the expression of TREM-1 in the renal interstitium is significantly correlated with the DN progression in human renal biopsies [32]. A recent study has shown that pioglitazone, a peroxisome proliferator activated receptor γ (PPARγ) agonist, promotes the M2 polarization; although it indicated that the process was not mediated by PPARγ, pioglitazone treatment in M2 cells did increase the expression of vascular endothelial cell growth factor receptor-3 (VEGFR3) via a PPARγ-dependent pathway [33]. Furthermore, VEGFR3 binds to vascular endothelial cell growth factor-C (VEGF-C) and VEGF-C has been identified to ameliorate renal interstitial fibrosis through lymphagiogenesis in UUO [34]. In another in vitro study, it has been established that advanced oxidation proteins products (AOPP) induces the transition of Mφ into dendritic-like-cells and reduces the cell surface thiol pool. Moreover, this effect was lost when the cells were pretreated with the antioxidant N-acetyl cysteine before AOPP exposure. Thus, it indicates that in an oxidizing environment, uremic toxins like plasma AOPP modifies the Mφ response by altering the thiol redox equilibrium [35].
The epithelial-to-mesenchymal transition (EMT) of tubular cells is also a feature of renal fibrosis. During EMT, epithelial markers are lost and mesenchymal genes overexpressed, consequently epithelial cells undergo phenotypic change, some involving myofibroblast transformation with the consequent extracellular matrix accumulation. Although the origin of myofibroblasts in fibrosis is still on debate, it seems that they were derived from residents cells that migrate, proliferate and transform. Some lineage-tracing studies have determined endogenous stromal cells (fibroblasts and pericytes) as the primary source [36,37], and tubular epithelium undergoing EMT as a plausible source in advanced stages where the basement membrane have suffered severe damage [38]. Nonetheless, subsequent studies have determined that Mφ are the main participants as revealed by the macrophage-to-myofibroblast transition occurring in active renal fibrosis [39,40], with the M2 phenotype being the predominant cell type [41]. Nevertheless, when Mφ becomes anti-inflammatory they support the resolution of inflammation. This process involves the expression of growth factors that promote both parenchymal and mesenchymal repair; these is the case of the anti-inflammatory cytokine TGF-β1 that activates mesenchymal repair mechanisms, but in addition to this is a profibrotic cytokine [42]. At the later stage after injury, Mφ contributes to the removal of fibrous tissue. Mediating the resolution phase of healing includes capillary regression and collagen remodeling. In this phase, Mφ can produce factors that terminate the repair response.
3. Macrophages and Diabetic Nephropathy
Mφ plays a significant role in the development of DN and is the cause of renal tissue stromal hyperplasia and irreversible pathological changes of glomeruli [43]. In experimental models of diabetes, it has been found that Mφ infiltration occurs at an early stage of the disease and correlates with renal injury. In addition, therapeutic strategies targeted to reduce monocyte infiltration attenuate the development of kidney injury [44].
Mφ depletion using diphtheria toxin (DT) in the CD11b–DT receptor (CD11b-DTR) transgenic mice confirmed the direct role of these cells in DN progression. This study showed that treatment with DT in order to deplete Mφ after induction of diabetes significantly reduced albuminuria, kidney macrophage recruitment and glomerular histological changes [45].
Increased expression of ICAM-1 by renal tubular cells responding to high circulating levels of glucose and advanced glycation end products (AGEs) in diabetic kidney enhances the recruitment of Mφ [46,47]. Once recruited, Mφ responds to local high levels of glucose, AGEs and oxidized low-density lipoprotein (Ox-LDL) to execute inflammatory cytokine secretion. Stimulation of the production of reactive oxygen species (ROS) and proteases continuously provoke Mφ stimulation, leading to tissue damage and renal fibrosis [12].
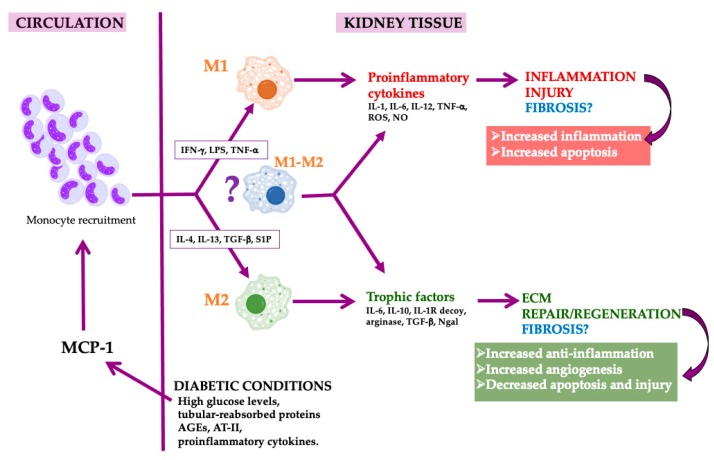
A key mediator in the infiltration of Mφ is the monocyte chemoattractant protein-1 (MCP-1) [48] (Figure 1). In fact, MCP-1 deficient diabetic mice do not develop albuminuria and is protected from an increase in plasma creatinine [49]. Additionally, urinary MCP-1 is upregulated in inflammatory renal disease and diabetic nephropathy [50]. Upregulated renal MCP-1 levels released from kidney tubular cells, smooth muscle cells, mesangial cells and podocytes are induced by elevations in glucose levels, tubular-reabsorbed protein, AGEs, angiotensin-II (AT-II) and in response to proinflammatory cytokines such as interleukin-1, TNF-α and interferon-γ [45]. In addition to Mφ accumulation, MCP-1 is thought to be indirectly involved in the recruitment of neutrophils through an intermediate mediator leukotriene B4 [51].
Figure 1.
Macrophages in diabetic nephropathy. High glucose levels, tubular-reabsorbed protein, advanced glycation end products (AGEs), angiotensin-II (AT-II) and proinflammatory cytokines induce the production of MCP-1 by kidney tubular cells and podocytes. As a consequence, kidney monocyte recruitment is provoked. In the tissue, monocytes are converted in macrophages that acquire the M1, M2 or mixed phenotype depending on the inflammatory milieu and the molecules released by these different macrophage populations. M1 provokes injury and M2 are proresolution and regenerative macrophages that could induce fibrosis, while mixed macrophages could acquire different roles depending on the milieu. Macrophage image by Mikael Häggström, used with permission.
The P2X7 receptor is expressed on Mφ and mediates proinflammatory signaling pathways. In human mesangial cells exposed to a high glucose environment, P2X7 receptors have increased the release of MCP-1 [52]. Furthermore, mice with a knockdown of the P2X7 receptor had reduced glomerular macrophage recruitment and collagen IV deposition. The use of NOX-E36 (emapticap pegol), which binds and inhibits MCP-1, resulted in a renal function improvement in patients with type 2 diabetes [53].
MCP-1 binds to C–C chemokine receptor 2 (CCR2) and treatment with the CCR2 antagonist CCX140-B resulted in decreased albuminuria, lessen glomerular hypertrophy and increased podocyte density in transgenic human CCR2 knockin mice rendered diabetic [54]. In addition, CCX140-B treatment in patients with type 2 diabetes and nephropathy stemmed a reduced albuminuria, highlighting their renoprotective role when given in addition to standard care [55]. Phase 3 clinical trials are now planned for the CCR2 antagonist CCX140-B.
Thus, it seems that reduction of Mφ recruitment by the use of agents able to reduce its accumulation in diabetic kidneys, improves renal function and fibrosis. In this sense, therapeutic approaches with beneficial renal outcomes in DN can also focus in antibodies against proteins involved in macrophage infiltration (Table 1).
Table 1.
Diabetic nephropathy therapies with macrophage implications.
| Drug/mAbs | Mechanism of Action | Macrophage Implication | Outcome | Ref. |
|---|---|---|---|---|
| Alantolactone | Inhibition of TNF-α and IL-6 | Reduces Mφ infiltration | In diabetic mice: reduced creatinine and urea nitrogen serum levels. | [56] |
| Tectorigenin | Improve vascular endothelium dysfunction through AdipoR1/2 pathway | Reduces Mφ infiltration and M1 polarization | In diabetic mice: reduced endothelia damage through lipotoxicity, improved insulin sensitivity, attenuated Mφ-induced inflammation | [57] |
| Emapticap Pegol | Inhibition of MCP-1 | Reduces Mφ infiltration | In diabetic patients: reduced HbA1c and urinary albumin/creatinine ratio. | [53] |
| Pentraxin-3 | Increase numbers of Mφ expressing Arg1 - CD206 | Promotes M2 polarization | In diabetic mice: increased expression of nephrin, acetylated nephrin, and WT-1. | [58] |
| Enalapril | Increase T cells number and promotes Mφ differentiation towards M1-like | Promotes M1-like polarization | In diabetic patients: reduced albuminuria without modulating the HbA1c %. | [59] |
| Monoclonal Antibodies Against CD148 | Prevents reduction of podocyte and nephrin expression and decreased glomerular fibronectin expression | Reduces Mφ infiltration | In diabetic mice: decreased albuminuria and mesangial expansion without altering hyperglycemia and blood pressure | [60] |
| Monoclonal Antibody against IL-17 | Blocks the NF-κB cascade, TGF-β and fibronectin. | Reduces Mφ infiltration | In diabetic mice: reduced albuminuria, glomerular damage, Mφ accumulation and renal fibrosis | [61] |
AdipoR1/2: adiponectin receptor 1/2. WT1: Wilms’ tumor-1 protein. Arg1: arginase 1. CD206: mannose receptor. HbA1c: hemoglobin A1c.
In addition, Mφ could be activated by inflammatory mediators released by activated lymphocytes. Activated T lymphocytes could secrete IFN-γ and/or TNF-α, both activating macrophages and inducing renal fibrosis [62]. It is known that cell adhesion molecules and inflammatory cytokines provoke the migration of T lymphocytes to the kidney and abnormal activation and migration of T lymphocytes contributed to the development of renal fibrosis in DN [62]. Pharmacological maneuvers able to provoke a reduction in the infiltration of T lymphocytes by inhibiting the production of MCP-1 and the proliferation of T lymphocytes, but promoting their apoptosis in diabetic kidneys of animal models or DN, induces anti-inflammation and antioxidant effects and finally reduces proteinuria and renal fibrosis [63].
In contrast with the above findings, pointing to a harmful role of lymphocytes in DN, there is also evidence that accumulation of IL-17A producing T helper cells (Th17 cells) in diabetic kidneys may help to limit the progression of diabetic nephropathy [64,65]. In this sense, mice with an IL-17A gene defect develop more serious renal injury of DN, while wild-type (WT) diabetic mice receiving a low dose of IL-17A are protected against DN [64]. Notably, the IL-17A treatment was related to the reduction of the Mφ infiltration, proinflammatory cytokines (MCP-1, IL-10, IL-6 and TNF-α) and STAT3 activation, thus revealing an anti-inflammatory effect in diabetic mice. Moreover, in diabetic patients, the urine level of IL-17A was increased in the presence of microalbuminuria but decreased in the presence of macroalbuminuria [65]. Therefore, IL-17A can protect DN by alleviating the inflammatory response.
Another study assessed the role of T-regulatory cells in the development of diabetic nephropathy in db/db mice. This study showed that depleting T-regulatory cells with anti-CD25 mAb could exacerbate diabetic renal injury and adoptively transferring CD4+FoxP3+cells into mice could reduce diabetic renal injury [66].
4. Macrophage Phenotype and Diabetic Nephropathy
As mentioned, macrophage plasticity allows them to acquire different phenotypes [67]. M1 and M2 play opposing roles since M1 is important for antigen presentation and immune inflammatory effects, whereas M2 mainly release cytokines that inhibit inflammation and exert anti-inflammatory effects and proresolution mechanisms [68].
Mφ located at the site of diabetic kidney injury are predominantly M1. The relevance of M1 to the development of DN has been shown using mice with Mφ cyclooxygenase-2 (Cox-2) deletion. These mice demonstrate increased M1 polarization, which is associated with increased renal injury [69]. Although other authors found that Mφ in the kidneys of streptozotocin-induced DN rat are characterized by an elevated expression of galectin-3 and TGF-β, suggesting an M2 dominance [59]. Adoptive transfer of proresolution M2 to streptozotocin-induced type 1 diabetic mice led to decreased macrophage infiltration to the kidney along with decreased renal damage including tubular atrophy, glomerular hypertrophy and interstitial expansion [70]. Agents that promote M2 polarization like Pentraxin-3 are able to attenuate renal damage in DN by promoting M2 macrophage differentiation [58]. Zhang et al. suggested that the inhibition of M1 macrophage activation and the promotion of M2 macrophage transformation prevented podocyte injury [71]. Similarly, activation of M2 by Sirt6 protected podocytes against injury in a mimicked diabetic kidney microenvironment [72].
In addition, the activated microglia/macrophage WAP (AMWAP) domain protein, known to have anti-inflammatory activity by turning macrophages to the M2 phenotype, protects the kidney against diabetic nephropathy, reduced albuminuria and glomerulosclerosis possibly through regulation of the macrophage phenotype [64].
However, enalapril treatment causing a repolarization of the macrophage towards a M1-like phenotype appears to inhibit the progression of kidney damage in DN experimental models [59].
Hence, beneficial kidney macrophage in the diabetic kidney may be similar to a phenotype described as a resolution macrophage, but the substances that define this proresolution phenotype are not established. These resolution Mφ do not always express markers that characterize them as either classically nor alternatively activated but are a hybrid of both phenotypes. Further studies will be necessary to characterize the functions of Mφ in the diabetic kidney. For example, although increased COX-2 expression in Mφ is often cited as a characteristic of a proinflammatory, M1 phenotype, other studies indicate that its expression may actually mitigate against detrimental effects in DN [73]. It has been found that administration of a heme oxygenase suppressed M1 and restored M2 in association with decreases in proinflammatory cytokine/chemokine, reduction of extracellular matrix/profibrotic protein and improvement of kidney function and histology [73]. These restoring macrophages are characterized by increased COX-2 expression, and maintenance of this phenotype is dependent upon macrophage-derived cAMP production. These data suggest that therapeutic strategies, which reduce the M1 phenotype and promote the proresolution phenotype in the kidney could have significant potential in the treatment and management DN. The time course of the different phenotypes is not clear.
In general, in the process of kidney fibrosis, first, resident immune cells, including Mφ produce chemoattractant substances that enhance inflammatory responses by recruiting more M1, after that, the change in tissue environment shapes the macrophage phenotype towards M2, with functional properties that meet the tissues need to address the danger. At the later stage after injury, M2 contribute to the removal of fibrous tissue by phagocytosis, mediating the resolution phase of healing that includes collagen remodeling. However, these healing M2 could become profibrotic, but little is known about how this particular population of Mφ terminates the repair response and becomes profibrotic. In experimental models of kidney fibrosis, comparing different M2 therapies, it has been found that not all M2 therapies were effective, only therapies using genetically modified Mφ, able to maintain a stable M2 phenotype were effective. The rest of M2 therapies become at some point profibrotic if they do not have a stable phenotype and were not antifibrotic [74]. These genetically modified Mφ overexpressed neutrophil gelatinase associated lipocalin (NGAL) being genetically stable and capable of preserving their anti-inflammatory and antifibrotic phenotype even when placed in a proinflammatory and profibrotic environment.
5. Macrophages Phenotype under the Control of Bioactive Molecules Generated in the Kidney
Apart from the classical process of activation and recruitment of M1 that initiate the inflammatory process, depending on the degree of inflammation and/or the environment, Mφ can become proresolution cells. These proresolution Mφ could become profibrotic and moreover, there are Mφ with mixed phenotypes that will also be part of these populations.
Depending on what Mφ synthesize or express and on the molecules of the environment capable of modifying its secretome, they can change the phenotype and express mixed phenotypes or profibrotic phenotypes.
Among the bioactive molecules capable of changing the phenotype, sphingosine 1-phosphate (S1P) has been described. Sphingolipids are pleiotropic regulators of cellular physiology that modulate diverse pathways of cell death, inflammation and immunity. Phosphorylation of sphingosine by sphingosine kinases (SK1) leads to the formation of S1P. Long term hyperglycemia and oxidative stress could activate SK1 and increase the production of S1P, eliciting expression of proinflammatory adhesion molecules in endothelial cells, vascular smooth muscle cell and glomerular mesangial cell proliferation. Living in the environment of hyperglycemia and oxidative stress, as occurs in diabetes, for a long time, activate SK1 and increase S1P synthesis [75]. Yagobian et al. demonstrates that inhibitors of the formation of S1P reduce tubulointerstitial renal inflammation and fibrosis in diabetic nephropathy [76]. By the contrary, other studies have shown that FTY720, a S1P agonist selectively inhibits lymphocyte migration and alleviates ischemia–reperfusion injury after renal fibrosis by reducing the release of ECM and reducing the progression of glomerulosclerosis [77]. Thus, while the increased expression of S1P under diabetic conditions seems clear, results on its involvement in diabetes progression does not seem so clear and its potential involvement in macrophage phenotype under diabetic conditions remains unexplored.
In inflammation associated to kidney ischemia reperfusion injury, we evidenced that apoptotic cell-derived S1P or exogenously administered S1P analogue FTY720 activates Mφ to support the proliferation and healing of renal epithelium trough the production of NGAL. Both suppression of inflammation and renal regeneration might require S1P receptor 3 (S1P3) signaling and downstream release of NGAL from Mφ [78]. Despite being controversial, S1P may increase the production of proresolution Mφ. It is not known in fibrosis associated to diabetic nephropathy whether this mechanism operates or is overcome or altered by others.
NGAL is also involved in the development of the kidney, inducing the differentiation of kidney progenitors in the metanephric mesenchyme into renal epithelia [79]. NGAL can be produced in multiple organs, including kidney, liver, heart, gut and various populations of immune cells, such as Mφ or dendritic cells [80,81] and may also be one of the key potential modulators of macrophage phenotype.
Systemic delivery of NGAL has been shown to have protective effects in ischemic AKI by inhibition of tubular cell death and induction of antioxidant genes [82]. NGAL plays a crucial role in epithelial proliferation by attenuating activation of PPARγ and in the induction of epithelial marker expression via activating megalin and the downstream activation of PI3K/Akt signaling pathway [83].
In addition, Mφ overexpressing NGAL could induce intrinsic resistance to ischemia, causing protection from kidney ischemia–reperfusion injury [84]. Moreover, IL-10–overexpressing Mφ could protect kidney from ischemic AKI and improve tissue repair through the induction of NGAL [85]. Similar to this study, it has been found that NGAL KO mice had worse renal damage after IR-induced AKI [86]. Furthermore, NGAL therapy is effective in DN [87]. In this study, macrophage NGAL cell therapy increased the anti-inflammatory molecule IL-10 and decreased the proinflammatory molecule TNF-α, indicating that NGAL overexpressing macrophage therapy could reduce inflammation in DN. This anti-inflammatory effect may have both a direct therapeutic effect on DN and at the same time could contribute to M2 phenotype stabilization.
The NLRP3 inflammasome is the best studied among the inflammasomes, multiprotein cytosolic complexes that form part of the NLR family of PRR [88,89]. NLRP3 is involved in chronic kidney disease pathogenesis [90,91]. In tissue from human renal biopsies, increased expression of NLRP3 mRNA was detected in diabetic kidney diseases. In addition, NLRP3 was associated with renal function and inhibition of NLRP3 inflammasome activation prevents renal inflammation and fibrosis at least in part via suppression of oxidative stress in diabetic nephropathy [92].
Activation of NLRP3 inflammasome plays a critical role in macrophage M1 polarization [93,94]. NF-κB activation is a hallmark of M1 and it has been shown that it promotes renal injury in crescentic glomerulonephritis [95]. Additionally, if NF-κB activation is inhibited in Mφ, they acquire an anti-inflammatory phenotype and suppress renal injury when transferred it in a rat model of nephrotoxic nephritis [96]. Conversely, NLRP3 is also involved in M2 polarization since NLRP3 transactivate IL-4 promoter to increase IL-4 expression (characteristic of M2 phenotype) in peripheral blood monocyte-derived Mφ [97]. In addition, it has been found in UUO mice that NLRP3 inflammasome activates NF-κB and IL-4/STAT6 signaling pathways and promotes M1 and M2 macrophage infiltration and renal injury [98]. Hence, indicating that targeting NLRP3 inflammasome in diabetes could act on Mφ in diabetic nephropathy.
6. Conclusions
Although the role of the different Mφ phenotypes in fibrosis and the effectivity of Mφ therapies with a stable M2 phenotype (genetically modified) were studied, it remains unclear how a M2 phenotype could become profibrotic and what is the influence of the environment.
Factors that modulate S1P, NGAL, or NLPR3 could be effective in the context of diabetic nephropathy, because of its ability to modify Mφ phenotype or the ratio between proresolution and inflammatory Mφ.
Acknowledgments
This study was supported by grants from Ministerio de Economiía y Competitividad, reference SAF2015–67770-R, awarded to G.H.
Author Contributions
G.H. researched the data, contributed to the conclusion and wrote the manuscript. P.C. researched the data and wrote the manuscript. G.H. is the guarantor of this work and takes the responsibility for the integrity of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Collins A.J., Foley R.N., Chavers B., Gilbertson D., Herzog C., Johansen K., Kasiske B., Kutner N., Liu J., St. Peter W., et al. United States renal data system 2011 annual data report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am. J. Kidney Dis. 2012;59:e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen D., Ping F., Mu W., Hill P., Atkins R.C., Chadban S.J. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology. 2006;11:226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 3.Klessens C.Q.F., Zandbergen M., Wolterbeek R., Bruijn J.A., Rabelink T.J., Bajema I.M., IJpelaar D.H.T. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant. 2017;32:1322–1329. doi: 10.1093/ndt/gfw260. [DOI] [PubMed] [Google Scholar]
- 4.Furuta T., Saito T., Ootaka T., Soma J., Obara K., Abe K., Yoshinaga K. The role of macrophages in diabetic glomerulosclerosis. Am. J. Kidney Dis. 1993;21:480–485. doi: 10.1016/S0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- 5.Hirata K., Shikata K., Matsuda M., Akiyama K., Sugimoto H., Kushiro M., Makino H. Increased expression of selectins in kidneys of patients with diabetic nephropathy. Diabetologia. 1998;41:185–192. doi: 10.1007/s001250050888. [DOI] [PubMed] [Google Scholar]
- 6.Bohle A., Wehrmann M., Bogenschütz O., Batz C., Müller C.A., Müller G.A. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol. Res. Pract. 1991;187:251–259. doi: 10.1016/S0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T.Q., Tarnow L., Andersen S., Hovind P., Parving H.-H., Goldschmeding R., van Nieuwenhoven F.A. Urinary Connective Tissue Growth Factor Excretion Correlates with Clinical Markers of Renal Disease in a Large Population of Type 1 Diabetic Patients with Diabetic Nephropathy. Diabetes Care. 2006;29:83–88. doi: 10.2337/diacare.29.01.06.dc05-1670. [DOI] [PubMed] [Google Scholar]
- 8.Yonemoto S., Machiguchi T., Nomura K., Minakata T., Nanno M., Yoshida H. Correlations of tissue macrophages and cytoskeletal protein expression with renal fibrosis in patients with diabetes mellitus. Clin. Exp. Nephrol. 2006;10:186–192. doi: 10.1007/s10157-006-0426-7. [DOI] [PubMed] [Google Scholar]
- 9.Cummings B.S., McHowat J., Schnellmann R.G. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J. Pharmacol. Exp. Ther. 2004;308:921–928. doi: 10.1124/jpet.103.060541. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury P., Sacks S.H., Sheerin N.S. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin. Exp. Immunol. 2006;145:346–356. doi: 10.1111/j.1365-2249.2006.03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad A.S., Kinsey G.R., Khutsishvili K., Gao T., Bolton W.K., Okusa M.D. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am. J. Physiol. Ren. Physiol. 2011;301:F1358–F1366. doi: 10.1152/ajprenal.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesch G.H. Role of macrophages in complications of type 2 diabetes. Clin. Exp. Pharmacol. Physiol. 2007;34:1016–1019. doi: 10.1111/j.1440-1681.2007.04729.x. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa G., Nakano K., Sawada M., Uno K., Shibayama Y., Ienaga K., Kondo M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991;40:1007–1012. doi: 10.1038/ki.1991.308. [DOI] [PubMed] [Google Scholar]
- 14.Kalantarinia K., Awad A.S., Siragy H.M. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64:1208–1213. doi: 10.1046/j.1523-1755.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 15.Awad A.S., You H., Gao T., Cooper T.K., Nedospasov S.A., Vacher J., Wilkinson P.F., Farrell F.X., Brian Reeves W. Macrophage-derived Tumor Necrosis Factor-α mediates diabetic renal injury. Kidney Int. 2015;88:722–733. doi: 10.1038/ki.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro-González J.F., Mora-Fernández C., Muros De Fuentes M., Chahin J., Méndez M.L., Gallego E., Macía M., Del Castillo N., Rivero A., Getino M.A., et al. Effect of Pentoxifylline on Renal Function and Urinary Albumin Excretion in Patients with Diabetic Kidney Disease: The PREDIAN Trial. J. Am. Soc. Nephrol. 2015;26:220–229. doi: 10.1681/ASN.2014010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gohda T., Niewczas M.A., Ficociello L.H., Walker W.H., Skupien J., Rosetti F., Cullere X., Johnson A.C., Crabtree G., Smiles A.M., et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J. Am. Soc. Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niewczas M.A., Gohda T., Skupien J., Smiles A.M., Walker W.H., Rosetti F., Cullere X., Eckfeldt J.H., Doria A., Mayadas T.N., et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng L.-F., Xiao Y., Sun L. A Glimpse of the Mechanisms Related to Renal Fibrosis in Diabetic Nephropathy. Adv. Exp. Med. Biol. 2019;1165:49–79. doi: 10.1007/978-981-13-8871-2_4. [DOI] [PubMed] [Google Scholar]
- 20.Brosius F.C. New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev. Endocr. Metab. Disord. 2008;9:245–254. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usui H.K., Shikata K., Sasaki M., Okada S., Matsuda M., Shikata Y., Ogawa D., Kido Y., Nagase R., Yozai K., et al. Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes. 2007;56:363–372. doi: 10.2337/db06-0359. [DOI] [PubMed] [Google Scholar]
- 22.Distler J.H.W., Györfi A.H., Ramanujam M., Whitfield M.L., Königshoff M., Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019;15:705–730. doi: 10.1038/s41584-019-0322-7. [DOI] [PubMed] [Google Scholar]
- 23.Djudjaj S., Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019;65:16–36. doi: 10.1016/j.mam.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Ginhoux F., Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Viehmann S.F., Böhner A.M.C., Kurts C., Brähler S. The multifaceted role of the renal mononuclear phagocyte system. Cell. Immunol. 2018;330:97–104. doi: 10.1016/j.cellimm.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Cao Q., Harris D.C.H., Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology. 2015;30:183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 27.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wynn T.A., Vannella K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puranik A.S., Leaf I.A., Jensen M.A., Hedayat A.F., Saad A., Kim K.W., Saadalla A.M., Woollard J.R., Kashyap S., Textor S.C., et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci. Rep. 2018;8:13948. doi: 10.1038/s41598-018-31887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sogawa Y., Nagasu H., Iwase S., Ihoriya C., Itano S., Uchida A., Kidokoro K., Taniguchi S., Takahashi M., Satoh M., et al. Infiltration of M1, but not M2, macrophages is impaired after unilateral ureter obstruction in Nrf2-deficient mice. Sci. Rep. 2017;7:8801. doi: 10.1038/s41598-017-08054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y., Wang Y., Ma P., An D., Zhao J., Liang S., Qin H. Myeloid-specific targeting of Notch ameliorates murine renal fibrosis via reduced infiltration and activation of bone marrow-derived macrophage. Protein Cell. 2019;10:196–210. doi: 10.1007/s13238-018-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X., Yang Y., Zhao Y. Macrophage Phenotype and Its Relationship with Renal Function in Human Diabetic Nephropathy. PLoS ONE. 2019;14:e0221991. doi: 10.1371/journal.pone.0221991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C., Zhang Y., Zhang C., Liu Y., Liu Y., Xu G. Pioglitazone increases VEGFR3 expression and promotes activation of M2 macrophages via the peroxisome proliferator-activated receptor γ. Mol. Med. Rep. 2019;19:2740–2748. doi: 10.3892/mmr.2019.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa S., Nakano T., Torisu K., Tsuchimoto A., Eriguchi M., Haruyama N., Masutani K., Kazuhiko Tsuruya K., Kitazono T. Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction. Lab. Investig. 2017;97:1439–1452. doi: 10.1038/labinvest.2017.77. [DOI] [PubMed] [Google Scholar]
- 35.Garibaldi S., Barisione C., Marengo B., Ameri P., Brunelli C., Balbi M., Ghigliotti G. Advanced oxidation protein products-modified albumin induces differentiation of RAW264.7 macrophages into dendritic-like cells which is modulated by cell surface thiols. Toxins. 2017;9:27. doi: 10.3390/toxins9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falke L.L., Gholizadeh S., Goldschmeding R., Kok R.J., Nguyen T.Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 2015;11:233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 37.Lebleu V.S., Taduri G., O’Connell J., Teng Y., Cooke V.G., Woda C., Sugimoto H., Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eddy A.A. The origin of scar-forming kidney myofibroblasts. Nat. Med. 2013;19:964–966. doi: 10.1038/nm.3299. [DOI] [PubMed] [Google Scholar]
- 39.Ming-Kuen Tang P., Zhou S., Li C.-J., Liao J., Xiao J., Wang Q.-M., Lian G.-Y., Li J., Huang X.-R., To K.-F., et al. The proto-oncogene tyrosine protein kinase Src is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int. 2018 doi: 10.1016/j.kint.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y.Y., Jiang H., Pan J., Huang X.R., Wang Y.C., Huang H.F., To K.F., Nikolic-Paterson D.J., Lan H.Y., Chen J.H. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J. Am. Soc. Nephrol. 2017;28:2053–2067. doi: 10.1681/ASN.2016050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng X.M., Wang S., Huang X.R., Yang C., Xiao J., Zhang Y., To K.F., Nikolic-Paterson D.J., Lan H.Y. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016;7:e2495. doi: 10.1038/cddis.2016.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y., Feng X., Liu X., Wang Y., Hu M., Cao Q., Zhang Z., Zhao L., Zhang J., Guo R., et al. Fate alteration of bone marrow-derived macrophages ameliorates kidney fibrosis in murine model of unilateral ureteral obstruction. Nephrol. Dial. Transplant. 2019;34:1657–1668. doi: 10.1093/ndt/gfy381. [DOI] [PubMed] [Google Scholar]
- 43.Chow F., Ozols E., Nikolic-Paterson D.J., Atkins R.C., Tesch G.H. Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 44.Okada S., Shikata K., Matsuda M., Ogawa D., Usui H., Kido Y., Nagase R., Wada J., Shikata Y., Makino H. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52:2586–2593. doi: 10.2337/diabetes.52.10.2586. [DOI] [PubMed] [Google Scholar]
- 45.You H., Gao T., Cooper T.K., Brian Reeves W., Awad A.S. Macrophages directly mediate diabetic renal injury. Am. J. Physiol. Ren. Physiol. 2013;305:F1719–F1727. doi: 10.1152/ajprenal.00141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chow F.Y., Nikolic-Paterson D.J., Ozols E., Atkins R.C., Tesch G.H. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J. Am. Soc. Nephrol. 2005;16:1711–1722. doi: 10.1681/ASN.2004070612. [DOI] [PubMed] [Google Scholar]
- 47.Chow F.Y., Nikolic-Paterson D.J., Ma F.Y., Ozols E., Rollins B.J., Tesch G.H. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 48.Haller H., Bertram A., Nadrowitz F., Menne J. Monocyte chemoattractant protein-1 and the kidney. Curr. Opin. Nephrol. Hypertens. 2016;25:42–49. doi: 10.1097/MNH.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 49.Chow F.Y., Nikolic-Paterson D.J., Ozols E., Atkins R.C., Rollin B.J., Tesch G.H. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 50.Tashiro K., Koyanagi I., Saitoh A., Shimizu A., Shike T., Ishiguro C., Koizumi M., Funabiki K., Horikoshi S., Shirato I., et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsukawa A., Hogaboam C.M., Lukacs N.W., Lincoln P.M., Strieter R.M., Kunkel S.L. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: Cross-talk between MCP-1 and leukot-riene B4. J. Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- 52.Menzies R.I., Booth J.W.R., Mullins J.J., Bailey M.A., Tam F.W.K., Norman J.T., Unwin R.J. Hyperglycemia-induced renal P2X7 receptor activation enhances diabetes-related Injury. EBioMedicine. 2017;19:73–83. doi: 10.1016/j.ebiom.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menne J., Eulberg D., Beyer D., Baumann M., Saudek F., Valkusz Z., Rcek A.W., Haller H. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol. Dial. Transplant. 2016;32:307–315. doi: 10.1093/ndt/gfv459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sullivan T., Miao Z., Dairaghi D.J., Krasinski A., Wang Y., Zhao B.N., Baumgart T., Ertl L.S., Pennell A., Seitz L., et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am. J. Physiol. Ren. Physiol. 2013;305:F1288–F1297. doi: 10.1152/ajprenal.00316.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minton K. Macrophages: A transcription factor to call their own. Nat. Rev. Immunol. 2011;11:74. doi: 10.1038/nri2934. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y., Ling Y., Wang X. Alantolactone mitigates renal injury induced by diabetes via inhibition of high glucose-mediated inflammatory response and macrophage infiltration. Immunopharmacol. Immunotoxicol. 2020;42:84–92. doi: 10.1080/08923973.2020.1725039. [DOI] [PubMed] [Google Scholar]
- 57.Yang S., Ma C., Wu H., Zhang H., Yuan F., Yang G., Yang Q., Jia L., Liang Z., Kang L. Tectorigenin attenuates diabetic nephropathy by improving vascular endothelium dysfunction through activating AdipoR1/2 pathway. Pharmacol. Res. 2020;153:104678. doi: 10.1016/j.phrs.2020.104678. [DOI] [PubMed] [Google Scholar]
- 58.Sun H., Tian J., Xian W., Xie T., Yang X. Pentraxin-3 attenuates renal damage in diabetic nephropathy by promoting M2 macrophage differentiation. Inflammation. 2015;38:1739–1747. doi: 10.1007/s10753-015-0151-z. [DOI] [PubMed] [Google Scholar]
- 59.Cucak H., Nielsen Fink L., Højgaard Pedersen M., Rosendahl A. Enalapril treatment increases T cell number and promotes polarization towards M1-like macrophages locally in diabetic nephropathy. Int. Immunopharmacol. 2015;25:30–42. doi: 10.1016/j.intimp.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi K., Kim R.H., Pasic L., He L., Nagasaka S., Katagiri D., May T., Shimizu A., Harris R.C., Mernaugh R.L. Agonistic anti-CD148 monoclonal antibody attenuates diabetic nephropathy in mice. Am. J. Physiol. Ren. Physiol. 2020;318:F647–F659. doi: 10.1152/ajprenal.00288.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma J., Li Y.J., Chen X., Kwan T., Chadban S.J., Wu H. Interleukin 17A promotes diabetic kidney injury. Sci. Rep. 2019;9:2264. doi: 10.1038/s41598-019-38811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C., Sytwu H.K., Lu K.C., Lin Y.F. Role of T cells in type 2 diabetic nephropathy. Exp. Diabetes Res. 2011;2011:514738. doi: 10.1155/2011/514738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Q., Shen W., Qin W., Zheng C., Zhang M., Zeng C., Wang S., Wang J., Zhu X., Liu Z. Treatment of db/db diabetic mice with triptolide: A novel therapy for diabetic nephropathy. Nephrol. Dial. Transplant. 2010;25:3539–3547. doi: 10.1093/ndt/gfq245. [DOI] [PubMed] [Google Scholar]
- 64.Mohamed R., Jayakumar C., Chen F., Fulton D., Stepp D., Gansevoort R.T., Ramesh G. Low-Dose IL-17 Therapy Prevents and Reverses Diabetic Nephropathy, Metabolic Syndrome, and Associated Organ Fibrosis. J. Am. Soc. Nephrol. 2016;27:745–765. doi: 10.1681/ASN.2014111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S.M., Lee S.H., Lee A., Kim D.J., Kim Y.G., Kim S.Y., Jeong K.H., Lee T.W., Ihm C.G., Lim S.J., et al. Targeting T helper 17 by mycophenolate mofetil attenuates diabetic nephropathy progression. Transl. Res. J. Lab. Clin. Med. 2015;166:375–383. doi: 10.1016/j.trsl.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Eller K., Kirsch A., Wolf A.M., Sopper S., Tagwerker A., Stanzl U., Wolf D., Patsch W., Rosenkranz A.R., Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hofkens W., Storm G., Berg W.V.D., Lent P.V. Inhibition of M1 macrophage activation in favour of M2 differentiation by liposomal targeting of glucocorticoids to the synovial lining during experimental arthritis. Ann. Rheum. Dis. 2011;70:70. doi: 10.1136/ard.2010.148973.11. [DOI] [Google Scholar]
- 68.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 69.Wang X., Yao B., Wang Y., Fan X., Wang S., Niu A., Yang H., Fogo A., Zhang M.Z., Harris R.C. Macrophage Cyclooxygenase-2 Protects Against Development of Diabetic Nephropathy. Diabetes. 2017;66:494–504. doi: 10.2337/db16-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng D., Wang Y., Cao Q., Lee V.W.S., Zheng G., Sun Y., Tan T.K., Wang Y., Alexander S.I., Harris D.C.H. Transfused macrophages ameliorate pancreatic and renal injury in muri ne diabetes mellitus. Nephron Exp. Nephrol. 2011;118:e87–e99. doi: 10.1159/000321034. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X.L., Guo Y.F., Song Z.X., Zhou M. Vitamin D prevents podocyte injury via regulation of macrophage M1/M2 phenotype in diabetic nephropathy rats. Endocrinology. 2014;155:4939–4950. doi: 10.1210/en.2014-1020. [DOI] [PubMed] [Google Scholar]
- 72.Ji L., Chen Y., Wang H., Zhang W., He L., Wu J., Liu Y. Overexpression of sirt6 promotes M2 macrophage transformation, alleviating renal injuryin diabetic nephropathy. Int. J. Oncol. 2019;55:103–115. doi: 10.3892/ijo.2019.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ndisang J.F., Jadhav A. Hemin therapy improves kidney function in male streptozotocin-induced diabetic rats: Role of the heme oxygenase/atrial natriuretic peptide/adiponectin axis. Endocrinology. 2014;155:215–229. doi: 10.1210/en.2013-1050. [DOI] [PubMed] [Google Scholar]
- 74.Guiteras R., Sola A., Flaquer M., Hotter G., Torras J., Grinyó J.M., Cruzado J.M. Macrophage Overexpressing NGAL Ameliorated Kidney Fibrosis in the UUO Mice Model. Cell. Physiol. Biochem. 2017;42:1945–1960. doi: 10.1159/000479835. [DOI] [PubMed] [Google Scholar]
- 75.Wang L., Xing X.P., Holmes A., Wadham C., Gamble J.R., Vadas M.A., Xia P. Activation of the sphingosine kinase-signaling pathway by high glucose mediates the proinflammatory phenotype of endothelial cells. Circ. Res. 2005;97:891–899. doi: 10.1161/01.RES.0000187469.82595.15. [DOI] [PubMed] [Google Scholar]
- 76.Yaghobian D., Don Anthony S., Yaghobian S., Chen X., Pollock C.A., Saad S. Increased sphingosine 1-phosphate mediates inflammation and fibrosis in tubular injury in diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2016;43:56–66. doi: 10.1111/1440-1681.12494. [DOI] [PubMed] [Google Scholar]
- 77.Delbridge M.S., Shrestha B.M., Raftery A.T., El Nahas A.M., Haylor J.L. Reduction of ischemia-reperfusion injury in the rat kidney by FTY720, a synthetic derivative of sphingosine. Transplantation. 2007;84:187–195. doi: 10.1097/01.tp.0000269794.74990.da. [DOI] [PubMed] [Google Scholar]
- 78.Sola A., Weigert A., Jung M., Vinuesa E., Brecht K., Weis N., Brüne B., Borregaard N., Hotter G. Sphingosine-1-phosphate signalling induces the production of Lcn-2 by macrophages to promote kidney regeneration. J. Pathol. 2011;225:597–608. doi: 10.1002/path.2982. [DOI] [PubMed] [Google Scholar]
- 79.Yang J., Goetz D., Li J.Y., Wang W., Mori K., Setlik D., Du T., Erdjument-Bromage H., Tempst P., Strong R., et al. An iron delivery pathway mediated by a lipocalin. Mol. Cell. 2002;10:1045–1056. doi: 10.1016/S1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 80.Thorsvik S., Bakke I., van Beelen Granlund A., Røyset E.S., Damås J.K., Østvik A.E., Sandvik A. KExpression of neutrophil gelatinaseassociated lipocalin (NGAL) in the gut in Crohn’s disease. Cell. Tissue Res. 2018;374:339–348. doi: 10.1007/s00441-018-2860-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakraborty S., Kaur S., Guha S., Batra S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mori K., Lee H.T., Rapoport D., Drexler I.R., Foster K., Yang J., Schmidt-Ott K.M., Chen X., Jau Y.L., Weiss S., et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemiareperfusion injury. J. Clin. Investig. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jung M., Brüne B., von Knethen A., Guiteras R., Cruzado J.M., Hotter G., Sola A. Lipocalin-2 abrogates epithelial cell cycle arrest by PPARγ inhibition. Lab. Investig. 2018;98:1408–1422. doi: 10.1038/s41374-018-0098-4. [DOI] [PubMed] [Google Scholar]
- 84.Jung M., Brüne B., Hotter G., Sola A. Macrophage-derived Lipocalin-2 contributes to ischemic resistance mechanisms by protecting from renal injury. Sci. Rep. 2016;6:21950. doi: 10.1038/srep21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung M., Sola A., Hughes J., Kluth D.C., Vinuesa E., Viñas J.L., Pérez-Ladaga A., Hotter G. Infusion of IL-10-expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 2012;81:969–982. doi: 10.1038/ki.2011.446. [DOI] [PubMed] [Google Scholar]
- 86.Lee S.A., Noel S., Kurzhagen J.T., Sadasivam M., Pierorazio P.M., Arend L.J., Hamad A.R., Rabb H. CD4+ T Cell-Derived NGAL Modifies the Outcome of Ischemic Acute Kidney Injury. J. Immunol. 2020;204:586–595. doi: 10.4049/jimmunol.1900677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guiteras R., Sola A., Flaquer M., Manonelles A., Hotter G., Cruzado J.M. Exploring macrophage cell therapy on Diabetic Kidney Disease. J. Cell. Mol. Med. 2019;23:841–851. doi: 10.1111/jcmm.13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musso G., De Michieli F., Bongiovanni D., Parente R., Framarin L., Leone N., Berrutti M., Gambino R., Cassader M., Cohney S., et al. New Pharmacologic Agents That Target Inflammation and Fibrosis in Non-Alcoholic Steatohepatitis-related Kidney Disease. Clin. Gastroenterol. Hepatol. 2017;15:972–985. doi: 10.1016/j.cgh.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Ślusarczyk J., Trojan E., Głombik K., Piotrowska A., Budziszewska B., Kubera M., Popiołek-Barczyk K., Lasoń W., Mika J., Basta-Kaim A. Targeting the NLRP3 inflammasome-related pathways via tianeptine treatment-suppressed microglia polarization to the M1 phenotype in lipopolysaccharide-stimulated cultures. Int. J. Mol. Sci. 2018;19:1965. doi: 10.3390/ijms19071965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vilaysane A., Chun J., Seamone M.E., Wang W., Chin R., Hirota S., Li Y., Clark S.A., Tschopp J., Trpkov K., et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anders H.J., Suarez-Alvarez B., Grigorescu M., Foresto-Neto O., Steiger S., Desai J., Marschner J.A., Honarpisheh M., Shi C., Jordan J., et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 2018;93:656–669. doi: 10.1016/j.kint.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 92.Masood H., Che R., Zhang A. Inflammasomes in the pathophysiology of Kidney Diseases. Kidney Dis. 2015;1:187–193. doi: 10.1159/000438843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Conos S.A., Chen K.W., De Nardo D., Hara H., Whitehead L., Núñez G., Masters S.L., Murphy J.M., Schroder K., Vaux D.L., et al. Active MLKL triggers the NLRP3 inflammasome in a cell-trinsic manner. Proc. Natl. Acad. Sci. USA. 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L., Fan Y., Su H., Wu L., Huang Y., Zhao L., Han B., Shu G., Xiang M., Yang J.M. Chlorogenic acid methyl ester exerts strong anti-inflammatory effects via inhibiting the COX-2/NLRP3/NF-kappaB pathway. Food Funct. 2018;9:6155–6164. doi: 10.1039/C8FO01281D. [DOI] [PubMed] [Google Scholar]
- 95.Tomita N., Morishita R., Lan H.Y., Yamamoto K., Hashizume M., Notake M., Toyosawa K., Fujitani B., Mu W., Nikolic-Paterson D.J., et al. In vivo administration of a nuclear transcription factor-kappaB decoy suppresses experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2000;11:1244–1252. doi: 10.1681/ASN.V1171244. [DOI] [PubMed] [Google Scholar]
- 96.Wilson H.M., Chettibi S., Jobin C., Walbaum D., Rees A.J., Kluth D.C. Inhibition of macrophage nuclear factor-κb leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am. J. Pathol. 2005;167:27–37. doi: 10.1016/S0002-9440(10)62950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y., Gao X., Miao Y., Wang Y., Wang H., Cheng Z., Wang X., Jing X., Jia L., Dai L., et al. NLRP3 regulates macrophage M2 polarization through up-regulation of IL-4 in asthma. Biochem. J. 2018;475:1995–2008. doi: 10.1042/BCJ20180086. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Y., Zhu X., Wang X., Peng Y., Du J., Yin H., Yang H., Ni X., Zhang W. H2S alleviates renal injury and fibrosis in response to unilateral ureteral obstruction by regulating macrophage infiltration via inhibition of NLRP3 signaling. Exp. Cell Res. 2020;387:111779. doi: 10.1016/j.yexcr.2019.111779. [DOI] [PubMed] [Google Scholar]