Abstract
Tungsten oxide nanostructures were modified by oxygen vacancies through hydrothermal treatment. Both the crystalline structure and morphological appearance were completely changed. Spherical WO3·H2O was prepared from tungstic acid solution by aging at room temperature, while rod-like WO3·0.33H2O was prepared by hydrothermal treatment of tungstic acid solution at 120 °C. These structures embedded in sodium alginate (SA)/polyvinylpyrrolidone (PVP) were synthesized as novel porous beads by gelation method into calcium chloride solution. The performance of the prepared materials as photocatalysts is examined for methylene blue (MB) degradation in aqueous solutions. Different operation parameters affecting the dye degradation process, such as light intensity, illumination time, and photocatalyst dosage are investigated. Results revealed that the photocatalytic activity of novel nanocomposite changed with the change in WO3 morphology. Namely, the beads with rod nanostructure of WO3 have shown better effectiveness in MB removal than the beads containing WO3 in spherical form. The maximum degradation efficiency was found to be 98% for WO3 nanorods structure embedded beads, while the maximum removal of WO3 nanospheres structure embedded beads was 91%. The cycling-ability and reuse results recommend both prepared structures to be used as effective tools for treating MB dye-contaminated wastewaters. The results show that the novel SA/PVP/WO3 nanocomposite beads are eco-friendly nanocomposite materials that can be applied as photocatalysts for the degradation of cationic dyes in contaminated water.
Keywords: cationic dye, photodegradation, nanocomposite hydrogel beads, sodium alginate, tungsten oxide nanostructures, polyvinylpyrrolidone
1. Introduction
Dyes are used broadly in many different industries, including leather, paper-making, cosmetics, tanning, printing, and plastics industries, as well as in the textile and dyeing industries [1,2]. The release of dye-contaminated wastewaters into the environment leads to intensive aquatic defects, which in turn affect the human race. Generally, dyes and their byproducts are considered to be toxic or mutagenic agents [3]. Different strategies are utilized to remove dyes from contaminated waters, such as biological methods [4], coagulation and flocculation [5], photocatalysis [6], and adsorption using natural or synthetic materials [7,8].
Nowadays, degradation by photocatalysis has become a promising technique for the removal of several organic [9] and inorganic [10] pollutants from wastewaters by converting them into non-hazardous substances. Several semiconductor materials have been used as photocatalysts to remove different types of pollutants from wastewaters. One of them is tungsten oxide (WO3) [11,12], which has a lower light energy conversion efficiency than the widely used TiO2 photocatalyst [13]. WO3 has the advantage of being non-toxic, cost-effective, with a high physicochemical stability under irradiation, and a wide range photocatalytic activity in the visible region [14]. It also has good preparation availability of various WO3 structures, for instance, orthorhombic WO3 has terminal oxygen, which increases its catalytic activity [15]. However, pure WO3 has limited photocatalytic activity due to its slow charge transfer and rapid recombination of the photogenerated electron-hole pairs [16]. Several studies have been carried out to tackle this problem. Kim et al. [13] have used thermal evaporation methods to produce WO3 nanorods on tungsten substrates. The obtained materials were used for photodegradation of methylene blue (MB) dyes [13]. Jeevitha et al. [17] prepared nanocomposites including both tungsten oxide and graphene oxide as efficient photocatalysts against MB dye. Ma et al. synthesized WO3/SnNb2O6 hybrid nanosheet heterojunctions as efficient Z-scheme photocatalysts via a simple hydrothermal co-assembly method [18]. Ke et al. enhanced the photocatalytic performance of WO3 nanosheets for photocatalytic water oxidation by using ion doping (Ag+) [19]. Zhang et al. used the co-deposition of noble metals (e.g., Pt) to ameliorate the charge transport between CdS and WO3 in CdS/WO3 nanojunction [20].
The problem regarding the larger-scale operation of photocatalysis is slow separation and recycling of photocatalyst during the wastewater treatment. This problem can be solved by supporting the photocatalyst onto a polymeric matrix.
Silver embedded in ZnO and polystyrene matrix film was prepared as a floating photocatalyst to remove MB with an efficiency of 97% [21]. ZnO and TiO2 nanoparticles were also incorporated into calcium alginate beads as a photocatalyst for the removal of copper ions [10]. A photocatalyst based on TiO2 immobilized in calcium alginate beads exhibited an increase in MB degradation efficiency when the beads were reused [22].
A natural polysaccharide polymer, sodium alginate (SA), derived from brown seaweeds, is composed of two acids: α-L-guluronic and β-D-mannuronic acids [23]. SA is biocompatible, non-toxic, biodegradable, gelable polysaccharide, and chelating able, suitable for chemical modification [24] and often used as a polymeric matrix that can act as a catalyst support [10]. SA can be formed as hydrogel beads by cross-linking the α-L-guluronic acid units with poly- or di-valent cations. However, it has some disadvantages as a natural polymer, such as microbial degradation and poor mechanical strength. For improving its usability, it should be blended with synthetic polymer(s) for semi-interpenetrating polymer network hydrogel. Furthermore, the presence of the active functional groups on the natural and synthetic polymers in the polymeric network, allows the obtained hydrogel beads to be used effectively as adsorbents [25].
In this study, polyvinylpyrrolidone (PVP) was blended with SA as a natural pore-former polymer, in order to increase the porosity of the formed beads [26]. In addition, in-situ hydrothermal assembly of WO3 nanospheres and nanorods was carried out and the formed nanostructures WO3 were embedded with SA/PVP polymer blend to form novel SA/PVP/WO3 nanocomposites blend hydrogel beads through a crosslinking method with calcium chloride as the cross-linker. This study aims to (i) prepare a photocatalyst composite by a facile, economic, and efficient method and (ii) investigate the photocatalytic performance of SA/PVP/WO3 nanocomposite beads on the removal efficiency of cationic dyes, such as methylene blue. This work focused on doing the performance comparison of WO3 photocatalyst with different morphologies and incorporated in porous polymeric beads to form novel hybrid nanocomposites. The obtained nanocomposites were used to remove the organic dye by two mechanisms: Adsorption and photocatalysis.
2. Materials and Methods
2.1. Materials
Sodium alginate (Sigma Aldrich, St. Louis (MO), USA), polyvinylpyrrolidone (Sigma Aldrich, St. Louis, Missouri, USA), and sodium tungstate hydrate (Na2WO4·2H2O > 99%) was acquired from Kanto Chemicals Co. (Tokyo, Japan). A strong acid type cation-exchange resin (Diaion 25 PK228LH, ion-exchange capacity > 2.05 meq mL−1) was purchased from Mitsubishi Chemical Co. (Tokyo, Japan). All chemicals were used without further purification. Deionized water was used for preparing the solutions.
2.2. Preparation of WO3 Nanostructures
The ion exchange process was done in a glass column with a height of 150 mm and a diameter of 24.6 mm. The glass column was packed with 30 mL of the ion-exchange resin. A flow of 10 mL of water was passed through the column to wash the resin and this washing step was repeated five additional times. 0.5 M of Na2WO4 solution was prepared by dissolving Na2WO4·2H2O powder in deionized water followed by loading 10 mL of this Na2WO4 solution on the glass column. The acidified tungstic acid (H2WO4) solution was recovered by elution with deionized water, with the resulting solution being yellowish and transparent. Inductively coupled plasma atomic emission spectrometry (ICP-AES, Optima 4300DV, Perkin Elmer, Waltham, Massachusetts, USA) was used to determine that the Na+ concentration was 1.7 ± 0.6 ppm (n = 3). This ion-exchange precursor was aged at room temperature for 24 h to produce spherical WO3 nanoparticles. On the other hand, the ion-exchange precursor was hydrothermally treated at 120 °C for 24 h to produce the WO3 nanorods.
2.3. Preparation of Adsorbent Beads
Each polymer was dissolved separately in distilled water at room temperature and mixed with percentage containing 90 wt.% SA, 7 wt.% PVP, and 3 wt.% WO3 for 2 h to form homogenous solutions. Then, 50 mL of polymer solutions was added dropwise using a syringe into the 200 mL solution containing 2% (w/v) of calcium chloride. It was allowed to harden for 30 min (during stirring) and then the polymer beads were rinsed three times with distilled water.
2.4. Characterization of the WO3 Nanostructures and Polymeric Beads
X-ray powder diffraction (XRD, Cu-Kα radiation, Shimadzu-7000, Kyoto, Japan) was used to determine the crystallographic phase of the prepared samples. Their morphology was examined by scanning electron microscopy (SU-70, Hitachi, Tokyo, Japan) combined with energy dispersive X-ray analysis (EDX) for the elements identifications. Fourier transform infrared (FTIR) analysis was done with a Bruker ALFA spectrometer (Bruker Corporation, Ettlingen, Germany).
2.5. Photocatalytic Decay Experiments
The evaluation of photocatalytic degradation of MB dye using different prepared SA/PVP/WO3 nanocomposite samples, either with WO3 nanorods or WO3 nanospheres, as a photocatalyst under the illumination of unfiltered and commercially available LED visible light was carried out using a Plexiglas cylindrical reactor with 15 cm diameter and 20 cm height, as shown in Figure S1 (Supplementary Information). The glass surface of the reactor was covered with aluminum foil. In addition, the reactor had two 12 W lamps with a light intensity of 1200 lm (Bareeq, Cairo, Egypt) fixed on the top as the radiation source.
Typically, 0.5 g L−1 of the catalyst was suspended in a model wastewater of MB dye solution. The suspension was magnetically stirred at room temperature and illuminated with visible light, with the samples being collected at a regular interval of time. The residual MB concentration after irradiation was monitored using UV–Vis spectrophotometer (Shimadzu UV-2600, Kyoto, Japan) at 665 nm by sampling 2 mL of the reaction mixture. The photocatalytic degradation of MB using different WO3 morphologies was calculated using the formulae,
| photodegradation % = [(C0 − C)/C0] × 100 | (1) |
where C0 and C are the initial and final dye concentrations, respectively.
The photocatalytic efficiency of the two different prepared composites beads on MB was studied at pH 7 of the dye solution. The pH was adjusted to seven by adding 0.1 M HCl or 0.1 M NaOH. Habib et al. [27] have reported that a pH value of seven is the most suitable for photocatalytic degradation activity.
2.6. Kinetic Models
The pseudo-first-order model and the pseudo-second-order model are the most common kinetic models used. The pseudo-first-order equation was stated as follows [28],
| (2) |
where qe and qt are the amounts of MB adsorbed or degraded (mg g−1) at equilibrium and at time t (sec), respectively, and k1 is the rate constant (sec−1). Values of K1 were calculated from the plots of log (qe − qt) versus t. The pseudo-second-order model can be expressed by the following expression [8],
| (3) |
where K2 (g mg−1 s−1) is the rate constant of the second-order model. The plot of t/q versus t should show a linear relationship when the second-order kinetics is applicable. qe and K2 can be determined from the slope and intercept of the plot. This procedure is probably predicting the behavior over the whole range of processes.
3. Results and Discussion
3.1. Characterization of the SA/PVP/WO3 Nanocomposite
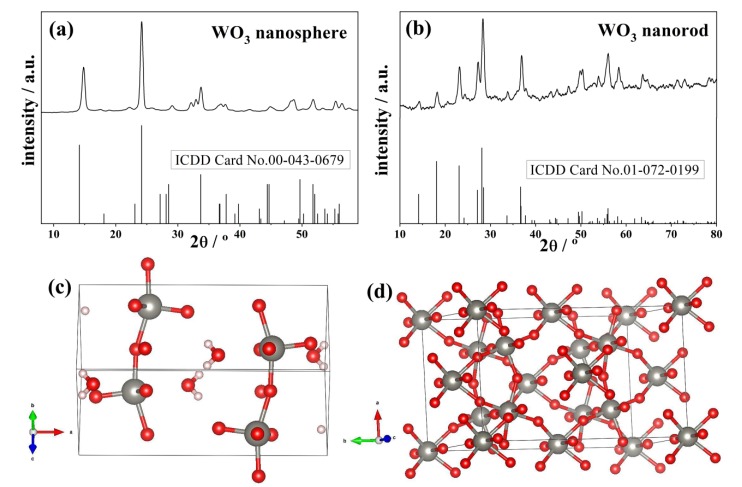
The crystal structure of the synthesized WO3 nanospheres and nanorods was checked by XRD analysis. The XRD patterns of both WO3 nanoparticles show highly crystalline features and it is close to the literature data of the orthorhombic WO3·H2O phase [29,30]. The WO3 nanosphere was in orthorhombic WO3·H2O phase with space group of Pmnb (62) and lattice parameters of a = 5.2380 Å, b = 10.7040 Å, and c = 5.1200 Å (ICDD Card No. 00-043-0679) (Figure 1a). On the other hand, the WO3 nanorods were in orthorhombic WO3·0.33H2O phase, with a space group of Fmm2 (42) and lattice parameters of a = 7.3590 Å, b =12.75130 Å, and c = 7.7040 Å (ICDD Card No. 01-072-0199) (Figure 1b). For both crystals, there was no secondary phase detected, although the WO3·0.33H2O nanorods contain more oxygen vacancies than WO3·H2O nanospheres, as shown in Figure 1c,d.
Figure 1.
XRD patterns of (a) the WO3 nanospheres (ICDD Card No. 00-043-0679) and (b) WO3 nanorods (ICDD Card No. 01-072-0199) and 3D chemical structure for the (c) WO3 nanospheres and (d) WO3 nanorods.
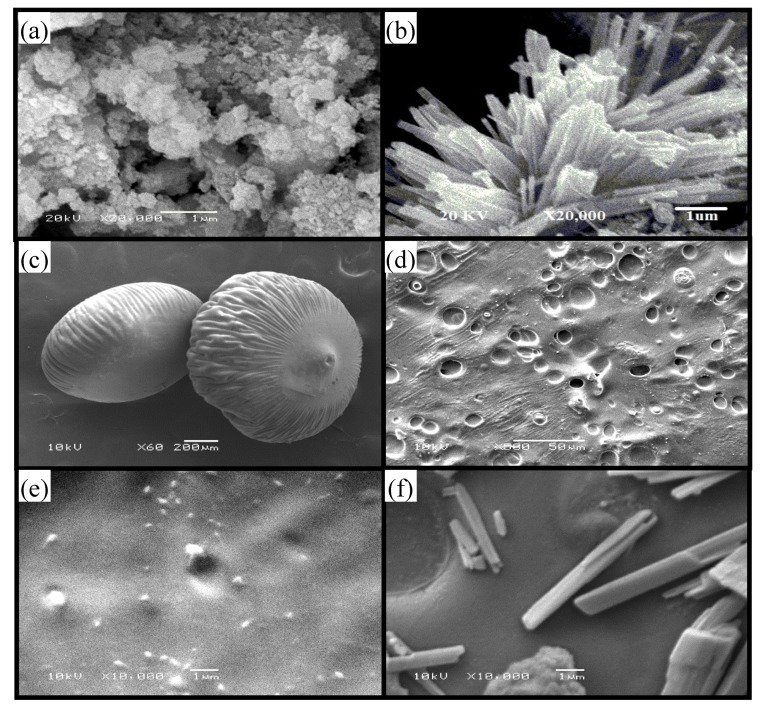
The SEM images in Figure 2a,b show the morphology of the synthesized WO3 nanoparticles as agglomerated or individual nanospheres and nanorods, respectively. It is noticeable from the presented XRD and SEM results that both the morphology and the crystalline structure agree with previous researches [30,31]. After embedding the WO3 nanoparticles into the blended SA/PVP polymers to obtain SA/PVP/WO3 nanocomposite beads, the SEM images of the beads were found to be rough and wrinkled (Figure 2c) with visible pores (Figure 2d). Meanwhile, by taking a high magnification inside the bead, a good dispersion of the WO3 nanospheres or nanorods was observed (Figure 2e,f, respectively).
Figure 2.
SEM images of (a) the WO3 nanospheres, (b) the WO3 nanorods, (c) spherical-shaped beads, and high magnification of (d) the bead surface and the inside of (e) the nanosphere and (f) the nanorod nanocomposite beads.
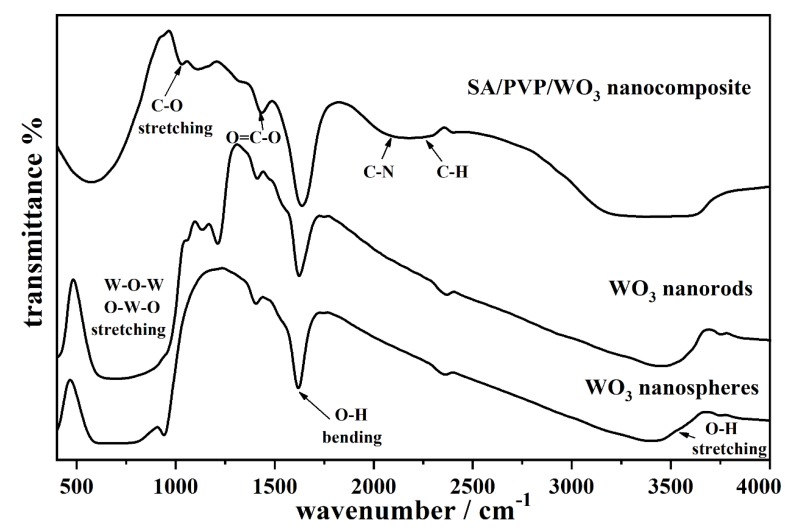
Figure 3 shows the FTIR spectra of WO3 nanospheres, WO3 nanorods, and SA/PVP/WO3 nanocomposite. The FTIR measurements for WO3 nanosphere and WO3 nanorod reveal, in general, that their patterns are composed of a bond between W and O, and in particular that the band in the 500–1000 cm−1 range is characteristic of the W-O-W and O-W-O stretching vibration modes [32]. Moreover, all samples show bands around 1600 and 3500 cm−1 that are attributed to the O-H stretching bending modes and the H2O bending vibration modes, respectively. On the other hand, the FTIR spectrum for SA/PVP/WO3 nanocomposite shows absorption bands at 1419 cm−1, which is characteristic of symmetric stretching vibration of (COO) groups for SA. The band at 1030 cm−1 represents skeletal stretching of (C–O) [33], the band at 2178 cm−1 corresponds to (C-N) bond of PVP, and the band at 2170–2300 cm−1 is due to the (C-H) bonds of the polymers [26]. The WO3 vibrations were found at the 600–1000 cm−1 region.
Figure 3.
FTIR spectra of the WO3 nanospheres, WO3 nanorods, and sodium alginate (SA)/polyvinylpyrrolidone (PVP)/WO3 nanocomposite.
3.2. Adsorption and Photocatalysis Removal of Methylene Blue
3.2.1. Effect of Time on MB Decay
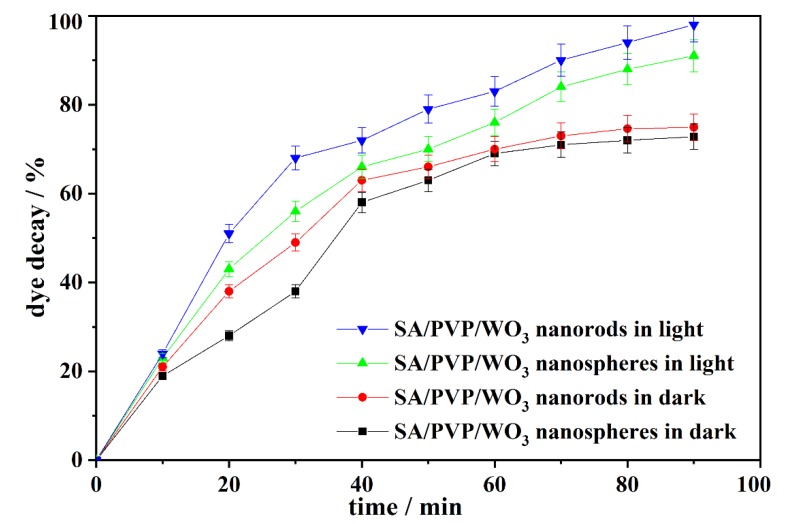
The adsorption behavior of SA/PVP/WO3 nanocomposites was studied in the dark to determine the adsorption extent for MB dye onto the beads. That information is required to assess the photocatalytic activity of the SA/PVP/WO3 nanocomposite beads for removing MB dye in the presence of light. The illumination time was performed using 0.5 g L−1 of the two different composite beads with 500 mL of 50 mg L−1 dye solution at solution pH equal to seven, in dark and under visible light at different time intervals. As shown in Figure 4 and illustrated in Table S1 in the Supplementary Information, dark adsorption increased with the increasing time, and then almost plateaued after 60 min, which may be due to the porous nature of the SA/PVP blended polymer. Additionally, the most abundant functional groups in SA polymer are carboxylic groups, which also enhance the adsorption of cationic dye molecules.
Figure 4.
The relation between illumination time and methylene blue (MB) decay (%) using the SA/PVP/WO3 nanorods and the SA/PVP/WO3 nanospheres nanocomposites.
However, under visible light, the results reveal that there was an increase in the photocatalytic activity with the increase in illumination time reaching 98% after 90 min of illumination using SA/PVP/WO3 nanorods composite and 91% after 90 min using SA/PVP/WO3 nanospheres composite.
3.2.2. Effect of the Light Intensity on the MB Photocatalytic Decay
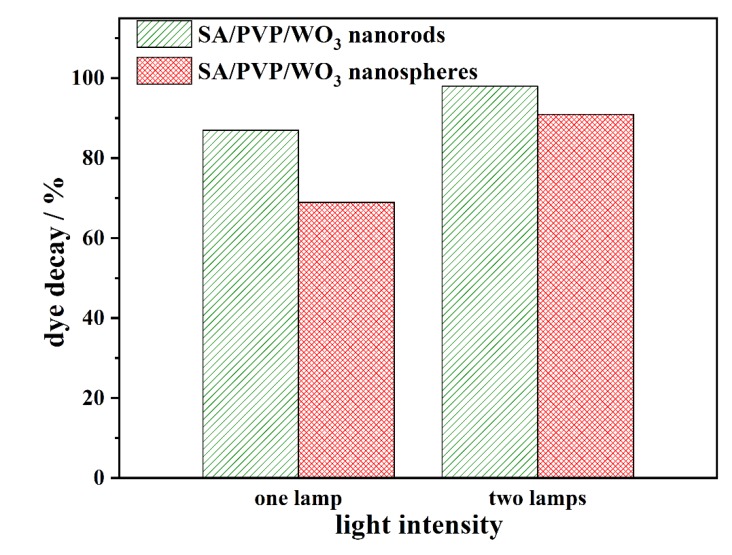
The effect of different light intensities on the effectiveness of photocatalytic dye decay using the composites with the two different morphologies of WO3 was assessed. The effect of UV light intensity on the efficiency of the system was evaluated by fixing two lamps on the reactor cover instead of one lamp. The results presented in Figure 5 indicate that increasing the light intensity increased the efficiency of the system after 90 min. This is because the increase of the light intensity increases the quantity of light received by the photocatalyst particles, which increases electron stimulation and enhances the system’s effectiveness [34].
Figure 5.
Effect of light intensity on the photocatalytic MB decay using SA/PVP/WO3 nanocomposites (pH 7; 90 min of illumination time; and 50 mg L−1 as the initial MB concentration).
3.2.3. Effect of Initial Dye Concentration on MB Photocatalytic Decay
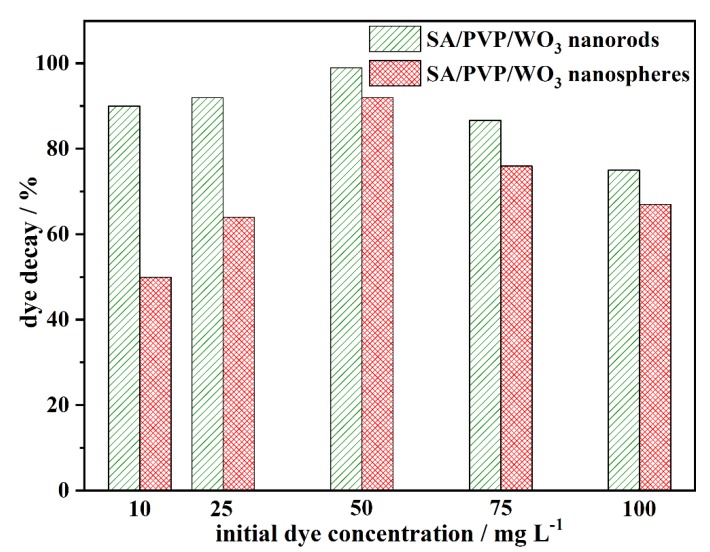
Figure 6 shows the effect of initial dye concentration on the MB decay using the two prepared photocatalysts. In a typical process, 0.5 g L−1 (fixed) of catalysts were added to the different solutions of dye with different concentration (10, 25, 50, and 100 mg L−1) maintaining the dye solution at pH 7 with a contact time of 90 min and light intensity of 1200 lm. However, the results reveal that the MB dye decay generally increases with the increasing initial MB concentration, with the maximum decay being obtained at 50 mg L−1. Further increase in the MB dye concentration has a negative effect on its decay.
Figure 6.
Effect of initial MB dye concentration on photocatalytic decay process using the two prepared nanocomposite photocatalysts (pH 7; 90 min contact time; and 1200 lm of light intensity).
This can be returned to the fact that dyes photodegradation rate depends mainly on the possibility of formation of hydroxyl free radicals OH• on the catalyst surface and the reaction of the dye molecules with the formed radicals. The initial increase in the decay rate with the increase in initial dye concentration might be attributed to an increase in the probability of the reaction between the dye molecules and OH• [35,36]. The proposed mechanism for MB degradation can be summarized in the following steps. First, visible light irradiation allows electrons in the valence band to transfer into the conduction band. Hence, holes (h) and electrons (e−) are formed at the surface of the WO3 photocatalyst. Then, the holes react with hydroxide ion, while electrons react with dissolved oxygen for the production of OH•, which degrade MB dye into non-toxic gases, such as carbon dioxide, and water. Furthermore, hydrogen peroxide reacts with electrons for the production of more OH• for enhancing the degradation of the dye [37,38,39].
| WO3 + visible light → h+ (hole) + e− (electron) | (4) |
| h+ + H2O → H+ + OH− | (5) |
| h+ + OH− →OH• | (6) |
| e− + O2 → O2•− | (7) |
| O2•− + e− + 2H+ → H2O2 | (8) |
| O2•− + H2O2 → OH• + OH− + O2 | (9) |
| OH• + dye → nontoxic products + CO2↑ | (10) |
| O2•− + dye → nontoxic products + CO2↑ | (11) |
However, a further rise in the MB concentration decreases the activity of the photocatalyst. This might be due to the inhibition of the reaction between the MB dye molecules and OH•, as more MB molecules are adsorbed on the catalyst surface at high dye concentration thereby reducing the formation of OH• [40]. Moreover, with increased color intensity, gaps pertaining to the entry of photons get restricted from reaching the surface of the photocatalyst limiting the radical attack (photodegradation) of pollutants [39]. Hence, initial MB concentration was found to be optimum at 50 mg L−1 exhibiting 98% photodegradation efficiency using SA/PVP/WO3 nanorods nanocomposite.
3.2.4. Effect of WO3 concentration in SA/PVP matrix
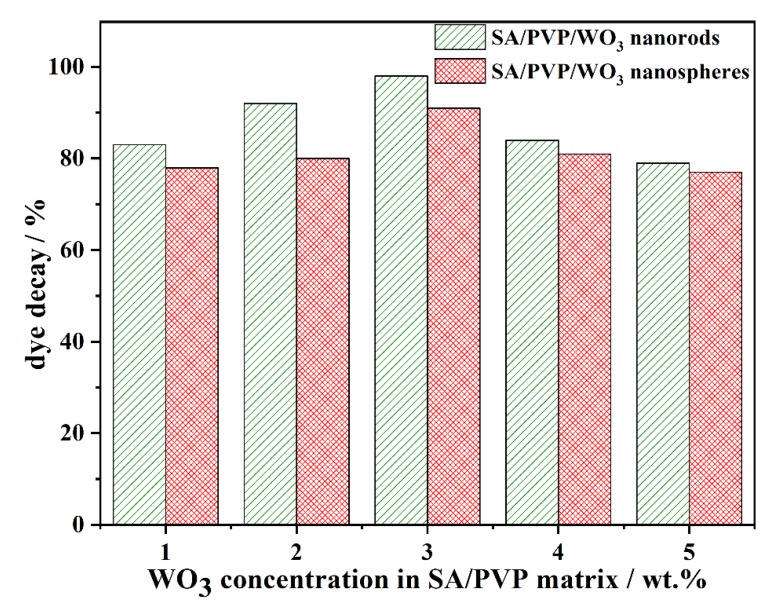
The effect of photocatalyst concentration on MB decay was studied by varying the prepared WO3 photocatalysts concentration in SA/PVP matrix between 1 to 5 wt.%. From Figure 7 it can be concluded that, with increasing catalyst load from 1 to 3 wt.%, the adsorption efficiency of the system decreases and photodegradation efficiency increases. This may be justified by the fact that at very low catalyst load more porous vacant sites are available on the outer surface of beads to absorb the dye molecules, in addition to the polymers functional groups (COO−), which react with the cationic dyes by electrostatic attraction but the available active sites for the photocatalytic reaction are not sufficient. On the other hand, by increasing the catalyst load to 3 wt.% more active sites for the photocatalytic reaction are available providing more chances for the hydroxyl ions adsorption onto the surface producing superoxide radicals. Meanwhile, at higher catalyst loading the photocatalytic activity was reduced, with the further increment in catalyst loading hampering the dye decay rate due to a shortage of light penetration [41]. Though both forms of WO3 have terminal oxygen, the highest photocatalytic activity of WO3·0.33H2O nanorod may be due to higher oxygen vacancies. Y. Li et al. reported that the more oxygen vacancies WO3 crystal has, the higher the photocatalytic activity will be [42].
Figure 7.
Effect of catalyst loading on the dye decay (pH 7; 90 min illumination time; 50 mg L−1 initial MB concentration; and 1200 lm of light intensity).
However, the catalyst load in SA/PVP/WO3 nanocomposite played a major role in the removal efficiency of dye contaminants. In other words, it will define that either adsorption or photocatalysis will dominate on the removal process, and it should be optimized to achieve better removal from the system and allow being reused several times without losing catalytic activity in the photocatalytic dye removal.
3.3. Analysis of the Reaction Kinetics and Proposed Mechanism
3.3.1. Kinetic Models
Pseudo-first-order and pseudo-second-order kinetic models are applied for the investigation of the reaction mechanism of SA/PVP/WO3 nanocomposite with MB. Generally, kinetic models describe the reaction rates while the order for the reaction defines the dependence of the reaction rates on the concentrations of reacting species [43]. Herein, two sets of experiments were done, in the dark and under light irradiation. Depending on the obtained results, as illustrated in Table 1, it was noticed that there are differences between the two kinetic models and their correlation coefficient (R2) values in the dark and in light irradiation. In dark mode, the rate constants K2 value of the pseudo-second-order model is high for SA/PVP/WO3 rod-like, which indicates the chemisorption nature for the adsorption process of MB [44]. Under light irradiation, the R2 value of the pseudo-first and second-orders model are the same for the rod-like WO3, which may be due to the high chemical stability of the prepared catalyst under light irradiation conditions.
Table 1.
Pseudo-first order and pseudo-second-order kinetic parameters.
| Nanocomposite Material | Pseudo-First-Order | Pseudo-Second-Order | |||
|---|---|---|---|---|---|
| qe mg g−1 |
K1 sec−1 |
R2 | K2 g mg−1 s−1 |
R2 | |
| SA/PVP/WO3 Sphere Nanocomposite in Dark | 72.8 | 0.057 | 0.954 | 0.000398238 | 0.902 |
| SA/PVP/WO3 Rods Nanocomposite in Dark | 74.9 | 0.062 | 0.920 | 0.000533691 | 0.979 |
| SA/PVP/WO3 Sphere Nanocomposite in Light | 91.0 | 0.039 | 0.948 | 0.000358546 | 0.992 |
| SA/PVP/WO3 Rods Nanocomposite in Light | 98.0 | 0.037 | 0.973 | 0.000368839 | 0.973 |
3.3.2. Proposed Reaction Mechanism of SA/PVP/WO3 Nanocomposite with MB
In general, the orthorhombic structure of WO3 contains terminal oxygen atoms as “unsteady state atoms”, and at pH region of three to seven, the surface of WO3 has a negative charge. Consequently, these “unsteady state” oxygen atoms interact with nitrogen atoms in MB molecules exhibiting faster adsorption property to MB [15]. Under light irradiation, electron-hole pairs are formed and concentrated on the surface [45]. On the oxygenated media, OH− and O2− radicals are produced (Figure S2 in Supplementary Materials). Finally, these superoxide radicals ion or the hydroxyl radicals degrade the MB dye into small molecular fragments, e.g., CO2, H2O, and H+, as the final products (Equations (4)–(11)). In summary, the degradation mechanism starts with the MB dye adsorption on the nanocomposite surface followed by its photodegradation [46].
The herein prepared photocatalysts are compared in Table 2 with other WO3-based nanocomposites previously tested for the removal of different organic dyes from water. The removal efficiency of tungsten oxide nanorod embedded in sodium alginate/polyvinylpyrrolidone composite was higher than that of the tungsten oxide-based counterpart. The comparison is very clear between the prepared composite and the aligned WO3 [47], tungsten-loaded TiO2 [48], and mesoporous WO3/TiO2 [49], where the removal efficiency percentages are up to 98%, up to 94%, 90%, and up to 88%, respectively. Although the removal efficiency is closer to that of WO3-graphene oxide (WO3-GO) [17] and WO3 nanorods on reduced graphene oxide [11], the cost and methodology of preparation of these materials is unfavorable when compared to the herein prepared nanocomposite beads, which recommends its practical application.
Table 2.
Tungsten oxide based nanocomposites and its photodegradation behavior against organic dyes.
| Materials | Structure | Morphology | Dye | Efficiency | Ref. |
|---|---|---|---|---|---|
| tungsten oxide embedded in sodium alginate/polyvinylpyrrolidone composite beads | orthorhombic crystalline WO3 | spherical, nanorods | MB | up to 98% | current work |
| aligned WO3 | triclinic, orthorhombic and monoclinic | nanorods and nanosheets | MB | up to 94% | [47] |
| tungsten-loaded TiO2 | crystalline WO3 at higher loadings (>12 mol%) | aggregation | MB | 90% | [48] |
| mesoporous WO3/TiO2 | crystalline | mesoporous | rhodamine B | up to 88% | [49] |
| MWCNT/WO3 | hexagonal and orthorhombic | aggregation | rhodamine B | up to 92% | [50] |
| α-Fe2O3/WO3 composite | crystalline | spherical-shaped α-Fe2O3 nanoparticles and WO3 nanorods | MB | up to 91% | [51] |
| tungsten oxide-graphene oxide (WO3-GO) | monoclinic | aggregation | MB | 97% | [17] |
| WO3 nanorods on reduced graphene oxide sheets | hexagonal wurtzite phase | flower-like | methylthionine chloride | 94% | [11] |
3.4. Recycling of SA/PVP/WO3 Nanocomposite
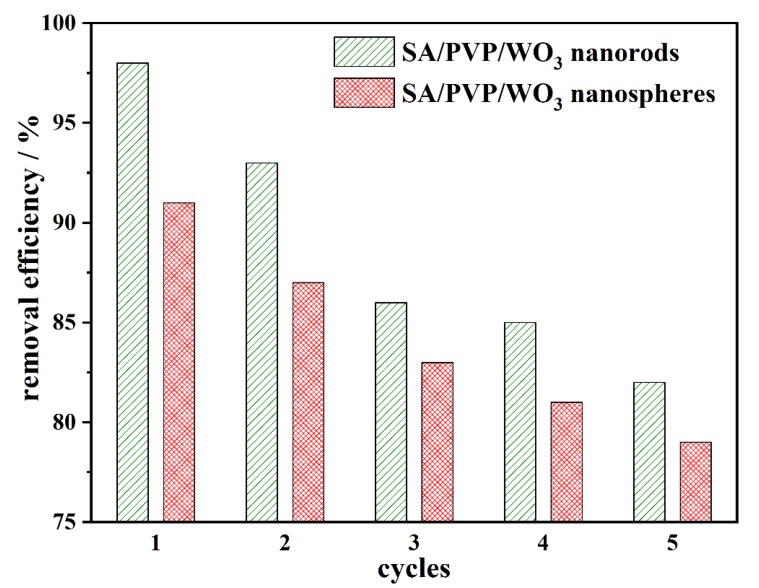
To study the reusability of SA/PVP/WO3 nanocomposite, which allows the process to be regarded as cost-effective, five experimental runs were carried out at optimized conditions using the same beads and degradation efficiency for MB (Figure 8). The SA/PVP/WO3 nanocomposites were recovered and washed with 0.1 M HCl solution and used for five times. The obtained results show that the efficiency decreases from 98% to 82% for used WO3 nanorods and decreases from 91% to 79% for used WO3 nanospheres. This may be attributed to the fouling of the porous surface of the composite [9].
Figure 8.
The recycling efficiency of SA/PVP/WO3 nanocomposites during MB removal for 5 consecutive cycles (pH 7; 90 min illumination time; and 50 mg L−1 initial MB concentration).
4. Conclusions
Tungsten oxide with two different morphologies was embedded in sodium alginate/ polyvinylpyrrolidone as blended polymers. The prepared SA/PVP/WO3 nanocomposite beads were employed for the removal of methylene blue dye in aqueous solutions under visible light. From the obtained MB removal profiles, SA/PVP/WO3 nanorods composite beads have performed better than SA/PVP/WO3 nanospheres composite beads. The removal of MB dye is not solely driven by the adsorption capability of the nanocomposite beads, but it is also attributed to the photocatalytic properties of the WO3. The mechanisms of MB dye removal can be explained in the following steps. The initial adsorption of MB dye molecules onto the SA/PVP/WO3 beads is followed by the photocatalytic degradation of the adsorbed dye molecules by WO3 nanoparticles. Thus, adsorption and photocatalysis are proposed as the main steps in the removal of MB dye and the composite beads can remove the cationic dye molecules by using the concept of “absorb and degrade”. From this study, it is demonstrated that the prepared composite beads can be easily recovered and reused as effective tools for treating MB dye-contaminated wastewaters.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/8/1905/s1, Figure S1: The used photocatalytic reactor, Figure S2: (a) Schematic illustration of the interaction between “unsteady state” oxygen atoms on WO3 and nitrogen atoms in MB molecules and (b) the suggested MB photodegradation mechanism, Table S1: Average experimental values* of the relation between illumination time and MB degradation (%) using the SA/PVP/WO3 nanorods and SA/PVP/WO3 nanospheres nanocomposites.
Author Contributions
E.M.E. carried out performance tests of the prepared materials as photocatalysts and analyzed the experimental data. M.S.E. carried out tungsten oxide preparation, characterization and simulated its structure using VESTA software. M.H.G. proposed the polymeric composite, beads preparation, and characterization. N.A.E. proposed the project and wrote the manuscript with input from the other coauthors. D.M.F.S. supervised and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vijayaraghavan K., Yun Y.S. Biosorption of C.I. reactive black 5 from aqueous solution using acid treated biomass of brown seaweed Laminaria sp. Dye. Pigment. 2008;76:726–732. doi: 10.1016/j.dyepig.2007.01.013. [DOI] [Google Scholar]
- 2.Aksu Z. Application of biosorption for the removal of organic pollutants: A review. Process. Biochem. 2005;40:997–1026. doi: 10.1016/j.procbio.2004.04.008. [DOI] [Google Scholar]
- 3.Sadettin S., Donmez G. Bioaccumulation of reactive dyes by thermophilic cyanobacteria. Process. Biochem. 2006;41:836–841. doi: 10.1016/j.procbio.2005.10.031. [DOI] [Google Scholar]
- 4.Aksu Z., Tezer S. Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process. Biochem. 2005;40:1347–1361. doi: 10.1016/j.procbio.2004.06.007. [DOI] [Google Scholar]
- 5.Guibal E., Roussy J. Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan) React. Funct. Polym. 2007;67:33–42. doi: 10.1016/j.reactfunctpolym.2006.08.008. [DOI] [Google Scholar]
- 6.Salama A., Mohamed A., Aboamera N.M., Osman T.A., Khattab A. Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Appl. Nanosci. 2018;8:155–161. doi: 10.1007/s13204-018-0660-9. [DOI] [Google Scholar]
- 7.Mohy Eldin M.S., Gouda M.H., Abu-Saied M.A., Youssef M.E., El-Shazly Y.M.S., Farag. H.A. Removal of methylene blue by amidoxime polyacrylonitrile-grafted cotton fabrics: Kinetic, equilibrium, and simulation studies. Fiber. Polym. 2016;17:1884–1897. doi: 10.1007/s12221-016-6373-3. [DOI] [Google Scholar]
- 8.El Essawy N.A., Ali S.M., Farag H.A., Konsowa A.H., Elnouby M., Hamad H.A. Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol. Environ. Saf. 2017;145:57–68. doi: 10.1016/j.ecoenv.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar S., Chakraborty S., Bhattacharjee C. Photocatalytic degradation of pharmaceutical wastes by alginate supported TiO2 nanoparticles in packed bed photoreactor (PBPR) Ecotoxicol. Environ. Saf. 2015;121:263–270. doi: 10.1016/j.ecoenv.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Kanakaraju D., Ravichandar S., Lim Y.C. Combined effects of adsorption and photocatalysis by hybrid TiO2/ZnO-calcium alginate beads for the removal of copper. J. Environ. Sci. 2017;55:214–223. doi: 10.1016/j.jes.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Tie L., Yu C., Zhao Y., Chen H., Yang S., Sun J., Dong S., Sun J. Fabrication of WO3 nanorods on reduced graphene oxide sheets with augmented visible light photocatalytic activity for efficient mineralization of dye. J. Alloys Compd. 2018;769:83–91. doi: 10.1016/j.jallcom.2018.07.176. [DOI] [Google Scholar]
- 12.Singh J., Kaur H., Rawat M. A novel green approach for the synthesis of tungsten oxide nanorods and its efficient potential towards photocatalytic degradation of reactive green 19 dye. J. Mater. Sci. Mater. Electron. 2018;29:13715–13722. doi: 10.1007/s10854-018-9501-6. [DOI] [Google Scholar]
- 13.Kim H., Senthil K., Yong K. Photoelectrochemical and photocatalytic properties of tungsten oxide nanorods grown by thermal evaporation. Mater. Chem. Phys. 2010;120:452–455. doi: 10.1016/j.matchemphys.2009.11.042. [DOI] [Google Scholar]
- 14.DePuccio D.P., Botella P., O’Rourke B., Landry C.C. Degradation of methylene blue using porous WO3, SiO2–WO3, and their Au-loaded analogs: Adsorption and photocatalytic studies. Acs Appl. Mater. Interfaces. 2015;7:1987–1996. doi: 10.1021/am507806a. [DOI] [PubMed] [Google Scholar]
- 15.Yassin A.M., Elnouby M., El-Deeb N.M., Hafez E.E. Tungsten oxide nanoplates; the novelty in targeting metalloproteinase-7 gene in both cervix and colon cancer cells. Appl. Biochem. Biotechnol. 2016;180:623–637. doi: 10.1007/s12010-016-2120-x. [DOI] [PubMed] [Google Scholar]
- 16.Singh S., Srivastava V.C., Lo S.L. Surface modification or doping of WO3 for enhancing the photocatalytic degradation of organic pollutant containing wastewaters: A review. Mater. Sci. Forum. 2016;855:105–126. doi: 10.4028/www.scientific.net/MSF.855.105. [DOI] [Google Scholar]
- 17.Jeevitha G., Abhinayaa R., Mangalaraj D., Ponpandian N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids. 2018;116:137–147. doi: 10.1016/j.jpcs.2018.01.021. [DOI] [Google Scholar]
- 18.Ma X., Ma W., Jiang D., Li D., Meng S., Chen M. Construction of novel WO3/SnNb2O6 hybrid nanosheet heterojunctions as efficient Z-scheme photocatalysts for pollutant degradation. J. Colloid Interface Sci. 2017;506:93–101. doi: 10.1016/j.jcis.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Ke J., Zhou H., Liu J., Duan X., Zhang H., Liu S., Wang S. Crystal transformation of 2D tungstic acid H2WO4 to WO3 for enhanced photocatalytic water oxidation. J. Colloid Interface Sci. 2018;514:576–583. doi: 10.1016/j.jcis.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L.J., Li S., Liu B.K., Wang D.J., Xie T.F. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light. ACS Catal. 2014;4:3724–3729. doi: 10.1021/cs500794j. [DOI] [Google Scholar]
- 21.Naji H.K., Oda A.M., Abdulaljeleel W., Abdilkadhim H., Hefdhi R. ZNO-Ag/PS and ZnO/PS films for photocatalytic degradation of methylene blue. Indones. J. Chem. 2020;20:314–323. doi: 10.22146/ijc.41347. [DOI] [Google Scholar]
- 22.Albarelli J.Q., Santos D.T., Murphy S., Oelgemoller M. Use of Ca–alginate as a novel support for TiO2 immobilization in methylene blue decolorisation. Water Sci. Technol. 2009;60:1081–1087. doi: 10.2166/wst.2009.459. [DOI] [PubMed] [Google Scholar]
- 23.Babu. V.R., Sairam M., Hosamani K.M., Aminabhavi T.M. Preparation of sodium alginate–methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr. Polym. 2007;69:241–250. doi: 10.1016/j.carbpol.2006.09.027. [DOI] [Google Scholar]
- 24.Akamatsu K., Maruyama K., Chen W., Nakao A., Nakao S. Drastic difference in porous structure of calcium alginate microspheres prepared with fresh or hydrolyzed sodium alginate. J. Colloid Interface Sci. 2011;363:707–710. doi: 10.1016/j.jcis.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya R., Ray S.K. Adsorption of industrial dyes by semi-IPN hydrogels of acrylic copolymers and sodium alginate. J. Ind. Eng. Chem. 2015;22:92–102. doi: 10.1016/j.jiec.2014.06.029. [DOI] [Google Scholar]
- 26.Gouda M.H., Gouveia W., Afonso M.L., Šljukić B., El Essawy N.A., Nassr A.B.A.A., Santos D.M.F. Poly(vinyl alcohol)-based crosslinked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J. Power Sources. 2019;432:92–101. doi: 10.1016/j.jpowsour.2019.05.078. [DOI] [Google Scholar]
- 27.Habib M.A., Shahadat M.T., Bahadur N.M., Ismail I.M.I., Mahmood A.J. Synthesis and characterization of ZnO-TiO2 nanocomposites and their application as photocatalyst. Int. Nano Lett. 2013;3:5. doi: 10.1186/2228-5326-3-5. [DOI] [Google Scholar]
- 28.Elkady M.F., Hassan H.S., Salama E. Sorption profile of phosphorus ions onto ZnO nanorods synthesized via sonic technique. J. Eng. 2016;2016:1–9. doi: 10.1155/2016/2308560. [DOI] [Google Scholar]
- 29.Liang Y.C., Chang C.W. Preparation of orthorhombic WO3 thin films and their crystal quality-dependent dye photodegradation ability. Coatings. 2019;9:90. doi: 10.3390/coatings9020090. [DOI] [Google Scholar]
- 30.Han B., Popov A.L., Shekunova T.O., Kozlov D.A., Ivanova O.S., Rumyantsev A.A., Shcherbakov A.B., Popova N.R., Baranchikov A.E., Ivanov V.K. Highly crystalline WO3 nanoparticles are nontoxic to stem cells and cancer cells. J. Nanomater. 2019;2019:1–13. doi: 10.1155/2019/5384132. [DOI] [Google Scholar]
- 31.Paula B., Sharma S., Purkayastha D.D., Dhar S.S., Bal R. Facile synthesis of size-controlled Ag supported on WO3 nanorods and their application as novel and active catalyst in oxidant-free dehydrogenation of benzyl alcohols. Catal. Commun. 2019;132:105804. doi: 10.1016/j.catcom.2019.105804. [DOI] [Google Scholar]
- 32.Soliman H.M.A., Kashyout A.B., El Nouby M.S., Abosehly A.M. Effect of hydrogen peroxide and oxalic acid on electrochromic nanostructured tungsten oxide thin films. Int. J. Electrochem. Sci. 2012;7:258–271. [Google Scholar]
- 33.Li K., Zhu J., Guan G., Wu H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019;122:485–498. doi: 10.1016/j.ijbiomac.2018.10.188. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahimi R., Maleki A., Zandsalimi Y., Ghanbari R., Shahmoradi B., Rezaee R., Safari M., Joo S.W., Daraei H., Puttaiah S.H., et al. Photocatalytic degradation of organic dyes using WO3-doped ZnO nanoparticles fixed on a glass surface in aqueous solution. J. Ind. Eng. Chem. 2019;73:297–305. doi: 10.1016/j.jiec.2019.01.041. [DOI] [Google Scholar]
- 35.Şahin Ö., Kaya M., Saka C. Plasma-surface modification on bentonite clay to improve the performance of adsorption of methylene blue. Appl. Clay Sci. 2015;116:46–53. doi: 10.1016/j.clay.2015.08.015. [DOI] [Google Scholar]
- 36.Dutta D., Thakur D., Bahadur D. SnO2 quantum dots decorated silica nanoparticles for fast removal of cationic dye (methylene blue) from wastewater. Chem. Eng. J. 2015;281:482–490. doi: 10.1016/j.cej.2015.06.110. [DOI] [Google Scholar]
- 37.Abdelrahman E.A., Hegazey R.M., Kotp Y.H., Alharbi A. Facile synthesis of Fe2O3 nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of methylene blue and crystal violet dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019;222:117195. doi: 10.1016/j.saa.2019.117195. [DOI] [PubMed] [Google Scholar]
- 38.Abdelrahman E.A., Hegazey R.M. Facile synthesis of HgO nanoparticles using hydrothermal method for efficient photocatalytic degradation of crystal violet dye under UV and sunlight irradiation. J. Inorg. Organomet. Polym. Mater. 2019;29:346–358. doi: 10.1007/s10904-018-1005-6. [DOI] [Google Scholar]
- 39.Nassar M.Y., Aly H.M., Abdelrahman E.A., Moustafa M.E. Synthesis, characterization, and biological activity of some novel Schiff bases and their Co(II) and Ni(II) complexes: A new route for Co3O4 and NiO nanoparticles for photocatalytic degradation of methylene blue dye. J. Mol. Struct. 2017;1143:462–471. doi: 10.1016/j.molstruc.2017.04.118. [DOI] [Google Scholar]
- 40.Yang Y., Zhang C., Huang D., Zeng G., Huang J., Lai C., Xiong W. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B. 2019;245:87–99. doi: 10.1016/j.apcatb.2018.12.049. [DOI] [Google Scholar]
- 41.Liu Z., Liu R., Yi Y., Han W., Kong F., Wang S. Photocatalytic degradation of dyes over a xylan/PVA/TiO2 composite under visible light irradiation. Carbohydr. Polym. 2019;223:115081. doi: 10.1016/j.carbpol.2019.115081. [DOI] [PubMed] [Google Scholar]
- 42.Li Y., Tang Z., Zhang J., Zhang Z. Enhanced photocatalytic performance of tungsten oxide through tuning exposed facets and introducing oxygen vacancies. J. Alloy. Compd. 2017;708:358–366. doi: 10.1016/j.jallcom.2017.03.046. [DOI] [Google Scholar]
- 43.Liu S., Tang W., Chou P. Microwave-assisted synthesis of triple 2D g-C3N4/Bi2WO6/rGO composites for ibuprofen photodegradation: Kinetics, mechanism and toxicity evaluation of degradation products. Chem. Eng. J. 2020;387:124098. doi: 10.1016/j.cej.2020.124098. [DOI] [Google Scholar]
- 44.Bhavsar K.S., Labhane P.K., Dhake R.B., Sonawane G.H. Solvothermal synthesis of activated carbon loaded CdS nanoflowers: Boosted photodegradation of dye by adsorption and photocatalysis synergy. Chem. Phys. Lett. 2020;744:137202. doi: 10.1016/j.cplett.2020.137202. [DOI] [Google Scholar]
- 45.Liu S., Lin W.A. Simple method to prepare g-C3N4-TiO2/waste zeolites as visible-light responsive photocatalytic coatings for degradation of indoor formaldehyde. J. Hazard. Mater. 2019;368:468–476. doi: 10.1016/j.jhazmat.2019.01.082. [DOI] [PubMed] [Google Scholar]
- 46.Ismail A.A., Faisal M., Al-Haddad A. Mesoporous WO-graphene photocatalyst for photocatalytic degradation of methylene blue dye under visible light illumination. J. Environ. Sci. 2018;66:328–337. doi: 10.1016/j.jes.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed B., Kumar S., Ojha A.K., Donfack P., Materny A. Facile and controlled synthesis of aligned WO3 nanorods and nanosheets as an efficient photocatalyst material. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017;175:250–261. doi: 10.1016/j.saa.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 48.Abdullah M.A., Chong F.K. Preparation and characterization of tungsten-loaded titanium dioxide photocatalyst for enhanced dye degradation. J. Hazard. Mater. 2010;176:451–458. doi: 10.1016/j.jhazmat.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 49.Yan X., Zong X., Lu G., Wang L. Ordered mesoporous tungsten oxide and titanium oxide composites and their photocatalytic degradation behavior. Prog. Nat. Sci. Mater. Int. 2012;22:654–660. doi: 10.1016/j.pnsc.2012.11.016. [DOI] [Google Scholar]
- 50.Saleha T.A., Gupta V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interf. Sci. 2011;362:337–344. doi: 10.1016/j.jcis.2011.06.081. [DOI] [PubMed] [Google Scholar]
- 51.Senthil R.A., Priya A., Theerthagiri J., Selvi A., Nithyadharseni P., Madhavan J. Facile synthesis of α-Fe2O3/WO3 composite with an enhanced photocatalytic and photo-electrochemical performance. Ionics. 2018;24:3673–3684. doi: 10.1007/s11581-018-2473-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.