Abstract
BACKGROUND
Mammalian target of rapamycin (mTOR) inhibitors have been shown to reduce the risk of tumour recurrence after liver transplantation for hepatocellular carcinoma (HCC). However, their role in established post-transplant HCC recurrence is uncertain.
AIM
To investigate whether mTOR inhibitor offers a survival benefit in post-transplant HCC recurrence.
METHODS
A retrospective study of 143 patients who developed HCC recurrence after liver transplantation was performed. They were divided into 2 groups based on whether they had received mTOR inhibitor-based immunosuppression. The primary endpoint was post-recurrence survival.
RESULTS
Seventy-nine (55%) patients received an mTOR inhibitor-based immunosuppressive regime, while 64 (45%) patients did not. The mTOR inhibitor group had a lower number of recurrent tumours (2 vs 5, P = 0.02) and received more active treatments including radiotherapy (39 vs 22%, P = 0.03) and targeted therapy (59 vs 23%, P < 0.001). The median post-recurrence survival was 21.0 ± 4.1 mo in the mTOR inhibitor group and 11.2 ± 2.5 mo in the control group. Multivariate Cox regression analysis confirmed that mTOR inhibitor therapy was independently associated with improved post-recurrence survival (P = 0.04, OR = 0.482, 95%CI: 0.241-0.966). The number of recurrent tumours and use of other treatment modalities did not affect survival. No survival difference was observed between mTOR inhibitor monotherapy and combination therapy with calcineurin inhibitor.
CONCLUSION
mTOR inhibitors prolonged survival after post-transplant HCC recurrence.
Keywords: Mammalian target of rapamycin inhibitor, Hepatocellular carcinoma, Recurrence, Liver transplant, Survival, Outcomes
Core tip: Mammalian target of rapamycin (mTOR) inhibitors have been shown to reduce the risk of tumour recurrence after liver transplantation for hepatocellular carcinoma (HCC). However, their role in established post-transplant HCC recurrence is uncertain. A retrospective study of 143 patients who developed HCC recurrence after liver transplantation was performed. Seventy-nine (55%) patients received an mTOR inhibitor-based immunosuppressive regime, while 64 (45%) patients did not. The median post-recurrence survival was 21.0 ± 4.1 mo in the mTOR inhibitor group and 11.2 ± 2.5 mo in the control group. Multivariate Cox regression analysis confirmed that mTOR inhibitor therapy was independently associated with improved post-recurrence survival (P = 0.04, OR = 0.482, 95%CI: 0.241-0.966).
INTRODUCTION
Calcineurin inhibitors (CNI) form the cornerstone of immunosuppressive therapy after liver transplantation. However, CNIs promote cancerous growth[1] and studies have demonstrated a dose-dependent relationship with tumour recurrence in patients transplanted for hepatocellular carcinoma (HCC)[2,3]. In contrast, mammalian target of rapamycin (mTOR) inhibitors, such as sirolimus and everolimus, are suggested to have anti-tumour effects by suppressing angiogenesis[4] and cellular proliferation[5]. mTOR inhibitors have been given to patients engrafted for HCC with encouraging results. Their oncological benefits were supported by the findings in numerous retrospective[6-10] and prospective studies[11,12] showing a reduced risk of recurrence.
In theory, patients with established recurrence or at risk of recurrence i.e., more advanced tumour at transplant benefit most from the oncological advantages offered by mTOR inhibitors. These patients are also candidates for mTOR inhibitor-based regimes in our centre. However, evidence supporting mTOR inhibitor therapy following HCC recurrence is limited. It is unknown whether mTOR inhibitors still confer survival benefits in this late course of the disease. Recommendations for mTOR inhibitors in this context are based on expert opinions[13]. To address this knowledge gap in the literature, the current study was undertaken to quantify survival following post-transplant HCC recurrence with regard to the administration of mTOR inhibitors.
MATERIALS AND METHODS
Patients
A retrospective study was conducted at Queen Mary Hospital, the University of Hong Kong, which is a tertiary referral centre and the only liver transplant centre in Hong Kong. Outpatient follow-up was arranged every 3 mo for patients transplanted for HCC, during which clinical examination and blood tests for liver function and alpha-fetoprotein (AFP) were performed. A contrast-enhanced computed tomography (CT) scan of the thorax and abdomen was performed at 6-month intervals. The diagnosis of recurrent HCC was primarily radiological. All consecutive patients diagnosed with recurrent HCC after liver transplantation between 2000 and 2019 were included in this study. The patients were divided into two groups based on whether they received an mTOR inhibitor (sirolimus or everolimus) after recurrence. Abnormal liver function during follow-up was investigated with a CT scan and/or liver biopsy as appropriate. Clinical suspicion of acute rejection was confirmed by liver biopsy.
Treatment
Upon recurrence, immunosuppression was tapered to the lowest effective dose. Considerations were given to an mTOR-based regime, with or without a reduced dose of CNI (tacrolimus with trough level < 5 μg/L). The decision was individualized based on the patients’ general status, liver function and tumour status. In this study, combination therapy was defined as patients receiving both mTOR inhibitor and CNI for more than 50% of the time.
Comprehensive staging was performed by dual-tracer positron emission tomography or a combination of contrast CT scan of the thorax and abdomen with a bone scan. Patients with disseminated recurrence were reviewed for targeted therapy e.g., sorafenib. Patients with oligo-recurrence i.e., recurrent disease limited in number and location were selected for loco-regional treatments including surgery, trans-arterial chemoembolization and radiotherapy[13]. The treatment decisions were discussed in a multidisciplinary tumour board among transplant surgeons, transplant hepatologists, radiation oncologists and medical oncologists.
Data collection, outcomes and statistics
Data were retrieved from a prospectively maintained database. Patients were compared in terms of pre-transplant status, characteristics of recurrence and the treatments they received. The characteristics of recurrence included pattern (intra-, extra-hepatic or both), location, tumour load (number and size) and serum level of AFP. The primary outcome was post-recurrence survival. Categorical variables were compared with the χ2 test. Continuous variables are presented as median and interquartile range. Parametric and non-parametric variables were compared using the t-test and Mann-Whitney U test, respectively. Survival was assessed by the Kaplan-Meier method. Potential confounding factors were compared with univariate and multivariate Cox-regression analysis. Data were analyzed using the Statistical Package for the Social Sciences 16.0 (SPSS) for Windows (SPSS Inc., Chicago, IL, United States). Statistical significance was defined as a P value < 0.05.
RESULTS
During the study period from January 2000 to December 2019, 143 patients were diagnosed with post-transplant HCC recurrence and they formed the basis of this study. Of these patients, 59 (41%) received liver transplantation in our centre, while 84 (59%) underwent the procedure elsewhere. Following the diagnosis of recurrence, 79 (55%) patients received an mTOR inhibitor-based immunosuppressive regime, while 64 (45%) patients did not.
Pre-transplant characteristics
The pre-transplant characteristics were comparable between the two groups (Table 1). There was a male predominance and the subjects primarily had hepatitis B virus induced liver disease (95% and 89%, respectively). The number of salvage transplantations i.e., liver transplantation performed for recurrent HCC after primary liver resection was similar (38% vs 33%, P = 0.52). Tumour status at the time of transplant was comparable in terms of number of tumours (2 vs 2, P = 0.85), size of largest tumour (4.3 vs 4.0 cm, P = 0.68), and serum level of AFP (144 vs 111 ng/mL, P = 0.51). The proportion of patients compliant with Milan (27 vs 22%, P = 0.54) and UCSF criteria (33% vs 23%, P = 0.22) were similar.
Table 1.
Baseline characteristics at the time of liver transplantation
| mTOR inhibitor (n = 79) | No mTOR inhibitor (n = 64) | P value | |
| Age at transplant (years) | 57 (50-62) | 53 (46-59) | 0.07 |
| Gender (%M) | 75 (95%) | 57 (89%) | 0.19 |
| Etiology | 0.28 | ||
| Cryptogenic | 1 (1%) | 4 (6%) | |
| HBV | 72 (91%) | 59 (92%) | |
| HCV | 4 (5%) | 1 (2%) | |
| Alcoholic liver disease | 3 (4%) | 2 (3%) | |
| Primary/salvage transplant | 49/30 | 43/21 | 0.52 |
| Cadaveric/living related | 53/26 | 47/17 | 0.41 |
| Whole graft/partial graft | 53/26 | 47/17 | 0.41 |
| No. of tumours | 2 (1-5) | 2 (1-6) | 0.85 |
| Size of largest tumour (cm) | 4.3 (2.9-6.6) | 4.0 (2.5-6.5) | 0.68 |
| AFP (ng/mL) | 144 (14-1388) | 111 (19-817) | 0.51 |
| Within Milan criteria | 21 (27%) | 14 (22%) | 0.54 |
| Within UCSF criteria | 26 (33%) | 15 (23%) | 0.22 |
AFP: Alpha-fetoprotein; HBV: Hepatitis B virus; HCV: Hepatitis C virus; mTOR: Mammalian target of rapamycin.
Recurrence status and treatment
The recurrence status is summarized in Table 2. Recurrence occurred later in calendar years in the mTOR inhibitor group (7/2013 vs 3/2008, P < 0.001). However, the timing was similar in terms of age (58 vs 55, P = 0.06) and time from transplant (12 vs 12 mo, P = 0.73). The mTOR inhibitor group had a lower number of recurrent tumours (2 vs 5, P = 0.02). Otherwise the disease status upon recurrence was comparable in terms of numbers of involved organs (1 vs 1, P = 0.50) and size of largest tumour (2.0 vs 2.1 cm, P = 0.74). There were more bone recurrences in the mTOR inhibitor group (22 vs 9%, P = 0.049).
Table 2.
Recurrence characteristics
| mTOR inhibitor (n = 79) | No mTOR inhibitor (n = 64) | P value | |
| Date of recurrence | 7/2013 | 3/2008 | < 0.001 |
| Age at recurrence (years) | 58 (52-64) | 55 (46-61) | 0.06 |
| Time from transplant (mo) | 12 (6-24) | 12 (5-25) | 0.73 |
| Number of tumours | 2 (1-5) | 5 (1-9) | 0.02 |
| Size of largest tumour (cm) | 2.0 (1.1-3.2) | 2.1 (1.1-3.9) | 0.74 |
| Number of organs involved | 1 (1-1) | 1 (1-2) | 0.50 |
| Site of recurrence | |||
| Liver | 34 (43%) | 28 (44%) | 0.93 |
| Lung | 36 (46%) | 35 (55%) | 0.33 |
| Bone | 17 (22%) | 6 (9%) | 0.049 |
| Peritoneum | 4 (5%) | 8 (13%) | 0.11 |
| Adrenal | 5 (6%) | 6 (9%) | 0.50 |
| Lymph node | 6 (8%) | 4 (6%) | 0.75 |
| AFP upon recurrence (ng/mL) | 14 (4-139) | 32 (6-855) | 0.19 |
| Immunosuppression | |||
| Calcineurin Inhibitor | 49 (62%) | 62 (97%) | < 0.001 |
| Tacrolimus level | 3.0 (0-4.9) | 5.2 (3.7-6.1) | 0.03 |
| Treatment | |||
| Surgery | 22 (28%) | 11 (17%) | 0.13 |
| RFA | 7 (9%) | 6 (9%) | 0.89 |
| TACE | 19 (24%) | 10 (16%) | 0.23 |
| Radiotherapy | 31 (39%) | 14 (22%) | 0.03 |
| Targeted therapy | 47 (59%) | 15 (23%) | < 0.001 |
| Immunotherapy | 2 (3%) | 0 (0%) | 0.18 |
| Supportive | 3 (4%) | 23 (36%) | < 0.001 |
AFP: Alpha-fetoprotein; RFA: Radiofrequency ablation; TACE: Trans-arterial chemoembolization; mTOR: Mammalian target of rapamycin.
Fewer patients in the mTOR inhibitor group received supportive care (4% vs 36%, P < 0.001) and more active treatments were undertaken, including radiotherapy (39 vs 22%, P = 0.03) and targeted therapy (59 vs 23%, P < 0.001).
Immunosuppression after recurrence
In the mTOR inhibitor group, 48 (61%) patients received sirolimus and 29 (37%) received everolimus. The remaining 2 patients (3%) were initially started on sirolimus but were subsequently converted to everolimus. The majority of them (80%, n = 63) were commenced on mTOR inhibitor after diagnosis of recurrence. Thirty-one of these patients (39%) were maintained on mTOR inhibitor only, while 48 (61%) received a combination of mTOR inhibitor and CNI. As a result, there was lower CNI usage (62 vs 97%, P < 0.001) and lower median tacrolimus levels (3.0 vs 5.2 μg/L, P = 0.03) in the mTOR inhibitor group.
Outcomes
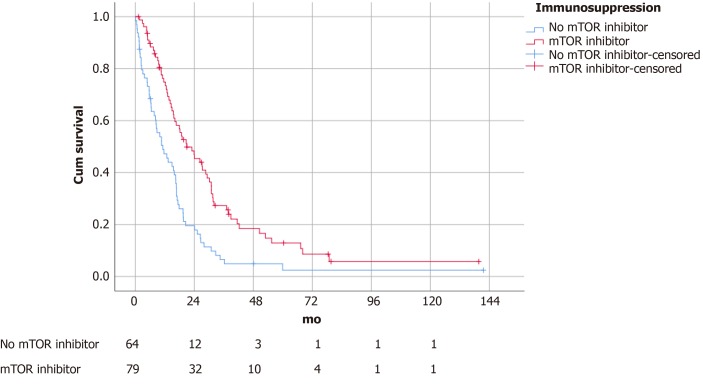
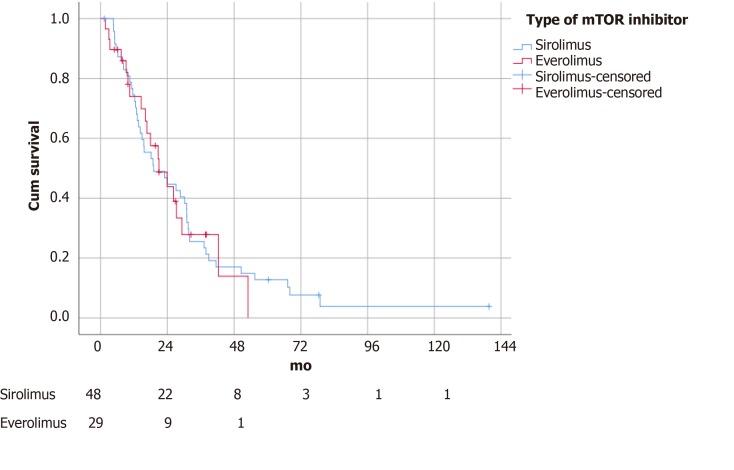
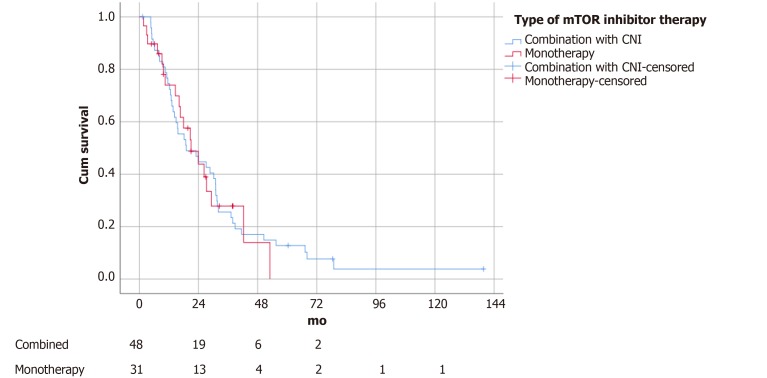
The median follow-up time was 14.2 mo. Patients with an mTOR inhibitor included in the immunosuppressive regime survived significantly longer (21.0 ± 4.1 vs 11.2 ± 2.5 mo, P = 0.04) (Figure 1). There was no difference in survival outcomes between patients receiving sirolimus and everolimus (19.1 ± 5.7 vs 21.0 ± 4.4 mo, P = 0.88) (Figure 2). Single agent immunosuppression did not affect survival (single vs combination: 26.3 ± 8.0 vs 17.9 ± 5.3 mo, P = 0.59) (Figure 3) or rejection rate (0 vs 4.2%, P = 0.25).
Figure 1.
Survival of patients with mammalian target of rapamycin inhibitor vs no mammalian target of rapamycin inhibitor (survival 21.0 ± 4.1 vs 11.2 ± 2.5 mo, P = 0.04). mTOR: Mammalian target of rapamycin.
Figure 2.
Survival of patients stratified by sirolimus vs everolimus (P = 0.88). mTOR: Mammalian target of rapamycin.
Figure 3.
Survival of patients stratified by mammalian target of rapamycin inhibitor monotherapy vs combination therapy with calcineurin inhibitor (P = 0.59). mTOR: Mammalian target of rapamycin.
As shown in Table 3, multivariate analysis confirmed that immunosuppression with mTOR inhibitor was independently associated with improved survival from recurrence (P = 0.04, OR = 0.482). Early recurrence (P = 0.001, OR = 0.977), liver recurrence (P = 0.01, OR = 1.92), larger tumour (P = 0.02, OR = 1.13), and higher AFP level (P = 0.02, OR = 1.00) were predictors of poor survival. The trough level of tacrolimus (P = 0.16), date of recurrence (P = 0.79) and number of recurrent tumours (P = 0.33) did not predict survival.
Table 3.
Survival analysis
|
Univariate |
Multivariate |
|||
| P value | OR (95%CI) | P value | OR (95%CI) | |
| Date of recurrence | 0.006 | 1.00 (1.00-1.00) | 0.79 | |
| Age at recurrence (years) | 0.93 | |||
| Time from transplant | < 0.001 | 0.977 (0.966-0.988) | 0.001 | 0.977 (0.963-0.991) |
| Number of tumours | < 0.001 | 1.01 (1.01-1.02) | 0.33 | |
| Size of largest tumour | 0.02 | 1.11 (1.02-1.20) | 0.02 | 1.13 (1.02-1.24) |
| Number of organs involved | 0.01 | 1.14 (1.11-1.89) | 0.27 | |
| Site of recurrence | ||||
| Liver | 0.01 | 1.62 (1.13-2.31) | 0.01 | 1.92 (1.14-3.25) |
| Lung | 0.43 | |||
| Bone | 0.17 | |||
| Peritoneal | 0.46 | |||
| Adrenal | 0.52 | |||
| Lymph node | 0.49 | |||
| AFP upon recurrence | 0.02 | 1.00 (1.00-1.00) | 0.02 | 1.00 (1.00-1.00) |
| Immunosuppression after recurrence | ||||
| mTOR inhibitor | < 0.001 | 0.485 (0.339-0.695) | 0.04 | 0.482 (0.241-0.966) |
| Calcineurin Inhibitor | 0.07 | |||
| Tacrolimus trough (μg/L) | 0.002 | 1.13 (1.04-1.22) | 0.16 | |
| Treatment | ||||
| Surgery | < 0.001 | 0.380 (0.240-0.601) | 0.22 | |
| RFA | 0.16 | |||
| TACE | 0.32 | |||
| Radiotherapy | 0.90 | |||
| Targeted therapy | 0.97 | |||
| Immunotherapy | 0.80 | |||
| Supportive | < 0.001 | 2.34 (1.49-3.67) | 0.73 | |
AFP: Alpha-fetoprotein; RFA: Radiofrequency ablation; TACE: Trans-arterial chemoembolization; mTOR: Mammalian target of rapamycin.
DISCUSSION
The results from our study suggested that incorporation of an mTOR inhibitor into the immunosuppressive regime of liver transplant recipients with recurrent HCC was associated with improved survival after recurrence (median survival 21.0 ± 4.1 vs 11.2 ± 2.5 mo, P = 0.04).
In this cohort, several differences in recurrence status were highlighted between both arms. Firstly, recurrence in the mTOR inhibitor group occurred later in calendar years (7/2013 vs 3/2008, P < 0.001). mTOR inhibitor was first administered in 2004 in our series and was only considered for patients from that time onwards. There was a fundamental time effect while improvements in medical and surgical treatment contributed to better survival outcomes[14-19]. Indeed, more patients in the mTOR inhibitor group received targeted therapy e.g., sorafenib (59 vs 23%, P < 0.001) and radiotherapy (39 vs 22%, P = 0.03). Stereotactic body radiotherapy was applied for patients with limited intrahepatic recurrence for local control.
Secondly, the mTOR inhibitor group had earlier disease with fewer tumours (2 vs 5, P = 0.02). This probably resulted due to selection bias. Clinicians could have avoided aggressive therapy in patients with widespread disease due to fear of futility. This also explained why more patients in the control group received supportive treatment (4 vs 36%, P < 0.001). Last but not least, mTOR inhibitors were used with reduced or spared CNI, which could have contributed to their protective effect[2,3]. Therefore, survival associations were estimated using multivariate Cox regression taking into account these potential confounders. Our results showed that mTOR inhibitor maintained a robust association with improved survival. Data suggested that the oncological advantages of mTOR inhibitors in patients with post-transplant HCC recurrence were independent of the CNI sparing effect. The other clinical differences, including date of recurrence, number of recurrent tumours, use of targeted therapy and decision for supportive treatment, did not contribute to the disparity in outcomes.
The prognosis after post-transplant HCC is dismal. The median survival after recurrence ranges from 8 to 19 mo[19-21]. Not surprisingly, most studies on mTOR inhibitors have focused on prevention rather than control of recurrence. The protective effects against recurrence have been illustrated by numerous retrospective[6-10] and prospective studies[11,12]. Interestingly, Geissler et al[12] pointed out from the SiLVER trial that survival benefits due to sirolimus were confined to low risk patients, as defined by those receiving a primary transplant for tumours within the Milan criteria[22]. Sirolimus was unable to alter the imminent disease course in patients with more advanced tumours. Along this line, the efficacy of mTOR inhibitor in established recurrence was questioned. This is the first report in the literature directed at this question. Apart from confirming the survival benefits, we reported a median post-recurrence survival of 21 mo associated with mTOR inhibitors. We did not manage all post-transplant HCC recurrences with palliative intent. We adapted the concept of oligo-recurrence[13,23], in which patients with recurrent disease limited in number and location were given the therapeutic opportunity of cure with a combination of systemic (including mTOR blockade) and loco-regional therapy. Twenty-seven patients (35%) in the study arm received curative treatment. The 3-year post-recurrence survival reached 27.1% in the entire mTOR inhibitor group. The results in this study indicate that long-term survival is not impossible and reinforces our treatment strategy.
Another consideration is whether mTOR inhibitor monotherapy offers superior survival outcomes over combination with CNI. Results from the SiLVER study revealed that patients receiving sirolimus monotherapy had fewer recurrences than those receiving combination therapy. The major concern with the CNI sparing regime is the risk of acute rejection. A previous study showed that mTOR inhibitor monotherapy was associated with a significantly higher rejection rate despite combination with CNI up to 4 mo after transplant[24]. Rejection might become less of a problem at the time of recurrence (median time from transplant 12 mo). Our study results were produced with a case mix of monotherapy and combination therapy. Two episodes of biopsy proven acute rejection occurred in the combination therapy group and none occurred in the monotherapy group. However, we did not perform protocol biopsy and mild episodes of rejection were not studied. Given the low incidence of acute rejection, the current study would be underpowered to detect any differences. The sample size might well be insufficient to study any differences in survival. We can not provide any recommendations regarding monotherapy versus combination therapy.
The current study was limited by its retrospective nature. Selection bias was inevitable. The non-mTOR inhibitor group had modestly more advanced disease. The performance status of our patients was not quantified in our pre-existing database. Patients with inferior performance status could be poorer candidates for mTOR inhibitor therapy due to potential side effects. The decision to administer mTOR inhibitor was primarily based on clinical judgement and was not protocol driven. Our data could not provide recommendations for patient selection. Results from previous studies showed that the mTOR pathway was not universally upregulated in all patients transplanted for HCC[25,26]. Whether a subgroup of patients benefit more from mTOR blockade remains to be answered by future studies. In summary, the current study adds to the literature confirming the clear survival benefits of mTOR inhibitor-based immunosuppression, and provides a foundation for this therapy in post-transplant HCC recurrence. It is not too late to offer mTOR blockade following the development of recurrence.
ARTICLE HIGHLIGHTS
Research background
Mammalian target of rapamycin (mTOR) inhibitors have been shown to reduce the risk of tumour recurrence after liver transplantation for hepatocellular carcinoma (HCC). However, their role in established post-transplant HCC recurrence is uncertain.
Research motivation
It is unknown whether mTOR inhibitor still confers survival benefits following HCC recurrence. Recommendations for mTOR inhibitor under this context are based on expert opinions. To address this knowledge gap in the literature, the current study was undertaken to quantify survival following post-transplant HCC recurrence with regard to the administration of mTOR inhibitors.
Research objectives
The objective was to ascertain any survival benefits conferred by mTOR inhibitors following HCC recurrence after liver transplantation.
Research methods
A retrospective study of 143 patients who developed HCC recurrence after liver transplantation was performed. The patients were divided into 2 groups based on whether they had received mTOR inhibitor-based immunosuppression. The primary endpoint was post-recurrence survival.
Research results
Seventy-nine (55%) patients received an mTOR inhibitor-based immunosuppressive regime, while 64 (45%) patients did not. The mTOR inhibitor group had a lower number of recurrent tumours (2 vs 5, P = 0.02) and received more active treatments including radiotherapy (39 vs 22%, P = 0.03) and targeted therapy (59 vs 23%, P < 0.001). The median post-recurrence survival was 21.0 ± 4.1 mo in the mTOR inhibitor group and 11.2 ± 2.5 mo in the control group. Multivariate Cox regression analysis confirmed that mTOR inhibitor therapy was independently associated with improved post-recurrence survival (P = 0.04, OR 0.482, 95%CI: 0.241-0.966). The number of recurrent tumours and use of other treatment modalities did not affect survival. There were no survival differences between patients treated with mTOR inhibitor monotherapy and combination therapy with calcineurin inhibitor.
Research conclusions
mTOR inhibitors prolonged survival after post-transplant HCC recurrence.
Research perspectives
The role of mTOR inhibitor therapy in post-transplant HCC recurrences should be confirmed with further prospective randomized studies. A further area of study should include patient selection for mTOR inhibitor treatment following HCC recurrence.
Footnotes
Institutional review board statement: Institutional review board approval was not required for this study as it was a retrospective analysis of data and thus treatments given to patients were not affected by the study.
Informed consent statement: No informed consent forms were required as this was a retrospective cohort study.
Conflict-of-interest statement: None of the authors has any conflict of interest.
STROBE statement: The guidelines of the STROBE Statement have been adopted.
Manuscript source: Invited manuscript
Peer-review started: December 30, 2019
First decision: January 28, 2020
Article in press: March 26, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corrales FJ, Mizuguchi T S-Editor: Dou Y L-Editor: Webster JR E-Editor: Xing YX
Contributor Information
Kin Pan Au, Department of Surgery, Queen Mary Hospital, Hong Kong 999077, China.
Kenneth Siu Ho Chok, Department of Surgery and State Key Laboratory for Liver Research, The University of Hong Kong, Hong Kong 999077, China. chok6275@hku.hk.
Data sharing statement
No additional data are available.
References
- 1.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 2.Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, Pinna AD. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005;11:497–503. doi: 10.1002/lt.20391. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, Thorburn D, Briceño J, De la Mata M, Burroughs AK. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193–1199. doi: 10.1016/j.jhep.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 6.Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:411–419. doi: 10.1111/apt.12185. [DOI] [PubMed] [Google Scholar]
- 7.Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, Guo Z, He X. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 8.Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, Onaca N, Goldstein R, Levy M, Klintmalm GB. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 9.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 10.Schnitzbauer AA, Schlitt HJ, Geissler EK. Influence of immunosuppressive drugs on the recurrence of hepatocellular carcinoma after liver transplantation: a gap between basic science and clinical evidence. Transplantation. 2011;91:1173–1176. doi: 10.1097/TP.0b013e318215e72b. [DOI] [PubMed] [Google Scholar]
- 11.Schnitzbauer AA, Zuelke C, Graeb C, Rochon J, Bilbao I, Burra P, de Jong KP, Duvoux C, Kneteman NM, Adam R, Bechstein WO, Becker T, Beckebaum S, Chazouillères O, Cillo U, Colledan M, Fändrich F, Gugenheim J, Hauss JP, Heise M, Hidalgo E, Jamieson N, Königsrainer A, Lamby PE, Lerut JP, Mäkisalo H, Margreiter R, Mazzaferro V, Mutzbauer I, Otto G, Pageaux GP, Pinna AD, Pirenne J, Rizell M, Rossi G, Rostaing L, Roy A, Turrion VS, Schmidt J, Troisi RI, van Hoek B, Valente U, Wolf P, Wolters H, Mirza DF, Scholz T, Steininger R, Soderdahl G, Strasser SI, Jauch KW, Neuhaus P, Schlitt HJ, Geissler EK. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer. 2010;10:190. doi: 10.1186/1471-2407-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Turrión VS, Jamieson N, Scholz T, Colledan M, Fändrich F, Becker T, Söderdahl G, Chazouillères O, Mäkisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Königsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116–125. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au KP, Chok KSH. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: A proposed management algorithm. World J Gastroenterol. 2018;24:5081–5094. doi: 10.3748/wjg.v24.i45.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piñero F, Marciano S, Anders M. Sorafenib for Recurrent Hepatocellular Carcinoma after Liver Transplantation: A South American Experience. Acta Gastroenterol Latinoam. 2016;46:300–309. [Google Scholar]
- 15.Fallah-Rad N, Cao Y, Knox JJ, Jang RWJ, Dhani NC, Sapisochin G, Grant D, Greig PD, Lilly L, Chen E. Sorafenib treatment in recurrent hepatocellular carcinoma post liver transplantation. J Clin Oncol. 2017;35:479–479. [Google Scholar]
- 16.Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275–280. doi: 10.1016/j.ejso.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C, Grant DR, Lázaro JL, Caralt M, Ghanekar A, McGilvray ID, Lilly L, Cattral MS, Selzner M, Charco R, Greig PD. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286–2294. doi: 10.1245/s10434-014-4273-6. [DOI] [PubMed] [Google Scholar]
- 18.Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg. 2017;266:118–125. doi: 10.1097/SLA.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440–447. doi: 10.1002/lt.24742. [DOI] [PubMed] [Google Scholar]
- 20.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–8; discussion 188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 22.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 23.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, Duvoux C, Nevens F, Fung JJ, Dong G, Rauer B, Junge G H2304 Study Group. Three-year Outcomes in De Novo Liver Transplant Patients Receiving Everolimus With Reduced Tacrolimus: Follow-Up Results From a Randomized, Multicenter Study. Transplantation. 2015;99:1455–1462. doi: 10.1097/TP.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 25.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 26.Sieghart W, Fuereder T, Schmid K, Cejka D, Werzowa J, Wrba F, Wang X, Gruber D, Rasoul-Rockenschaub S, Peck-Radosavljevic M, Wacheck V. Mammalian target of rapamycin pathway activity in hepatocellular carcinomas of patients undergoing liver transplantation. Transplantation. 2007;83:425–432. doi: 10.1097/01.tp.0000252780.42104.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.