Abstract
The imbalanced regulation of metabolic homeostasis and energy production is highly associated with inflammation, tumor growth, metastasis and cancer progression. Both glycolysis and oxidative phosphorylation maintain metabolic homeostasis and energy production in cells. Long noncoding RNAs (lncRNAs) are a class of non-protein-coding transcripts longer than 200 nucleotides. Furthermore, lncRNAs can function as either tumor suppressors or oncogenes in cancer. Dysregulated lncRNAs reportedly regulate cancer hallmarks such as tumor growth, metabolism and metastasis. Accordingly, uncovering the interaction between lncRNAs and cellular metabolism has become a necessity when attempting to identify effective therapeutic and preventive strategies in cancer progression. This review summarizes important knowledge of the actions of known lncRNAs-mediated cancer metabolism.
Keywords: lncRNA, glycolysis, mitochondria, cancer, therapeutic target
1. Introduction
Cancer cells can reprogram cellular metabolism to adapt and survive in nutritionally restricted environments [1]. Most cancer cells probably exhibit comprehensive reprogramming of glucose metabolism. Cancer cells prefer glycolysis over oxidative phosphorylation to generate adenosine triphosphate (ATP), regardless of oxygen abundance. Several studies have reported that tumor suppressors and oncogenes, such as p53, hypoxia-inducible factor-1α (HIF-1α) and Myc, can reprogram cellular metabolism in cancer cells [2,3,4]. Hexokinase 2 (HK2) is a well-known key gene for regulating glycolysis [5]. Multiple studies have indicated that HK2 is highly expressed in tumors and acts as a poor prognostic factor [6]. DeWaal and colleagues demonstrated that the inhibition of HK2 repressed glycolysis and induced oxidative phosphorylation in hepatomas [7]. Furthermore, the deletion of HK2 synergized with sorafenib to reduce tumor growth. These findings suggest that glycolysis is positively associated with cancer progression.
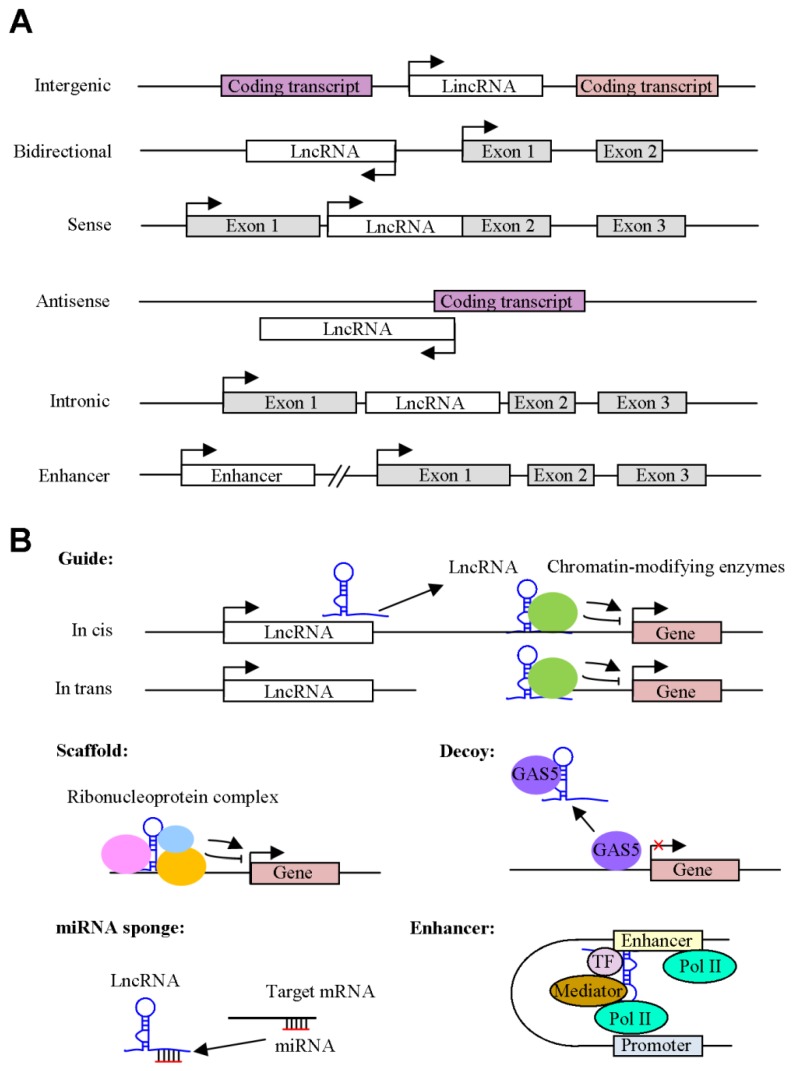
Long noncoding RNAs (lncRNAs) are a class of non-protein-coding transcripts longer than 200 nucleotides that are involved in multiple major biological functions, such as proliferation, apoptosis, metastasis, and cell metabolism [8]. LncRNAs can be transcribed by RNA polymerase II and undergo the splicing of multiple exons. LncRNAs can be categorized into six types depending on the origin of their expression (Figure 1A): (1) lncRNAs that can be transcribed between two coding transcripts (intergenic lncRNAs); (2) lncRNAs that can be transcribed from the promoter of a protein-coding gene but in the opposite direction (bidirectional lncRNAs); (3) lncRNAs that are expressed from the sense or antisense RNA strand of a protein coding gene (sense lncRNA or antisense lncRNA). These transcripts can entirely or partially overlap the exons of coding transcripts. Notably, natural antisense transcripts (NATs), a subset of lncRNAs, are transcribed from coding transcripts, including protein-coding genes and non-coding genes. NATs can be complementary to and overlap with coding transcripts; (4) lncRNAs that are expressed from the intron of a protein coding gene and do not overlap with any exon (intronic lncRNA); and (5) Enhancer RNA (eRNA) is an another class of nonconding RNA, which is transcribed from the enhancer. Moreover, lncRNA can fold and adopt the complex structures, resulting in interacting with DNA, RNA and proteins. A previous study reported that the 5′-end and 3′-end of HOX transcript antisense RNA (HOTAIR) can associate with polycomb repressive complex 2 (PRC2) and LSD1/CoREST/REST (RE1-silencing transcription factor) complex, respectively [9]. The structures of Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1), including hairpin regions, were specifically able to bind hnRNPG and hnRNPC proteins, which modulated gene expressions [10]. Collectively, the complex structures of lncRNA have flexibility, plasticity, and enable themself to regulate cellular functions. Functionally, five general mechanisms have emerged for broadly classifying lncRNA function (Figure 1B): (1) Guides are lncRNA transcripts that regulate target gene expression by binding to regulatory proteins, such as transcription factors and chromatin modifiers, to direct them to precise locations in the genome by cis or trans regulation. HOTAIR can guide the chromatin-modifying enzymes, such as PRC2, to bind to the target genes and regulates their expressions [11]. (2) Scaffolds are lncRNAs that act as the organizer and facilitate association with specific regulatory cofactors. The lncRNA, associated with the ribonucleoprotein (RNP) complex, is often important for RNP’s function. The scaffold lncRNAs, including MALAT1, TUG1 and ANRIL, interact with PRC1 or PRC2 and modulate target gene expression [12]. (3) Decoys are a class of lncRNAs that regulates specific gene expression by sequestering transcriptional regulators away from their binding site. Notably, growth arrest-specific 5 (GAS5) functions as decoy of nuclear protein, including glucocorticoid receptor and p53, and subsequently alter downstream target gene expression [13,14]. (4) The effect of lncRNA on miRNA sponges is that lncRNA regulates cellular function via complementary interacting with miRNA. The overexpression of lncRNA SNHG7 promoted cell proliferation, migration and invasion via sponging miR-216b [15]. (5) The eRNAs serve as trans-activating RNA and are transcribed from enhancer sequences, which cooperate with the promoter to modulate gene transcription. Zhang et al. [16] demonstrated that an eRNA, NET1-associated eRNA (NET1e), was upregulated in breast cancer. The overexpression of NET1e promoted cell proliferation. These observations indicated that NET1e acted as an oncogenic eRNA in breast cancer.
Figure 1.
Classifications and actions of Long noncoding RNAs (lncRNAs) in cancer. (A) A schematic diagram indicating the classification of lncRNAs according to their orientation and position, including intergenic, bidirectional sense, antisense, intronic lncRNAs and enhancer RNAs (eRNAs). The arrow represents the transcription direction. (B) The mechanisms of lncRNAs. Guides are lncRNAs that can recruit specific proteins to target genes, either in cis or in trans. Scaffolds are lncRNAs that can associate with multiple proteins to form ribonucleoprotein complexes. This complex may modulate histone modifications such as methylation. Decoys are lncRNAs that can bind to transcription factors or other proteins and are subsequently removed from a specific location. LncRNAs can function as microRNA (miRNA ) sponges to regulate cellular function. Enhancers act as cis-acting elements and contribute to increase the target genes expression.
Crosstalk between lncRNAs and cellular metabolism has been implicated in cancer progression, and it is important to explore the nature of this connection. Interestingly, the energy stress-related lncRNA NBR2 (neighbor of BRCA1 gene 2) is induced by the liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) axis [17]. The knockdown of lncRNA NBR2 inhibits AMPKα activity, resulting in unchecked cell cycle progression, altered autophagy/apoptosis response and enhanced tumor formation. Our group demonstrated that taurine upregulated gene 1 (TUG1) is highly expressed in hepatocellular carcinoma (HCC) specimens compared to adjacent normal tissues [18]. The knockdown of TUG1 promoted the marked inhibition of cell metastasis and glycolysis through the modulation of microRNA (miR)-455-3p. These findings suggest that lncRNAs are responsible for regulating cellular metabolism. In this review, we focus on how lncRNAs affect cellular metabolism through the modulation of specific pathways in cancers.
2. The Effects of lncRNAs on Glucose Metabolism in Cancer Cells
Glucose metabolism, including glucose uptake, oxidative phosphorylation and lactate production, is one of the energy sources of tumor cells [19]. Cancer cells reprogram glucose metabolism to enhance cell growth, survival, and drug resistance [20]. Under normal conditions, mitochondrial oxidative phosphorylation is the main source of ATP and produces 30 to 32 molecules of ATP. Notably, tumor cells tend to have increased glycolysis, which only produces two molecules of ATP, even under sufficient oxygen conditions. An important feature of this phenotype, the Warburg effect, is increased glucose uptake and fermentation of glucose to lactate [21]. ATP is formed faster than oxidative phosphorylation. Here, we summarize how lncRNAs modulate glucose metabolism via the regulation of glucose transporters (GLUTs) and glycolytic genes and how the lncRNA/GLUT or lncRNA/glycolytic gene axis controls tumorigenesis.
2.1. Glucose Transporter and lncRNA
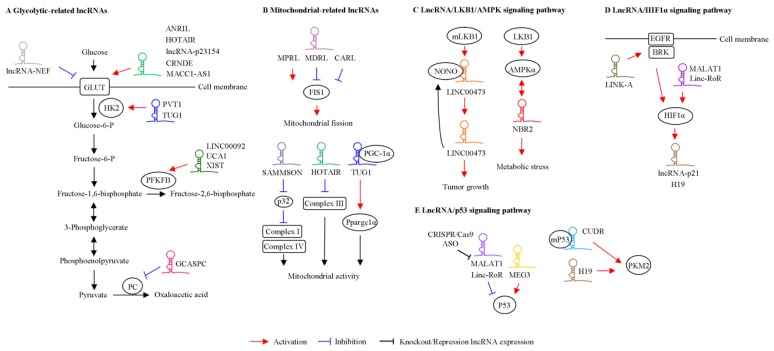
GLUTs play an important role in regulating glucose metabolism in tumor cells [22]. Several lncRNAs regulate the glycolytic pathway through the interaction of GLUTs (Figure 2A and Table 1). A novel large antisense noncoding RNA (ANRIL) is highly expressed in nasopharyngeal carcinoma (NPC) [23]. ANRIL promotes GLUT1 and lactate dehydrogenase A (LDHA) expression, resulting in the upregulation of glucose uptake and the promotion of cancer progression via the AKT/mTOR pathway. In HCC, HOTAIR promotes glycolysis by inducing GLUT1 expression and activating the mTOR signaling pathway [24]. Wang et al. demonstrated that lncRNA-p23154 promotes cell metastasis and glycolysis through the modulation of GLUT1 in oral squamous cell carcinoma [25]. In addition, the depletion of lncRNA NBR2 attenuates phenformin-induced glucose uptake and GLUT1 expression [17]. LncRNA-NEF is downregulated in patients with non-small-cell lung cancer [26]. The ectopic expression of lncRNA-NEF suppresses cell growth and glucose uptake via the inhibition of GLUT1 expression. Colorectal neoplasia differentially expressed (CRNDE) is upregulated in colorectal cancer and enhances GLUT4 expression and glucose uptake [27]. These findings indicate that the expression of GLUTs is regulated by lncRNAs and subsequently results in alterations in glycolysis. In addition to modulating the expression of GLUT1, lncRNAs also regulate the distribution of GLUT1 in tumor cells. LncRNA MACC1-AS1 is highly expressed under metabolic stress in gastric cancer cells [28,29]. MACC1-AS1 promotes the expression of GLUT1 surrounding the cell membrane. This finding suggests that MACC1-AS1 promotes glucose uptake and glycolysis by increasing the distribution of GLUT1 in the vicinity of the cell membrane. Collectively, lncRNA regulates glucose metabolism through the modulation of GLUTs expression in tumor cells. Moreover, understanding lncRNA-mediated regulation of GLUT protein subcellular localization is important not only in uncovering the function of individual proteins but also in developing therapeutic agents.
Figure 2.
Functional roles of lncRNA in tumor metabolism. LncRNAs regulate target gene-mediated glucose metabolism (A) and mitochondrial function (B) in cancer. The role of lncRNA-mediated liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) pathways (C), hypoxia-inducible factor 1α (HIF1α) (D), and p53 (E) in tumor cells was shown.
Table 1.
Metabolic-regulated lncRNAs and their potential mechanisms in cancers.
| Gene Name | Principal Functions | Molecules and Signaling Pathways Involveda | Cancer Development | Prognostic Markers in Cancerb | Up- or Downregulationc | Cancer/Cell Types | Reference |
|---|---|---|---|---|---|---|---|
| ANRIL | Glucose uptake | GLUT1, LDHA, AKT/mTOR | Progression | ✓ | Up | NPC | [23] |
| HOTAIR | Glycolysis Mitochondrial function Apoptosis |
GLUT1, mTOR, vimentin, MICU1 | Progression | ✓ | Up | HCC, HeLa cell, Head and neck squamous cell carcinoma | [24,53,54] |
| LncRNA-p23154 | Glycolysis Metastasis |
GLUT1 | Progression | ✓ | Up | Oral squamous cell carcinoma | [25] |
| LncRNA NBR2 | Glucose uptake Tumor growth Apoptosis |
GLUT1, AMPK activity, mTORC1 | Regression | ✓ | Down | 786-O, MDA-MB-231 | [17] |
| LncRNA-NEF | Cell growth Glycolysis |
GLUT1 | Regression | ✓ | Down | Non-small-cell lung cancer | [26] |
| CRNDE | Glucose uptake Warburg effect |
GLUT4, insulin/IGF axis | Progression | ✓ | Up | Colorectal cancer | [27] |
| MACC1-AS1 | Glycolysis Cell viability Stemness |
AMPK/Lin28, TGFβ1, miR-145-5p | Progression | ✓ | Up | Gastric cancer | [28,29] |
| TUG1 | Tumor formation Glycolysis Metastasis OXPHOS |
HK2, miR-455-3p, AMPKβ2, PGC-1α | Progression | ✓ | Up | HCC, Immortalized mouse podocytes | [18,51] |
| PVT1 | Glycolysis Cell growth Cell cycle Invasion |
miR-497, HK2 | Progression | ✓ | Up | Osteosarcoma | [35] |
| H19 | Warburg effect Drug resistance Glutathione metabolism |
miR-675, PKM2, EGR pathway NRF2, miR-657 |
Dual role | ✓ | Dual role | Liver cancer, diabetic mouse model | [68,82] |
| GCASPC | Cell growth Tumor formation |
miR-17-3p, PC | Regression | ✓ | Down | Gallbladder cancer | [39] |
| LINC00092 | Glycolysis Migration |
PFKFB2, CXCL14 | Progression | ✓ | Up | Ovarian cancer | [40] |
| LncRNA XIST | - | PFKFB2, miR-212-3p, miR-122-5p, AMPK | - | - | - | Acute kidney injury | [42] |
| MPRL | Mitochondrial fission Apoptosis |
E2F1, miR-483-5p, FIS1 | Regression | ✓ | Up (in chemosensitive patient) | Tongue squamous cell carcinoma | [46] |
| MDRL | Mitochondrial fission Drug resistance Apoptosis |
miR-484, miR-361 | - | - | - | Mouse cardiomyocyte | [47] |
| CARL | Mitochondrial fission Apoptosis |
miR-539, PHB2 | - | - | - | Mouse cardiomyocyte | [48] |
| UCA1 | Mitochondrial function Colony formation Tumor growth |
miR-195/ARL2 | Progression | ✓ | Up | Bladder cancer | [55] |
| SAMMSON | Mitochondrial homeostasis Colony formation |
P32, MAPK, complex I/IV | Progression | ✓ | Up | Melanoma | [56] |
| RMRP | Oxygen consumption Mitochondrial DNA replication |
HuR, GRSF1 | - | - | - | Hela, HEK293 cells | [59] |
| ASncmtRNA | Tumor growth Cell death |
Cyclin B1, cyclin D1, CDK1, CDK4, survivin | Progression | - | - | Breast cancer | [62] |
| NEAT1 | Oxidative stress | miR-204, NFκB, miR-181d-5p/CDKN3 axis | Progression | ✓ | Up | Rat mesangial cells, endothelial cells | [66,70] |
| MALAT1 | Oxidative stress Antioxidant Lipid peroxidation Apoptosis |
KEAP1, NRF2, p38/MAPK | Progression | ✓ | Up | HUVEC, lens epithelial cells | [69,70] |
| LncRNA-p21 | Hypoxia Glycolysis Warburg effect Tumor formation |
HIF-1α, VHL | Progression | ✓ | Up | HeLa, MCF7, H1299, IMR90 | [80] |
| LINK-A | Metabolic reprogramming | BRK, EGFR, GPNMB | Progression | ✓ | Up | Triple-negative breast cancer | [83] |
| Linc-RoR | Hypoxia | RPS6KB1, PDK1, HIF-1α, miR-145, p53 | Progression | ✓ | Up | Liver cancer | [84,91] |
| LINC00473 | Tumor growth | LKB1, CRTC1, CREB, NONO | Progression | ✓ | Up | Lung cancer | [75] |
| lncRNA CUDR | Tumor growth | PIM1, PKM2, p53 | Progression | Up | Liver cancer | [86] |
a: Downstream molecules and signaling pathways involved in lncRNA-mediated functions. b: ✓: Target gene acts as a prognostic marker in cancer. -: Information is unavailable. c: Up: LncRNA is upregulated in cancer compared with adjacent normal tissues. Down: LncRNA is downregulated in cancer compared with adjacent normal tissues.
2.2. Glycolytic Enzyme and lncRNA
In addition to modulating GLUT expression or localization, several glycolytic genes are regulated by lncRNAs (Figure 2A and Table 1). HK can catalyze the first and irreversible step of glycolysis [30]. Five HK isoforms encoded by separate genes in mammalian cells have been identified [31]. HK1 is ubiquitously expressed in adult tissues, while HK2 is a more regulated form expressed in few adult tissues, including skeletal and cardiac muscle and adipose tissues [32], but it is highly expressed in cancer cells. In fact, HK2 acts as a key regulator of the Warburg effect in tumor cells. Lin et al. demonstrated that HK2 expression is regulated by the TUG1/miR-455-3p/AMPKβ2 axis and is involved in TUG1-mediated function in HCC [18]. As indicated by a previous study, TUG1 is associated with HK2-mediated glycolysis in osteosarcoma [33]. LncRNA PVT1 plays an oncogenic role in multiple cancers [34]. The overexpression of PVT1 enhances glucose metabolism via modulation of the miR-497/HK2 axis in osteosarcoma [35]. Pyruvate kinase M2 (PKM2) is a metabolic enzyme that plays a crucial role in cellular metabolism and tumor growth [36]. Li et al. demonstrated that miR-675 promotes lncRNA H19 expression through the activation of the early growth response protein 1 (EGR) pathway in hepatoma cells [37]. Moreover, H19 induces PKM2 expression, which is involved in the Warburg effect during cancer progression. Pyruvate carboxylase (PC) is involved in modulating cellular metabolism, including glucose metabolism, gluconeogenesis and de novo fatty acid synthesis [38]. LncRNA gallbladder cancer–associated suppressor of pyruvate carboxylase (GCASPC) is downregulated in gallbladder cancer specimens and correlated with cancer progression [39]. Furthermore, GCASPC can interact with PC and then inhibit its protein expression via the modulation of protein stability. The expression levels of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 (PFKFB2), which is involved in glucose metabolism, are regulated by lncRNAs, including LINC00092, UCA1, and X inactive-specific transcript (XIST) [40,41,42]. For example, a high level of CXCL14 in cancer-associated fibroblasts (CAFs) mediates the upregulation of LINC00092 in ovarian cancer cells [40]. Mechanistically, LINC00092 is associated with PFKFB2, thereby inducing cell metastasis by modulating glycolysis in cancer-associated fibroblasts (CAFs). These findings suggest that crosstalk between tumor cells and stromal cells mediated by LINC00092 is important in cell metastasis and glycolysis. Recently, lncRNA XIST was found to act as a competing endogenous RNA (ceRNA) that regulates PFKFB2 expression by sponging miR-212-3p and miR-122-5p [42]. Accordingly, lncRNA-mediated glucose metabolism may occur through three different mechanisms: (1) alteration of the expression levels of GLUT/glycolytic enzymes, (2) alteration of the distribution of GLUTs, or (3) interactions with glycolytic genes and the modulation of their activity. These observations suggest that lncRNAs act as upstream regulators of glucose metabolism and that they may be used for novel therapeutic target development to prevent cancer progression.
3. The Effects of lncRNAs on Mitochondrial Function in Cancer Cells
Mitochondria contain a membrane bilayer that consists of an inner and an outer mitochondrial membrane. Mitochondria are responsible for producing the majority of cellular energy (ATP) through the process of oxidative phosphorylation (OXPHOS), Krebs cycle and the synthesis of biosynthetic precursors, such as nucleotides, amino acids, lipids, heme and nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) [43,44]. Accordingly, mitochondrial quality control is pivotal for cancer maintenance and progression. Mitochondria are highly dynamic organelles accompanied by fusion and fission, which modulate mitochondrial shape and function. Thirteen structural subunits of complexes I, III, IV, and V, 2 rRNAs, and 22 tRNAs are encoded by the mitochondrial genome [45]. The other components of OXPHOS are encoded by the nuclear genome. Increasing evidence indicates that lncRNAs encoded by nuclear DNA and mitochondrial DNA are correlated with mitochondrial functions. These associations are described below and shown in Figure 2B and Table 1.
3.1. Nuclear DNA-Encoded lncRNAs and Mitochondrial Function
MiRNA processing-related lncRNA (MPRL) is highly expressed in tongue squamous cell carcinoma cell lines after cisplatin treatment [46]. MPRL alters mitochondrial fission and cisplatin sensitivity via the interaction of pre-miR-483. This association represses the expression of the mature form of miR-483-5p and then upregulates the miR-483-5p target gene mitochondrial fission 1 (FIS1), which is responsible for regulating mitochondrial fission. Mitochondrial dynamic related lncRNA (MDRL) reduces miR-361 expression and indirectly induces the expression of miR-484, a negative regulator of FIS1 protein expression [47]. Mechanistically, miR-361 associates with pri-miR-484 and suppresses its maturation via Drosha in the nucleus, resulting in increased FIS1 expression and cell apoptosis. Thus, mitochondrial fission and apoptosis are indirectly regulated by MDRL. Another study indicated that lncRNA, named cardiac apoptosis-related lncRNA (CARL), repressed mitochondrial fission and apoptosis by targeting miR-539 and PHB2 [48]. Thus, lncRNA can impact mitochondria quality control involved in cancer progression through the modulation of miRNA expressions.
Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) is an important transcriptional coactivator that modulates mitochondrial biogenesis and mtDNA replication [49,50]. Ppargc1α gene is upregulated by TUG1 in podocytes [51]. Mechanistically, TUG1 directly interacts with PGC-1α protein and contributes to induce the binding of PGC-1α to its own promoter. Furthermore, the TUG1 binding region upstream of the Ppargc1α gene was identified by genome-wide chromatin isolation by RNA purification sequencing. This finding indicates the important regulatory mechanism of lncRNA and mitochondria in a murine model. Additionally, several lncRNAs have been reported to control OXPHOS activity through the modulation of the OXPHOS complex subunit. HOTAIR, involved in OXPHOS activity, is highly expressed and an unfavorable prognosis in a variety of cancers [52]. The knockdown of HOTAIR leads to altered mitochondrial morphology in HeLa cells, characterized by mitochondrial swelling and loss of cristae. Additionally, HOTAIR can reduce OXPHOS complex III subunit VII protein expression, which functions as a regulator of OXPHOS [53]. Kong and colleagues demonstrated that the depletion of HOTAIR promoted the apoptosis of head and neck squamous cell carcinoma through the induction of mitochondrial calcium uptake 1 (MICU1) [54]. Mitochondrial membrane potential was altered by HOTAIR blockage. UCA1 enhanced mitochondrial function through the upregulation of ARL2, which plays an important role in mitochondrial activity [55]. Survival associated mitochondrial melanoma specific oncogenic non-coding RNA (SAMMSON) is an intergenic lncRNA that is particularly expressed in melanoma cells [56]. SAMMSON is expressed in the cytoplasm and mitochondria. SAMMSON can bind to complement C1q-binding protein p32, which is required for mitochondrial pre-rRNA processing [57]. Functionally, SAMMSON silencing reduced p32 expression, leading to a decrease in OXPHOS complex I and IV activity and mitochondrial membrane depolarization. The knockdown of p32 leads to a fragmented mitochondrial network and shifts tumor cell metabolism from OXPHOS to glycolysis [58]. In line with activity assays, complex I and complex IV subunit protein levels were reported to be reduced, while complex II subunit levels were unchanged. The A lncRNA RNA component of the RNA processing endoribonuclease (RMRP) can be transcribed by nuclear DNA and associated with RNA binding proteins (HuR and GRSF1) and translocate it to mitochondria [59]. The depletion of GRSF1 reduces the mitochondrial levels of RMRP, resulting in decreased oxygen consumption rates and mitochondrial DNA replication. These investigations provide a new insight into how RNA binding proteins influence the localization of lncRNAs, where they exert their normal function.
3.2. Mitochondrial DNA-Encoded lncRNAs and Mitochondrial Function
Mitochondria are able to take up lncRNAs encoded by nuclear DNA but can also encode lncRNAs with their own DNA. Three lncRNAs, lncND5, lncND6 and lncCytB, transcribed from mtDNA, have been reported by deep-sequencing results [60]. A previous study reported that mitochondrial RNase P protein 1 (MRPP1) and pentatricopeptide repeat domain protein 2 (PTCD2) are involved in the regulation of the expression levels of the mature form of tRNA, rRNA and mitochondrial gene expression [60]. Notably, MRPP1 and PTCD2 can regulate lncND5, lncND6 and lncCytB expression. These observations suggest that important contributions of these nuclear-encoded proteins impact mitochondrial-related lncRNAs. However, the detailed regulatory mechanism should be fully addressed. Chimeric lncRNAs containing mtDNA-encoded genes have been characterized. Sense mitochondrial ncRNA (SncmtRNA) is expressed in proliferating cells, including normal and cancer cells, but not in resting cells [61,62]. SncmtRNA has a characteristic RNAse-resistant double-stranded structure with a 40-nucleotide loop. This association between cell growth and the expression of SncmtRNA-1 suggests a functional role for this transcript in cell cycle progression. Recently, anti-sense SncmtRNA (ASncmtRNA) is downregulated by HPV-16 or 18 in human immortalized keratinocytes. Moreover, the E2 oncogene is involved in this regulation [61]. Fitzpatrick et al. demonstrated that the knockdown of ASncmtRNA induced cell death and inhibited tumor growth via cell cycle-related genes, such as cyclin B1, cyclin D1, CDK1, CDK4, and surviving in breast cancer cell lines [62]. Thus, these findings shed light on the functional role of ASncmtRNA in cancer progression.
4. The Effects of lncRNAs on Oxidative Stress in Cancer Cells
Oxidative stress (OS) is indicated by the homeostatic imbalance of antioxidant and free radicals in cells [63]. In particular, free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) act as contributor to control OS. Indisputably, OS contributes to cell death and physiological dysfunction, which could be ascribed to DNA damage and inflammation. Recent studies have revealed that lncRNAs function as mediators for negatively or positively regulating OS in cancer cells (Table 1). The nuclear factor erythroid 2-related factor 2/Kelch-like ECH-associated protein 1/antioxidant response element (Nrf2/Keap1/ARE) axis is an important executer in response to OS to maintain the balance of the oxidation/antioxidant system [64]. Chen’s group indicated that NEAT1 was highly expressed in sepsis-induced acute kidney injury (AKI) patients. The knockdown of NEAT1 alleviated lipopolysaccharide-induced injury in rat mesangial cells [65]. Additionally, NEAT1 repressed OS-induced vascular endothelial cell injury by activating the miR-181d-5p/CDKN3 pathway [66]. Several lncRNAs have been shown to be associated with cisplatin resistance. Zhang et al. demonstrated that H19 was upregulated in cisplatin-resistant cells compared with parental cells [67]. The knockdown of H19 in cisplatin-resistant cells resulted in the recovery of cisplatin sensitivity in vitro and in vivo. Moreover, NRF2-regulated genes, including NQO1, GSR, G6PD, GCLC, GCLM and GSTP1, which are involved in the glutathione metabolism pathway, were repressed in H19-depleted cells. Thus, the crosstalk of H19 and glutathione metabolism may regulate cancer-drug resistance. In addition, the ectopic expression of H19 suppressed inflammation and OS in a rat model of diabetic cardiomyopathy (DCM). Moreover, H19 expression was suppressed in high-glucose conditions. The depletion of H19 resulted in decreased miR-657 expression. The expression of voltage-dependent anion channel 1 (VDAC1) was upregulated in the inhibition of miR-657 cell lines, which contributed to enhance cell apoptosis [68]. Notably, the overexpression of H19 suppressed VDAC1 expression and repressed apoptosis under a high glucose condition. These findings indicated that the H19/miR-657/VDAC1 axis is responsible for modulating high glucose-induced apoptosis, suggesting that it may establish a novel therapeutic target for DCM. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) plays a negative or positive role under OS. The overexpression of MALAT1 plays a positive role in the antioxidant pathway by inducing hydrogen peroxide (H2O2) in human umbilical vein endothelial cells (HUVECs) [69]. Mechanistically, Keap1 expression levels are repressed by MALAT1, leading to the activation and stabilization of the Nrf2 protein, resulting in the attenuation of OS-mediated damage and lipid peroxidation in H2O2-treated HUVECs. MALAT1 has also been identified as an Nrf2 regulator that associates with Nrf2 [70]. In addition, MALAT1 modulates apoptosis and oxidative stress via the p38MAPK pathway in human lens epithelial cells [71]. These findings suggest that actions on the Nrf2/Keap1/ARE pathway might be an important strategy for the lncRNA-mediated regulation of OS.
5. The Effects of lncRNAs/Signal Transduction Pathways on Cellular Metabolism in Cancer Cells
Numerous studies indicate that cellular metabolism is regulated by lncRNAs and their downstream signaling pathways, including the LKB1/AMPK, HIFα and p53 pathways. This crosstalk is described below and shown in Figure 2C–E.
5.1. LKB1/AMPK Signaling Pathway
AMPK is an energy sensor and is required for glucose homeostasis [72]. Upon the activation of AMPK, it enhances the activation of the TSC2 complex, leading to the inhibition of the mTOR-activated GTP-binding protein Rheb. Under metabolic stress, the AMPK-mediated phosphorylation of TSC2 protects cells from apoptosis. Liver kinase B1 (LKB1), known as a protein kinase, acts as a tumor suppressor that regulates cell proliferation and energy metabolism by regulating the activity of mTOR [73]. LKB1 is an upstream regulator of AMPKα, and therapy induces the phosphorylation of AMPK. The knockdown of LKB1 enhances tumor growth, glucose uptake, ATP production, and macromolecules synthesis. Notably, in LKB1-deficient cancer cell lines, the metabolic reprogramming effect is regulated by HIF-1α, which executes its antagonism by suppressing mTORC1 [74]. LINC00473 is upregulated in human non-small cell lung cancer and is significantly correlated with LKB1 activity [75]. Mechanistically, nuclear LINC00473 associates with non-POU domain-containing octamer-binding protein (NONO), which is involved in the cAMP signaling pathway. Glucose starvation promotes the activation of AMPK or acetyl-CoA carboxylase [76]. LncRNA NBR2 is induced by the LKB1/AMPK signaling pathway under metabolic stress (glucose starvation) [17]. NBR2 is a tumor suppressor that enhances the activity of AMPKα. The depletion of NBR2 significantly attenuates the phosphorylation of AMPK and mTORC1 inactivation, suggesting the regulation of the NBR2/AMPKα feedback loop mechanism. Notably, these lncRNAs were involved in facilitating metabolic plasticity by regulating the LKB1/AMPKα axis.
5.2. Hypoxia
HIF acts as a transcription factor that is expressed by tumor cells adapting to hypoxic environments [77]. The activation of HIF-1α induces the Warburg effect, partly through the upregulation of GLUTs and glycolytic enzymes or the suppression of OXPHOS [78,79]. Under hypoxic conditions, lincRNA-p21 is upregulated by HIF-1α and then enhances HIF-1α protein stability, establishing a positive feedback regulation [80]. Functionally, lincRNA-p21 is involved in hypoxia-mediated glycolysis. In normal liver cell line L02, the glycolytic genes (GLUT4, HK2 and ENO-1), MALAT1 and HIF-α are upregulated by arsenite treatment [81]. Moreover, MALAT1 enhances arsenite-induced glycolysis by inducing the disassociation of HIF-1α from VHL, preventing the VHL-mediated ubiquitination of HIF-1α. In addition, hypoxia induces lncRNA H19 expression, which is involved in hypoxia-induced signal transduction processes in cancer cells, resulting in modulating glucose metabolism [82]. Lin et al. demonstrated that an lncRNA in the cytoplasm, long intergenic noncoding RNA for kinase activation (LINK-A), is responsible for regulating metabolic reprogramming in triple-negative breast cancer [83]. Mechanistically, LINK-A induces the recruitment of BRK to the EGFR-GPNMB complex and subsequently enhances the breast tumor kinase (BRK) activity. Moreover, the phosphorylation of HIF-1α at tyrosine 565 by BRK and then stabilizes HIF-1α via interfering with the hydroxylation of proline 564. Takahashi and colleagues reported that linc-ROR is associated with hypoxic conditions and acts as a molecular sponge of miR-145 to regulate HIF-1α expression [84]. The underlying crosstalk of lncRNA and HIF-1α in cancer progression may lead to clinical applications.
5.3. p53 Signaling Pathway
The loss of p53 in the cell can lead to mitochondrial respiratory damage and increased glycolysis [85]. Glucose transporters, such as GLUT1 and GLUT4, and glycolytic genes, including phosphoglycerate mutase (PGM), TP53-induced glycolysis and apoptosis regulator (TIGAR) and 6-phosphofructokinase 1 (PFK1), are regulated by p53. Several lncRNAs are directly or indirectly regulated by p53. Wu et al. demonstrated that mutant p53 (N340Q/L344R) facilitates the progression of HCC by upregulating PKM2 [86]. LncRNA CUDR is associated with the mutated form of p53. This complex binds to the promoter regions of PKM2 and enhances its gene expression. The overexpression of maternally expressed gene 3 (MEG3) induces p53 expression and the activation of p53 downstream target genes [87,88]. Notably, an antisense transcript of p53 (wrap53) modulates p53 expression through targeting the 5′ UTR of p53 [89]. Tripathi et al. reported that the knockout of MALAT1 in fibroblasts stimulates DNA damage repair and results in the activation of p53 and its downstream target genes [90]. Upon DNA damage, lincRNA ROR acts as a repressor of p53 [91]. Accordingly, the p53-mediated glucose metabolism in cancer progression is manifested in the suppression of glycolysis and the promotion of oxidative phosphorylation and tricarboxylic acid (TCA) cycle.
6. Application of lncRNA for the Treatment of Cancer
Tumor formation is a complicated process. The approaches in cancer treatment can be performed by chemotherapy, targeted therapy and immunotherapy. Therefore, the cancer treatment requires understanding target genes involved in cancer progression for successful intervention. As mentioned above, several lncRNAs expressions are dysregulared in cancers and involved in metabolic reprogramming and cancer progression. Because of the tissue-specific characteristics of lncRNAs, they could be the next generation of biomarkers for human cancer or disease. LncRNAs may be promising targets for treating cancer. Although the therapeutic approach of lncRNAs still remains at the laboratory stage, several types of approaches targeting lncRNAs in cancer treatment are described below.
6.1. CRISPR/Cas9 Genome Editing Technique
Recently, CRISPR/Cas9 has also received extensive attention in the treatment of cancer [92]. One of the limitations for knockout lncRNAs by CRISPR/Cas9 system is that nucleotide insertions/deletions mutation may not lead to a functional loss of lncRNAs, which lack an open reading frame. To solve this challenge, Ho et al. [93] demonstrated that, via the adopted unique selection system, a marker gene could be integrated into the genome via homologous recombination. In fact, lncRNA-21A, UCA1 and AK023948 were successfully knockout in cancer cell lines using CRISPR-Cas9. In addition, Daneshvar and co-workers demonstrated that a sequence encoding GFP followed by a poly (A) signal was inserted to the downstream of the Divergent to GSC, induced by TGF-β family signaling (DIGIT) transcription start site using CRISPR-Cas9 system [94]. The insertion of cassette allowed for the induction of transcription of GFP at the DIGIT locus but led to termination at the poly(A) signal, resulting in disrupting full length DIGIT expression. Several studies have shown that CRISPR/Cas9 can successfully inhibit lncRNA expression by targeting the transcriptional site of a gene promoter to repress transcription in tumor cells and animal models [95]. Another successful strategy for knockout lncRNA is that TATA box of NEAT1 was removed from the original region by CRISPR-Cas9-mediated homologous recombination [96]. An lncRNA named gastric cancer metastasis associated long noncoding RNA (GMAN) is highly expressed in gastric cancer and is associated with poor prognosis [97]. A well-designed animal experiment indicated that targeting GMAN via the CRISPR/Cas9 system significantly repressed the metastasis of gastric cancer cells and improved overall survival in a mouse model. Alternatively, CRISPR activation (CRISPRa) can selectively induce a gene of interest from its original chromosomal locus [98]. This methodology was applied to activate protein-coding and noncoding genes to explore their biological function in cells. This system requires catalytically dead Cas9 (dCas9) protein fused to the VP64 transcriptional activator and a single-guide RNA (sgRNA) targeted to the promoter region of the gene of interest. The activation of TUG1 by CRISPRa partially restores the thyroid hormone-inhibited cell growth effect in hepatoma cell lines [99]. Some lncRNAs are expressed specifically in different tissues and even different people. Thus, personalized treatment depending on the situation of patients can be performed in the future. So far, CRISPR/Cas9 has broad adaptability and target specificity. However, the off-target effects of CRISPR/Cas9 still exist in practical applications. Therefore, oncologists should be more cautious in designing gene-editing therapies. Currently, the clinical application of the CRISPR/Cas9 system in targeting lncRNAs to treat cancer is not well understood. Moreover, developing more specific gene-editing tools is important.
6.2. Antisense Oligonucleotides
Antisense oligonucleotides (ASOs) have been used in clinical applications to target mRNAs involved in cancer progression [100]. Basically, ASOs, which form a DNA–RNA structure with a target, trigger RNase-H-mediated RNA degradation. In addition, ASOs can bind to splicing regulatory sequences and modulate the splicing pathway. Notably, the exact sequences of lncRNAs expressed in tumor cells or tissues remain unclear. The variants of lncRNAs can be identified using the rapid amplification of cDNA ends (RACE) assay. Accordingly, targeting lncRNA by the ASO strategy may be a promising method for cancer therapy. In addition, ASOs have been shown to specifically alter RNA splicing events. These ASOs can bind to splicing regulatory sequences and modulate the splicing pathway. The therapeutic repression of NATs such as ANRIL and p21-AS by treating with a specific ASO can induce the overlapping tumor suppressor genes expression [101,102]. The knockdown of ASncmtRNA by ASO resulted in the dramatic inhibition of cell proliferation and tumor growth in vitro and in a xenograft model [62]. Gone et al. demonstrated that specific ASO-MALAT1 treatment reduced its expression in lung cancer cells and inhibited cell migration ability [103]. Similar effects were observed in breast cancer [104], providing ideas for the clinical treatment of cancer progression. Because of the poor membrane permeability of ASOs, ASOs are mainly confined to the cytoplasm. Thus, it is difficult for ASOs to modulate nuclear lncRNA expression.
6.3. Short Hairpin RNAs
Gene silencing via the RNA interference (RNAi) holds great potential for the treatment of various diseases, such as cancer [105]. The delivery methods can be executed by transfecting plasmid that express short hairpin RNAs (shRNAs). On the other hand, the delivery of shRNA-expressing plasmid by viral vector has the advantage of long-term treatment. There is much evidence on the use of shRNAs to target lncRNAs to prevent cancer progression. Our group demonstrated that the knockdown of TUG1 or BC200 with shRNAs in hepatoma reduced cell proliferation [18,106]. In addition, transfecting HOTAIR-specific shRNA via a retrovirus in a breast cancer cell line signifi-cantly inhibited tumor cell metastasis in vivo [107]. It was also found that the knockdown of lncRNA-PNUTS via an adenovirus system could reduce the epithelial–mesenchymal transition and metastases [108]. The knockdown of lncRNA-BCAR4 by lentiviral transfection significantly inhibited the formation of metastases in breast cancer in vivo in mice [109]. Adeno-associated viruses (AAVs) are structures of uncoated, single-stranded DNA [110]. The application of adenovirus vectors is far more extensive, and there are some clinical trials [111,112]. AAV-based vectors are an efficient gene delivery system, mainly because they are non-pathogenic, do not elicit an immune response and are stable in live cells [110]. After large-scale screening, ideal AAV systems have been developed to target human cancer cells [113]. AAVs have laid a solid foundation for the clinical treatment of tumors by targeting lncRNAs. So far, Patient-Derieved Xenograft Models (PDX) models are a useful model for pre-clinical investigation. Some studies reported that PDX has been applied to identify lncRNAs function from basic research to pre-clinical studies [114]. The therapeutic responses of SAMMSON in vivo were tested in PDX melanoma models (designated Mel006 and Mel010) [56]. The silence of SAMMSON in Mel006 and Mel010 xenografts significantly increased apoptosis and repressed the tumor growth. Notably, this assay in the PDX model did not cause weight loss or any relevant adverse effects. Thus, the depletion of SAMMSON is highly efficient and useful for anti-melanoma. Recently, the association of lncRNA-p21 and neuroendocrine prostate cancer cells (NEPC)-PDX has been uncovered [115]. The expression levels of lncRNA-p21 are highly expressed in NEPC. Moreover, enzalutamide (Enz) treatment enhanced the lncRNA-p21 to promote the neuroendocrine differentiation (NED). Furthermore, the treatment of the EZH2 inhibitor suppressed the Enz-induced NED in the PDX model. Together, these findings may provide some potential therapies of treating EZH2 inhibitors to target the lncRNA-p21/EZH2 signaling pathway. Several studies support that it is worth utilizing PDXs model to investigate targeted therapies for cancer progression with the integration of clinical observations.
7. Conclusions
Recently, reprogramming cellular metabolism has become a hallmark of cancer cells. Increasing studies have elucidated that lncRNAs, which were originally regarded as genomic junk, are pivotally involved in tumor growth, metastasis, metabolism and cancer progression. The lncRNA networks modulating cellular metabolism in cancer are comprehensively listed in Table 1. Theoretically, these effects are highly associated with metabolism, which can impact the regulation of cellular metabolism and energy homeostasis. In this review article, we highlight the lncRNAs associated with glucose metabolism, mitochondrial function and oxidative stress and present their potential mechanisms. However, some lncRNAs exert opposite roles in different studies, which might be due to the tissue-specific features of the lncRNAs. As discussed in this review, these findings suggest that these metabolic lncRNAs may provide a novel approach for the diagnosis and treatment of cancer progression. Further studies are required to address the detailed mechanisms of lncRNAs, and cellular metabolisms are required.
Funding
This work was supported by grants from Chang Gung Memorial Hospital, Taoyuan, Taiwan (CMRPG3H0721, CMRPG3H0722, CMRPG3H0723, NZRPG3G0171, NZRPG3G0172, NZRPG3G0173, NMRPG3H0561, NRRPG3J0141 to YHL) and the Ministry of Science and Technology of the Republic of China (MOST 106-2321-B-182A-004-MY3, MOST 107-2320-B-182A-028-, MOST 108-2320-B-182A-004- to YHL).
Conflicts of Interest
The author have no conflicts of interest to disclose.
References
- 1.Pavlova N.N., Thompson C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J., Zhang C., Hu W., Feng Z. Tumor suppressor p53 and metabolism. J. Mol. Cell Biol. 2019;11:284–292. doi: 10.1093/jmcb/mjy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stine Z.E., Walton Z.E., Altman B.J., Hsieh A.L., Dang C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015;5:1024–1039. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corcoran S.E., O’Neill L.A. HIF1alpha and metabolic reprogramming in inflammation. J. Clin. Investig. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf A., Agnihotri S., Micallef J., Mukherjee J., Sabha N., Cairns R., Hawkins C., Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeWaal D., Nogueira V., Terry A.R., Patra K.C., Jeon S.M., Guzman G., Au J., Long C.P., Antoniewicz M.R., Hay N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018;9:446. doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes J.C.R., Acuna S.M., Aoki J.I., Floeter-Winter L.M., Muxel S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somarowthu S., Legiewicz M., Chillon I., Marcia M., Liu F., Pyle A.M. HOTAIR forms an intricate and modular secondary structure. Mol. Cell. 2015;58:353–361. doi: 10.1016/j.molcel.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N., Zhou K.I., Parisien M., Dai Q., Diatchenko L., Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidovich C., Cech T.R. The recruitment of chromatin modifiers by long noncoding RNAs: Lessons from PRC2. RNA. 2015;21:2007–2022. doi: 10.1261/rna.053918.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balas M.M., Johnson A.M. Exploring the mechanisms behind long noncoding RNAs and cancer. Noncoding RNA Res. 2018;3:108–117. doi: 10.1016/j.ncrna.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anfossi S., Babayan A., Pantel K., Calin G.A. Clinical utility of circulating non-coding RNAs-an update. Nat. Rev. Clin. Oncol. 2018;15:541–563. doi: 10.1038/s41571-018-0035-x. [DOI] [PubMed] [Google Scholar]
- 14.Kino T., Hurt D.E., Ichijo T., Nader N., Chrousos G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan Y., Ma J., Pan Y., Hu J., Liu B., Jia L. LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis. 2018;9:722. doi: 10.1038/s41419-018-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z., Lee J.H., Ruan H., Ye Y., Krakowiak J., Hu Q., Xiang Y., Gong J., Zhou B., Wang L., et al. Transcriptional landscape and clinical utility of enhancer RNAs for eRNA-targeted therapy in cancer. Nat. Commun. 2019;10:4562. doi: 10.1038/s41467-019-12543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Xiao Z.D., Han L., Zhang J., Lee S.W., Wang W., Lee H., Zhuang L., Chen J., Lin H.K., et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat. Cell Biol. 2016;18:431–442. doi: 10.1038/ncb3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y.H., Wu M.H., Huang Y.H., Yeh C.T., Cheng M.L., Chi H.C., Tsai C.Y., Chung I.H., Chen C.Y., Lin K.H. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67:188–203. doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- 19.Kim S.Y. Cancer Energy Metabolism: Shutting Power off Cancer Factory. Biomol. Ther. (Seoul) 2018;26:39–44. doi: 10.4062/biomolther.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J., Xia L., Liang J., Han Y., Wang H., Oyang L., Tan S., Tian Y., Rao S., Chen X., et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J. Exp. Clin. Cancer Res. 2019;38:218. doi: 10.1186/s13046-019-1214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberti M.V., Locasale J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balon T.W. SGLT and GLUT: Are they teammates? Focus on “Mouse SGLT3a generates proton-activated currents but does not transport sugar”. Am. J. Physiol. Cell Physiol. 2012;302:C1071–C1072. doi: 10.1152/ajpcell.00054.2012. [DOI] [PubMed] [Google Scholar]
- 23.Zou Z.W., Ma C., Medoro L., Chen L., Wang B., Gupta R., Liu T., Yang X.Z., Chen T.T., Wang R.Z., et al. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7:61741–61754. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei S., Fan Q., Yang L., Zhang X., Ma Y., Zong Z., Hua X., Su D., Sun H., Li H., et al. Promotion of glycolysis by HOTAIR through GLUT1 upregulation via mTOR signaling. Oncol. Rep. 2017;38:1902–1908. doi: 10.3892/or.2017.5840. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Zhang X., Wang Z., Hu Q., Wu J., Li Y., Ren X., Wu T., Tao X., Chen X., et al. LncRNA-p23154 promotes the invasion-metastasis potential of oral squamous cell carcinoma by regulating Glut1-mediated glycolysis. Cancer Lett. 2018;434:172–183. doi: 10.1016/j.canlet.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Chang L., Xu W., Zhang Y., Gong F. Long non-coding RNA-NEF targets glucose transportation to inhibit the proliferation of non-small-cell lung cancer cells. Oncol. Lett. 2019;17:2795–2801. doi: 10.3892/ol.2019.9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis B.C., Graham L.D., Molloy P.L. CRNDE, a long non-coding RNA responsive to insulin/IGF signaling, regulates genes involved in central metabolism. Biochim. Biophys. Acta. 2014;1843:372–386. doi: 10.1016/j.bbamcr.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y., Liu Y., Lin L., Huang Q., He W., Zhang S., Dong S., Wen Z., Rao J., Liao W., et al. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol. Cancer. 2018;17:69. doi: 10.1186/s12943-018-0820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He W., Liang B., Wang C., Li S., Zhao Y., Huang Q., Liu Z., Yao Z., Wu Q., Liao W., et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38:4637–4654. doi: 10.1038/s41388-019-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanner L.B., Goglia A.G., Wei M.H., Sehgal T., Parsons L.R., Park J.O., White E., Toettcher J.E., Rabinowitz J.D. Four Key Steps Control Glycolytic Flux in Mammalian Cells. Cell Syst. 2018;7:49–62.e48. doi: 10.1016/j.cels.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson J.E. Isozymes of mammalian hexokinase: Structure, subcellular localization and metabolic function. J. Exp. Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 32.Robey R.B., Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 33.Han X., Yang Y., Sun Y., Qin L., Yang Y. LncRNA TUG1 affects cell viability by regulating glycolysis in osteosarcoma cells. Gene. 2018;674:87–92. doi: 10.1016/j.gene.2018.06.085. [DOI] [PubMed] [Google Scholar]
- 34.Onagoruwa O.T., Pal G., Ochu C., Ogunwobi O.O. Oncogenic Role of PVT1 and Therapeutic Implications. Front. Oncol. 2020;10:17. doi: 10.3389/fonc.2020.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song J., Wu X., Liu F., Li M., Sun Y., Wang Y., Wang C., Zhu K., Jia X., Wang B., et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys. Res. Commun. 2017;490:217–224. doi: 10.1016/j.bbrc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Hsu M.C., Hung W.C. Pyruvate kinase M2 fuels multiple aspects of cancer cells: From cellular metabolism, transcriptional regulation to extracellular signaling. Mol. Cancer. 2018;17:35. doi: 10.1186/s12943-018-0791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Li J., Jia S., Wu M., An J., Zheng Q., Zhang W., Lu D. miR675 upregulates long noncoding RNA H19 through activating EGR1 in human liver cancer. Oncotarget. 2015;6:31958–31984. doi: 10.18632/oncotarget.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lao-On U., Attwood P.V., Jitrapakdee S. Roles of pyruvate carboxylase in human diseases: From diabetes to cancers and infection. J. Mol. Med. (Berl) 2018;96:237–247. doi: 10.1007/s00109-018-1622-0. [DOI] [PubMed] [Google Scholar]
- 39.Ma M.Z., Zhang Y., Weng M.Z., Wang S.H., Hu Y., Hou Z.Y., Qin Y.Y., Gong W., Zhang Y.J., Kong X., et al. Long Noncoding RNA GCASPC, a Target of miR-17-3p, Negatively Regulates Pyruvate Carboxylase-Dependent Cell Proliferation in Gallbladder Cancer. Cancer Res. 2016;76:5361–5371. doi: 10.1158/0008-5472.CAN-15-3047. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L., Ji G., Le X., Wang C., Xu L., Feng M., Zhang Y., Yang H., Xuan Y., Yang Y., et al. Long Noncoding RNA LINC00092 Acts in Cancer-Associated Fibroblasts to Drive Glycolysis and Progression of Ovarian Cancer. Cancer Res. 2017;77:1369–1382. doi: 10.1158/0008-5472.CAN-16-1615. [DOI] [PubMed] [Google Scholar]
- 41.He Z., You C., Zhao D. Long non-coding RNA UCA1/miR-182/PFKFB2 axis modulates glioblastoma-associated stromal cells-mediated glycolysis and invasion of glioma cells. Biochem. Biophys. Res. Commun. 2018;500:569–576. doi: 10.1016/j.bbrc.2018.04.091. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Q., Wang L. LncRNA XIST serves as a ceRNA to regulate the expression of ASF1A, BRWD1M, and PFKFB2 in kidney transplant acute kidney injury via sponging hsa-miR-212-3p and hsa-miR-122-5p. Cell Cycle. 2020;19:290–299. doi: 10.1080/15384101.2019.1707454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11:102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hroudova J., Fisar Z. Control mechanisms in mitochondrial oxidative phosphorylation. Neural Regen. Res. 2013;8:363–375. doi: 10.3969/j.issn.1673-5374.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taanman J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta. 1999;1410:103–123. doi: 10.1016/S0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 46.Tian T., Lv X., Pan G., Lu Y., Chen W., He W., Lei X., Zhang H., Liu M., Sun S., et al. Long Noncoding RNA MPRL Promotes Mitochondrial Fission and Cisplatin Chemosensitivity via Disruption of Pre-miRNA Processing. Clin. Cancer Res. 2019;25:3673–3688. doi: 10.1158/1078-0432.CCR-18-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K., Sun T., Li N., Wang Y., Wang J.X., Zhou L.Y., Long B., Liu C.Y., Liu F., Li P.F. MDRL lncRNA regulates the processing of miR-484 primary transcript by targeting miR-361. PLoS Genet. 2014;10:e1004467. doi: 10.1371/journal.pgen.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K., Long B., Zhou L.Y., Liu F., Zhou Q.Y., Liu C.Y., Fan Y.Y., Li P.F. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat. Commun. 2014;5:3596. doi: 10.1038/ncomms4596. [DOI] [PubMed] [Google Scholar]
- 49.Scarpulla R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez-Marcos P.J., Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011;93:884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long J., Badal S.S., Ye Z., Wang Y., Ayanga B.A., Galvan D.L., Green N.H., Chang B.H., Overbeek P.A., Danesh F.R. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J. Clin. Investig. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai B., Song X.Q., Cai J.P., Zhang S. HOTAIR: A cancer-related long non-coding RNA. Neoplasma. 2014;61:379–391. doi: 10.4149/neo_2014_075. [DOI] [PubMed] [Google Scholar]
- 53.Zheng P., Xiong Q., Wu Y., Chen Y., Chen Z., Fleming J., Gao D., Bi L., Ge F. Quantitative Proteomics Analysis Reveals Novel Insights into Mechanisms of Action of Long Noncoding RNA Hox Transcript Antisense Intergenic RNA (HOTAIR) in HeLa Cells. Mol. Cell Proteomics. 2015;14:1447–1463. doi: 10.1074/mcp.M114.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong L., Zhou X., Wu Y., Wang Y., Chen L., Li P., Liu S., Sun S., Ren Y., Mei M., et al. Targeting HOTAIR Induces Mitochondria Related Apoptosis and Inhibits Tumor Growth in Head and Neck Squamous Cell Carcinoma in vitro and in vivo. Curr. Mol. Med. 2015;15:952–960. doi: 10.2174/1566524016666151123112716. [DOI] [PubMed] [Google Scholar]
- 55.Li H.J., Sun X.M., Li Z.K., Yin Q.W., Pang H., Pan J.J., Li X., Chen W. LncRNA UCA1 Promotes Mitochondrial Function of Bladder Cancer via the MiR-195/ARL2 Signaling Pathway. Cell Physiol. Biochem. 2017;43:2548–2561. doi: 10.1159/000484507. [DOI] [PubMed] [Google Scholar]
- 56.Leucci E., Vendramin R., Spinazzi M., Laurette P., Fiers M., Wouters J., Radaelli E., Eyckerman S., Leonelli C., Vanderheyden K., et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531:518–522. doi: 10.1038/nature17161. [DOI] [PubMed] [Google Scholar]
- 57.Wu H., Sun H., Liang X., Lima W.F., Crooke S.T. Human RNase H1 is associated with protein P32 and is involved in mitochondrial pre-rRNA processing. PLoS ONE. 2013;8:e71006. doi: 10.1371/journal.pone.0071006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fogal V., Richardson A.D., Karmali P.P., Scheffler I.E., Smith J.W., Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noh J.H., Kim K.M., Abdelmohsen K., Yoon J.H., Panda A.C., Munk R., Kim J., Curtis J., Moad C.A., Wohler C.M., et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 2016;30:1224–1239. doi: 10.1101/gad.276022.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rackham O., Shearwood A.M., Mercer T.R., Davies S.M., Mattick J.S., Filipovska A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA. 2011;17:2085–2093. doi: 10.1261/rna.029405.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villota C., Campos A., Vidaurre S., Oliveira-Cruz L., Boccardo E., Burzio V.A., Varas M., Villegas J., Villa L.L., Valenzuela P.D., et al. Expression of mitochondrial non-coding RNAs (ncRNAs) is modulated by high risk human papillomavirus (HPV) oncogenes. J. Biol. Chem. 2012;287:21303–21315. doi: 10.1074/jbc.M111.326694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fitzpatrick C., Bendek M.F., Briones M., Farfan N., Silva V.A., Nardocci G., Montecino M., Boland A., Deleuze J.F., Villegas J., et al. Mitochondrial ncRNA targeting induces cell cycle arrest and tumor growth inhibition of MDA-MB-231 breast cancer cells through reduction of key cell cycle progression factors. Cell Death Dis. 2019;10:423. doi: 10.1038/s41419-019-1649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin Y.H. MicroRNA Networks Modulate Oxidative Stress in Cancer. Int. J. Mol. Sci. 2019;20:4497. doi: 10.3390/ijms20184497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David J.A., Rifkin W.J., Rabbani P.S., Ceradini D.J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017;2017:4826724. doi: 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y., Qiu J., Chen B., Lin Y., Chen Y., Xie G., Qiu J., Tong H., Jiang D. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-kappaB pathway. Int. Immunopharmacol. 2018;59:252–260. doi: 10.1016/j.intimp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 66.Zhang M., Wang X., Yao J., Qiu Z. Long non-coding RNA NEAT1 inhibits oxidative stress-induced vascular endothelial cell injury by activating the miR-181d-5p/CDKN3 axis. Artif. Cells Nanomed. Biotechnol. 2019;47:3129–3137. doi: 10.1080/21691401.2019.1646264. [DOI] [PubMed] [Google Scholar]
- 67.Zheng Z.G., Xu H., Suo S.S., Xu X.L., Ni M.W., Gu L.H., Chen W., Wang L.Y., Zhao Y., Tian B., et al. The Essential Role of H19 Contributing to Cisplatin Resistance by Regulating Glutathione Metabolism in High-Grade Serous Ovarian Cancer. Sci. Rep. 2016;6:26093. doi: 10.1038/srep26093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun H.J., Hou B., Wang X., Zhu X.X., Li K.X., Qiu L.Y. Endothelial dysfunction and cardiometabolic diseases: Role of long non-coding RNAs. Life Sci. 2016;167:6–11. doi: 10.1016/j.lfs.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Zeng R., Zhang R., Song X., Ni L., Lai Z., Liu C., Ye W. The long non-coding RNA MALAT1 activates Nrf2 signaling to protect human umbilical vein endothelial cells from hydrogen peroxide. Biochem. Biophys. Res. Commun. 2018;495:2532–2538. doi: 10.1016/j.bbrc.2017.12.105. [DOI] [PubMed] [Google Scholar]
- 70.Chen J., Ke S., Zhong L., Wu J., Tseng A., Morpurgo B., Golovko A., Wang G., Cai J.J., Ma X., et al. Long noncoding RNA MALAT1 regulates generation of reactive oxygen species and the insulin responses in male mice. Biochem. Pharmacol. 2018;152:94–103. doi: 10.1016/j.bcp.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 71.Boilan E., Winant V., Dumortier E., Piret J.P., Bonfitto F., Osiewacz H.D., Debacq-Chainiaux F., Toussaint O. Role of p38MAPK and oxidative stress in copper-induced senescence. Age (Dordr) 2013;35:2255–2271. doi: 10.1007/s11357-013-9521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin S.C., Hardie D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen H.B., Babcock J.T., Wells C.D., Quilliam L.A. LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of Yap. Oncogene. 2013;32:4100–4109. doi: 10.1038/onc.2012.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shackelford D.B., Shaw R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Z., Li J.L., Lin S., Cao C., Gimbrone N.T., Yang R., Fu D.A., Carper M.B., Haura E.B., Schabath M.B., et al. cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer and mediates tumor growth. J. Clin. Investig. 2016;126:2267–2279. doi: 10.1172/JCI85250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren Y., Shen H.M. Critical role of AMPK in redox regulation under glucose starvation. Redox Biol. 2019;25:101154. doi: 10.1016/j.redox.2019.101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laitala A., Erler J.T. Hypoxic Signalling in Tumour Stroma. Front. Oncol. 2018;8:189. doi: 10.3389/fonc.2018.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park H.S., Kim J.H., Sun B.K., Song S.U., Suh W., Sung J.H. Hypoxia induces glucose uptake and metabolism of adiposederived stem cells. Mol. Med. Rep. 2016;14:4706–4714. doi: 10.3892/mmr.2016.5796. [DOI] [PubMed] [Google Scholar]
- 79.Sormendi S., Wielockx B. Hypoxia Pathway Proteins As Central Mediators of Metabolism in the Tumor Cells and Their Microenvironment. Front. Immunol. 2018;9:40. doi: 10.3389/fimmu.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang F., Zhang H., Mei Y., Wu M. Reciprocal regulation of HIF-1alpha and lincRNA-p21 modulates the Warburg effect. Mol. Cell. 2014;53:88–100. doi: 10.1016/j.molcel.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Luo F., Liu X., Ling M., Lu L., Shi L., Lu X., Li J., Zhang A., Liu Q. The lncRNA MALAT1, acting through HIF-1alpha stabilization, enhances arsenite-induced glycolysis in human hepatic L-02 cells. Biochim Biophys Acta. 2016;1862:1685–1695. doi: 10.1016/j.bbadis.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Wu W., Hu Q., Nie E., Yu T., Wu Y., Zhi T., Jiang K., Shen F., Wang Y., Zhang J., et al. Hypoxia induces H19 expression through direct and indirect Hif-1alpha activity, promoting oncogenic effects in glioblastoma. Sci. Rep. 2017;7:45029. doi: 10.1038/srep45029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin A., Li C., Xing Z., Hu Q., Liang K., Han L., Wang C., Hawke D.H., Wang S., Zhang Y., et al. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat. Cell Biol. 2016;18:213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takahashi K., Yan I.K., Haga H., Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J. Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puzio-Kuter A.M. The Role of p53 in Metabolic Regulation. Genes Cancer. 2011;2:385–391. doi: 10.1177/1947601911409738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu M., An J., Zheng Q., Xin X., Lin Z., Li X., Li H., Lu D. Double mutant P53 (N340Q/L344R) promotes hepatocarcinogenesis through upregulation of Pim1 mediated by PKM2 and LncRNA CUDR. Oncotarget. 2016;7:66525–66539. doi: 10.18632/oncotarget.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y., Zhong Y., Wang Y., Zhang X., Batista D.L., Gejman R., Ansell P.J., Zhao J., Weng C., Klibanski A. Activation of p53 by MEG3 non-coding RNA. J. Biol. Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 88.Zhu J., Liu S., Ye F., Shen Y., Tie Y., Zhu J., Wei L., Jin Y., Fu H., Wu Y., et al. Long Noncoding RNA MEG3 Interacts with p53 Protein and Regulates Partial p53 Target Genes in Hepatoma Cells. PLoS ONE. 2015;10:e0139790. doi: 10.1371/journal.pone.0139790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahmoudi S., Henriksson S., Corcoran M., Mendez-Vidal C., Wiman K.G., Farnebo M. Wrap53, a Natural p53 Antisense Transcript Required for p53 Induction upon DNA Damage. Mol. Cell. 2016;64:1009. doi: 10.1016/j.molcel.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 90.Tripathi V., Shen Z., Chakraborty A., Giri S., Freier S.M., Wu X., Zhang Y., Gorospe M., Prasanth S.G., Lal A., et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang A., Zhou N., Huang J., Liu Q., Fukuda K., Ma D., Lu Z., Bai C., Watabe K., Mo Y.Y. The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Res. 2013;23:340–350. doi: 10.1038/cr.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhan T., Rindtorff N., Betge J., Ebert M.P., Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin. Cancer Biol. 2019;55:106–119. doi: 10.1016/j.semcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 93.Ho T.T., Zhou N., Huang J., Koirala P., Xu M., Fung R., Wu F., Mo Y.Y. Targeting non-coding RNAs with the CRISPR/Cas9 system in human cell lines. Nucleic Acids Res. 2015;43:e17. doi: 10.1093/nar/gku1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daneshvar K., Pondick J.V., Kim B.M., Zhou C., York S.R., Macklin J.A., Abualteen A., Tan B., Sigova A.A., Marcho C., et al. DIGIT Is a Conserved Long Noncoding RNA that Regulates GSC Expression to Control Definitive Endoderm Differentiation of Embryonic Stem Cells. Cell Rep. 2016;17:353–365. doi: 10.1016/j.celrep.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhen S., Li X. Application of CRISPR-Cas9 for Long Noncoding RNA Genes in Cancer Research. Hum. Gene Ther. 2019;30:3–9. doi: 10.1089/hum.2018.063. [DOI] [PubMed] [Google Scholar]
- 96.Li R., Harvey A.R., Hodgetts S.I., Fox A.H. Functional dissection of NEAT1 using genome editing reveals substantial localization of the NEAT1_1 isoform outside paraspeckles. RNA. 2017;23:872–881. doi: 10.1261/rna.059477.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhuo W., Liu Y., Li S., Guo D., Sun Q., Jin J., Rao X., Li M., Sun M., Jiang M., et al. Long Noncoding RNA GMAN, Up-regulated in Gastric Cancer Tissues, Is Associated With Metastasis in Patients and Promotes Translation of Ephrin A1 by Competitively Binding GMAN-AS. Gastroenterology. 2019;156:676–691.e611. doi: 10.1053/j.gastro.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 98.Hilton I.B., D’Ippolito A.M., Vockley C.M., Thakore P.I., Crawford G.E., Reddy T.E., Gersbach C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015;33:510–517. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin Y.H., Wu M.H., Huang Y.H., Yeh C.T., Lin K.H. TUG1 Is a Regulator of AFP and Serves as Prognostic Marker in Non-Hepatitis B Non-Hepatitis C Hepatocellular Carcinoma. Cells. 2020;9:262. doi: 10.3390/cells9020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takakura K., Kawamura A., Torisu Y., Koido S., Yahagi N., Saruta M. The Clinical Potential of Oligonucleotide Therapeutics against Pancreatic Cancer. Int. J. Mol. Sci. 2019;20:3331. doi: 10.3390/ijms20133331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yap K.L., Li S., Munoz-Cabello A.M., Raguz S., Zeng L., Mujtaba S., Gil J., Walsh M.J., Zhou M.M. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dimitrova N., Zamudio J.R., Jong R.M., Soukup D., Resnick R., Sarma K., Ward A.J., Raj A., Lee J.T., Sharp P.A., et al. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol. Cell. 2014;54:777–790. doi: 10.1016/j.molcel.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gong N., Teng X., Li J., Liang X.J. Antisense Oligonucleotide-Conjugated Nanostructure-Targeting lncRNA MALAT1 Inhibits Cancer Metastasis. ACS Appl. Mater. Interfaces. 2019;11:37–42. doi: 10.1021/acsami.8b18288. [DOI] [PubMed] [Google Scholar]
- 104.Arun G., Diermeier S., Akerman M., Chang K.C., Wilkinson J.E., Hearn S., Kim Y., MacLeod A.R., Krainer A.R., Norton L., et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koornneef A., Van Logtenstein R., Timmermans E., Pisas L., Blits B., Abad X., Fortes P., Petry H., Konstantinova P., Ritsema T. AAV-mediated in vivo knockdown of luciferase using combinatorial RNAi and U1i. Gene Ther. 2011;18:929–935. doi: 10.1038/gt.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin Y.H., Wu M.H., Huang Y.H., Yeh C.T., Chi H.C., Tsai C.Y., Chuang W.Y., Yu C.J., Chung I.H., Chen C.Y., et al. Thyroid hormone negatively regulates tumorigenesis through suppression of BC200. Endocr. Relat. Cancer. 2018;25:967–979. doi: 10.1530/ERC-18-0176. [DOI] [PubMed] [Google Scholar]
- 107.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grelet S., Link L.A., Howley B., Obellianne C., Palanisamy V., Gangaraju V.K., Diehl J.A., Howe P.H. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat. Cell Biol. 2017;19:1105–1115. doi: 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xing Z., Park P.K., Lin C., Yang L. LncRNA BCAR4 wires up signaling transduction in breast cancer. RNA Biol. 2015;12:681–689. doi: 10.1080/15476286.2015.1053687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Naso M.F., Tomkowicz B., Perry W.L., 3rd, Strohl W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gaudet D., Methot J., Dery S., Brisson D., Essiembre C., Tremblay G., Tremblay K., De Wal J., Twisk J., Van den Bulk N., et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: An open-label trial. Gene Ther. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodrigues G.A., Shalaev E., Karami T.K., Cunningham J., Slater N.K.H., Rivers H.M. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm. Res. 2018;36:29. doi: 10.1007/s11095-018-2554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lisowski L., Dane A.P., Chu K., Zhang Y., Cunningham S.C., Wilson E.M., Nygaard S., Grompe M., Alexander I.E., Kay M.A. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arun G., Diermeier S.D., Spector D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo J., Wang K., Yeh S., Sun Y., Liang L., Xiao Y., Xu W., Niu Y., Cheng L., Maity S.N., et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat. Commun. 2019;10:2571. doi: 10.1038/s41467-019-09784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]