Abstract
The development of the lung involves a diverse group of molecules that regulate cellular processes, organ formation, and maturation. The various stages of lung development are marked by accumulation of small RNAs that promote or repress underlying mechanisms, depending on the physiological environment in utero and postnatally. To some extent, the pathogenesis of various lung diseases is regulated by small RNAs. In this review, we discussed miRNAs regulation of lung development and diseases, that is, COPD, asthma, pulmonary fibrosis, and pulmonary arterial hypertension, and also highlighted possible connotations for human lung health.
Keywords: lung development, lung diseases, miRNA, human, mouse
1. Introduction
The direct interaction between the environment and the mammalian respiratory structures plays a critical role in the stimulation of several pathological conditions that disrupt lung function [1,2,3]. Moreover, molecular alterations in the embryonic lung [4,5] are detrimental to normal organ formation. Advances in scientific methods have so far helped explain some of the complex signaling networks in the developing lung [6,7] and also explored pulmonary stem cells for the purpose of tissue regeneration in lung diseases [8]. The functions of small non-coding RNAs in cell differentiation and organ regeneration [9,10] also underscore their value in lung development and diseases.
The developing mammalian lung undergoes a series of sophisticated stages [11], and the expression of specific miRNAs at each level in utero and extra-uterine life may influence organ morphogenesis, maturation, and pathogenesis of diseases. The role of miRNAs in lung development became evident when Dicer1, a gene involved in encoding an enzyme that supports the biogenesis of small interfering RNAs and miRNAs, was inactivated in the mouse lung epithelium [12]. There were decreased branching morphogenesis (E12.5 and E13.5) and increased mesenchymal Fgf10 as well as epithelial Spry2 and Bmp4 levels at E12.5 [12]. Following this publication, several lines of evidence reported various levels of miRNAs expression in the lung and their contribution to biological processes during organ development and pathogenesis of some lung diseases.
2. Consequences of miRNAs Expression in the Developing Lung
The development of human and mouse lungs are characterized by similar divisions spanning from pseudoglandular, canalicular, terminal saccular, to a final alveolar stage [11]. Regarding miRNAs involvement in lung morphogenesis, we discussed what is known in humans, mice, and rats (see Figure 1). Furthermore, profound genetic similarities have been reported between humans and the two rodents [13,14].
Figure 1.
Summary of the role of miRNAs at various stages of human, mouse, and rat lung development as indicated in cited articles.
After fertilization in the mouse, total miRNA in the two-cell-stage embryo was demonstrated to be significantly lower than the levels in one-cell zygote [15]. Notably, total miRNA in the four-cell-stage embryo was higher than the levels in the two-cell-stage embryo [15]. In the early mouse embryo, miR-127 was upregulated at E6.5 and E7.5 [16]. Overexpression of miR-127 in mouse embryonic stem cells, which differentiated into embryoid bodies, increased the mRNA levels of Gsc, Foxa2, and Brachyury (mesendoderm markers), but elicited no change in Pax6 and Otx2 (ectoderm markers) three days after transfection. Inhibition of miR-127 showed opposite regulation of Gsc, Foxa2, and Brachyury. miR-127, moreover, was suggested to control mesendoderm differentiation [16]. miR-326 regulated sonic hedgehog signaling by targeting Smo and Gli2 [17]. The inhibition of smoothened in cultured explanted E12 mouse lung resulted in the expansion of distal epithelium, and disruption of normal branching pattern and mesenchymal integrity [17]. Another study described the functional role of miR-142-3p in the mouse embryonic lung mesenchyme. The miRNA was shown to regulate adenomatous polyposis coli to further modulate Wnt signaling [18]. Taken together, miR-142-3p regulated the proliferation and differentiation of mesenchymal progenitors in the mouse embryonic lung [18].
In the developing mouse lung, the level of miR-17 was stable (E11.5–E16.5), predominantly at the pseudoglandular stage, with higher epithelial levels at E12.5 [19]. Simultaneous knockdown of miR-17 and its paralogs, miR-20a and miR-106b, for 72 h in epithelial lung explants altered branching, even in FGF10-treated condition [19]. In the human fetal lung, miR-449a was upregulated at 18-20 weeks (canalicular stage), and in the mouse embryonic lung, miR-449a level was increased from E15.5 to E18.5 [20]. The inhibition of miR-449a in E16.5 mouse lung culture (end of pseudoglandular stage) increased the mRNA levels of Mycn and Sox9, and the protein levels of Ki-67 and SOX9 particularly in the distal epithelial region [20].
Knockout of miR-26a-1/miR-26a-2 in the mouse promoted the formation of dilated lumens and aerated regions at the beginning of the canalicular stage (E16.5) of lung development [21]. Results at the saccular stage (E18.5) also indicated large lamellar bodies, and increased SP-A, SP-B, and SP-C protein levels in miR-26a knockout mice. The study further proposed a role for miR-26a in the synthesis of pulmonary surfactant [21]. The loss of epithelial histone deacetylase 3 in E18.5 mouse lung resulted in increased levels of miR-17-92 cluster and miRNAs in the Dlk1-Dio3 locus [22]. Overexpression of miR-17-92 in the epithelium of embryonic mouse lung revealed remarkably packed AT1 cells. Together, the study showed that histone deacetylase 3 regulated the level of miR-17-92, which further modulated TGF-β signaling and subsequently, AT1 cell spreading as well as saccular formation [22]. In the fetal rat lung, the highest level of miR-127 was reported at E21 [23]. Furthermore, overexpression of miR-127 in fetal rat lung (E14) culture increased terminal and internal bud sizes.
Regarding alveolarization, several studies have been done in bronchopulmonary dysplasia (BPD) mouse models. For example, hyperoxia-induced impairment in alveolarization was partially mitigated in miR-34a−/− mice [24]. In another study, the administration of miR-29b, nonetheless, improved alveolarization and attenuated fibrotic signaling in postnatal day 28 mice (exposed to 85% O2) whose mothers were intraperitoneally administered with LPS during pregnancy [25]. The administration of miR-876-3p mimic to postnatal day 14 mice exposed to hyperoxia restored alveolar structures and reduced neutrophilic inflammation [26]. After hyperoxia exposure, miR-30a-3p and -5p levels increased significantly in female mice lungs on postnatal days 7 and 21 [27]. Similarly, in neonatal human pulmonary microvascular endothelial cells exposed to hyperoxia, samples obtained from females expressed significantly higher levels of miR-30a-3p and -5p compared to levels in male samples [27]. Altogether, there were significant decreases in miR-30a-3p and -5p levels, and increased mRNA and protein levels of DLL4 in human BPD lung samples. [27]. In another set of experiments carried in newborn mice, hyperoxia disrupted alveolar architecture and the injection of an inhibitor for miR-421 attenuated the process [28]. Collectively, miR-421 regulated Fgf10, induced apoptosis in the lung, and increased inflammatory response in a BPD mouse model [28]. Details on the expression profile of miRNAs at various stages of late lung development in experimental BPD models have been summarized by Nardiello and Morty [29]. Moreover, the studies discussed so far implicate miRNAs in the regulation of alveolarization.
3. The Role of miRNAs in Some Developmental Milestones of the Lung
Besides data on specific epochs of lung development, some studies have reported the expression and role of miRNAs at various levels in the fetal and postnatal lungs.
A profile of miRNAs in the developing lung revealed an increased level of miR-17-92 cluster at E11.5, which gradually decreased until adult life [30]. Overexpression of miR-17-92 cluster in the lung epithelium of transgenic embryos impaired normal lung development [30]. At E16, E19, and E21 of rat lung development, a wide diversity of miRNAs were regulated [31]. The following miRNAs, let-7a, miR-93, miR-125b-5p, miR-146b, miR-296, miR-1949, and miR-3560 were shown to be consistently regulated in all three stages investigated in the rat embryonic lungs [31]. Between the three stages, miR-1949, miR-125b-5p, miR-296, and miR-93 were downregulated whereas let-7a, miR-146b, and miR-3560 were upregulated. The seven miRNAs were further implicated in rat fetal lung development [31].
During the differentiation of epithelial cells (stimulated with 1mM Bt2cAMP) isolated from midgestational human fetal lungs, the level of miR-29 family was significantly elevated [9]. This coincided with increased mRNA level of SFTPA in epithelial cell at 48 h and 72 h of culture in the presence of 1mM Bt2cAMP. Similar regulatory patterns were observed in epithelial cells isolated from mouse fetal lungs (E15.5–E18.5). Knockdown of miR-29 family promoted TGF-β signaling, which in turn decreased the protein level of SP-A in human fetal lung epithelial cells [9]. The inhibition of miR-200 family in type II cells isolated from human fetal lungs decreased protein levels of SP-A, SP-B and pro-SP-C, and furthermore, reduced phospholipid-containing lamellar inclusions [32]. Similarly, in another study, knockout of miR-200b resulted in the disruption of surfactant properties, decreased lung septation, and thickened alveolar walls [33].
In the developing mouse lung, the relevance of miR-302/367 cluster was demonstrated in endoderm progenitors [34]. Overexpression of miR-302/367 cluster increased the thickness of epithelial lining and reduced sacculation in the embryonic lung (E18.5) [34]. Moreover, in the study, miR-302/367 regulated the proliferation of lung progenitors by repressing Cdkn1a and Rbl2. The miRNA cluster further modulated lung endodermal progenitor cell polarity by downregulating Tiam1 and Lis1 mRNA and protein levels [34].
4. miRNAs in Lung Diseases
Clinical evidence has indicated decline in lung function of some adults who survived BPD during early life [35,36]. It is speculative that the resolution of impairments at the alveolar stage of human lung development may leave quiescent imprints that could influence respiratory activities and molecular pathogenesis of lung diseases later in life. We have explained the role of some miRNAs in lung development. Nevertheless, there exists an extensive literature on the regulatory effects of miRNAs in lung diseases. Moreover, the molecular mechanisms that regulate the risk of developing lung diseases in later life, in some neonates/infants who survived respiratory disorders, remain unclear. We propose that the role of miRNAs in signaling pathways that stimulate lung diseases after neonatal/infant life could provide hints for studies intending to bridge these separate mechanisms. Additionally, the epigenetic hallmarks of miRNAs isolate them as one of the classes of molecules that require attention for future studies.
In this review, we focused on the following lung diseases, that is, chronic obstructive pulmonary disease (COPD), asthma, pulmonary fibrosis, and pulmonary arterial hypertension (PAH), because of their impact on global morbidity, mortality, and the common presence of inflammation and tissue remodeling during their pathogenesis. We also considered their pathological associations and availability of experimental models and data, as in the case of PAH (group 1 pulmonary hypertension). It was reported that noncommunicable diseases accounted for 71% of global deaths with chronic respiratory diseases (CRDs) (7%) ranking second to cancers (16%) and third to cardiovascular diseases (31%) [37]. Among the CRDs, COPD and asthma constituted the highest disease burden [38]. Despite obvious pathological differences between asthma and fibrosis, fibroblast accumulation was observed in the proximal airways of patients with severe persistent asthma [39]. Additionally, perichondrial fibrosis was confirmed in the airways of autopsied lungs from patients with bronchial asthma, and was also suggested in the lungs of COPD patients [40]. Asthma exhibits three pathological features with PAH, and this was related to the activation of NFAT in both diseases [41]. Lastly, pulmonary hypertension is common in COPD and idiopathic pulmonary fibrosis [42,43,44].
4.1. COPD
COPD is characterized by progressive loss of lung function provoked by chronic bronchitis and emphysema [45]. The pathogenesis of COPD is associated with abnormal inflammatory response in the airways, and recent studies have reported the importance of miRNAs in regulating this process. For example, besides miR-145-5p role in decreasing nuclear translocation of NF-κB p65, the miRNA targeted KLF5 to inhibit inflammation and apoptosis in cigarette smoke extract (CSE)-treated human bronchial epithelial cells (HBECs) [46]. The transfection of miR-29b mimic into HBE4-E6/E7 cell line downregulated the mRNA and protein levels of BRD4 [47]. miR-29b significantly decreased the mRNA level of IL-8 by regulating BRD4 in CSE-treated HBE4-E6/E7 cells [47]. In another line of evidence, treatment of CSE to murine monocytic cell line (THP-1) increased the concentrations of IL-1β and TNF-α, protein levels of TLR-4 and NF-κB p65, and decreased the level of miR-149-3p [48]. Transfection of miR-149-3p mimic into CSE-induced THP-1 cells, nonetheless, decreased the level of the proteins mentioned [48]. Bronchial biopsies from COPD patients who were treated with corticosteroids for 6 and 30 months showed increased levels of miR-320d and miR-339-3p, and decreased levels of miR-708 and miR-155 [49]. CSE stimulation of primary bronchial epithelial and BEAS-2B cells overexpressing miR-320d decreased the release of CXCL8 [49]. Additionally, treatment of IL-1β to BEAS-2B cells overexpressing miR-320d downregulated the activation of NF-κB [49]. In CSE-induced human bronchial epithelial (HBE) cells, miR-218 decreased the level of mRNA and release of IL-6 and IL-8 [50]. Similarly, the protein and mRNA levels, as well as the release of MUC5AC by CSE-induced HBE cells were also reduced as a result of miR-218 overexpression. Taken together, miR-218 regulated TNFR1/NF-κB signaling to modulate MUC5AC production and inflammation in CSE-stimulated HBE cells [50]. The inhibition of miR-3202 increased the levels of IFN-γ and TNF-α in a co-culture of T lymphocytes and HBE cells [51]. Furthermore, in a COPD rat model, cigarette smoke (CS) exposure elevated the concentrations of IFN-γ and TNF-α, increased the protein level of FAIM2, disrupted the lung architecture, and downregulated the level of miR-3202 [51]. In another set of experiments, miR-223 was suggested to induce CX3CL1 mRNA level by regulating histone deacetylase 2 in human pulmonary artery endothelial cells (HPAECs) [52]. In human lung tissues from two cohorts of COPD patients, there were negative correlations between miR-223 and histone deacetylase 2 levels [52]. In another study, miR-181c regulated CCN1, which was upregulated in the lung tissues of CS-exposed mice as well as COPD patients [53]. Nevertheless, the inhibition of miR-181c increased the mRNA levels and release of IL-6 and IL-8 in CSE-treated HBECs and CS-exposed mice.
As a form of pre-clinical therapeutic approach, the inhibition of some miRNAs was demonstrated to alleviate inflammatory phenotypes in COPD. In CS-exposed mice, anti-miR-130a decreased the BAL macrophage, neutrophil, dendritic cell, CD4+ T cell, and CD8+ T cell counts [54]. Furthermore, the levels of IL-1β, TNF-α, and IL-6 were reduced in BALF. The inhibition of miR-130a in CSE-treated BEAS-2B cells upregulated the protein levels of WNT1, β-catenin, and LEF1 [54]. In an earlier study, intranasal administration of anti-miR-195 to mice mitigated CS-induced disruption of the lungs [55]. Anti-miR-195 increased the protein level of PHLPP2, and decreased p-AKT in the lungs of CS-exposed mice. Altogether, miR-195 targeted PHLPP2 and also promoted the release of IL-6 and TNF-α, an observation similar to knockdown of PHLPP2 in BEAS-2B cells [55].
There have been general reports of miRNA regulation of various molecules in experimental COPD models. In serum samples of COPD patients as well as CSE-treated NCI-H292 cells, miR-212-5p level was shown to be reduced [56]. Nonetheless, miR-212-5p increased p-AKT and downregulated the mRNA and protein levels of IGFBP3. Moreover, opposite observations were reported in CSE-stimulated NCI-H292 cells [56]. The authors could not show the possible role of miR-212-5p in CSE-treated conditions. There is evidence that peripheral blood mononuclear cells and lung tissues obtained from COPD patients exhibited a higher level of miR-664a-3p compared to smokers [57]. Additionally, overexpression of miR-664a-3p in BEAS-2B cells negatively regulated FHL1 mRNA and protein levels. The authors suggested further work to elucidate the roles of miR-664a-3p and FHL1 in COPD [57].
4.2. Asthma
Asthma is marked by recurrent episodes of symptoms (for example, breathlessness, cough and wheezing), airway obstruction, inflammation, and bronchial hyper-responsiveness to various forms of stimuli [58]. The role of miRNAs in asthma-related signaling pathways have been clearly explained by Mousavi et al. [59] and Taka et al. [60]. Nonetheless, the following are studies that emerged in recent months. In severe asthmatic bronchial epithelial cells obtained from patients, the level of miR-744 was decreased compared to its expression in normal control cells [61]. miR-744 downregulated the mRNA and protein levels of TGFB1, and in TGF-β1-stimulated normal and severe asthmatic bronchial epithelial cells, miR-744 mimic decreased p-SMAD3 and also increased the protein level of SARA. miR-744 decreased the proliferation of bronchial epithelial cells, suggesting the importance of the miRNA in epithelial functions in asthma [61]. miR-943-3p was shown to regulate the level of SFRP4 [62]. Inhibition of miR-943-3p, however, reduced OVA-induced subepithelial fibrosis and smooth muscle thickness in the mouse lung. miR-943-3p repression of SFRP4 was reported to support remodeling of the airway in OVA-induced mice [62]. Lung tissues obtained from asthma patients showed increased and decreased protein levels of Beclin-1 and P62, respectively [63]. Furthermore, it was demonstrated that miR-30a regulated ATG5 to inhibit autophagy-induced airway fibrosis [63].
4.3. Pulmonary Fibrosis
Pulmonary fibrosis usually occurs in diseases such as rheumatoid arthritis and systemic sclerosis. Fibrosis of the lung is devastating when there are no established underlying causes, as manifested in idiopathic pulmonary fibrosis (IPF), an interstitial lung disease with poor prognosis and median survival from 2.5 to 3.5 years [64,65]. Until now, experimental models are being developed to recapitulate the progressive pathophysiology of IPF for accurate molecular studies. Nevertheless, the profibrotic impact of transforming growth factor-β (TGF-β) in pulmonary fibrosis has been widely reported in past years. Many in vitro and in vivo studies have employed TGF-β1 and bleomycin treatments as experimental models for pulmonary fibrosis. In this review, we discussed miRNAs and their regulation of TGF-β signaling, fibroblast differentiation, collagen production, apoptosis, and some phenotypes of pulmonary fibrosis.
Treatments of TGF-β1 and bleomycin downregulated the level of miR-18a-5p, and inhibition of TGF-β1 in bleomycin-stimulated pleural mesothelial cells potentially increased the level of the miRNA [66]. In the study, miR-18a-5p targeted TGFBR2 and inhibition of miR-18a-5p increased p-SMAD2/3, disrupted mouse lung architecture, and stimulated sub-pleural fibrosis [66]. Congruence to this experimental data, miR-153 was also shown to regulate TGFBR2 [67]. miR-153 inhibited the phosphorylation of SMAD2/3 and consequently, decreased the protein levels of fibronectin and α-SMA. Thus, the miRNA regulated fibrogenesis in the human pulmonary fibroblast line MRC-5 [67]. Similarly, miR-1343 suppressed TGFBR1 and TGFBR2, and also reduced the levels of markers of fibrosis (collagen type I and α-SMA) in TGF-β1-stimulated primary lung fibroblasts [68]. In another study, the mRNA and protein levels of TGFBR2 and NOX4, as well as p-SMAD2 level, were decreased by miR-9-5p in human fetal lung fibroblasts (HFL-1) [69]. Similar observation was reported in the mouse. Orotracheal administration of bleomycin following lenti-miR-9 treatment interrupted structural damage to the lungs, hence prevented pulmonary fibrosis in mice [69]. miR-101 decreased the mRNA and protein levels of TGFBR1, FZD4 and FZD6, and thus blocked NFATc2 and TGF-β1 signaling to attenuate fibroblast proliferation and activation [70].
In an in vitro model of pulmonary fibrosis, miR-486-5p mimic was shown to inhibit TGF-β1 activation of SMAD2 and further, decreased the protein levels of fibronectin, α-SMA and CTGF in mouse fibroblasts (NIH/3T3) [71]. Intratracheal administration of miR-486-5p in a lung fibrosis mouse model also decreased myofibroblast differentiation and collagen deposition. Besides SMAD2 binding, miR-323a-3p also targeted TGFA [72]. miR-323a-3p mimic repressed the mRNA and protein levels of SMAD2 and TGFA in human bronchial epithelial cells (16HBE14o-). miR-323a-3p also downregulated Caspase 3 to reduce the apoptosis of 16HBE14o- cells. Taken together, miR-323a-3p inhibited bleomycin-induced lung fibrosis in mice [72].
During the pathogenesis of pulmonary fibrosis, TGF-β1 usually stimulates the transdifferentiation of fibroblasts to myofibroblasts. Conversely, as shown in a study, miR-27a-3p inhibited TGF-β1 activation of human lung fibroblasts (MRC-5 line) [73]. It was suggested in another study that miR-489 targeted Myd88 to decrease protein levels of IL-1β and TGF-β1 in silica-induced lung fibrosis mouse model [74]. miR-489 attenuated fibroblast differentiation by blocking the phosphorylation of SMAD3 in TGF-β1-stimulated NIH/3T3 cells [74]. Similarly, miR-133a downregulated the protein level of CTGF, and the mRNA levels of COL1A1 and ACTA2 to inhibit pulmonary fibroblast differentiation and to prevent fibrosis phenotype in TGF-β1-treated conditions [75]. Another line of evidence reported that miR-542-5p regulated the mRNA and protein levels of ITGA6 in TGF-β1-stimulated NIH-3T3 cells [76]. Knockdown of Itga6 in TGF-β1-treated NIH-3T3 cells nonetheless, inhibited markers of fibrosis and also blocked the phosphorylation of FAK and AKT [76]. miR-542-5p was suggested to regulate Itga6 level, and thus attenuated fibroblast differentiation via the FAK signaling pathway.
In pulmonary fibrosis, the regulation of collagen production is important for the therapeutic treatment of the disease. miR-29a and miR-29c were demonstrated as examples of miRNAs that regulate collagen production in pulmonary fibrosis. miR-29a downregulated the mRNA and protein levels of LOXL2 and SERPINH1 in MRC-5 fibroblasts [77]. It is noteworthy that LOXL2 and SERPINH1 genes are involved in the synthesis of collagen. Therefore, miR-29a elicits anti-fibrotic properties. Knockdown of histone deacetylase C4 resulted in an increase in type I collagen and a decrease in miR-29c level in control human lung fibroblasts [78]. Additionally, in IPF fibroblasts overexpressing protein phosphatase 2A, the silencing of histone deacetylase C4 increased type I collagen and decreased the level of miR-29c [78].
Recent developments have shown the potential roles of miRNAs in the regulation of apoptosis in pulmonary fibrosis. A study demonstrated that TGF-β1 downregulated miR-29c and the protein level of cell surface death receptor, Fas, in HFL-1 cells [79]. miR-29c mimic however upregulated the protein abundance of Fas and also induced apoptosis in TGF-β1-stimulated HFL-1 cells [79]. Following this publication, another study suggested that knockout of mir-29c in mouse alveolar epithelial type II cells induced apoptosis and decreased cell renewal [80]. miR-29c regulated the level of Foxo3a to inhibit the apoptosis of alveolar epithelial type II cells. Overexpression of miR-29c in alveolar epithelial type II cells decreased the level of hydroxyproline in a bleomycin-induced lung fibrosis mouse model [80]. miR-34a−/− mice exhibited severe fibrosis phenotype after bleomycin instillation [81]. It was further revealed that primary lung fibroblasts isolated from bleomycin-treated miR-34a−/− mice became less senescent. Additionally, there was decreased cell apoptosis in the lungs of bleomycin treated miR-34a−/− mice [81].
In most studies, the regulatory effects of miRNAs in pulmonary fibrosis are demonstrated to target the alleviation of the disease. For example, miR-708-3p mimic regulated ADAM17 and also downregulated markers of fibrosis in TGF-β1-treated MRC-5 cells [82]. Administration of synthesized miR-708-3p agomir to mice treated with bleomycin resulted in thinner alveolar walls [82]. In another study, miR-338-5p attenuated bleomycin-induced fibrosis in mice [83]. Similarly, miR-338-5p regulated LPA1 protein level to decrease fibrosis phenotype in bleomycin-treated mouse lungs [84].
4.4. PAH
PAH is one of five classifications of pulmonary hypertension [85], and leads to right ventricular failure as well as premature death. One major pathological feature of PAH is vascular remodeling. There are a growing number of publications regarding the role of miRNAs in some categories of pulmonary hypertension. Jusic et al. [86] have recently reviewed what is already known in pulmonary arterial hypertension. However, since their publication, there is new evidence to expand knowledge in this field of active research.
Some studies have demonstrated that miRNAs contribute to the alleviation of PAH. For instance, the proliferation of human pulmonary arterial smooth muscle cells (hPASMC) was inhibited by miR-205-5p [87]. It was further explained that miR-205-5p regulated MICAL2, a protein that proliferated hPASMCs via the activation of ERK1/2 signaling [87]. The mRNA level of FGF-7 was upregulated in tissues obtained from PAH patients and in PAH-PASMCs [88]. miR-455-3p-1 alleviated PAH by regulating FGF7 and RAS/ERK signaling, respectively [88]. In a recent study, there were decreased levels of miR-181a-5p and miR-181b-5p, whereas the protein level of endocan was elevated in monocrotaline-induced PAH in a time-dependent manner [89]. miR-181a/b-5p decreased the protein level of endocan to mitigate inflammatory response in TNF-α-treated primary rat pulmonary arterial endothelial cells [89].
There is compelling evidence that some miRNAs promote the pathogenesis of PAH. It was recently shown that hypoxia exposure stimulated PAH and also increased miR-27a level in the rat pulmonary artery and in HPAECs [90]. Moreover, miR-27a targeted SMAD5 to promote hypoxia-induced endothelial to mesenchymal transition [90]. Chronic normobaric hypoxia stimulated PAH and also increased the level of miR-335-3p in the mouse lung [91]. The inhibition of miR-335-3p resulted in the attenuation of chronic normobaric hypoxia-induced vascular remodeling and PAH in mice [91]. A significant increase in miR-30a-5p level in the plasma of PAH patients was recently reported [92]. Hypoxia exposure to HPAECs progressively downregulated miR-30a-5p and upregulated YKL-40 levels in a time-dependent manner. Furthermore, it was evidently indicated that miR-30a-5p targets YKL-40. To investigate the functional role of the miRNA, HPAECs were exposed to hypoxia after transfection with miR-30a-5p mimic. Consequently, miR-30a-5p enhanced the proliferation and attenuated the apoptosis of HPAECs [92].
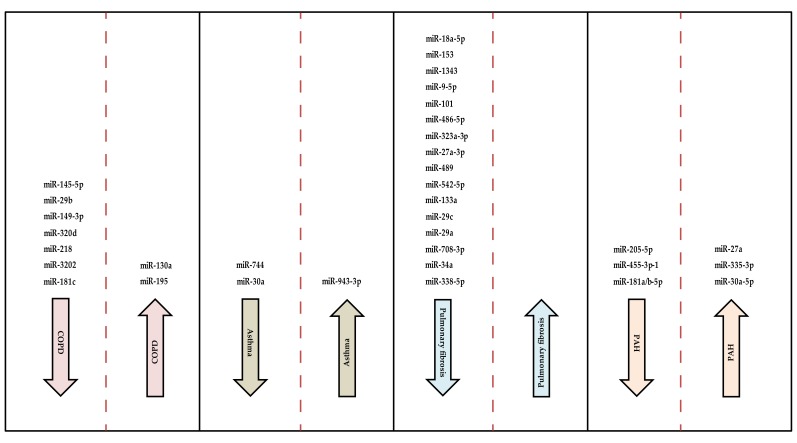
Figure 2 shows a summary of miRNAs involved in pulmonary arterial hypertension and the other lung diseases discussed. We have also summarized the miRNAs and their target genes in lung development and diseases (Table 1).
Figure 2.
Schematic representation of miRNAs in pulmonary diseases as already cited from published articles. Arrows pointing downwards indicate the inhibition of the pathogenesis of the diseases by the miRNAs in the column. miRNAs in columns with arrows pointing upwards progress the development of the diseases. No miRNA was found to be involved in the progression of pulmonary fibrosis in this review.
Table 1.
miRNAs and their target genes in lung development and diseases, as shown in cited articles.
| miRNA | Molecular Target(s) |
|---|---|
| Lung Development | |
| miR-326 | Smo and Gli2 |
| miR-142-3p | Apc |
| miR-449a | MYCN |
| miR-421 | Fgf10 |
| miR-302/367 | Cdkn1a, RbI2, Tiam1, and Lis1 |
| Lung Diseases | |
| COPD | |
| miR-145-5p | KLF5 |
| miR-149-3p | TLR-4 |
| miR-218 | TNFR1 |
| miR-3202 | FAIM2 |
| miR-223 | HDAC2 |
| miR-181c | CCN1 |
| miR-195 | PHLPP2 |
| miR-664a-3p | FHL1 |
| Asthma | |
| miR-744 | TGFB1 |
| miR-943-3p | SFRP4 |
| miR-30a | ATG5 |
| Pulmonary Fibrosis | |
| miR-18a-5p | TGFBR2 |
| miR-153 | TGFBR2 |
| miR-1343 | TGFBR1 and TGFBR2 |
| miR-9-5p | TGFBR2 and NOX4 |
| miR-101 | TGFBR1, FZD4, and FZD6 |
| miR-486-5p | SMAD2 |
| miR-323a-3p | SMAD2 and TGFA |
| miR-489 | Smad3 |
| miR-133a | TGFBR1, CTGF, and Col1a1 |
| miR-542-5p | Itga6 |
| miR-29a | LOXL2 and SERPINH1 |
| miR-708-3p | ADAM17 |
| miR-338-5p | LPA1 |
| PAH | |
| miR-205-5p | MICAL2 |
| miR-455-3p-1 | FGF7 |
| miR-181a/b-5p | endocan |
| miR-27a | Smad5 |
| miR-30a-5p | YKL-40 |
5. Comparative Role of miRNAs in Lung Development and Diseases
Some review articles have mentioned the involvements of TGF-β/BMP, Wnt, sonic hedgehog, and FGF10 signaling in lung development and diseases [93,94,95,96,97]. The signaling pathways of these molecules are regulated by miRNAs. Considering the role of miRNAs in lung development and the pathogenesis of some diseases, it is crucial to identify specific miRNAs and their regulatory influence in the two biological processes.
We observed a dual function of miR-34a, specifically in the late stage of lung development and pathogenesis of IPF. In newborn mice, miR-34a impaired alveolarization, and in adult mice, the miRNA alleviated the progression of pulmonary fibrosis [24,81]. Figure 3 illustrates the functions of miR-34a in the mouse lung.
Figure 3.
miR-34a regulates lung development and the pathogenesis of pulmonary fibrosis.
Furthermore, depending on targeting sites and the pathogenesis of the disease, some categories of miRNAs may exhibit various regulatory functions in the lung. For example, miR-27a-3p attenuated pulmonary fibrosis, and in another report, miR-27a supported PAH [73,90]. Similarly, miR-30a interrupted the development of asthma, and on the other hand, miR-30a-5p promoted PAH [63,92]. miR-29a and miR-29c inhibited the pathogenesis of IPF, and miR-29b regulated inflammation in COPD [47,77,79,80]. miR-181c attenuated the pathogenesis of COPD and miR-181a/b-5p were involved in the regulation of PAH [53,89].
6. Perspective
As a recommendation, miRNAs are receiving remarkable attention to be used as biomarkers and clinical diagnosis of lung diseases. For example, recent attempts to find biomarkers in the serum samples of children suffering from asthma revealed associations between some miRNAs and functional metrics of the lungs [98]. Among the numerous circulating miRNAs detected in serum samples of research participants, 22 were associated with FEV1/FVC, eight with FVC%, and four with FEV1% [98]. Apart from asthma, the concept of using miRNAs as biomarkers in COPD was recently described by Salimian et al. [99]. Other lung diseases such as sarcoidosis and cancer have also been connected with numerous miRNAs [100]. Conversely, the circulating miRNAs in patients with lung diseases may only indicate the presence of the conditions and not necessarily their progression. We suggest the screening of miRNAs at various pathophysiological stages of pulmonary diseases to address this concern. Moreover, to ensure healthy human lung, further work is required to standardize miRNAs for the diagnosis of lung diseases.
Beyond their utility as biomarkers, miRNAs are important to understand the mechanistic underpinnings of disease pathophysiology in the context of lung development. Thus, miRNAs are essential for lung development with particular interest in the alveolar stage, which has notable examples in BPD patients. In a recent study, intravenous administration of miR-302b/c mimic after Streptococcus pneumoniae infection in mice proliferated AT1 and AT2 cells, and also increased survival [10]. let-7d was transduced into bone marrow-derived mesenchymal stem cells (let-7d), which were intravenously injected into bleomycin-treated mice [101]. This caused decreased inflammatory cells in the lungs, and mice began to recover from weight loss induced by bleomycin instillation two days after let-7d treatment [101]. Reports on the role of miRNAs in the alveolar stage of lung development could support efforts made to promote normal tissue repair and regeneration after injury. We also propose the characterization of miRNAs expressed in stem cells and progenitor cells of the lung to support the programming of regeneration and restoration of normal organ function after severe tissue damage.
In summary, the prospects of acquiring lung diseases in early life have been partly associated with molecular factors in utero. The expression of miRNAs at various stages of lung development and diseases widely project their contribution to human lung health. We speculate that the involvement of miRNAs in the pseudoglandular and canalicular stages of lung development suggests their relevance in reepithelialisation in some human lung diseases. Additionally, the influence of miRNAs in the late stage of lung development could enlighten efforts being made toward alveolar repair in lung diseases.
Acknowledgments
Many thanks to our colleagues Draginja Kovacevic and Huan Ma. We also express our gratitude to Leibniz Lung Center, member of the German Center for Lung Research (DZL), Borstel, Germany, for supporting this review.
Author Contributions
E.B. and S.K.-E. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bayram H., Sapsford R.J., Abdelaziz M.M., Khair O.A. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. J. Allergy Clin. Immunol. 2001;107:287–294. doi: 10.1067/mai.2001.111141. [DOI] [PubMed] [Google Scholar]
- 2.Peters J.M., Avol E., Gauderman W.J., Linn W.S., Navidi W., London S.J., Margolis H., Rappaport E., Vora H., Gong H., Jr., et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am. J. Respir. Crit. Care Med. 1999;159:768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 3.Michaudel C., Fauconnier L., Jule Y., Ryffel B. Functional and morphological differences of the lung upon acute and chronic ozone exposure in mice. Sci. Rep. 2018;8:10611. doi: 10.1038/s41598-018-28261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzaga S., Henriques-Coelho T., Davey M., Zoltick P.W., Leite-Moreira A.F., Correia-Pinto J., Flake A.W. Cystic adenomatoid malformations are induced by localized FGF10 overexpression in fetal rat lung. Am. J. Respir. Cell Mol. Biol. 2008;39:346–355. doi: 10.1165/rcmb.2007-0290OC. [DOI] [PubMed] [Google Scholar]
- 5.Okubo T., Hogan B.L. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J. Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss A.M., Tian Y., Tsukiyama T., Cohen E.D., Zhou D., Lu M.M., Yamaguchi T.P., Morrisey E.E. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domyan E.T., Ferretti E., Throckmorton K., Mishina Y., Nicolis S.K., Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotton D.N., Morrisey E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W., Benlhabib H., Mendelson C.R. The MicroRNA 29 Family Promotes Type II Cell Differentiation in Developing Lung. Mol. Cell. Biol. 2016;36:2141. doi: 10.1128/MCB.00096-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Li Y., Zhang P., Baker S.T., Wolfson M.R., Weiser J.N., Tian Y., Shen H. Regenerative therapy based on miRNA-302 mimics for enhancing host recovery from pneumonia caused by Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA. 2019;116:8493–8498. doi: 10.1073/pnas.1818522116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warburton D., El-Hashash A., Carraro G., Tiozzo C., Sala F., Rogers O., De Langhe S., Kemp P.J., Riccardi D., Torday J., et al. Lung organogenesis. Curr. Top. Dev. Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris K.S., Zhang Z., McManus M.T., Harfe B.D., Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc. Natl. Acad. Sci. USA. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouse Genome Sequencing C., Waterston R.H., Lindblad-Toh K., Birney E., Rogers J., Abril J.F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs R.A., Weinstock G.M., Metzker M.L., Muzny D.M., Sodergren E.J., Scherer S., Scott G., Steffen D., Worley K.C., Burch P.E., et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 15.Tang F., Kaneda M., O’Carroll D., Hajkova P., Barton S.C., Sun Y.A., Lee C., Tarakhovsky A., Lao K., Surani M.A. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–648. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma H., Lin Y., Zhao Z.A., Lu X., Yu Y., Zhang X., Wang Q., Li L. MicroRNA-127 Promotes Mesendoderm Differentiation of Mouse Embryonic Stem Cells by Targeting Left-Right Determination Factor 2. J. Biol. Chem. 2016;291:12126–12135. doi: 10.1074/jbc.M116.723247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Z., Cushing L., Ai X., Lu J. miR-326 is downstream of Sonic hedgehog signaling and regulates the expression of Gli2 and smoothened. Am. J. Respir. Cell Mol. Biol. 2014;51:273–283. doi: 10.1165/rcmb.2013-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carraro G., Shrestha A., Rostkovius J., Contreras A., Chao C.M., El Agha E., Mackenzie B., Dilai S., Guidolin D., Taketo M.M., et al. miR-142-3p balances proliferation and differentiation of mesenchymal cells during lung development. Development. 2014;141:1272–1281. doi: 10.1242/dev.105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carraro G., El-Hashash A., Guidolin D., Tiozzo C., Turcatel G., Young B.M., De Langhe S.P., Bellusci S., Shi W., Parnigotto P.P., et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev. Biol. 2009;333:238–250. doi: 10.1016/j.ydbio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanford E.L., Choy K.W., Donahoe P.K., Tracy A.A., Hila R., Loscertales M., Longoni M. MiR-449a Affects Epithelial Proliferation during the Pseudoglandular and Canalicular Phases of Avian and Mammal Lung Development. PLoS ONE. 2016;11:e0149425. doi: 10.1371/journal.pone.0149425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y.F., Kan Q., Yang Y., Zhang Y.H., Shen J.X., Zhang C., Zhou X.Y. Knockout of microRNA26a promotes lung development and pulmonary surfactant synthesis. Mol. Med. Rep. 2018;17:5988–5995. doi: 10.3892/mmr.2018.8602. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Frank D.B., Morley M.P., Zhou S., Wang X., Lu M.M., Lazar M.A., Morrisey E.E. HDAC3-Dependent Epigenetic Pathway Controls Lung Alveolar Epithelial Cell Remodeling and Spreading via miR-17-92 and TGF-beta Signaling Regulation. Dev. Cell. 2016;36:303–315. doi: 10.1016/j.devcel.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskaran M., Wang Y., Zhang H., Weng T., Baviskar P., Guo Y., Gou D., Liu L. MicroRNA-127 modulates fetal lung development. Physiol. Genom. 2009;37:268–278. doi: 10.1152/physiolgenomics.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Camp J., Quantius J., Lignelli E., Arndt P.F., Palumbo F., Nardiello C., Surate Solaligue D.E., Sakkas E., Mizikova I., Rodriguez-Castillo J.A., et al. Targeting miR-34a/Pdgfra interactions partially corrects alveologenesis in experimental bronchopulmonary dysplasia. EMBO Mol. Med. 2019;11:e9448. doi: 10.15252/emmm.201809448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durrani-Kolarik S., Pool C.A., Gray A., Heyob K.M., Cismowski M.J., Pryhuber G., Lee L.J., Yang Z., Tipple T.E., Rogers L.K. miR-29b supplementation decreases expression of matrix proteins and improves alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L339–L349. doi: 10.1152/ajplung.00273.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal C.V., Olave N., Travers C., Rezonzew G., Dolma K., Simpson A., Halloran B., Aghai Z., Das P., Sharma N., et al. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight. 2018;3:93994. doi: 10.1172/jci.insight.93994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Coarfa C., Dong X., Jiang W., Hayward-Piatkovskyi B., Gleghorn J.P., Lingappan K. MicroRNA-30a as a candidate underlying sex-specific differences in neonatal hyperoxic lung injury: Implications for BPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316:L144–L156. doi: 10.1152/ajplung.00372.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan H.S., Xiong D.Q., Huang F., Cui J., Luo H. MicroRNA-421 inhibition alleviates bronchopulmonary dysplasia in a mouse model via targeting Fgf10. J. Cell. Biochem. 2019;120:16876–16887. doi: 10.1002/jcb.28945. [DOI] [PubMed] [Google Scholar]
- 29.Nardiello C., Morty R.E. MicroRNA in late lung development and bronchopulmonary dysplasia: The need to demonstrate causality. Mol. Cell. Pediatr. 2016;3:19. doi: 10.1186/s40348-016-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y., Thomson J.M., Wong H.Y., Hammond S.M., Hogan B.L. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev. Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y., Kai G., Pu X.D., Qing K., Guo X.R., Zhou X.Y. Expression profile of microRNAs in fetal lung development of Sprague-Dawley rats. Int. J. Mol. Med. 2012;29:393–402. doi: 10.3892/ijmm.2011.855. [DOI] [PubMed] [Google Scholar]
- 32.Benlhabib H., Guo W., Pierce B.M., Mendelson C.R. The miR-200 family and its targets regulate type II cell differentiation in human fetal lung. J. Biol. Chem. 2015;290:22409–22422. doi: 10.1074/jbc.M114.636068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoshgoo N., Visser R., Falk L., Day C.A., Ameis D., Iwasiow B.M., Zhu F., Ozturk A., Basu S., Pind M., et al. MicroRNA-200b regulates distal airway development by maintaining epithelial integrity. Sci. Rep. 2017;7:6382. doi: 10.1038/s41598-017-05412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y., Zhang Y., Hurd L., Hannenhalli S., Liu F., Lu M.M., Morrisey E.E. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245. doi: 10.1242/dev.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gough A., Linden M., Spence D., Patterson C.C., Halliday H.L., McGarvey L.P. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur. Respir. J. 2014;43:808–816. doi: 10.1183/09031936.00039513. [DOI] [PubMed] [Google Scholar]
- 36.Yang J., Kingsford R.A., Horwood J., Epton M.J., Swanney M.P., Stanton J., Darlow B.A. Lung Function of Adults Born at Very Low Birth Weight. Pediatrics. 2020;145:e20192359. doi: 10.1542/peds.2019-2359. [DOI] [PubMed] [Google Scholar]
- 37.Noncommunicable Diseases Country Profiles 2018. [(accessed on 6 April 2020)]; Available online: https://www.who.int/nmh/publications/ncd-profiles-2018/en/
- 38.Xie M., Liu X., Cao X., Guo M., Li X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020;21:49. doi: 10.1186/s12931-020-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benayoun L., Druilhe A., Dombret M.C., Aubier M., Pretolani M. Airway structural alterations selectively associated with severe asthma. Am. J. Respir. Crit. Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 40.Haraguchi M., Shimura S., Shirato K. Morphometric analysis of bronchial cartilage in chronic obstructive pulmonary disease and bronchial asthma. Am. J. Respir. Crit. Care Med. 1999;159:1005–1013. doi: 10.1164/ajrccm.159.3.9712144. [DOI] [PubMed] [Google Scholar]
- 41.Said S.I., Hamidi S.A., Gonzalez Bosc L. Asthma and pulmonary arterial hypertension: Do they share a key mechanism of pathogenesis? Eur. Respir. J. 2010;35:730–734. doi: 10.1183/09031936.00097109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersen K.H., Iversen M., Kjaergaard J., Mortensen J., Nielsen-Kudsk J.E., Bendstrup E., Videbaek R., Carlsen J. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J. Heart Lung Transplant. 2012;31:373–380. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Cuttica M.J., Kalhan R., Shlobin O.A., Ahmad S., Gladwin M., Machado R.F., Barnett S.D., Nathan S.D. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir. Med. 2010;104:1877–1882. doi: 10.1016/j.rmed.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Shorr A.F., Wainright J.L., Cors C.S., Lettieri C.J., Nathan S.D. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur. Respir. J. 2007;30:715–721. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 45.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease American Thoracic Society. Am. J. Respir. Crit. Care Med. 1995;152:S77–S121. [PubMed] [Google Scholar]
- 46.Dang X., Yang L., Guo J., Hu H., Li F., Liu Y., Pang Y. miR-145-5p is associated with smoke-related chronic obstructive pulmonary disease via targeting KLF5. Chem. Biol. Interact. 2019;300:82–90. doi: 10.1016/j.cbi.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Tang K., Zhao J., Xie J., Wang J. Decreased miR-29b expression is associated with airway inflammation in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316:L621–L629. doi: 10.1152/ajplung.00436.2018. [DOI] [PubMed] [Google Scholar]
- 48.Shen W., Liu J., Zhao G., Fan M., Song G., Zhang Y., Weng Z., Zhang Y. Repression of Toll-like receptor-4 by microRNA-149-3p is associated with smoking-related COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:705–715. doi: 10.2147/COPD.S128031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faiz A., Steiling K., Roffel M.P., Postma D.S., Spira A., Lenburg M.E., Borggrewe M., Eijgenraam T.R., Jonker M.R., Koppelman G.H., et al. Effect of long-term corticosteroid treatment on microRNA and gene-expression profiles in COPD. Eur. Respir. J. 2019;53:1801202. doi: 10.1183/13993003.01202-2018. [DOI] [PubMed] [Google Scholar]
- 50.Xu H., Sun Q., Lu L., Luo F., Zhou L., Liu J., Cao L., Wang Q., Xue J., Yang Q., et al. MicroRNA-218 acts by repressing TNFR1-mediated activation of NF-kappaB, which is involved in MUC5AC hyper-production and inflammation in smoking-induced bronchiolitis of COPD. Toxicol. Lett. 2017;280:171–180. doi: 10.1016/j.toxlet.2017.08.079. [DOI] [PubMed] [Google Scholar]
- 51.Shen W., Liu J., Fan M., Wang S., Zhang Y., Wen L., Wang R., Wei W., Li N., Zhang Y., et al. MiR-3202 protects smokers from chronic obstructive pulmonary disease through inhibiting FAIM2: An in vivo and in vitro study. Exp. Cell Res. 2018;362:370–377. doi: 10.1016/j.yexcr.2017.11.038. [DOI] [PubMed] [Google Scholar]
- 52.Leuenberger C., Schuoler C., Bye H., Mignan C., Rechsteiner T., Hillinger S., Opitz I., Marsland B., Faiz A., Hiemstra P.S., et al. MicroRNA-223 controls the expression of histone deacetylase 2: A novel axis in COPD. J. Mol. Med. 2016;94:725–734. doi: 10.1007/s00109-016-1388-1. [DOI] [PubMed] [Google Scholar]
- 53.Du Y., Ding Y., Chen X., Mei Z., Ding H., Wu Y., Jie Z. MicroRNA-181c inhibits cigarette smoke-induced chronic obstructive pulmonary disease by regulating CCN1 expression. Respir. Res. 2017;18:155. doi: 10.1186/s12931-017-0639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y., Guan S., Ge Y., Yang Y., Cao Y., Zhou J. Cigarette smoke promotes chronic obstructive pulmonary disease (COPD) through the miR-130a/Wnt1 axis. Toxicol. Vitro. 2020;65:104770. doi: 10.1016/j.tiv.2020.104770. [DOI] [PubMed] [Google Scholar]
- 55.Gu W., Yuan Y., Yang H., Wu H., Wang L., Tang Z., Li Q. Role of miR-195 in cigarette smoke-induced chronic obstructive pulmonary disease. Int. Immunopharmacol. 2018;55:49–54. doi: 10.1016/j.intimp.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 56.Jia Q., Chang J., Hong Q., Zhang J.J., Zhou H., Chen F.H. MiR-212-5p exerts a protective effect in chronic obstructive pulmonary disease. Discov. Med. 2018;26:173–183. [PubMed] [Google Scholar]
- 57.Zhong S., Chen C., Liu N., Yang L., Hu Z., Duan P., Shuai D., Zhang Q., Wang Y. Overexpression Of hsa-miR-664a-3p Is Associated With Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease Via Targeting FHL1. Int. J. Chron. Obstruct. Pulmon. Dis. 2019;14:2319–2329. doi: 10.2147/COPD.S224763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Asthma E., Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 59.Mousavi S.R., Ahmadi A., Jamalkandi S.A., Salimian J. Involvement of microRNAs in physiological and pathological processes in asthma. J. Cell Physiol. 2019;234:21547–21559. doi: 10.1002/jcp.28781. [DOI] [PubMed] [Google Scholar]
- 60.Taka S., Tzani-Tzanopoulou P., Wanstall H., Papadopoulos N.G. MicroRNAs in Asthma and Respiratory Infections: Identifying Common Pathways. Allergy Asthma Immunol. Res. 2020;12:4–23. doi: 10.4168/aair.2020.12.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang H., Lu H., Liang L., Zhi Y., Huo B., Wu L., Xu L., Shen Z. MicroRNA-744 Inhibits Proliferation of Bronchial Epithelial Cells by Regulating Smad3 Pathway via Targeting Transforming Growth Factor-beta1 (TGF-beta1) in Severe Asthma. Med. Sci. Monit. 2019;25:2159–2168. doi: 10.12659/MSM.912412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen J., Zhao J., Ye Q.Y., Gu X.D. Interference of miR-943-3p with secreted frizzled-related proteins4 (SFRP4) in an asthma mouse model. Cell Tissue Res. 2019;378:67–80. doi: 10.1007/s00441-019-03026-6. [DOI] [PubMed] [Google Scholar]
- 63.Li B.B., Chen Y.L., Pang F. MicroRNA-30a Targets ATG5 and Attenuates Airway Fibrosis in Asthma by Suppressing Autophagy. Inflammation. 2020;43:44–53. doi: 10.1007/s10753-019-01076-0. [DOI] [PubMed] [Google Scholar]
- 64.American Thoracic Society Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am. J. Respir. Crit. Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 65.American Thoracic S., European Respiratory S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Q., Ye H., Xiang F., Song L.J., Zhou L.L., Cai P.C., Zhang J.C., Yu F., Shi H.Z., Su Y., et al. miR-18a-5p Inhibits Sub-pleural Pulmonary Fibrosis by Targeting TGF-beta Receptor II. Mol. Ther. 2017;25:728–738. doi: 10.1016/j.ymthe.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang C., Li X., Zhang L., Cui D., Quan X., Yang W. The anti-fibrotic effects of microRNA-153 by targeting TGFBR-2 in pulmonary fibrosis. Exp. Mol. Pathol. 2015;99:279–285. doi: 10.1016/j.yexmp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Stolzenburg L.R., Wachtel S., Dang H., Harris A. miR-1343 attenuates pathways of fibrosis by targeting the TGF-beta receptors. Biochem. J. 2016;473:245–256. doi: 10.1042/BJ20150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fierro-Fernandez M., Busnadiego O., Sandoval P., Espinosa-Diez C., Blanco-Ruiz E., Rodriguez M., Pian H., Ramos R., Lopez-Cabrera M., Garcia-Bermejo M.L., et al. miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX4 and TGFBR2. EMBO Rep. 2015;16:1358–1377. doi: 10.15252/embr.201540750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang C., Xiao X., Yang Y., Mishra A., Liang Y., Zeng X., Yang X., Xu D., Blackburn M.R., Henke C.A., et al. MicroRNA-101 attenuates pulmonary fibrosis by inhibiting fibroblast proliferation and activation. J. Biol. Chem. 2017;292:16420–16439. doi: 10.1074/jbc.M117.805747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ji X., Wu B., Fan J., Han R., Luo C., Wang T., Yang J., Han L., Zhu B., Wei D., et al. The Anti-fibrotic Effects and Mechanisms of MicroRNA-486-5p in Pulmonary Fibrosis. Sci. Rep. 2015;5:14131. doi: 10.1038/srep14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ge L., Habiel D.M., Hansbro P.M., Kim R.Y., Gharib S.A., Edelman J.D., Konigshoff M., Parimon T., Brauer R., Huang Y., et al. miR-323a-3p regulates lung fibrosis by targeting multiple profibrotic pathways. JCI Insight. 2016;1:e90301. doi: 10.1172/jci.insight.90301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui H., Banerjee S., Xie N., Ge J., Liu R.M., Matalon S., Thannickal V.J., Liu G. MicroRNA-27a-3p Is a Negative Regulator of Lung Fibrosis by Targeting Myofibroblast Differentiation. Am. J. Respir. Cell Mol. Biol. 2016;54:843–852. doi: 10.1165/rcmb.2015-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Q., Han L., Yan W., Ji X., Han R., Yang J., Yuan J., Ni C. miR-489 inhibits silica-induced pulmonary fibrosis by targeting MyD88 and Smad3 and is negatively regulated by lncRNA CHRF. Sci. Rep. 2016;6:30921. doi: 10.1038/srep30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei P., Xie Y., Abel P.W., Huang Y., Ma Q., Li L., Hao J., Wolff D.W., Wei T., Tu Y. Transforming growth factor (TGF)-beta1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death Dis. 2019;10:670. doi: 10.1038/s41419-019-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan J., Li P., Pan H., Li Y., Xu Q., Xu T., Ji X., Liu Y., Yao W., Han L., et al. miR-542-5p Attenuates Fibroblast Activation by Targeting Integrin alpha6 in Silica-Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2018;19:3717. doi: 10.3390/ijms19123717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamikawaji K., Seki N., Watanabe M., Mataki H., Kumamoto T., Takagi K., Mizuno K., Inoue H. Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis. J. Hum. Genet. 2016;61:985–993. doi: 10.1038/jhg.2016.99. [DOI] [PubMed] [Google Scholar]
- 78.Khalil W., Xia H., Bodempudi V., Kahm J., Hergert P., Smith K., Peterson M., Parker M., Herrera J., Bitterman P.B., et al. Pathologic Regulation of Collagen I by an Aberrant Protein Phosphatase 2A/Histone Deacetylase C4/MicroRNA-29 Signal Axis in Idiopathic Pulmonary Fibrosis Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2015;53:391–399. doi: 10.1165/rcmb.2014-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsushima S., Ishiyama J. MicroRNA-29c regulates apoptosis sensitivity via modulation of the cell-surface death receptor, Fas, in lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;311:L1050–L1061. doi: 10.1152/ajplung.00252.2016. [DOI] [PubMed] [Google Scholar]
- 80.Xie T., Liang J., Geng Y., Liu N., Kurkciyan A., Kulur V., Leng D., Deng N., Liu Z., Song J., et al. MicroRNA-29c Prevents Pulmonary Fibrosis by Regulating Epithelial Cell Renewal and Apoptosis. Am. J. Respir. Cell Mol. Biol. 2017;57:721–732. doi: 10.1165/rcmb.2017-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cui H., Ge J., Xie N., Banerjee S., Zhou Y., Antony V.B., Thannickal V.J., Liu G. miR-34a Inhibits Lung Fibrosis by Inducing Lung Fibroblast Senescence. Am. J. Respir. Cell Mol. Biol. 2017;56:168–178. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu B., Li R., Zhang J., Meng C., Zhang J., Song X., Lv C. MicroRNA-708-3p as a potential therapeutic target via the ADAM17-GATA/STAT3 axis in idiopathic pulmonary fibrosis. Exp. Mol. Med. 2018;50:e465. doi: 10.1038/emm.2017.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhuang Y., Dai J., Wang Y., Zhang H., Li X., Wang C., Cao M., Liu Y., Ding J., Cai H., et al. MiR-338* targeting smoothened to inhibit pulmonary fibrosis by epithelial-mesenchymal transition. Am. J. Transl. Res. 2016;8:3206–3213. [PMC free article] [PubMed] [Google Scholar]
- 84.Zhuang Y., Dai J., Wang Y., Zhang H., Li X., Wang C., Cao M., Liu Y., Cai H., Zhang D., et al. MiR-338* suppresses fibrotic pathogenesis in pulmonary fibrosis through targeting LPA1. Am. J. Transl. Res. 2016;8:3197–3205. [PMC free article] [PubMed] [Google Scholar]
- 85.Simonneau G., Gatzoulis M.A., Adatia I., Celermajer D., Denton C., Ghofrani A., Gomez Sanchez M.A., Krishna Kumar R., Landzberg M., Machado R.F., et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 86.Jusic A., Devaux Y., Action E.U.-C.C. Noncoding RNAs in Hypertension. Hypertension. 2019;74:477–492. doi: 10.1161/HYPERTENSIONAHA.119.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tao W., Sun W., Zhu H., Zhang J. miR-205-5p suppresses pulmonary vascular smooth muscle cell proliferation by targeting MICAL2-mediated Erk1/2 signaling. Microvasc. Res. 2019;124:43–50. doi: 10.1016/j.mvr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Zhou C., Chen Y., Kang W., Lv H., Fang Z., Yan F., Li L., Zhang W., Shi J. Mir-455-3p-1 represses FGF7 expression to inhibit pulmonary arterial hypertension through inhibiting the RAS/ERK signaling pathway. J. Mol. Cell Cardiol. 2019;130:23–35. doi: 10.1016/j.yjmcc.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Zhao H., Guo Y., Sun Y., Zhang N., Wang X. miR-181a/b-5p ameliorates inflammatory response in monocrotaline-induced pulmonary arterial hypertension by targeting endocan. J. Cell Physiol. 2020;235:4422–4433. doi: 10.1002/jcp.29318. [DOI] [PubMed] [Google Scholar]
- 90.Liu T., Zou X.Z., Huang N., Ge X.Y., Yao M.Z., Liu H., Zhang Z., Hu C.P. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci. 2019;227:64–73. doi: 10.1016/j.lfs.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 91.Fan J., Fan X., Guang H., Shan X., Tian Q., Zhang F., Chen R., Ye F., Quan H., Zhang H., et al. Upregulation of miR-335-3p by NF-kappaB Transcriptional Regulation Contributes to the Induction of Pulmonary Arterial Hypertension via APJ during Hypoxia. Int. J. Biol. Sci. 2020;16:515–528. doi: 10.7150/ijbs.34517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan H., Yao H., Lie Z., Chen G., Lin S., Zhang Y. MicroRNA30a5p promotes proliferation and inhibits apoptosis of human pulmonary artery endothelial cells under hypoxia by targeting YKL40. Mol. Med. Rep. 2019;20:236–244. doi: 10.3892/mmr.2019.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartram U., Speer C.P. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 94.Danopoulos S., Shiosaki J., Al Alam D. FGF Signaling in Lung Development and Disease: Human Versus Mouse. Front. Genet. 2019;10:170. doi: 10.3389/fgene.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kugler M.C., Joyner A.L., Loomis C.A., Munger J.S. Sonic hedgehog signaling in the lung. From development to disease. Am. J. Respir. Cell Mol. Biol. 2015;52:1–13. doi: 10.1165/rcmb.2014-0132TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pongracz J.E., Stockley R.A. Wnt signalling in lung development and diseases. Respir. Res. 2006;7:15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang R.N., Green J., Wang Z., Deng Y., Qiao M., Peabody M., Zhang Q., Ye J., Yan Z., Denduluri S., et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kho A.T., Sharma S., Davis J.S., Spina J., Howard D., McEnroy K., Moore K., Sylvia J., Qiu W., Weiss S.T., et al. Circulating MicroRNAs: Association with Lung Function in Asthma. PLoS ONE. 2016;11:e0157998. doi: 10.1371/journal.pone.0157998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salimian J., Mirzaei H., Moridikia A., Harchegani A.B., Sahebkar A., Salehi H. Chronic obstructive pulmonary disease: MicroRNAs and exosomes as new diagnostic and therapeutic biomarkers. J. Res. Med. Sci. 2018;23:27. doi: 10.4103/jrms.JRMS_1054_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alipoor S.D., Adcock I.M., Garssen J., Mortaz E., Varahram M., Mirsaeidi M., Velayati A. The roles of miRNAs as potential biomarkers in lung diseases. Eur. J. Pharmacol. 2016;791:395–404. doi: 10.1016/j.ejphar.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huleihel L., Sellares J., Cardenes N., Alvarez D., Faner R., Sakamoto K., Yu G., Kapetanaki M.G., Kaminski N., Rojas M. Modified mesenchymal stem cells using miRNA transduction alter lung injury in a bleomycin model. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L92–L103. doi: 10.1152/ajplung.00323.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]