Abstract
BACKGROUND
Although surgical resection is associated with the best long-term outcomes for neuroendocrine liver metastases (NELM), the current indications for and outcomes of surgery for NELM from a population perspective are not well understood.
AIM
To determine the current indications for and outcomes of liver resection (LR) for NELM using a population-based cohort.
METHODS
A retrospective review of the 2014-2017 American College of Surgeons National Surgical Quality Improvement Program and targeted hepatectomy databases was performed to identify patients who underwent LR for NELM. Perioperative characteristics and 30-d morbidity and mortality were analyzed.
RESULTS
Among 669 patients who underwent LR for NELM, the median age was 60 (interquartile range: 51-67) and 51% were male. While the number of metastases resected ranged from 1 to 9, the most common (45%) number of tumors resected was one. The majority (68%) of patients had a largest tumor size of < 5 cm. Most patients underwent partial hepatectomy (71%) while fewer underwent a right or left hepatectomy or trisectionectomy. The majority of operations were open (82%) versus laparoscopic (17%) or robotic (1%). In addition, 30% of patients underwent intraoperative ablation while 45% had another concomitant operation including cholecystectomy (28.8%), bowel resection (20.2%), or partial pancreatectomy (3.4%). Overall 30-d morbidity and mortality was 29% and 1.3%, respectively. On multivariate analysis, American Society of Anesthesiologists class ≥ 3 [odds ratios (OR), OR = 2.089, 95% confidence intervals (CI): 1.197-3.645], open approach (OR = 1.867, 95%CI: 1.148-3.036), right hepatectomy (OR = 1.618, 95%CI: 1.014-2.582), and prolonged operative time of > 230 min (OR = 1.731, 95%CI: 1.168-2.565) were associated with higher 30-d morbidity while intraoperative ablation and concomitant procedures were not.
CONCLUSION
LR for NELM was performed with relatively low postoperative morbidity and mortality. Concomitant procedures performed at the time of LR did not increase morbidity.
Keywords: Carcinoid, Neuroendocrine tumor, Primary tumor resection, Intraoperative ablation, Cholecystectomy, Small bowel resection
Core tip: Surgical resection of neuroendocrine liver metastases is associated with the best long-term outcomes, however the current indications for and outcomes of surgery are not well understood. In this study, we performed a retrospective review of the 2014-2017 American College of Surgeons National Surgical Quality Improvement Program to identify 669 patients who underwent liver resection to define characteristics associated with increased 30-d postoperative morbidity and mortality. Overall morbidity and mortality were relatively low at 29% and 1.3% respectively. Factors associated with increased 30-d morbidity included open and prolonged cases (> 230 min), right hepatectomy, and American Society of Anesthesiologists class ≥ 3 while concomitant procedures including intraoperative ablation did not influence morbidity.
INTRODUCTION
Neuroendocrine tumors (NET) are a heterogeneous group of neoplasms that can occur anywhere in the body but commonly arise from the gastrointestinal tract. While relatively rare, the incidence and prevalence of NETs are steadily increasing, at least in part due to improved imaging and diagnostic techniques[1,2]. Despite their low grade nature, a substantial proportion (60%-80%) of well-differentiated NETs are diagnosed with or will develop neuroendocrine liver metastases (NELM), which is one of the strongest prognostic factors among patients with NETs[3]. For example, the 5-year overall survival of patients with untreated NELM range from 13% to 54%, compared with 61% to 79% among individuals who undergo treatment[4-8].
Multiple treatments exist for patients with NELM including surgical resection, ablative techniques, transarterial therapies, somatostatin analogs, cytotoxic chemotherapy, targeted therapies, and peptide receptor radionuclide therapy[4]. Other novel systemic and targeted therapies are rapidly emerging[9,10]. Despite the absence of level I evidence, surgical resection is associated with the best long-term outcomes based on retrospective cohort studies and meta-analyses[6,11,12]. Indeed, even cytoreductive surgery (i.e., surgical debulking) of NELM has been associated with improved overall survival if residual disease less than 10%-30% can be achieved[5,13-15]. Based on these data, surgical resection of NELM has been recommended as the preferred initial approach, when feasible, by the European Neuroendocrine Tumors Society and North American Neuroendocrine Tumors Society[16,17].
Previous studies evaluating the short-term outcomes of surgery for NELM have frequently been limited by their retrospective, single-institution nature[18-22]. Other more recent multi-institutional studies have been limited to high-volume institutions and conducted over long study periods[6,14,23]. Thus, there is a need for an evaluation of contemporary practice patterns and outcomes from a population-based perspective. Such information would inform patient selection and facilitate patient education and the informed consent process. Therefore, we utilized the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) targeted hepatectomy database to analyze contemporary characteristics of patients with NELM who underwent liver resection (LR) in the United States and evaluate factors associated with postoperative morbidity and mortality.
MATERIALS AND METHODS
Data acquisition and study population
The ACS-NSQIP data set is a national validated and risk adjusted outcomes based database that includes demographic, clinical, perioperative, and 30-d postoperative details of patients undergoing surgery from 600 eligible hospitals across the United States. The ACS-NSQIP uses a systemic sampling process to ensure that each case has an equal chance of selection and it is frequently monitored to minimize sample bias. A retrospective review of the 2014-2017 ACS-NSQIP and targeted hepatectomy databases were performed and patients were matched based on case ID numbers. All adult patients undergoing LR were identified using Current Procedural Terminology codes 47120, 47122, 47125, and 47130.
Study variables and outcomes
Independent variables included demographic, preoperative health status, relevant comorbidities, operative, and postoperative outcomes. Demographics included age and gender. Preoperative health included American Society of Anesthesiologists (ASA) classification, body mass index, weight loss within 6 mo from surgery, smoking, chronic steroid use, preoperative sepsis (systemic inflammatory response syndrome or septic shock) and preoperative transfusion. Comorbidities included diabetes mellitus, chronic obstructive pulmonary disease, hypertension requiring medications, bleeding disorders, congestive heart failure. Targeted hepatectomy variables included neoadjuvant therapy within 90 d of surgery, ascites, viral hepatitis, and pre-operative biliary stent placement. Operative variables consisted of operative approach (open, laparoscopic, robotic), type of resection (trisegmentectomy, total right or left hemihepatectomy, or partial hepatectomy), concomitant procedures (cholecystectomy, intestinal resection, partial pancreatectomy), size of metastatic lesion (< 2, 2-5, or > 5 cm) number of metastatic lesions (1-2, 3-5, or > 5), number of concurrent partial hepatectomies (0, 1-5, 6-9, or > 10), liver texture (normal, congested, cirrhotic or fatty), operative time, pringle maneuver during resection, concomitant intraoperative ablation (IA), and biliary reconstruction.
Overall morbidity included each of the following within 30 d from the date of surgery: Superficial, deep and organ/space surgical site infection, sepsis, respiratory complications including pneumonia or reintubation, pulmonary embolism, deep vein thrombosis, myocardial infarct, cardiac arrest, stroke, renal complications such as renal insufficiency or urinary tract infection, hemorrhage requiring at least 4 U of packed red blood cells, bile leak, liver intervention post hepatectomy and pos-toperative liver failure. Perioperative mortality was also measured and defined as death within 30 d after LR. Length of stay, discharge disposition, 30-d readmission and reoperation were also among the postoperative measures assessed.
Statistical analysis
Descriptive statistics were reported as percentages of the total number of patients in the study. Univariate and multivariate analyses were used to identify factors associated with the development of overall morbidity and mortality within 30 d of surgery. Logistic regression analysis was used for univariate analysis. Stepwise logistic regression analysis was used for multivariable analysis and included all non-collinear variables. Results are reported as odds ratios (OR) and 95% confidence intervals (CI). Analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, United States). P values < 0.05 were considered statistically significant. All statistics were performed by an experienced biostatistician.
RESULTS
Patient characteristics
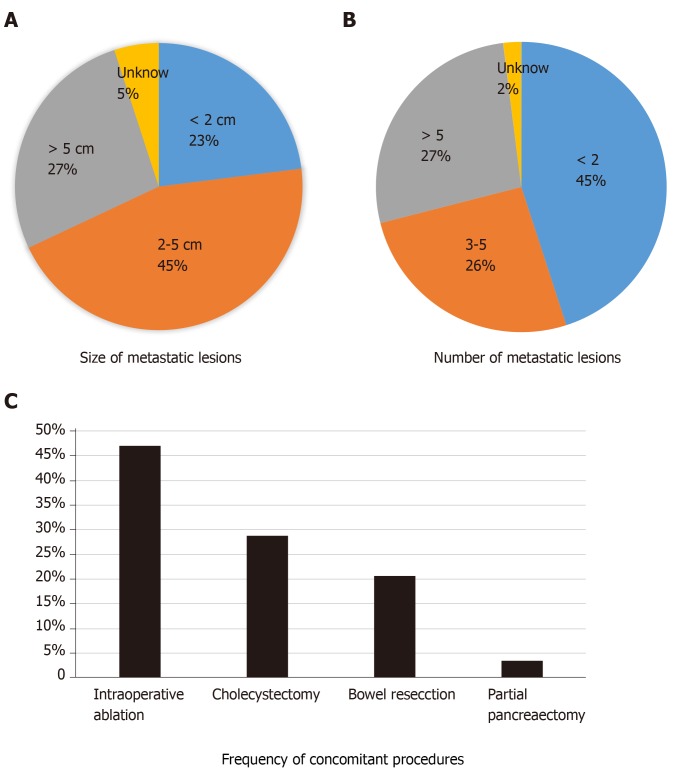
Between 2014-2017, 669 patients were identified within the ACS-NSQIP database who underwent LR for NELM. Complete demographic and clinicopathologic criteria of the cohort are listed in Table 1. The median age was 60 [interquartile range (IQR): 51-67], 51% were male and the mean body mass index was 27.4 (IQR: 24-32). Most patients underwent partial hepatectomy (71%) while fewer underwent hemihepatectomy (23%) or trisegmentectomy (6%). The majority of operations were open (82%); fewer were laparoscopic (17%) or robotic (1%). The mean number of lesions resected was 3.8 (range 1-9) and the most common size of the largest tumor was 2-5 cm (45%) (Figure 1A and B). Among all patients, 30.6% underwent concomitant IA in conjunction with a LR. In addition, 45.4% of patients underwent a combined resection in addition to the LR. The most common concomitant procedure was a cholecystectomy (28.8%), followed by intestinal resection (20.2%) and pancreatectomy (3.4%) (Figure 1C).
Table 1.
Demographic, clinical, and operative characteristics of patients with neuroendocrine liver metastases undergoing resection
| NELM (n = 669) | |
| Median age in years, n (IQR) | 60 (51-67) |
| Male gender, n (%) | 341 (51) |
| ASA classification, n (%) | |
| I | 2 (0.3) |
| II | 143 (21) |
| III | 459 (69) |
| IV | 61 (9) |
| Median BMI (kg/m2), n (IQR) | 27.4 (24-32) |
| Comorbidities/preoperative | |
| > 10% loss body weight in last 6 mo, n (%) | 30 (4.4) |
| Diabetes mellitus with oral agents or insulin, n (%) | 115 (17) |
| Current smoker within one yr, n (%) | 79 (12) |
| Severe chronic obstructive pulmonary disease, n (%) | 9 (0.3) |
| Congestive heart failure in 30 d before surgery, n (%) | 5 (0.7) |
| Hypertension requiring medications, n (%) | 326 (49) |
| Viral hepatitis, n (%) | 13 (1.9) |
| Preoperative biliary stent, n (%) | 13 (1.9) |
| Ascites within 30 d, n (%) | 3 (0.4) |
| Preoperative sepsis, n (%) | 2 (0.3) |
| Steroid use for a chronic condition, n (%) | 20 (3) |
| Bleeding disorders, n (%) | 18 (2.6) |
| Preoperative transfusion, n (%) | 3 (0.4) |
| Neoadjuvant therapy, n (%) | 119 (17.7) |
| Patients with concomitant procedure (%) | 304 (45.4) |
| Total number of cholecystectomy | 193 (28.8) |
| Total number of small/large bowel resection | 135 (20.2) |
| Total number of partial pancreatectomy | 23 (3.4) |
| Operative approach, n (%) | |
| Open | 546 (82) |
| Laparoscopic | 113 (17) |
| Robotic | 10 (1) |
| Liver resection type, n (%) | |
| Trisegmentectomy | 42 (6) |
| Right hepatectomy | 99 (15) |
| Left hepatectomy | 52 (8) |
| Partial lobectomy | 476 (71) |
| Size of metastatic lesion, n (%) | |
| < 2 cm | 152 (23) |
| 2-5 cm | 303 (45) |
| > 5 cm | 181 (27) |
| Unknown | 33 (5) |
| Number of metastatic lesions, n (%) | |
| < 2 | 298 (45) |
| 3-5 | 168 (26) |
| > 5 | 166 (27) |
| Unknown | 37 (2) |
| Concurrent partial liver resections, n (%) | |
| 0 | 205 (30.6) |
| 1-5 | 385 (57.5) |
| 6-9 | 44 (6.6) |
| > 10 | 5 (0.7) |
| Unknown | 30 (4.5) |
| Liver texture, n (%) | |
| Normal | 190 (28) |
| Congested | 8 (1) |
| Cirrhotic | 12 (2) |
| Fatty | 57 (9) |
| Unknown | 402 (60) |
| Median optime in minutes, n (IQR) | 232 (179-297) |
| Pringle maneuver during resection, n (%) | 161 (24) |
| Biliary reconstruction, n (%) | 14 (2) |
| Intraoperative ablation, n (%) | 205 (30) |
| Drain placement | 253 (38) |
NELM: Neuroendocrine liver metastases; IQR: Interquartile range; BMI: Body mass index; ASA: American Society of Anesthesiology.
Figure 1.
Indications for liver resection for neuroendocrine liver metastases. Overall size (A) and number (B) of neuroendocrine liver metastases and frequency of concomitant procedures including intraoperative ablation (C) among patients undergoing liver resection for neuroendocrine liver metastases.
Perioperative outcomes
Table 2 reports the postoperative outcomes of patients undergoing resection of NELM. Median operative time was 232 min (IQR: 179-297) and the mean length of stay was 6 (IQR: 4-8) d. The overall 30-d complication rate was 29% with the most common perioperative complications being perioperative transfusion (15.5%), intraabdominal infection (7.3%), bile leakage (5.9%), sepsis (5.6%), surgical site infection (3.5%), reoperation (3.1%), pneumonia (2.9%), liver failure (2.6%), and pulmonary embolism (2%). Of note, serious adverse events such as stroke, cardiac arrest, and myocardial infarction occurred in less than 1% of all patients undergoing resection for NELM. Postoperative mortality occurred in 1.3%. The vast majority (95%) of patients were able to be discharged home and readmission was required in 11.2% of the patients.
Table 2.
Postoperative outcomes of patients with neuroendocrine liver metastases undergoing liver resection
| NELM (n = 669) | |
| Post-hepatectomy | |
| Bile leakage, n (%) | 40 (5.9) |
| Post hepatectomy invasive intervention, n (%) | 65 (9.7) |
| Post hepatectomy liver failure, n (%) | 18 (2.6) |
| Specific complications | |
| Superficial surgical site infection, n (%) | 24 (3.5) |
| Deep incisional surgical site infection, n (%) | 4 (0.6) |
| Organ/space surgical site infection, n (%) | 49 (7.3) |
| Bleeding requiring transfusion, n (%) | 104 (15.5) |
| Unplanned re-intubation, n (%) | 9 (1.3) |
| Pneumonia, n (%) | 20 (2.9) |
| Pulmonary embolism, n (%) | 14 (2) |
| Progressive renal insufficiency, n (%) | 7 (1.1) |
| Urinary tract infection, n (%) | 14 (2) |
| Stroke, n (%) | 1 (0.1) |
| Cardiac arrest, n (%) | 2 (0.3) |
| Myocardial infarction, n (%) | 3 (0.4) |
| Deep venous thrombosis/thrombophlebitis, n (%) | 9 (1.4) |
| Sepsis, n (%) | 38 (5.6) |
| Overall | |
| Median length of hospital stay in days, n (IQR) | 6 (4-8) |
| Discharge destination to home, n (%) | 637 (95.2) |
| 30-d readmission, n (%) | 75 (11.2) |
| Reoperation, n (%) | 21 (3.1) |
| 30-d overall morbidity, n (%) | 194 (29) |
| Mortality, n (%) | 9 (1.3) |
NELM: Neuroendocrine liver metastases; IQR: Interquartile range.
Predictors of postoperative morbidity
Factors associated with 30-d morbidity on univariate analysis are reported in Table 3. ASA class of ≥ 3 (OR = 2.418, 95%CI: 1.422-4.113, P = 0.0011), open approach (OR = 1.943, 95%CI: 1.218-3.102, P = 0.0053), right, left or trisection hepatectomy (OR = 1.660, 95%CI 1.169-2.355, P = 0.0046), and operative time (> 230 min, OR = 2.403, 95%CI: 1.407-2.968, P = 0.0002) were all associated with increased morbidity while IA was associated with a decrease in perioperative morbidity (OR = 0.686, 95%CI: 0.476-0.988, P = 0.0431). Interestingly, the use of concomitant procedures (including bowel resections, cholecystectomy, or pancreatectomy), as well as the size or number of tumors were not associated with postoperative morbidity.
Table 3.
Significant predictors of 30-d overall morbidity among patients undergoing hepatectomy for neuroendocrine liver metastases based on univariate logistic regression analysis
| OR | 95%CI | P value | |
| Age > 60 | 0.931 | 0.658-1.316 | 0.6835 |
| Male gender | 1.232 | 0.890-1.707 | 0.2092 |
| ASA class ≥ 3 | 2.418 | 1.422-4.113 | 0.0011 |
| BMI > 27 | 1.030 | 0.743-1.428 | 0.8596 |
| Preop biliary stent | 2.562 | 0.850-7.719 | 0.0946 |
| Viral hepatitis | 2.538 | 0.841-7.660 | 0.0983 |
| Concomitant bowel resection | 1.278 | 0.885-1.844 | 0.1906 |
| Concomitant cholecystectomy | 1.094 | 0.749-1.598 | 0.6431 |
| Concomitant pancreatectomy | 1.579 | 0.626-3.984 | 0.3335 |
| Open approach | 1.943 | 1.218-3.102 | 0.0053 |
| Size < 2 cm (ref) | |||
| Size 2-5 cm | 0.989 | 0.645-1.516 | 0.9591 |
| Size > 5 cm | 1.397 | 0.882-2.215 | 0.1546 |
| Number of tumors > 1 | 0.984 | 0.681-1.422 | 0.9317 |
| Right/left/triseg hepatectomy | 1.660 | 1.169-2.355 | 0.0046 |
| Abnormal liver texture | 1.340 | 0.818-2.193 | 0.2447 |
| Intraoperative ablation | 0.686 | 0.476-0.988 | 0.0431 |
| Biliary reconstruction | 3.979 | 1.317-12.023 | 0.0144 |
| Operative time > 230 min | 2.043 | 1.407-2.968 | 0.0002 |
| Pringle | 1.429 | 0.986-2.070 | 0.0593 |
OR: Odds ratio; CI: Confidence intervals; ASA: American Society of Anesthesiologists; BMI: Body mass index.
On multivariable logistic regression, ASA class of ≥ 3 (OR = 2.089, 95%CI: 1.197-3.645, P = 0.0095), open approach (OR = 1.867, 95%CI: 1.148-3.036, P = 0.0118), right, left or trisegmental hepatectomy (OR = 1.618, 95%CI: 1.014-2.582, P = 0.0437), and operative time > 230 min (OR = 1.731, 95%CI: 1.168-2.565, P = 0.0062) were independently associated with increased morbidity while normal liver texture was protective of overall morbidity (OR = 0.641, 95%CI: 0.433-0.950, P = 0.0266) (Table 4).
Table 4.
Significant predictors of 30-d overall morbidity among patients undergoing hepatectomy for neuroendocrine liver metastases based on multivariate stepwise logistic regression analysis
| OR | 95%CI | P value | |
| ASA class ≥ 3 | 2.089 | 1.197-3.645 | 0.0095 |
| Normal liver texture | 0.641 | 0.433-0.950 | 0.0266 |
| Open approach | 1.867 | 1.148-3.036 | 0.0118 |
| Right hepatectomy | 1.618 | 1.014-2.582 | 0.0437 |
| Intraoperative ablation | 0.697 | 0.473-1.029 | 0.0697 |
| Biliary reconstruction | 2.802 | 0.870-9.021 | 0.0842 |
| Operative time > 230 min | 1.731 | 1.168-2.565 | 0.0062 |
OR: Odds ratio; CI: Confidence intervals; ASA: American Society of Anesthesiologists.
DISCUSSION
The incidence of NETs is increasing worldwide and a majority of patients will present with metastatic disease in their liver[1]. NELM is a strong negative prognostic factor for survival and is associated with significant reductions in patient quality of life[24]. While several systemic and liver-directed therapies are available, surgical resection is typically recommended when feasible[25]. In this paper, we used a contemporary, population-based, prospective database to define the characteristics and outcomes of patients undergoing surgery for NELM in the United States. These results highlight several important findings. First, the majority of operations are being performed for small tumors in the setting of multifocal disease and typically are minor resections. Second, a significant proportion of cases are being performed concomitant with another operation, either liver IA, cholecystectomy, or (presumably) primary tumor resection. Finally, modern surgery for NELM can be performed with relatively minimal postoperative morbidity (29%) and mortality (1.3%). These results are critical for informed preoperative discussions with patients as well as future comparative effectiveness research with other liver-directed treatments.
Recent advances in the perioperative management of patients undergoing LR have improved the safety of hepatectomy and expanded criteria for selecting patients for surgery. Indeed, a recent study by Cloyd et al[26] evaluated nearly 4000 patients who underwent LR over two decades and noted steady improvements in postoperative morbidity despite increases in case complexity. Improvements in the outcomes of LR are likely multifactorial but improved patient selection, evaluation and optimization, are paramount. Accurate liver volumetry and future liver remnant augmentation have been important strategies for minimizing post hepatectomy and postoperative liver failure[27,28]. Improved perioperative and anesthetic care, including reduced intravenous fluid administration and less blood loss, have similarly been critical advances in contemporary hepatic surgery. The introduction of enhanced recovery after surgery processes have further contributed to reduced morbidity following surgery and are now commonly routinized at major medical centers[29]. Finally, implementation of laparoscopic and robotic approaches for surgery have led to reduced postoperative pain, blood loss, and length of hospital stay with similar outcomes compared with open approaches[30-33].
Prior studies evaluating the role of IA for NELM have demonstrated that this therapeutic approach is generally well tolerated and is indicated for patients whose tumors are not amenable to resection[6,34] though the safety of IA during surgery for NELM has not been thoroughly evaluated[34-36]. In this study, we noted that IA was associated with decreased 30-d morbidity in univariate analysis though this association did not persist on multivariate analysis. These findings are consistent with a recent study evaluating IA during resection of colorectal liver metastasis which found lower overall morbidity, hospital length of stay, and readmission rates in patients who underwent LR and IA compared to patients who underwent LR alone[37]. Based on these results and others, IA appears to be a safe and effective strategy to expand the surgical options for patients with multifocal NELM.
While the role of primary tumor resection in the setting of unresectable NELM remains controversial, resection of the primary NET is indicated when liver metastases are resectable[19,38-41]. The current study suggests that resection of the primary (e.g., pancreatectomy, intestinal resection) can be performed safely and is not associated with increased postoperative morbidity. These findings are consistent with the large body of literature which suggests that most LRs for colorectal liver metastasis can be performed safely in a combined fashion with standard colorectal resections[42,43].
While the ACS-NSQIP targeted hepatectomy database has the advantage of containing hepatectomy-specific perioperative variables, a limitation of the current database was the lack of cancer- and patient-specific information. For example, the database lacked relevant information such as the symptomatic status of patients, functional status of tumors, tumor grade, or presence of extra-hepatic disease. In addition, it lacked Furthermore, as the study was limited to the 30-d postoperative period, we were unable to describe the long-term efficacy of LR for NELM. However, the purpose of the current study was to evaluate the indications for and short-term outcomes of surgery for NELM. Multiple prior studies have found that LR for NELM is associated with good long-term survival[4,6,44]. Similarly, the ACS-NSQIP database did not have information on carcinoid crisis, however, previous studies have shown this to be a relatively rare event[45,46]. This study had several other limitations, primarily related to its retrospective nature and the fact that data were limited to the 30-d postoperative period which may be insufficient to capture all complications.
In conclusion, in this contemporary population-based analysis, we demonstrated that LR can be performed for NELM with relatively low postoperative morbidity and mortality. Concomitant operations such as cholecystectomy, bowel resection, pancreatectomy, and IA can safely be performed and do not contribute to increased morbidity. Careful patient selection, minimizing operative time, and utilizing minimally invasive surgical approaches may help reduce postoperative morbidity. While multiple therapeutic options exist for NELM, given the excellent long-term outcomes observed in the literature and the satisfactory short-term outcomes demonstrated herein, surgical resection should remain the standard of care when feasible.
ARTICLE HIGHLIGHTS
Research background
Multiple liver-directed therapies, including hepatic resection, exist for patients with neuroendocrine liver metastases (NELM). While surgical resection is associated with the best long-term outcomes, the current indications for and outcomes of surgery for NELM from a population perspective are not well understood.
Research motivation
A better understanding of the frequency and predictors of postoperative complications will improve shared-decision making for patients with NELM, especially given the expanding number of liver-directed and systemic therapies available.
Research objectives
The purpose of the current study was to define the current indications for surgery for NELM, characterize the short-term outcomes of patients undergoing surgery, and evaluate predictors of complications using a population-based approach.
Research methods
A retrospective review of the 2014-2017 American College of Surgeons National Surgical Quality Improvement Program targeted hepatectomy database was performed to identify patients who underwent hepatic resection for NELM. Perioperative characteristics and 30-d morbidity and mortality were analyzed.
Research results
Among 669 patients who underwent liver resection for NELM, the number of metastases resected ranged from 1 to 9 though the most common (45%) number of tumors resected was one. The majority (68%) of patients had a largest tumor size of < 5 cm and most patients underwent partial hepatectomy (71%). The majority of operations were open (82%) versus laparoscopic (17%) or robotic (1%). In addition, 30% of patients underwent intraoperative ablation while 45% had another concomitant operation including cholecystectomy (28.8%), bowel resection (20.2%), or partial pancreatectomy (3.4%). Overall 30-d morbidity and mortality was 29% and 1.3%, respectively. On multivariate analysis, American Society of Anesthesiologists class ≥ 3, open approach, formal hemi-hepatectomy or trisectionectomy, and prolonged operative time were associated with higher 30-d morbidity. Concomitant procedures including intraoperative ablation, small bowel resection, or pancreatectomy were not independently associated with higher morbidity.
Research conclusions
In this contemporary population-based analysis, we demonstrated that hepatic resection can be performed with relatively low postoperative morbidity and mortality for patients with NELM. Concomitant operations such as cholecystectomy, bowel resection, pancreatectomy, and liver ablation can safely be performed and do not contribute to increased morbidity. Careful patient selection, minimizing operative time, and utilizing minimally invasive approaches may help reduce postoperative morbidity. While multiple therapeutic options exist for NELM, given the excellent long-term outcomes observed in the literature and the satisfactory short-term outcomes demonstrated in the current study, surgical resection should remain the standard of care when feasible.
Research perspectives
This study highlights the current population-based indications for liver resection for patients with neuroendocrine liver metastases and confirms satisfactory short-term outcomes. In light of these findings, future research should focus on expanding the indications for hepatic resection particularly given the increasing number of liver-directed and systemic therapy options available. Future prospective studies should evaluate the optimal sequencing of liver-directed therapies including neoadjuvant and adjuvant strategies to improve long-term outcomes.
Footnotes
Institutional review board statement: Not applicable as data was de-identified and publicly available.
Informed consent statement: Not applicable as data was de-identified and publicly available.
Conflict-of-interest statement: We have no financial relationships to disclose.
Manuscript source: Invited manuscript
Peer-review started: November 21, 2019
First decision: December 13, 2019
Article in press: March 5, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Campana D, Nacak M, Vagholkar K, Mrzljak A S-Editor: Zhou JJ L-Editor: A E-Editor: Wu YXJ
Contributor Information
Steven D Scoville, Department of Surgery, Division of Surgical Oncology at The Ohio State University, James Cancer Center, Columbus, OH 43210, United States; The Arthur G James Comprehensive Cancer Center and Solove Research Institute, The Ohio State University, Columbus, OH 43210, United States.
Dimitrios Xourafas, Department of Surgery, Division of Surgical Oncology at The Ohio State University, James Cancer Center, Columbus, OH 43210, United States; Department of Surgery, Harvard Medical School, Department of Surgery, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, United States.
Aslam M Ejaz, Department of Surgery, Division of Surgical Oncology at The Ohio State University, James Cancer Center, Columbus, OH 43210, United States; Department of Surgery, The Ohio State University, Columbus, OH 43210, United States.
Allan Tsung, Department of Surgery, Division of Surgical Oncology at The Ohio State University, James Cancer Center, Columbus, OH 43210, United States; Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Timothy Pawlik, Department of Surgery, Division of Surgical Oncology at The Ohio State University, James Cancer Center, Columbus, OH 43210, United States; Department of Surgery, The Ohio State University, Columbus, OH 43210, United States.
Jordan M Cloyd, Department of Surgery, Division of Surgical Oncology at The Ohio State University, James Cancer Center, Columbus, OH 43210, United States; Department of Surgery, The Ohio State University, Columbus, OH 43210, United States; Department of Surgery, Division of Surgical Oncology, The Ohio State University Wexner Medical Center, Columbus, OH 43210, United States. jordan.cloyd@osumc.edu.
Data sharing statement
No additional data are available.
References
- 1.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MR, Harris C, Baeg KJ, Aronson A, Wisnivesky JP, Kim MK. Incidence Trends of Gastroenteropancreatic Neuroendocrine Tumors in the United States. Clin Gastroenterol Hepatol. 2019;17:2212–2217.e1. doi: 10.1016/j.cgh.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Spolverato G, Bagante F, Wagner D, Buettner S, Gupta R, Kim Y, Maqsood H, Pawlik TM. Quality of life after treatment of neuroendocrine liver metastasis. J Surg Res. 2015;198:155–164. doi: 10.1016/j.jss.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Farley HA, Pommier RF. Treatment of Neuroendocrine Liver Metastases. Surg Oncol Clin N Am. 2016;25:217–225. doi: 10.1016/j.soc.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 6.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB, Chu CK, Ferrero A, Schulick RD, Choti MA, Mentha G, Strub J, Bauer TW, Adams RB, Aldrighetti L, Capussotti L, Pawlik TM. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 8.Bacchetti S, Bertozzi S, Londero AP, Uzzau A, Pasqual EM. Surgical treatment and survival in patients with liver metastases from neuroendocrine tumors: a meta-analysis of observational studies. Int J Hepatol. 2013;2013:235040. doi: 10.1155/2013/235040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloyd JM, Konda B, Shah MH, Pawlik TM. The emerging role of targeted therapies for advanced well-differentiated gastroenteropancreatic neuroendocrine tumors. Expert Rev Clin Pharmacol. 2019;12:101–108. doi: 10.1080/17512433.2019.1561273. [DOI] [PubMed] [Google Scholar]
- 10.Scoville SD, Cloyd JM, Pawlik TM. New and emerging systemic therapy options for well-differentiated gastroenteropancreatic neuroendocrine tumors. Expert Opin Pharmacother. 2020;21:183–191. doi: 10.1080/14656566.2019.1694003. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather M, Swanson R, Wang J, Brais LK, Dutton T, Kulke MH, Clancy TE. Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann Surg Oncol. 2017;24:2319–2325. doi: 10.1245/s10434-017-5839-x. [DOI] [PubMed] [Google Scholar]
- 12.Woltering EA, Voros BA, Beyer DT, Wang YZ, Thiagarajan R, Ryan P, Wright A, Ramirez RA, Ricks MJ, Boudreaux JP. Aggressive Surgical Approach to the Management of Neuroendocrine Tumors: A Report of 1,000 Surgical Cytoreductions by a Single Institution. J Am Coll Surg. 2017;224:434–447. doi: 10.1016/j.jamcollsurg.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Morgan RE, Pommier SJ, Pommier RF. Expanded criteria for debulking of liver metastasis also apply to pancreatic neuroendocrine tumors. Surgery. 2018;163:218–225. doi: 10.1016/j.surg.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Ejaz A, Reames BN, Maithel S, Poultsides GA, Bauer TW, Fields RC, Weiss MJ, Marques HP, Aldrighetti L, Pawlik TM. Cytoreductive debulking surgery among patients with neuroendocrine liver metastasis: a multi-institutional analysis. HPB (Oxford) 2018;20:277–284. doi: 10.1016/j.hpb.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Scott AT, Breheny PJ, Keck KJ, Bellizzi AM, Dillon JS, O'Dorisio TM, Howe JR. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs) Surgery. 2019;165:166–175. doi: 10.1016/j.surg.2018.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Toole D, Kianmanesh R, Caplin M. ENETS 2016 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology. 2016;103:117–118. doi: 10.1159/000443169. [DOI] [PubMed] [Google Scholar]
- 17.Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, Law CH, Liu EH, Kim MK, Menda Y, Morse BG, Bergsland EK, Strosberg JR, Nakakura EK, Pommier RF. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46:715–731. doi: 10.1097/MPA.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen EH, Kvols L, McLoughlin JM, Lewis JM, Alvarado MD, Yeatman T, Malafa M, Shibata D. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol. 2007;14:780–785. doi: 10.1245/s10434-006-9148-z. [DOI] [PubMed] [Google Scholar]
- 19.Boudreaux JP, Wang YZ, Diebold AE, Frey DJ, Anthony L, Uhlhorn AP, Ryan P, Woltering EA. A single institution's experience with surgical cytoreduction of stage IV, well-differentiated, small bowel neuroendocrine tumors. J Am Coll Surg. 2014;218:837–844. doi: 10.1016/j.jamcollsurg.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 20.de Mestier L, Neuzillet C, Hentic O, Kianmanesh R, Hammel P, Ruszniewski P. Prolonged survival in a patient with neuroendocrine tumor of the cecum and diffuse peritoneal carcinomatosis. Case Rep Gastroenterol. 2012;6:205–210. doi: 10.1159/000338740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–51; discussion 651-653. doi: 10.1016/j.surg.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Chung MH, Pisegna J, Spirt M, Giuliano AE, Ye W, Ramming KP, Bilchik AJ. Hepatic cytoreduction followed by a novel long-acting somatostatin analog: a paradigm for intractable neuroendocrine tumors metastatic to the liver. Surgery. 2001;130:954–962. doi: 10.1067/msy.2001.118388. [DOI] [PubMed] [Google Scholar]
- 23.Sham JG, Ejaz A, Gage MM, Bagante F, Reames BN, Maithel S, Poultsides GA, Bauer TW, Fields RC, Weiss MJ, Marques HP, Aldrighetti L, Pawlik TM, He J. The Impact of Extent of Liver Resection Among Patients with Neuroendocrine Liver Metastasis: an International Multi-institutional Study. J Gastrointest Surg. 2019;23:484–491. doi: 10.1007/s11605-018-3862-2. [DOI] [PubMed] [Google Scholar]
- 24.Frilling A, Sotiropoulos GC, Li J, Kornasiewicz O, Plöckinger U. Multimodal management of neuroendocrine liver metastases. HPB (Oxford) 2010;12:361–379. doi: 10.1111/j.1477-2574.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, Davidson B, Siegler M, Caplin M, Solcia E, Schilsky R Working Group on Neuroendocrine Liver Metastases. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–21. doi: 10.1016/S1470-2045(13)70362-0. [DOI] [PubMed] [Google Scholar]
- 26.Cloyd JM, Mizuno T, Kawaguchi Y, Lillemoe HA, Karagkounis G, Omichi K, Chun YS, Conrad C, Tzeng CD, Odisio BC, Huang SY, Hicks M, Wei SH, Aloia TA, Vauthey JN. Comprehensive Complication Index Validates Improved Outcomes Over Time Despite Increased Complexity in 3707 Consecutive Hepatectomies. Ann Surg. 2020;271(4):724–731. doi: 10.1097/SLA.0000000000003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rassam F, Olthof PB, Bennink RJ, van Gulik TM. Current Modalities for the Assessment of Future Remnant Liver Function. Visc Med. 2017;33:442–448. doi: 10.1159/000480385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahnemai-Azar AA, Cloyd JM, Weber SM, Dillhoff M, Schmidt C, Winslow ER, Pawlik TM. Update on Liver Failure Following Hepatic Resection: Strategies for Prediction and Avoidance of Post-operative Liver Insufficiency. J Clin Transl Hepatol. 2018;6:97–104. doi: 10.14218/JCTH.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Zheng G, Zhang W, Zhang F, Lv S, Wang A, Fang Z. Enhanced Recovery after Surgery Programs for Liver Resection: a Meta-analysis. J Gastrointest Surg. 2017;21:472–486. doi: 10.1007/s11605-017-3360-y. [DOI] [PubMed] [Google Scholar]
- 30.Jackson NR, Hauch A, Hu T, Buell JF, Slakey DP, Kandil E. The safety and efficacy of approaches to liver resection: a meta-analysis. JSLS. 2015;19:e2014.00186. doi: 10.4293/JSLS.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 32.Tsilimigras DI, Moris D, Vagios S, Merath K, Pawlik TM. Safety and oncologic outcomes of robotic liver resections: A systematic review. J Surg Oncol. 2018;117:1517–1530. doi: 10.1002/jso.25018. [DOI] [PubMed] [Google Scholar]
- 33.Gani F, Ejaz A, Dillhoff M, He J, Weiss M, Wolfgang CL, Cloyd J, Tsung A, Johnston FM, Pawlik TM. A national assessment of the utilization, quality and cost of laparoscopic liver resection. HPB (Oxford) 2019;21:1327–1335. doi: 10.1016/j.hpb.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Orgera G, Krokidis M, Cappucci M, Gourtsoyianni S, Tipaldi MA, Hatzidakis A, Rebonato A, Rossi M. Current status of Interventional Radiology in the management of Gastro-Entero-Pancreatic Neuroendocrine Tumours (GEP-NETs) Cardiovasc Intervent Radiol. 2015;38:13–24. doi: 10.1007/s00270-014-1005-z. [DOI] [PubMed] [Google Scholar]
- 36.Kose E, Kahramangil B, Aydin H, Donmez M, Takahashi H, Aucejo F, Siperstein A, Berber E. Outcomes of laparoscopic tumor ablation for neuroendocrine liver metastases: a 20-year experience. Surg Endosc. 2020;34:249–256. doi: 10.1007/s00464-019-06759-1. [DOI] [PubMed] [Google Scholar]
- 37.Xourafas D, Pawlik TM, Cloyd JM. Early Morbidity and Mortality after Minimally Invasive Liver Resection for Hepatocellular Carcinoma: a Propensity-Score Matched Comparison with Open Resection. J Gastrointest Surg. 2019;23:1435–1442. doi: 10.1007/s11605-018-4016-2. [DOI] [PubMed] [Google Scholar]
- 38.Hellman P, Lundström T, Ohrvall U, Eriksson B, Skogseid B, Oberg K, Tiensuu Janson E, Akerström G. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg. 2002;26:991–997. doi: 10.1007/s00268-002-6630-z. [DOI] [PubMed] [Google Scholar]
- 39.Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140:891–897; discussion 897-898. doi: 10.1016/j.surg.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 40.Tsilimigras DI, Ntanasis-Stathopoulos I, Kostakis ID, Moris D, Schizas D, Cloyd JM, Pawlik TM. Is Resection of Primary Midgut Neuroendocrine Tumors in Patients with Unresectable Metastatic Liver Disease Justified? A Systematic Review and Meta-Analysis. J Gastrointest Surg. 2019;23:1044–1054. doi: 10.1007/s11605-018-04094-9. [DOI] [PubMed] [Google Scholar]
- 41.Xiang JX, Zhang XF, Beal EW, Weiss M, Aldrighetti L, Poultsides GA, Bauer TW, Fields RC, Maithel SK, Marques HP, Pawlik TM. Hepatic Resection for Non-functional Neuroendocrine Liver Metastasis: Does the Presence of Unresected Primary Tumor or Extrahepatic Metastatic Disease Matter? Ann Surg Oncol. 2018;25:3928–3935. doi: 10.1245/s10434-018-6751-8. [DOI] [PubMed] [Google Scholar]
- 42.Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, Barbas AS, Abdalla EK, Choti MA, Vauthey JN, Ludwig KA, Mantyh CR, Morse MA, Clary BM. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 43.Idrees JJ, Bagante F, Gani F, Rosinski BF, Chen Q, Merath K, Dillhoff M, Cloyd J, Pawlik TM. Population level outcomes and costs of single stage colon and liver resection versus conventional two-stage approach for the resection of metastatic colorectal cancer. HPB (Oxford) 2019;21:456–464. doi: 10.1016/j.hpb.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010;12:427–433. doi: 10.1111/j.1477-2574.2010.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimbrough CW, Beal EW, Dillhoff ME, Schmidt CR, Pawlik TM, Lopez-Aguiar AG, Poultsides G, Makris E, Rocha FG, Crown A, Abbott DE, Fisher AV, Fields RC, Krasnick BA, Idrees K, Marincola-Smith P, Cho CS, Beems M, Maithel SK, Cloyd JM. Influence of carcinoid syndrome on the clinical characteristics and outcomes of patients with gastroenteropancreatic neuroendocrine tumors undergoing operative resection. Surgery. 2019;165:657–663. doi: 10.1016/j.surg.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrne RM, Pommier RF. Small Bowel and Colorectal Carcinoids. Clin Colon Rectal Surg. 2018;31:301–308. doi: 10.1055/s-0038-1642054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.