Summary:
The limitations of classical drugs have spurred the development of covalently-tethered photoswitchable ligands to control neuromodulatory receptors. However, a major shortcoming of tethered photopharmacology is the inability to obtain optical control with a comparable efficacy to the native ligand. To overcome this, we have developed a family of branched photoswitchable compounds to target metabotropic glutamate receptors (mGluRs). These compounds permit photo-agonism of Gi/o-coupled group II mGluRs with near-complete efficiency relative to glutamate when attached to receptors via a range of orthogonal, multiplexable modalities. Through a chimeric approach, branched ligands also allow efficient optical control of Gq-coupled mGluR5, which we use to probe the spatiotemporal properties of receptor-induced calcium oscillations. In addition, we report branched, photoswitch-fluorophore compounds for simultaneous receptor imaging and manipulation. Finally, we demonstrate this approach in vivo in mice where photoactivation of SNAP-mGluR2 in the medial prefrontal cortex reversibly modulates working memory in normal and disease-associated states.
eTOC Blurb:
Acosta -Ruiz et al. introduce branched tethered photoswitchable ligands which allow highly efficient optical control of G protein-coupled receptors with genetic targeting and high spatiotemporal resolution. These tools allow for a dissection of mGluR5-induced calcium oscillations and mGluR2-mediated modulation of working memory.
Introduction:
Pharmacological studies have provided a foundation for our understanding of the molecular basis of biological function, particularly with regard to membrane receptors (Katzung, 2004). However, shortcomings in the spatiotemporal precision at which drugs can be added and removed, the paucity of drugs which distinguish between molecular subtypes, and the inability to target drug action to genetically-defined cell types limits the mechanistic insight that can be gleaned from such studies. In recent years, photopharmacology has emerged as an alternative approach where light-dependent chemical cages, such as 4-methoxy-7-nitroindolinyl (MNI), or photoswitches, such as azobenzenes, are conjugated to established compounds to allow light to control ligand efficacy and thus afford the system with improved spatial and temporal precision (Hull et al., 2018).
When such chemical photoswitches are covalently tethered to a protein target they facilitate the highest degree of specificity, spatial and temporal control and, through genetic targeting of the protein target itself, allow drug action to effectively be limited to defined cell types within physiological systems (Leippe et al., 2017). By tethering the ligand to a specific tag, the subtype specificity problem is solved at the level of attachment rather than at the ligand binding site, enabling native ligands to serve as the functional group without concerns about off-target pharmacological effects. Genetic encoding of the target protein can also, in principle, facilitate the incorporation of receptor variants or mutants to test their role in a physiological context. While earlier studies used native nucleophiles (Lester et al., 1980) or engineered cysteines (Banghart et al., 2004; Levitz et al., 2013; Volgraf et al., 2006) or reactive unnatural amino acids (Tsai et al., 2015) for tethering, we recently introduced photoswitchable, orthogonal, remotely-tethered ligands (“PORTLs”) which attach to a genetically-encoded self-labeling tag (i.e. SNAP), enabling efficient and orthogonal labeling of the protein target (Broichhagen et al., 2015; Levitz et al., 2017).
G protein-coupled receptors (GPCRs), the largest family of membrane receptors in eukaryotes and the largest class of drug targets (Lagerstrom and Schioth, 2008; Wacker et al., 2017), are particularly well-suited to tethered photopharmacology. Many receptor subtypes for the same ligand often exist and due to their highly conserved binding sites, developing specific agonists and antagonists remains a major challenge. Furthermore, the cellular and physiological complexity of GPCR signaling, especially in the nervous system where they signal in spatially-delimited contexts such as the synapse, and in distinct cell types within neural circuits, highlights the limitations of traditional pharmacology. The metabotropic glutamate receptors (mGluRs) form a family of neuromodulatory GPCRs which respond to the excitatory neurotransmitter glutamate to control neuronal excitability and synaptic strength in many different brain regions (Reiner and Levitz, 2018). These central roles in neuronal signaling have allowed the eight mGluRs to emerge as attractive drug targets for disorders ranging from psychiatric and neurological disease to cancer (Nicoletti et al., 2011), but deciphering their underlying mechanisms has proven to be challenging. Azobenzene-glutamate PORTLs were initially developed for N-terminally SNAP-tagged mGluR2 (Broichhagen et al., 2015; Levitz et al., 2017) where they have been shown to effectively turn mGluR2 signaling on and off with sub-second precision for applications in cultured cells (Levitz et al., 2016) and in the retina of behaving mice (Berry et al., 2017). While this system represents the most efficient tethered photoswitch systems characterized to date, many limits still exist including incomplete efficacy relative to glutamate, the inability to visualize labeled receptors, and the lack of validation of the technique in intact brain tissue. In addition, extending the PORTL approach to other mGluR subtypes has been a major challenge that has so far limited this approach primarily to analysis of mGluR2.
Here we develop strategies to enhance the efficiency and applicability of PORTLs, with a focus on mGluRs. Following mechanistic characterization of the determinants of PORTL efficiency, we successfully design and characterize branched PORTLs for SNAP, CLIP, Halo and nanobody-based labeling strategies. The branching approach also allows for high-efficiency optical control of mGluR3, which previously showed minimal light responses with single-branch PORTLs (Levitz et al., 2017), and of mGluR5 through a chimera strategy. We use this approach for control and spatiotemporal analysis of mGluR5-induced calcium oscillations. In addition, the branching framework allows for the development of dual photoswitchable ligand/fluorophore compounds that permit simultaneous optical control and sensing of mGluR2. Finally, we use branched PORTLs in freely-moving mice, where we find that photoactivation of SNAP-mGluR2 in the subset of cells of the medial prefrontal cortex which natively express mGluR2 is sufficient to modulate a working-memory-related behavior under normal conditions and in a pharmacological model of psychosis.
Results:
SNAP-tag flexibility is a major determinant of tethered photoswitch efficiency
We initially focused on a mechanistic analysis of the prototypical system of benzylguanine-azobenzene-glutamate (“BGAGn” where n=number of PEG repeats between BG and azobenzene) conjugated to SNAP-mGluR2 (Figure 1A). We previously found that a similar 50–60% photoswitching efficiency was observed in compounds ranging from BGAG0 to BGAG12, but that beyond 12 PEG repeats the efficiency decreases (Levitz et al., 2017). The lack of clear length-dependence raised the questions of what other parameters determine photoswitch efficiency. We probed the role of the distance and orientation between PORTL attachment and ligand binding sites by modifying the linker between the self-labelling SNAP-tag and the mGluR2 ligand binding domain. For each construct we determined BGAG12 efficiency relative to glutamate by measuring G protein-coupled inward rectifier potassium (GIRK) channel activation in HEK 293T cells using whole cell patch clamp electrophysiology (Figure 1B; Figure S1A). The system tolerated either the complete removal of the short threonine-arginine linker or the addition of up to 4 flexible glycine-glycine-serine (“GGS”) repeats, but a modest decrease in efficiency was observed with 6 GGS repeats across a range of BGAG lengths (Figure S1B, C).
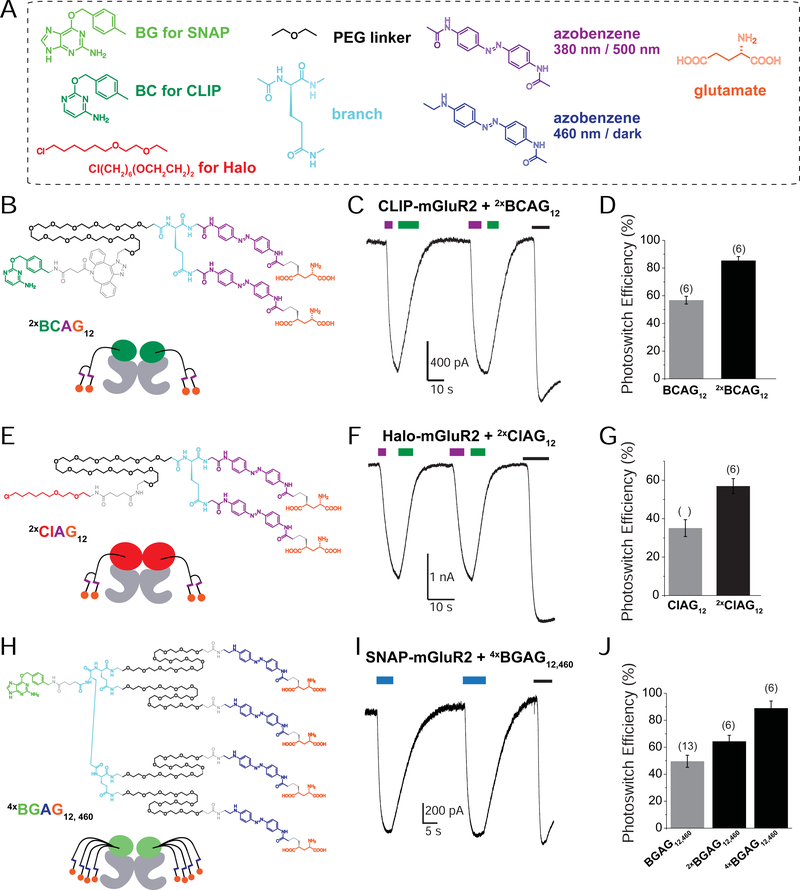
Figure 1. A Branched Photoswitchable Ligand Enables High Efficiency, Rapid Photo-activation and Photo-deactivation of mGluR2.
(A) Schematic showing photo-activation of SNAP-tagged mGluR2 with covalently-tethered “BGAG” photoswitches. Full-length mGluR2 is shown in grey and the genetically-encoded N-terminal SNAP-tag is shown in green. BGAG molecules contain an O6-benzylguanine moiety for SNAP labeling, an azobenzene photoswitch (magenta) and a 4’ tethered L-glutamate (orange circle).
(B) HEK 293T whole cell patch clamp trace showing BGAG12-mediated photoactivation of mGluR2 by 385 nm (magenta) and deactivation with 525 nm (green) compared to application of saturating glutamate.
(C) Schematic showing branched BGAG concept. The percentage of subunits with an active, cis-BGAG is calculated based on azobenzene photostationary states at 385 nm and the photoactivation efficiency is estimated based on the cooperativity of mGluR2 where agonist binding in one subunit activates 20% relative to binding in both subunits (see Figure S2).
(D) Chemical structure of 2xBGAG12.
(E-F) Representative whole cell patch clamp recording (E) and summary bar graph (F) showing high efficiency photoactivation of SNAP-mGluR2 via 2xBGAG12. * indicates statistical significance (unpaired t-test, p=0.0004).
(G) Kinetics of photo-activation and photo-deactivation of mGluR2 with BGAG12 versus 2xBGAG12. * indicates statistical significance (unpaired t-test, p=0.03).
(H-J) 2Representative traces from cortical neurons shows 2xBGAG12-mediated light-induced hyperpolarization (H) and action potential silencing (I) and summary bar graph (J) shows enhanced hyperpolarization for 2xBGAG12. * indicates statistical significance (unpaired t-test, p=0.02). The numbers of cells tested are shown in parentheses. Error bars show s.e.m.
We next turned to the SNAP-tag itself as a potential source of photoswitch modulation. We tested the “SNAPfast” (SNAPf) variant which is defined by an increased affinity for a broader substrate profile (Sun et al., 2011) and photoswitch efficiency was drastically reduced for BGAG12 (Figure S1D, E) and nearly abolished for BGAG0 (Figure S1F). Based on its increased intramolecular interactions (Sun et al., 2011), we hypothesized that SNAPf provides a less flexible attachment point which constrains the conformational landscape of BGAG. We tested this with single molecule Förster resonance energy transfer measurements of detergent-solubilized, surface-immobilized SNAP-mGluR2 or SNAPf-mGluR2 dimers and found evidence suggesting that SNAPf is indeed more rigid than SNAP (Figure S1G–L) (Vafabakhsh et al., 2015). Together these data point to flexibility of the labeling tag itself as a determinant of PORTL photoswitch efficiency.
Branched BGAGs enable near-complete optical control of SNAP-mGluR2
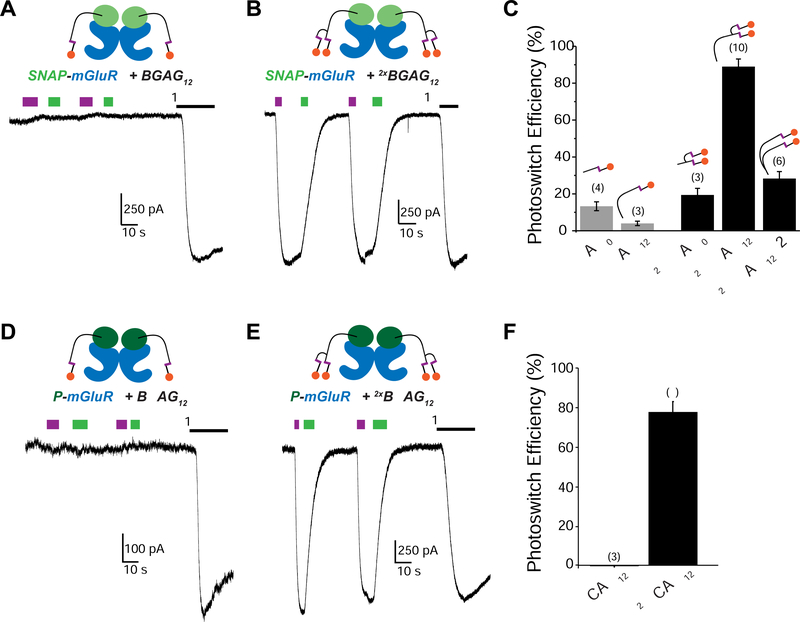
Despite optimization of BGAG length, inter-domain linkers and SNAP variants, a photoswitch efficiency of ~50–60% appeared to be an upper limit (Figure 1B). We sought another strategy to improve this efficiency. Following illumination, azobenzenes reach a photostationary state (PSS) with a certain proportion of molecules populating the high-energy cis-isomer. At optimal wavelengths, typically 80–90% of bis-acylated 4,4’-diaminoazobenzene scaffolds populate the cis-state (Gorostiza et al., 2007). When factoring in mGluR2 cooperativity (Levitz et al., 2016), this translates to ~60–80% photoswitch efficiency, even with 100% labeling efficiency (Figure S2A). Based on this, we reasoned that a molecule with two azobenzene groups and, thus, two independent chances of photoisomerization would increase efficiency. Since the azobenzene glutamate head group is the pharmacologically active part of BGAGs, we envisioned a branched molecule containing two azobenzene-glutamates (Figure 1C). Indeed, when we calculated the expected photoswitch efficiencies for hypothetical “doubleBGAG” molecules across a range of systems of different cooperativities we predicted clear improvements across all photostationary states and over all labeling efficiencies (Figure S2).
The design of branched BGAGs is based on previously described BGAG12 compounds with an additional branching amino acid to allow incorporation of a second azobenzene-glutamate moiety to produce “doubleBGAG12” (abbreviated as 2xBGAG12) (Figure 1D; Scheme S1, S2). 2xBGAG12 showed similar photophysical properties to BGAG12 (Figure S3A, B), efficient labeling of SNAP-mGluR2 (Figure S3C) and, most importantly, enabled optical control of SNAP-mGluR2 with nearly complete efficiency relative to saturating glutamate (Figure 1E, F). As predicted (Figure S2B), even at decreased labeling efficiencies 2xBGAG12 enhanced photoswitch efficiency (Figure S3D). Facile tuning of photocurrent amplitude was permitted by varying the illumination wavelength (Figure S3E). In addition to enhancing overall efficiency, 2xBGAG12 also produced faster optical activation of mGluR2 (Figure 1G) likely due to either a critical amount of active cis-conformers being reached more quickly or faster downstream signaling due to more complete population of the receptor’s active state.
To test if the enhanced efficiency of 2xBGAG12 would be maintained in a more complex, physiological environment with native effectors, we turned to cultures of cortical neurons. Following transfection of SNAP-mGluR2, photoactivation with either 2xBGAG12 or BGAG12 led to robust hyperpolarization and decreased neuronal firing, but the magnitude of the effect was larger for 2xBGAG12 (Figure 1H–J). 2xBGAG12-mediated photo-activation was bistable (Figure S3F) and faster in neurons compared to HEK 293T cells (τon=0.30±0.10 s and τoff =0.48±0.07 s in neurons, n=12 cells). Importantly, the group II mGluR antagonist LY341495 was able to fully abolish light-induced effects on membrane potential (Figure S3G). Together these experiments show that 2xBGAG12-mediated optical control of mGluR2 is well-suited to studies in the native neuronal context.
We decided to further characterize the branched BGAG approach and first asked if the presence of two azobenzene-glutamate moieties would increase basal activation of mGluR2 by trans-2xBGAG12 in the dark. Application of a saturating LY341495 concentration had a marginal effect on baseline current which was identical for unlabeled, BGAG12- or 2xBGAG12-labeled SNAP-mGluR2, indicating that there is minimal basal receptor activation in all systems (Figure 2A, B). We also titrated LY341495 to determine the concentration-dependence of the block of 2xBGAG12-mediated photoactivation which was comparable to what was observed with 100 μM glutamate (Figure S4A). We also photoisomerized 2xBGAG12 in the presence of glutamate and did not observe any current decrease (Figure S4B), consistent with 2xBGAG12 serving as a full agonist. We also measured glutamate dose response curves for SNAP-mGluR2 in the presence or absence of 2xBGAG12 labeling and found a small rightward shift in the presence of 2xBGAG12 (Figure S4C), which is consistent with previous analysis of BGAG12 (Broichhagen et al., 2015) and indicates that the trans form of the azobenzene-glutamate serves as a weak antagonist. We also analyzed photoswitching in SNAP-mGluR2-R57A, a low affinity variant that we previously characterized with BGAG0 and BGAG12 (Levitz et al., 2017). Whereas single chain BGAG variants showed no photoactivation in this mutant, small but detectable photoactivation was observed with 2xBGAG12 (Figure 2C, D) indicating that the local concentration is increased. Based on these data, we estimate that 2xBGAG12 mimics a local glutamate concentration of ~100 μM. In addition to weak photoactivation in the absence of glutamate, 2xBGAG12 also showed large photocurrents in the presence of sub-saturating 1 mM glutamate (Figure 2C, D), consistent with previous studies showing activation cooperativity (Levitz et al., 2016). Finally, we further tested the agonist sensitivity of SNAP-mGluR2 and found indistinguishable dose-response curves compared to mGluR2wt for the partial agonist DCG-IV (Figure S4D) and the full agonist LY379268 (Figure S4E).
Figure 2. Mechanistic Characterization of Branched BGAG-mediated Photoswitching of SNAP-mGluR2.
(A-B) trans-2xBGAG12 does not increase basal activation of mGluR2. Representative trace (A) shows that application of saturating LY341495 blocks photoactivation without substantially altering the baseline current level and summary bar graph (B) shows a similarly small effect in the absence or presence of BGAG12 or 2xBGAG12.
(C-D) Photoswitching of low affinity SNAP-mGluR2-R57A reveals an enhanced effective concentration of 2xBGAG12 relative to BGAG12. In the absence or presence of glutamate, photocurrents are observed for 2xBGAG12 but not BGAG12. * indicates statistical significance (unpaired t-test, p= 0.01).
(E-G) Role of branched BGAG length, branch location and number of branches. Photoswitching of SNAP-mGluR2 with both 2xBGAG12,v2 (E) and 4xBGAG 12 (F) shows high-efficiency photoactivation. Summary bar graph (G) shows photoswitch efficiency for SNAP-mGluR2 with 2xBGAG12, 2xBGAG12,v2, 4xBGAG12, and 2xBGAG0.
The numbers of cells tested are shown in parentheses. Error bars show s.e.m.
We next explored the specific composition of the branched PORTL by synthesizing the following branched BGAG variants: a short variant 2xBGAG0 (Scheme S3), 2xBGAG12,v2 (Scheme S4) where the branch point is placed just after the BG and, thus, two PEG12 chains are included in the compound, and 4xBGAG12 (Scheme S5) where four azobenzene-glutamates are added in parallel. 2xBGAG12,v2 permitted high-efficiency photoactivation of SNAP-mGluR2 to the same level as 2xBGAG12 (Figure 2E), indicating that the branch location is not a critical parameter. Similarly, 4xBGAG12 also showed high efficiency photoactivation but did not allow any further enhancement (Figure 2F), likely due to the fact that the proportion of subunits with a cis-azobenzene is already at saturation in 2xBGAGs (Figure S4B). In contrast, and despite the robust photoswitching seen with BGAG0, 2xBGAG0 provided photoactivation of SNAP-mGluR2 with only ~20% efficiency (Figure 2G). This result suggests that branching may restrict the conformational freedom of BGAGs and, in the case of the short 2xBGAG0, this entropic cost reduces the efficiency of ligand binding.
Together these data provide a strategy for enhancing optical control of SNAP-mGluR2. To see if this branching-based strategy would also improve a less optimal PORTL system, we tested 2xBGAG12 on SNAPf-mGluR2 and observed a clear improvement (Figure S4F, G). We next wondered if the strategy of branching could be generalized to other mGluR2-targeting PORTLs.
Generalization of branched PORTLs to other labeling modes and spectral variants
A major advantage of the PORTL system is the ability to design and synthesize PORTLs by flexible mix-and-matching of chemical moieties (Figure 3A). We hoped that this modularity would enable a toolset of photoswitchable mGluR2 variants optimized for different applications based on the desired attachment chemistry or spectral properties. To enable high efficiency optical control of mGluR2 tagged with CLIP, a variant of SNAP with orthogonal labeling (Gautier et al., 2008), we synthesized “doubleBCAG12” (2xBCAG12) (Scheme S6). Similar to 2xBGAG12, 2xBCAG12 enabled near-complete optical control of CLIP-mGluR2 (Figure 3B–D). Branching did not alter the specificity of 2xBCAG12, which showed no photocurrent when applied to cells expressing SNAP-mGluR2 (2.8 ± 0.3% relative to 1 mM glutamate, n=3 cells).
Figure 3. PORTL Branching Enhances mGluR2 Photoactivation in a Range of Modalities.
2(A) Toolset of chemical moieties for mix-and-match design of PORTLs for SNAP, CLIP and Halo-tagged receptors.
(B-D) 2xBCAG12 (B) enhances efficiency of CLIP-mGluR2 photoactivation compared to BCAG12. * indicates statistical significance (unpaired t-test, p=0.00008).
(E-G) 2xClAG12 (E) enhances efficiency of Halo-mGluR2 compared to ClAG12 (F, G). * indicates statistical significance (unpaired t-test, p=0.006).
(H-J) Branching enhances the efficiency of visible light-mediated (blue bar=460 nm) photoactivation of SNAP-mGluR2. * indicates statistical significance (unpaired t-test; p=0.02 between BGAG12,460 and 2xBGAG12,460 and p = 0.009 between 2xBGAG12,460 and 4xBGAG12,460).
The numbers of cells tested are shown in parentheses. Error bars show s.e.m.
With the goal of further expanding the repertoire of PORTLs to a third self-labelling, orthogonal suicide enzyme, we implemented the Halo-tag, which reacts specifically with alkyl chlorides (Los et al., 2008). We synthesized ClAG12 (Scheme S1, S7) and cloned an N-terminally Halo-tagged mGluR2 construct which showed normal glutamate sensitivity (Fig. S5B). Based on the hypothesis that branching Halo-targeting PORTLs would enhance photoswitching, we also synthesized “doubleClAG12” (2xClAG12) (Scheme S8; Figure 3E). Both PORTLs showed photoactivation of mGluR2 with identical spectral properties to BGAGs (Figure 3F), but the efficiency of photoactivation of Halo-mGluR2 was boosted by branching (Figure 3F, G). We also characterized the labeling efficiency of 2xClAG12 (Figure S5A) and found a similar concentration-dependence to BGAG labeling of SNAP tags where 1 μM labeling is sufficient for saturation. This result introduces the Halo-tag to the branched PORTL approach and expands the toolkit to three distinct, orthogonal protein tags.
A key advantage of azobenzene-based photoswitches is the ability to tune the photochemical properties of the compound. The previously reported red-shifted BGAG12,460 allows for visible-light induced, fast-relaxing photoactivation of mGluR2 which is advantageous in some settings, including for vision restoration applications (Berry et al., 2017). However, BGAG12,460 shows weaker activation of SNAP-mGluR2 than BGAG12 likely due to the decreased population of cis at the photostationary state (Hull et al., 2018). We synthesized “doubleBGAG12,460” and observed enhanced visible light photoactivation of SNAP-mGluR2 (Scheme S9; Figure S5B). Given the modest improvement of adding a second branch, we designed and synthesized 4xBGAG12,460 with four azobenzene-glutamates and were able to observe further enhancement to produce near-complete photoactivation of mGluR2 (Scheme S10; Figure 3H–J). In contrast to the bistable azobenzene of BGAG12, BGAG12,460-based PORTLs show fast-relaxation in the dark which allows the amplitude of photoactivation to be intensity-dependent (Figure S5C). Consistent with an enhancement of the ability to produce a critical population of cis-azobenzenes, branching increased the light-sensitivity of BGAG12,460 (Figure S5CD).
Finally, a long-term goal of tethered photopharmacology is to incorporate the optical control afforded by such compounds into antibody-mediated targeting of proteins. Along these lines, we recently reported “nanobody-photoswitch conjugates” (NPCs) consisting of a SNAP-tagged nanobody labeled with a PORTL. NPCs containing an anti-GFP nanobody are able to photoactivate GFP-tagged mGluR2, but with limited efficiency (Farrants et al., 2018). Similar to other systems tested, 2xBGAG12 doubled the photoswitch efficiency of NPC-mediated photoswitching of mGluR2 (Figure S5E, F). This result further confirms that branched PORTLs are an effective general strategy for improving photoswitch efficiency.
Branched PORTLs markedly enhance optical control of mGluR3
Given the molecular diversity of glutamate receptors (Reiner and Levitz, 2018), it is desirable to obtain the optical control of multiple mGluRs with high efficiency for comparative or multiplexed studies. Toward this goal we turned to mGluR3, the other member of the group II mGluR subfamily for which there is a paucity of subtype-specific drugs. Previous work with BGAG PORTLs has shown only weak ~20% photoactivation of SNAP-mGluR3 with BGAGs (Levitz et al., 2017). Unlike SNAP-mGluR2, SNAP-mGluR3 photoactivation shows steep length-dependence with the short variant, BGAG0, providing the highest efficiency. This suggests that mGluR3 photoactivation is limited, in part, by the local concentration of the azobenzene-glutamate moiety. We further characterized SNAP-mGluR3 photoswitching and found that neither introduction of SNAPf to produce SNAPf-mGluR3 (Figure S6A, B) nor addition of a flexible (GGS)2 linker between SNAP and mGluR3 enhanced photoswitching with BGAG0 or BGAG12 (Figure S6B).
We next turned to branched BGAGs in the hope that the increased valence of the system would enable efficient photoswitching. Indeed, 2xBGAG12 drastically improved photoactivation of SNAP-mGluR3 to levels comparable to SNAP-mGluR2 (Figure 4A–C). To our surprise, neither 2xBGAG0 or 2xBGAG12,v2 were able to enhance photoswitch efficiency (Figure 4C). It is difficult to account for the dramatically enhanced efficiency of branched PORTLs with either the increased proportion of subunits containing a cis-azobenzene or the modest, two-fold increase in local glutamate concentration. Furthermore, the dependence on specific branching pattern suggests that the branch point introduces secondary interactions with the mGluR3 LBD that provide some binding enthalpy. Reducing the glutamate affinity of SNAP-mGluR3 by introducing an alanine at arginine 64, the homologous position to R57 in mGluR2, decreased but did not abolish 2xBGAG12 photoswitch efficiency (Figure S6C, D). This result suggests that, similar to the case with mGluR2, the glutamate moiety is in an effective concentration range of ~100 μM. We also tested 2xBCAG12 on CLIP-mGluR3 and observed drastically enhanced photoswitch efficiency compared to BCAG12 (Figure 4D–F). Ultimately, the ability to optically control either mGluR2 or mGluR3 with either CLIP or SNAP-tags opens the door to dissecting their distinct functions in the nervous system.
Figure 4. Branched PORTLs Enable Efficient Optical Control of mGluR3.
(A-B) Traces showing photoactivation of SNAP-mGluR3 with BGAG12 (A) or 2xBGAG12 (B).
(C) Bar graph showing optimal photoswitching of SNAP-mGluR3 with 2xBGAG12, but not 2xBGAG12,v2 or 2xBGAG0.
(D-E) Traces showing photoactivation of CLIP-mGluR3 with BCAG12 (D) or 2xBCAG12 (E).
(F) Bar graph showing optimal photoswitching of CLIP-mGluR3 with 2xBCAG12. The numbers of cells tested are shown in parentheses. Error bars show s.e.m.
Efficient optical control of mGluR5 signaling with spatiotemporal precision
We next turned to mGluR5, a group I mGluR that is highly expressed throughout the central and peripheral nervous systems. mGluR5 is expressed in a wide range of cell types within the brain, including excitatory principal cells, inhibitory interneurons and astrocytes, making it difficult to dissect the role of specific mGluR5 populations with drug application or knock-out. The ability to photoactivate mGluR5 in defined cell types would allow one to approach such questions. Furthermore, mGluR5 has myriad signaling partners, interacts with an extensive network of scaffold proteins via its large C-terminal domain (CTD) and couples to a variety of effectors and regulators (Reiner and Levitz, 2018). PORTL-based control of mGluR5 would allow for the testing of receptor mutants in native systems to probe the role of specific interactions or regulatory sites. For example, mGluR5 activation of Gq is known to induce a unique form of calcium oscillations that are thought to be due to reversible protein kinase C (PKC)-mediated phosphorylation of a residue on the membrane-proximal part of the CTD (Kawabata et al., 1996; Kim et al., 2005).
To date, group I mGluRs have not been successfully photosensitized with PTLs or PORTLs, likely due to pharmacological incompatibility of the azobenzene-glutamate moiety. However, we and others have shown that extracellular domains of mGluRs are portable and can gate the TMDs of other mGluR subtypes (Levitz et al., 2016). We thus reasoned that a chimera between mGluR2 and mGluR5 that contains the entire TMD and CTD of mGluR5 would maintain the unique properties of mGluR5 signaling, while allowing efficient PORTL-mediated optical control. In contrast, other chimera-based optogenetic approaches (Rost et al., 2017; Spangler and Bruchas, 2017) employ the 7-TM core of rhodopsin along with the intracellular loops and CTDs of a given receptor which is unlikely to fully recapitulate the complex conformational and signaling dynamics of the target receptor. We thus engineered a SNAP-mGluR2–5 chimera (Figure 5A) and observed similar glutamate-evoked oscillatory calcium responses to mGluR5 in HEK 293T cells (Figure S7A). SNAP-mGluR2–5 showed strong surface expression and the expected glutamate dose-response (Figure S7A, B). Most importantly, subcellular 405 nm illumination produced BGAG-dependent, repeatable, robust calcium oscillations (Figure 5B; Figure S7C, D) of a similar frequency to glutamate, and light responses were more reliable for 2xBGAG12 versus BGAG12 (Figure 5C). Light-induced calcium oscillations were blocked by MPEP, an mGluR5 negative allosteric modulator (Figure S7E), or enhanced by VU 0360172, an mGluR5 positive allosteric modulator (Figure S7F), showing that the mGluR5 TMD maintains its native allosteric binding site in this chimera. To further test if mGluR5 identity is maintained in our system, we probed the role of the mGluR5 PKC phosphorylation site, Ser839, in induction of light-induced calcium oscillations. Consistent with the effects of the analogous mutation in mGluR5, mutation of Ser839 to alanine (S839A) decreased the oscillatory response to both glutamate (Figure S7G) and light (Figure S7H) in our construct.
Figure 5. Optical Control of mGluR5 Signaling via Branched PORTLs and a Chimera-Based Approach.
(A) Schematic showing chimera including an N-terminal SNAP tag, the extracellular domains of mGluR2 and the transmembrane and C-terminal domains of mGluR5.
(B) GCaMP6 calcium imaging in HEK 293T cells shows light-induced calcium oscillations mediated by SNAP-mGluR2–5 and 2xBGAG12. Right, corresponding images of cells before (top) and during (bottom) subcellular 405 nm illumination (purple dot). 488 nm imaging light is sufficient to rapidly de-activate 2xBGAG12 following 405 nm illumination.
(C) Summary of efficiency of optical control of SNAP-mGluR2–5 for BGAG12 versus 2xBGAG12. * indicates statistical significance (Pearson’s chi-square test, p=0.002).
(D-E) Increasing the subcellular area of photoactivation increases the probability of a single peak or an oscillatory light response. Data from 38 cells were included in this analysis.
(F-G) Increasing the subcellular area of photoactivation does not alter the frequency or amplitude of oscillatory light responses. The summary plot shows data for individual cells (grey lines; n=10 cells) and from an average of all tested cells (black).
(H-I) Representative trace shows photoactivation of SNAP-mGluR2–5 with 2xBGAG12 demonstrating an offset in Ca2+ response timing at two distinct ROIs, the photoactivation site in purple and a distal ROI in gray (H). Bar graph (I) shows quantification of Ca2+ wave velocity (d), where n=5 cells and error bars shows s.e.m.
(J) Targeted photoactivation of SNAP-mGluR2–5 with 2xBGAG12 leads to oscillatory responses with a simultaneous increase in cytosolic calcium (R-GECO, red) and decrease in endoplasmic reticulum calcium (ER-GCaMP6, green). Right, the response with both sensors spreads from the site of photoactivation to the distal part of the cell.
We used targeted photoactivation to small regions of the cell to probe the properties of evoked calcium oscillations. We first asked if the size of the illumination spot was a determinant of response probability and observed that, indeed, increasing the area of photoactivation increased the probability of a calcium response (Figure 5D, E). Figure 5E summarizes the photoactivation size-dependence of calcium responses and shows that ~25% of the cell was needed to have a 50% chance of a response. Consistent with previous work on mGluR5 (Nash et al., 2002), SNAP-mGluR2–5-induced calcium oscillation frequency was largely independent of the concentration of glutamate (Fig. S7J, K). Interestingly, in contrast to the weak effect of increasing glutamate concentration, it has been shown that increased receptor expression levels increase the frequency of calcium oscillations (Nash et al., 2002). Given this, we asked if the area of photoactivation of a cell would lead to alterations in the calcium oscillation frequency. We varied the size of the photoactivation area and found that the frequency was largely insensitive to the proportion of the cell that was targeted (Figure 5F, G). We also examined how calcium oscillations spread throughout the cell by comparing the calcium signal at the site of photoactivation to a distal part of the cell. Interestingly, both the temporal profile (Figure 5H) and calcium response amplitude (100.6 ±13.5 % of the amplitude at the activation site, n= 6 cells) were maintained at the distal site. Furthermore, we were able to use the offset in signals to calculate a calcium wave velocity for each cell of 10–20 μm/s (Figure 5I), which is comparable to prior measurements of spontaneous calcium waves in mammalian cells (Meyer, 1991).
Finally, we asked if photoactivation of SNAP-mGluR2–5 leads to oscillatory responses in endoplasmic reticulum (ER) calcium and if such responses would spread throughout the cell, as observed with cytosolic calcium (Figure 5I). We co-expressed SNAP-mGluR2–5 with the ER-targeted “ER-GCaMP6f” (de Juan-Sanz et al., 2017) and the cytosolic red calcium sensor R-GECO (Zhao et al., 2011) and observed light-induced, anti-correlated calcium oscillations in the cytosol and ER that spread from the site of activation (Figure 5J). This result confirms that cytosolic calcium oscillations are driven by cyclical release from and refilling of intracellular stores and indicates that ER calcium depletion spreads throughout the cell and is not maintained in a local subregion near the site of activation. This measurement also demonstrates the ability to multiplex branched PORTL-mediated photoactivation with simultaneous imaging of two different fluorescent sensors in the green (488 nm excitation) and red channels (560 nm excitation).
A key mechanism by which mGluR5 modulates neural activity is through signaling within astrocytes, especially in the developing brain (Cai et al., 2000; Sun et al., 2013). Astrocytic mGluR5 can respond to synaptic glutamate to control astrocyte calcium signaling dynamics, gliotransmission and morphology (Lavialle et al., 2011; Panatier and Robitaille, 2016; Petrelli and Bezzi, 2018), but the inability to isolate astrocytic mGluR5 signaling from neuronal mGluR5 signaling or to target mGluR5 signaling to specific subcellular compartments within an astrocyte has hampered progress on the underlying mechanisms of this form of neuromodulation. As previously reported, cultured astrocytes showed calcium elevations in responses to mGluR5 activation (Codazzi et al., 2001; Cornell-Bell et al., 1990; Kawabata et al., 1998), with a mix of single peaks and oscillations (Figure S8A–C). We next asked if our photoswitchable mGluR5 system could allow us to mimic native mGluR5 responses and permit the use of targeted photoactivation to probe this system. Photoactivation of 2xBGAG12-labeled SNAP-mGluR2–5 in astrocytes initiated either single peaks or calcium oscillations (Figure 6A; Figure S8D, E) of higher frequency than in HEK cells but of a similar frequency to native mGluR5-mediated DHPG responses (Figure S8F), supporting the ability of SNAP-mGluR2–5 to probe native mGluR5 signaling. In the absence of the receptor construct, no light responses were observed (Figure S8G). Targeted photoactivation to small areas revealed signal spreading heterogeneity between the soma and extended processes that was different from the spatially-homogenous HEK 293T cells. When photoactivation was targeted to the soma, oscillations were observed in the soma in ~55% of cells of those responding cells and oscillations were observed in at least one process in ~55% of those cells (Figure 6A, C). However, when photoactivation was targeted to a process, local oscillations were observed at the targeted process in ~75% of cells but were only seen in the soma in only 20% of those cells (Figure 6B, C; Figure S8H). This is consistent with previous observations of global or compartmentalized spontaneous or drug-induced calcium responses in astrocytes (Shigetomi et al., 2016), but provides further precision due to the ability to target the stimulus to a subcellular region. In addition, oscillations were of a higher frequency in the processes compared to in the soma (Figure 6D), suggesting underlying differences in the nature of mGluR5 signaling in these different cellular locations. Finally, we asked if locally-induced calcium responses were mediated by intracellular stores as has previously been shown with group I mGluR agonist responses in the soma (Nakahara et al., 1997). We performed local photoactivation of SNAP-mGluR2–5 in either processes or soma and found that calcium responses were maintained in the absence of extracellular calcium in both locations (Figure S8I). Together this work demonstrates the suitability of our approach for probing mGluR5 signaling dynamics in astrocytes and motivates future applications to dissect the contribution of neuronal versus astrocytic mGluR5 signaling to synaptic and circuit-level processes.
Figure 6. Photoactivation of mGluR5 signaling in astrocytes reveals subcellular confinement of receptor-induced calcium oscillations.
(A-B) Subcellular photoactivation of SNAP-mGluR2–5 with 2xBGAG12 in cultured astrocytes produces reliable calcium oscillations and allows for the visualization of subcellular calcium waves. Photoactivation occurred only at the purple circle in either the soma (A) or a process (B) . Inset highlights that, in this representative cell, calcium oscillations occur at the site of photoactivation, but not in distal sites.
(C) Bar graphs shows the range of calcium responses, organized by site of photoactivation and measurement. The number of cells measured are shown inscribed in each bar. A higher proportion of cells showed oscillations in response to photoactivation in processes versus the soma. * indicates statistical significance (comparison between distributions of bar graphs; Pearson’s Chi-Square Test, p=0.037).
(D) Calcium oscillation frequencies in response to photoactivation in the processes versus the soma. Lines connect values measured in the same cell with photoactivation in either location. * indicates statistical significance (paired t-test, p=0.017).
(E) Schematic showing the properties of calcium oscillations induced by photoactivation in the soma versus a process.
Dual photo-activation and imaging with branched PORTL/fluorophore compounds
Optogenetic actuators are powerful tools for dissecting the molecular basis of biological processes. However, a complete understanding of the underlying molecular events also requires precise sensing of protein localization and/or conformation. Combining the ability to optically manipulate and sense the same receptor population would be a particularly powerful means of obtaining a full picture of receptor function. Chemical conjugation of expressed proteins with organic fluorophores produces brighter fluorescence compared to fluorescent proteins, allows one to maintain flexibility for using different spectral variants with the same protein target and permits surface versus intracellular targeting (Xue et al., 2015). Combining attachment of fluorophores and photoswitch actuators to the same receptor population would enable the simultaneous study of receptor localization and/or conformational state while controlling its activity, a potentially very powerful technique.
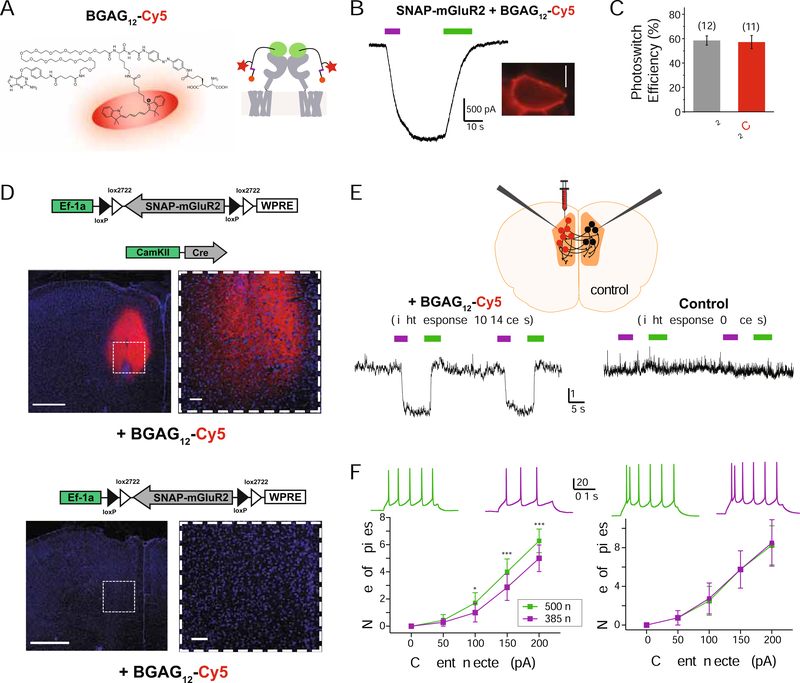
Given our demonstration that SNAP-tagged receptor targets tolerate PORTL branching, we decided to test if incorporation of a fluorophore into a branched BGAG would enable dual optical manipulation and detection. As such, we chose the same branching point endowed with a far-red, spectrally-orthogonal fluorophore instead of a second azobenzene-glutamate. Accordingly, a branched Cy5 was installed on BGAG12 to obtain “BGAG12-Cy5” (Figure 7A; Scheme S11). BGAG12-Cy5 showed the expected absorption spectrum (Figure S9A), efficient labeling of SNAP-mGluR2 (Figure S9B) and efficient optical control of SNAP-mGluR2 while also allowing imaging of the receptor on the plasma membrane of cells (Figure 7B,C; Figure S9B,C).
Figure 7. Branched Fluorophore-Containing BGAGs Allow Dual Photoactivation and Detection of mGluR2 following in vivo Labeling.
(A) Chemical structure, left, and schematic, right, showing BGAG12-Cy5, a PORTL for dual optical manipulation and sensing of mGluRs.
(B) Representative trace, left, and image, right, showing photoactivation and detection of SNAP-mGluR2 in a HEK 293T cell. Scale bars= 10 μm.
(C) Bar graph showing comparable photoswitch efficiency relative to saturating glutamate for BGAG12-Cy5 and BGAG12.
(D) Top, images showing SNAP-mGluR2 labeled with BGAG12-Cy5 following viral expression and in vivo PORTL injections. Bottom, images showing control slices from mice injected with BGAG12-Cy5 but not expressing SNAP-mGluR2. Scale bars= 500 μm (left) or 50 (right) μm.
(E) Top, schematic showing experiment where viral delivery and BGAG12-Cy5 injection is only done in one hemisphere of the medial prefrontal cortex. Bottom, current clamp traces showing light-induced hyperpolarization only in the fluorescent hemisphere and not in the non-fluorescent “control” hemisphere.
(F) Input-output curves showing current induced firing for cells in the fluorescent (left) and the non-fluorescent hemisphere (right). Inset shows representative spike firing traces following 525 nm (green) or 385 nm (purple) illumination. Error bars show s.e.m. * indicates statistical significance (2-way ANOVA; current, F(4,24) = 24.84, p = 0.00001; light, F(1,6) = 19.35, p = 0.005; current x light, F(4,24) = 5.65, p = 0.0024; Turkey’s MC test, 385 vs 500 [100 pA, p = 0.007; 150 pA, p = 0.003; 200 pA, p = 0.0007]).
Labeling with BGAG12-Cy5 should provide a real time view of which cells or tissue regions have labeled, photoswitchable receptors. This would both enhance the efficiency of identifying expressing, labeled cells as well as defining precise cellular or subcellular targeting experiments. To test this, we injected viruses (Cre-dependent FLEX-SNAP-mGluR2 and Cre recombinase under the CaMKII promoter) and BGAG12-Cy5 into the medial prefrontal cortex (mPFC) of mice in only one hemisphere. The mPFC was targeted because this is an area of native mGluR2 expression (Ferraguti and Shigemoto, 2006) and because of its involvement with many of the psychiatric disorders for which mGluRs are implicated. Confocal imaging showed clear targeting of SNAP-mGluR2 by BGAG12-Cy5 with minimal background in control mice (Figure 7D). We then performed patch clamp recordings from slices prepared from injected mice. The fluorescence from BGAG12-Cy5 allowed for the identification of the site of receptor expression and labeling. We recorded from fluorescent cells in the injected hemisphere and non-fluorescent cells in the control hemisphere and observed a light-induced hyperpolarization only in the fluorescent hemisphere (Figure 7E) that was similar in amplitude to pharmacological activation of native group II mGluRs (Figure S9D). In addition, photoactivation of mGluR2 produced a reversible, repeatable decrease in spike firing in response to current injection (Figure 7F). No light responses were observed in slices from wild type mice injected with BGAG12-Cy5 (Figure S9E) and expression and labeling of SNAP-mGluR2 did not alter the resting membrane potential of neurons (Figure S9F). Notably, previous drug application-based slice studies of GPCRs have provided minimal information on the kinetics and repeatability of receptor effects, likely due to long bath exchange and tissue penetration times. Here, photo-activation of SNAP-mGluR2 revealed rapid on and off kinetics on the hundreds of milliseconds time scale (τON = 330 ± 41 ms, τOFF= 687 ± 64 ms; n= 13 cells) and clear reproducibility over many cycles (Figure S9G, H). Together this demonstrates the utility of the bi-functional fluorophore/PORTL approach and provides a framework for using branching to further expand the world of PORTLs and PORTL-based applications.
in vivo photo-activation of SNAP-mGluR2 within the mPFC modulates a working memory-related behaviour
Having seen both the clear efficacy of branched BGAGs in brain slices following in vivo labeling and the robust ability of mGluR2 activation to modulate mPFC neurons (Figure 7), we asked if our approach could be used to modulate behavior. We turned to a readout of relevance to working memory, an important cognitive function that is altered in a wide range of disorders for which mGluRs have been proposed as drug targets (Millan et al., 2012). Prior work with working memory-related tasks has shown that global group II mGluR knockout in mice alters performance (De Filippis et al., 2015) and, similarly, injection of drugs that target mGluR2/3 can improve or impair performance depending on the context (Moghaddam and Adams, 1998)(Aultman and Moghaddam, 2001; Griebel et al., 2016). Typically, in wild type mice, mGluR2/3 agonists impair performance (Aultman and Moghaddam, 2001; Higgins et al., 2004; Schlumberger et al., 2009). Crucially, pinpointing the relevant brain subregions and cell types in which specific group II mGluRs mediate these effects has been challenging. We used a Y-maze to measure spontaneous spatial novelty preference, a behavior that depends on working memory to identify a new arm of the maze, and asked if targeted photoactivation of mGluR2 in pyramidal neurons of the mPFC was sufficient to modify performance. Adeno-Associated Viruses (AAV) for Cre-dependent FLEX-SNAP-mGluR2 and Cre recombinase under the CaMKII promoter were injected bilaterally into the mPFC to target expression to excitatory cells (Figure 8A, B; Figure S10A) and a dual fiber optic-cannula was implanted for bilateral optical control. Infusion of 2xBGAG12 was performed 12 to 16 hours before behavioral testing. Brief pulses of 385 nm light for activation of mGluR2 were delivered via optic fiber into the mPFC for 2 minutes in the home cage starting 2 minutes prior to and maintained during the entirety of the Y-maze task (Figure 8A). Control mice that only received the FLEX-SNAP-mGluR2, but not the CaMKII-Cre virus, and did receive 2xBGAG12 injection and 385 nm light, alternated between arms of the maze ~70% of the time. Similarly, control mice that received both viruses and 385 nm light but did not receive 2xBGAG12, also alternated between arms of the maze ~70% of the time. In contrast, experimental mice that received both viruses along with 2xBGAG12 and 385 nm light, showed a clear reduction in alternation to ~50% (Figure 8C). Importantly, all three groups showed indistinguishable total number of arm entries (Figure S10B).
Figure 8. in vivo Photoactivation of SNAP-mGluR2 in the Medial Prefrontal Cortex via 2xBGAG12 Reversibly Modulates Mouse Behavior in a Y-maze.
(A) Experimental design where, 6–8 weeks following dual AAV injection and implant placement, 2xBGAG12 is injected into the mPFC 12–16 hours prior to behavioral testing. 385 nm illumination is first applied two minutes before mice enter the Y-maze.
(B) Representative image showing bilateral SNAP-mGluR2 expression in the mPFC. To visualize receptors, mice were injected with the SNAP-reactive fluorophore BG-LD55 and slices were imaged. Scale bars= 500 μm (left) or 50 (right) μm.
(C) Summary of Y-maze behavioral analysis. The percentage of alternations between different arms of the maze was higher in control mice that either did not receive the 2xBGAG12 injection (“control 1”) or the CaMKII-Cre virus (“control 2”) compared to experimental mice that receive 2xBGAG12 and express SNAP-mGluR2. All mice received the 385 nm photoactivation protocol. * indicates statistical significance (1-way ANOVA; SA F(2,14) = 14.87, p = 0.0003; (Turkey’s MC test [Exp vs Ctrl1 p = 0.004; Exp vs Ctrl2 p = 0.0005]). The number of mice in each group is shown in parentheses.
(D-F) Miniature excitatory post-synaptic currents (mEPSCs) (D) recorded in layer 2/3 neurons of the mPFC in coronal slices taken from mice with bilateral expression of SNAP-mGluR2 and labeled with BGAG12-Cy5. Photoactivation leads to a decrease in the frequency (E), but no effect on the amplitude (F), of mEPSCs. * indicates statistical significance (1-way RM ANOVA; Freq, F(2,14) = 4.858, p = 0.02; Fischer’s LD test [BL vs 385, p = 0.01; 385 vs 515 p = 0.02])
(G) Similar effects on mEPSC frequency and amplitude are seen in wild-type mice treated with 100 nM LY379268 compared to SNAP-mGluR2 photoactivation. n=8 cells for each conditions.
(H) Representative images showing the expression pattern of tdTomato in Grm2-Cre mice. Scale bars= 10 mm (top) or 100 μm (bottom).
(I) Y-maze behavioral analysis in Grm2-Cre mice that express SNAP-mGluR2. The percentage of alternations between different arms of the maze was higher in control mice that did not receive the 2xBGAG12 injection (“control 1”) compared to experimental mice that receive 2xBGAG12 (unpaired T test, p= 0.04).
(J) Reversibility experiment demonstrating that delivery of 515 nm light midway through behavioral testing rescued the working memory impairment induced by SNAP-mGluR2 photoactivation in Grm2-Cre mice. * indicates statistical significance (paired t test, p=0.03).
(K) When Grm2-Cre mice expressing SNAP-mGluR2 were pre-treated with MK-801 to impair performance the percentage of alternations was higher in experimental mice that received 2xBGAG12 compared to control mice (unpaired T test, p=0.01).
The number of mice in each group is shown in parentheses. Error bars show s.e.m.
While this experiment demonstrates the suitability of the branched PORTL approach in vivo, we asked how well SNAP-mGluR2 photoactivation mimics the native signaling of mGluR2. Previous anatomical and electrophysiological work in rodents and primates has shown that mGluR2 in the mPFC is primarily presynaptic where it mediates forms of synaptic inhibition (Kiritoshi and Neugebauer, 2015) (Bocchio et al., 2018; Jin et al., 2018). We first confirmed this by measuring miniature excitatory post-synaptic currents (mEPSCs) in the mPFC in coronal slices from wild-type mice before, during and after LY37 application. LY37 led to a reversible ~20–30% reduction in mEPSC frequency but no clear effect on mEPSC amplitude (Figure S10C, D). We next turned to slices from mice that had been co-injected with AAV for FLEX-SNAP-mGluR2 and CaMKII-Cre and labeled with BGAG12-Cy5. We found that neurons in layer 2/3 showed a similar 20–40% reduction in mEPSC frequency, but not amplitude, following receptor photo-activation (Figure 8D–G). Importantly, the basal mEPSC frequency (2.1 ± 0.7 for wild-type versus 1.4 ± 0.4 for SNAP-mGluR2 + BGAG12-Cy5; unpaired t test, p=0.32) and amplitude (25.4 ± 2.4 for wild-type versus 21.3 ± 1.3 for SNAP-mGluR2 + BGAG12-Cy5; unpaired t test, p=0.27) were not altered in these mice. These results indicate that SNAP-mGluR2 expression does not substantially alter the synaptic properties of the mPFC and that photo-activation leads to similar effects at the synapse, likely via the same effectors as native mGluR2 activation.
Single cell RNA sequencing data supports the targeting of mGluR2 only to excitatory neurons (Zeisel et al., 2015), but we hypothesized that mGluR2 expression is likely further restricted to a subset of pyramidal cells. To target SNAP-mGluR2 expression precisely to the cells which natively express mGluR2, we turned to a bacterial artificial chromosome-based transgenic mouse which expresses Cre-recombinase under control of the mGluR2 promoter (“Grm2-Cre”). This mouse has previously been reported(Gerfen et al., 2013) but has not been fully characterized or used for study of mGluR2 localization and function. We first confirmed that this mouse reproduces the known expression profile of mGluR2 by crossing with a tdTomato reporter mouse (“FLEX:tdTomato”). We found fluorescent cells throughout the brain with a pattern that closely matched previous studies of mGluR2 (Ferraguti and Shigemoto, 2006), including strong fluorescence in dentate gyrus granule cells and Golgi cells of the cerebellum (Figure 8H; S10E). We also found tdTomato-positive cells throughout all layers of the mPFC but with enrichment in layers 2 and 3 (Figure S10G) and further confirmed the fidelity of the line using fluorescence in situ RNA hybridization (FISH) (Figure S10F).
Having established a means of targeting Cre-dependent expression to the mGluR2-positive subset of cells in the mPFC we asked if targeted photoactivation to these cells would be sufficient to produce a clear effect on working memory in the Y-maze. To do this, we injected AAV for FLEX-SNAP-mGluR2 into the mPFC with the same coordinates as used for the previous behavioral experiment. We first labeled SNAP-mGluR2 with a BG-conjugated fluorophore and observed clear expression with modest enrichment in layer 2/3 (Figure S10H). We next turned to our Y-maze behavioral assay where we found that photoactivation of SNAP-mGluR2 by 2xBGAG12 led to a clear decrease in performance (Figure 8I) without altering the total number of arm entries (Figure S10I). Given the fact that the effects of SNAP-mGluR2 photoactivation on synaptic transmission in the mPFC were rapidly reversible (Figure 8E), we asked if the behavioral effect could be reversed over the time scale of our measurements. We found that 515 nm illumination midway through the behavioral assay was able to reverse the inhibition of working memory which, in contrast, was maintained in mice that continued to receive 385 nm illumination (Figure 8J). Together these experiments indicate that targeting SNAP-mGluR2 photoactivation to mGluR2-expressing cells is sufficient to reproduce the effects of global treatment with mGluR2/3 agonists but with rapid onset and reversal.
We next decided to further probe the effects of SNAP-mGluR2 photoactivation in the mPFC in the context of a disease-relevant perturbation. Application of a non-competitive NMDA receptor antagonist, such as PCP or MK-801, is thought to model an NMDAR hypofunction-based model of the positive and negative symptoms of schizophrenia (Moghaddam and Javitt, 2012). It has previously been shown in both rodents and humans, that mGluR2/3 agonism can alleviate the effects of non-competitive NMDAR antagonists on measures of working memory (Krystal et al., 2005; Moghaddam and Adams, 1998). We asked if mGluR2 activation within the mPFC is sufficient to pinpoint this effect of global drug application. First, we confirmed that intra-peritoneal MK-801 injection into mice led to a clear impairment in Y-maze performance 45 minutes later (Figure S10J, K). We next performed the SNAP-mGluR2 photoactivation experiment in Grm2-Cre mice following injection of MK-801 and observed a clear improvement in performance (Figure 8K; Figure S10L). This measurement indicates that SNAP-mGluR2 activation solely within the subset of mGluR2-expressing cells in the mPFC is able to rapidly relieve the working memory impairment induced by global application of MK-801. Together these behavioral data show the suitability of branched PORTLs for studies of the circuit basis of GPCR-mediated neuromodulation and supports a central role for GPCRs within the mPFC in supporting working memory-related behavior and alleviation of working memory deficits associated with psychosis.
Discussion:
Branched PORTLs: A strategy for high efficiency optical control and dual control and detection
Despite their emergence as useful tools for studying membrane receptor signaling, the applicability and generalizability of tethered photoswitches has remained limited by both a lack of understanding of the underlying mechanisms and a limited number of strategies for enhancing optical control of a given target. Here we report a family of branched, tethered photoswitchable ligands that enhance the efficiency of existing photoswitchable receptors (i.e. mGluR2) and enable extension of the approach to other, related targets (i.e. mGluR3, mGluR5). As a complement to our implementation of branched PORTLs, we also provide new insight into the mechanisms of tethered photoswitches. Together our observations (Table S1) indicate that the efficiency of photoswitching is determined by the relative energetic contributions of the enthalpy change associated with binding of the active form of the functional group and the associated entropy loss of the protein tag and PORTL linker, similar to what has previously been described for a tethered enzyme inhibitor (Krishnamurthy et al., 2007). The effects of branching overcome the previous limitations of incomplete cis occupancy and insufficient local ligand concentration and should be useful for a wide range of probes, including soluble photochromic ligands (Hull et al., 2018) and non-photosensitive tethered ligands (Podewin et al., 2018; Shields et al., 2017). Critically, branching opens up many opportunities for PORTL fine-tuning via modification of linker composition, length and branch location and may allow the incorporation of both orthosteric and allosteric ligands for further tuning of optical control. Furthermore, we also introduce branched PORTLs that incorporate both a photoswitchable ligand and an organic fluorophore to enable dual manipulation and detection of the same receptor population. Together, this work demonstrates the design possibilities afforded by PORTL branching and should open the door to optical studies that link receptor activation to localization, mobility, and conformation with high temporal precision.
One major point of consideration when applying branched PORTL-mediated optical control of GPCRs are the relative advantages and disadvantages of heterologous expression. As demonstrated in this study, expression of full-length receptors allows for facile, flexible targeting of specific photoswitchable GPCRs to genetically-defined cellular populations. Furthermore, the use of full-length receptors provides the unique ability to incorporate mutations or variants to alter activation mechanism, effector coupling properties or receptor regulation (i.e. phosphorylation sites) to permit studies that connect specific aspects of receptor function to neurophysiology or behavior. However, while the use of full-length receptors should allow for a recapitulation of receptor-specific function, as demonstrated here with photoactivation of mGluR5 signaling in astrocytes (Figure 6) or mGluR2 signaling within the mPFC (Figure 7, 8), heterologous expression can lead to alterations in the system due to overexpression which can lead to non-physiological targeting or signaling. This issue, along with potential effects of labeling tags (i.e. SNAP, CLIP, or Halo) necessitate extensive controls in each new system in which these tools are applied. Alternatively, strategies including genetic knock-in or CRISPR-based gene modification to introduce labeling tags (Gao et al., 2019; Nishiyama et al., 2017), nanobody-mediated PORTL targeting (Figure S5) or membrane-tethering of PORTLs (Donthamsetti et al., 2019) may prove to be valuable approaches to target native receptors for PORTL-mediated optical control. In all configurations, the enhanced efficiency and dual imaging capabilities of branching should greatly facilitate the use of PORTLs to study neuromodulation with high precision.
Optical dissection of mGluR5-induced calcium signaling dynamics
We used the spatiotemporal precision of the branched PORTL system to probe the nature of mGluR5-induced calcium oscillations. Here we find that neither glutamate concentration, as previously reported (Nash et al., 2002), nor the proportion of the cell that is photoactivated alters the frequency of calcium oscillations. This suggests that beyond a response threshold, which we calculate as either ~1 μM glutamate or activation of ~25% of the cell, calcium oscillations occur at an intrinsic frequency for a given cell. This essentially turns mGluR5 into a binary switch rather than a dial, as receptor responses are normally modeled. What determines this intrinsic frequency? One possibility is that it is merely determined by the relative densities of receptors, G proteins, protein kinase C, phosphatases and other factors that shape G protein turnover and calcium handling. Altering the proportion of the cell that is activated or the glutamate concentration does not effectively change these ratios. This is consistent with a previous report that increasing the expression level of receptors, and likely receptor reserve, can increase the frequency of calcium oscillations (Nash et al., 2002). Future work is needed to understand the mechanisms that determine calcium oscillation properties and how these oscillations are decoded by downstream process, such as transcription.
We also demonstrate mGluR5 photoactivation in astrocytes, where we find similar oscillatory responses following receptor activation, but clear evidence that different sub-domains of the cell appear to confine calcium oscillations. Most strikingly, we find that activation of mGluR5 in astrocytic processes often produced local, higher-frequency calcium oscillations that did not travel to the soma. This suggests that synaptic glutamate may induce local calcium responses that do not spread globally throughout the cell, perhaps to maintain input specificity. Consistent with this notion, imaging of spontaneous signals in astrocytes has led to numerous examples of calcium waves which remain confined either within processes or smaller microdomains (Shigetomi et al., 2016). In the case of mGluR5 activation, determining the mechanism of signal confinement will require the identification and study of the relevant effectors, calcium sources and scaffolding proteins. Together, these experiments demonstrate the power of PORTL-based optical control for a quantitative dissection of receptor-induced cell signaling dynamics with a precision not afforded by soluble ligands.
Optical modulation of behavior via prefrontal cortex mGluR2 photoactivation
GPCRs in general and mGluRs, in particular, are well-known to modulate neural signaling and, ultimately, to control behavior. This behavioral control underlies the prominence of GPCRs as the molecular targets of drugs for neurological and psychiatric disorders. However, the aforementioned shortcomings of pharmacological approaches have made it difficult to pinpoint which receptor populations mediate behavioral effects in terms of brain regions, cell types and receptor subtypes. Of particular interest for mGluRs, is the prefrontal cortex (PFC) where alterations in glutamate levels and neuronal activity have been linked to a range of psychiatric disorders, all of which involve cognitive deficits (Millan et al., 2012). Group II mGluRs are highly-expressed in the PFC and the two subtypes show overlapping but distinct expression patterns (Gu et al., 2008). Within the cortex both are expressed in pyramidal cells, but only mGluR3 is likely to be expressed in astrocytes, and expression of either subtypes in interneurons has not been demonstrated (Zeisel et al., 2015). Notably, recent work in non-human primates has shown complex, dose-dependent modulation of working memory responses and single unit activity in the PFC to group II mGluR agonists, suggesting the existence of multiple receptor population with distinct roles (Jin et al., 2017). Consistent with their reported roles in the PFC and associated cognitive behaviors, group II mGluR agonists and positive allosteric modulators have emerged as potential anti-psychotic (Stansley and Conn, 2018) or anxiolytic drugs (Ferraguti, 2018).
Here we showthat branched PORTLs are well suited to interrogating the role of mGluR signaling in specific cell types and circuits in regulating behavior. Specifically, we show that SNAP-mGluR2 activation in pyramidal cells of the medial prefrontal cortex recapitulates the modest hyperpolarization and presynaptic inhibition of native mGluR2 and is sufficient to produce a large behavioral effect in the Y-maze. The robust light-induced decrease in mEPSC frequency suggests that branched PORTL-mediated control of mGluRs should be well-suited for optogenetic applications that require targeted presynaptic inhibition of specific long-range projections. Crucially, when we limit expression to the subset of mGluR2-positive cells within the mPFC using the Grm2-Cre mouse, this effect is maintained. Furthermore, when mice were treated with MK-801 to model the negative symptoms associated with schizophrenia, targeted SNAP-mGluR2 photoactivation in the mPFC was able to reverse the deficits in the Y-maze assay. Compared to previous pharmacological studies of mGluRs this result allows for a clearer interpretation of the brain subregion and cellular subtypes involved and is consistent with the widely-supported role of mPFC neurons in working memory (Constantinidis and Klingberg, 2016; Goldman-Rakic, 1995) and with optogenetic studies targeting the mPFC (Gilmartin et al., 2013; Kim et al., 2016; Liu et al., 2014). Furthermore, our data is consistent with previous studies showing that NMDAR inhibition leads to an imbalance in prefrontal excitation and inhibition (Homayoun and Moghaddam, 2007) and suggests that re-normalization of activity can occur by mGluR2-mediated presynaptic inhibition of glutamate release. Interestingly, our data provides three useful observations regarding the timing of effects. First, our experiment shows that the onset and offset of GPCR-mediated behavioral modulation is relatively rapid as light activation was started only 2 minutes prior to the behavioral measure and reversal of the effect was observed within our 2.5-minute analysis window. Second, in contrast to prior studies which injected group II mGluR agonists prior to or at the same time of NMDAR agonists, photoactivation was performed 45 minutes after MK-801 injection indicating that reversal can occur following onset of the effects of noncompetitive. NMDAR antagonism. Finally, labeling of SNAP-mGluR2 was performed 12–16 hours prior to the experiment indicating that mGluR2 is relatively stable on the surface of the cell over this time scale. Ultimately, these experiments motivate further study of the role of group II mGluRs within the microcircuitry of the PFC and indicates that the branched PORTL approach should contribute to our ability to dissect the mechanisms by which GPCRs mediate behavioral control and disease treatment in preclinical models.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Joshua Levitz (jtl2003@med.cornell.edu). All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Grm2-Cre and C57BL/6J mice
Wild-type mice were of strain C57BL/6J provided by Jackson Laboratory. Grm2-Cre founder wildtype and mutant animals were purchased from Mutant Mouse Resource & Research Center (MMRRC) under strain name STOCK Tg(Grm2-cre)MR90Gsat/Mmucd and stock number 034611-UCD, generated from The Gene Expression Nervous System Atlas - GENSAT – Project (NINDS Contracts N01NS02331 & HHSN271200723701C to The Rockefeller University, New York, NY). Founder wildtypes were crossed to mutants to generate heterozygous Grm2-Cre experimental mice. B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J mice, also known as Ai9 mice and referred to here as FLEX-tdTomato mice, were purchased from Jackson Laboratory. For FISH and Neuroanatomy experiments, Grm2-Cre heterozygous mice were crossed to mutant FLEX-tdTomato mice, and mice heterozygous for both Grm2-Cre and FLEX-tdTomato were used for experimental analysis. Animals were genotyped by TransnetYX, Inc. for Cre (Grm2-Cre mouse) (FP: TTAATCCATATTGGCAGAACGAAAACG; RP: CAGGCTAAGTGCCTTCTCTACA), tdRFP (FLEX-tdTomato) (FP: AGATCCACCAGGCCCTGAA; RP:GTCTTGAACTCCACCAGGTAGTG), and ROSA WT (FLEX-tdTomato mouse) (FP:TTCCCTCGTGATCTGCAACTC; RP: CTTTAAGCCTGCCCAGAAGACT). For all experiments, other than primary culture experiments, male mice between 8 – 10 weeks of age were used. All animal use procedures were performed in accordance with Weill Cornell Medicine Institution Animal Care & Use Committee (IACUC) guidelines under approved protocol (2017–0023).
Primary mouse cortical neuron cultures
Cortical neurons were isolated from P1–2 wild-type mice and plated at 50,000–75,000 cells per coverslip on poly-ornithine-coated coverslips (12 mm). Neurons were plated in media containing DMEM supplemented with 5 g/L glucose, 100 mg/L Transferrin (Millipore), 10% FBS, 2% B-27 (Thermo Fisher), 1% Glutamax (Thermo Fisher) and 0.25 g/L insulin. At DIV 3–4, 50% of the plating media was removed and exchanged for feeding media containing media supplemented with 4 μM cytosine β-d-arabinofuranoside (Ara-C).
Primary mouse astrocyte cultures
Mixed cultures of cortical and hippocampal astrocytes were prepared from P1–3 mice and plated on poly-D-lysine-coated coverslips (18 mm) in media containing DMEM supplemented with 20% FBS, 25 mM glucose, 2 mM Glutamax, and 1 mM sodium pyruvate. At DIV 3–4, cells were washed to remove debris and media was changed every 3–4 days. 3–5 days after transfection, a subset of coverslips were fixed in 4% paraformaldehyde for immunohistochemical analyses to probe the specificity of GCaMP6f expression for astrocytes relative to other cell types. This was determined by quantification of GCaMP6f colocalization with immunohistochemical markers for astrocytes (glutamine synthetase). Neu-N and Iba1 staining was performed on a subset of coverslips, but no positively labeled cells were observed. >90% of GCaMP6 positive cells showed co-labeling with glutamine synthetase, confirming their identity as astrocytes.
Cell cultures of HEK293T
HEK293T cells were purchased from ATCC (CRL-11268), authenticated by Bio-Synthesis, Inc. and tested negative for mycoplasma using a kit from Molecular Probes. Cells were maintained in DMEM (GIBCO) supplemented with 5% fetal bovine serum and passaged by trypsin/EDTA digestion upon reaching ~95% confluency.
METHOD DETAILS
General Chemical Methods
All reactions are outlined in Scheme S1–S11 in the Supporting Information. Solvents for chromatography and reactions were purchased dry over molecular sieves or in HPLC grade. Unless otherwise stated, all other reagents were used without further purification from commercial sources. LC-MS was performed on a Shimadzu MS2020 connected to a Nexera UHPLC system equipped with a Waters ACQUITY UPLC BEH C18 (1.7 μm, 50 × 2.1 mm). Buffer A: 0.1% FA in H2O Buffer B: acetonitrile. The typical gradient was from 10% B for 0.5 min ➔ gradient to 90% B over 4.5 min ➔ 90% B for 0.5 min ➔ gradient to 99% B over 0.5 min with 1 mL/min flow.
High resolution mass spectrometry (HRMS) was performed using a Bruker maXis II ETD hyphenated with a Shimadzu Nexera system. The instruments were controlled via Brukers otofControl 4.1 and Hystar 4.1 SR2 (4.1.31.1) software. The acquisition rate was set to 3 Hz and the following source parameters were used for positive mode electrospray ionization: End plate offset = 500 V; capillary voltage = 3800 V; nebulizer gas pressure = 45 psi; dry gas flow = 10 L/min; dry temperature = 250 °C. Transfer, quadrupole and collision cell settings are mass range dependent and were fine-adjusted with consideration of the respective analyte’s molecular weight. For internal calibration sodium format clusters were used. Samples were desalted via fast liquid chromatography. A Supelco Titan™ C18 UHPLC Column, 1.9 μm, 80 Å pore size, 20 × 2.1 mm and a 2 min gradient from 10 to 98% aqueous MeCN with 0.1% FA (H2O: Carl Roth GmbH + Co. KG ROTISOLV® Ultra LC-MS; MeCN: Merck KGaA LiChrosolv® Acetonitrile hypergrade for LC-MS; FA - Merck KGaA LiChropur® Formic acid 98%−100% for LC-MS) was used for separation. Sample dilution in 10% aqueous ACN (hyper grade) and injection volumes were chosen dependent of the analyte’s ionization efficiency. Hence, on-column loadings resulted betwee 0.25–5.0 ng. Automated internal re-calibration and data analysis of the recorded spectra were performed with Bruker’s DataAnalysis 4.4 SR1 software.
Preparative RP-HPLC was performed on a Waters e2695 system equipped with a 2998 PDA detector for product collection (at 220, 280, 360 or 460 nm) on a Supelco Ascentis® C18 HPLC Column (5 μm, 250 × 21.2 mm). Buffer A: 0.1% TFA in H2O Buffer B: acetonitrile. The typical gradient was from 10% B for 5 min ➔ gradient to 90% B over 45 min ➔ 90% B for 5 min ➔ gradient to 99% B over 5 min with 8 mL/min flow. Compounds 1, 2, 25 and BG-COOH, BC-DBCO were previously described (Broichhagen et al., 2015; Levitz et al., 2017).
Abbreviations:
DIPEA: N,N-diisopropylethylamine; DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene; DMF: N,N-dimethylformamide; DMSO: dimethylsulfoxide; FA: formic acid; Su: succinimidyl; TFA: trifluoroacetic acid; TSTU: O-(N-succinimidyl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate.
Notes and observations:
NHS ester stability: TSTU was the coupling reagent of choice used for the synthesis of BGAGs, converting an acid to its respective NHS-ester usually within minutes. While most NHS esters were used in situ without purification, they can be isolated by RP-HPLC (cf. compound 4) and immediate lyophilization. Aliquoting and storage at –20 °C is recommended to avoid repeated freeze-thaw cycles that lead to decomposition. Fmoc deprotection: Fmoc is a standard amine protecting group extensively used in solid phase peptide synthesis, where amide couplings (with activating agents in DMF) and subsequent deprotection (with piperidine in DMF) is performed iteratively in high yields. Inspired by this and with the aim to reduce labor and purification steps, peptide couplings were performed in DMF with TSTU as an activating agent, and after amide coupling was complete, 5 vol% of piperidine was added directly to the reaction mixture. This proved to work reliably in our hands with all blue-shifted azobenzene compounds based on structure 1, but lead to complex reaction mixtures when using this method with red-shifted azobenzene compounds based on structure 2. Why the reason for this was not further investigated, we chose to purify Fmoc-containing compounds by RP-HPLC mainly to remove DMF, DIPEA and urea side products from TSTU, and employed DBU in MeCN as a deprotection reagent. Indeed, this was tolerated very well by red-shifted compounds and is noted when used in the procedures below. Stability of BG, BC and Halo-congeners towards acid: BGAGs need final deprotection of the NHBoc group to the free amine and TFA is the deprotecting agent of choice, however, the O-benzylated guanine and cytosine bases were shown to be labile towards strong acids. As such, we investigated and found that TFA can be used with BG-containing compounds if kept on ice with pre-cooled TFA, and its removal is not done in a rotary evaporator but by applying a gentle stream of nitrogen in a well ventilated chemical hood. Unfortunately, BC-containing compounds to not survive this treatment unharmed, and this is the reason why the NHBoc group is deprotected beforehand and strain promoted alkyne azide click reaction is performed in another orthogonal way. The Halo-group, however, is inert towards neat TFA at r.t..
General protocol A to generate NHS esters:
A 1 mL vial was charged with 1.0 equiv. of acid dissolved in DMF (1 mL / 10 mg) and 4.0 equiv. of DIPEA was added before 1.1 equiv. of TSTU in one portion (for amounts <1 mg of TSTU, stock solutions were prepared as it is critical to not overload TSTU). The active NHS ester was allowed to form for 15 min and used without further purification.
General procedure B for peptide couplings and in situ Fmoc deprotection:
A 1 mL vial was charged with 1.0 equiv. amine dissolved in DMF (1 mL / 10 mg) and 4.0 equiv. DIPEA. The pre-formed NHS ester (section 1.3) was added drop-wise at and the reaction mixture was allowed to stir at r.t. Upon complete conversion according to LCMS (usually < 30 min), 5 vol% of piperdine was added to the reaction mixture and the reaction allowed to stir for additional 10 min, before it was quenched by addition of 5 vol% HOAc and 10 vol% water and subjected to RP-HPLC.
General procedure C for peptide couplings for branching:
A 1 mL vial was charged with 3.0 equiv. amine dissolved in DMF (1 mL / 10 mg) and 8.0 equiv. DIPEA. The bis NHS ester 4 (1.0 equiv.) was dissolved in the same amount of DMF and added slowly and dropwise under vigorous stirring. The order and speed of addition is crucial to afford minimal amounts of side-products (i.e. imids, mono amides of succinates). Upon complete conversion according to LCMS, the reaction was directly deprotected or quenched and subjected to RP-HPLC (see below).
General procedure D for Boc deprotection:
A 15 mL falcon tube was charged with Boc protected compound and put in an ice bath. Pre-cooled (4 °C) TFA was added neat. The reaction mixture was vortexed to ensure homogeneity and put back on ice for 15 min before all volatiles were removed under a gentle stream of nitrogen. The residue was taken up in DMF/water (9/1) and subjected to RP-HPLC. NOTE: Azobenzene-containing reaction mixtures turned deep red upon addition of TFA.
Synthesis
5-((2-(2-((6-Chlorohexyl)oxy)ethoxy)ethyl)amino)-5-oxopentanoic acid (Halo-COOH) A 4 mL dram vial was charged with 100 mg (310 μmol, 1.0 equiv.) HaloNHBoc and 1 mL neat TFA was added. The solution was allowed to stand at r.t. for 5 min before all volatiles were removed under a gentle stream of nitrogen. 1 mL DMF and 160 μL DIPEA were added, before 35.3 mg (310 μmol, 1.0 equiv.) of glutaric anhydride was added in one portion. The reaction mixture was incubated o.n., before it was quenched with 160 μL HOAc, diluted with water and subjected to RP-HPLC to obtain 92 mg (274 μmol) of the desired product as a clear oil after lyophilization in 88% yield. HRMS (ESI): calc. for C15H29ClNO5 [M+H]+: 338.1729, found: 338.1728.
Bis(2,5-dioxopyrrolidin-1-yl) (((9H-fluoren-9-yl)methoxy)carbonyl)-D-glutamate (4) A 4 mL dram vial was charged with 300 mg (812 μmol, 1.0 equiv.) of (((9H-fluoren-9-yl)methoxy)carbonyl-D-glutamic acid (3) dissolved in 3 mL DMSO and 850 μL DIPEA before 978 mg (3.25 mmol, 4.0 equiv.) TSTU was added in one portion. The reaction mixture was stirred vigorously for 1 h before it was quenched by the addition of 850 μL HOAc and 200 μL water and subjected to preparative RP-HPLC. The desired product was obtained as a white powder after lyophilization in 35% yield (161 mg, 286 μmol). NOTE: Immediate freeze-drying after elution from the HPLC system is highly recommended to suppress hydrolysis. The product was aliquoted to avoid multiple freeze-thaw cycles that also hydrolyzed the NHS esters. HRMS (ESI): calc. for C28H26N3O10 [M+H]+: 564.1613, found: 564.1614.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-(((2,2’-(((R)-2-Aminopentanedioyl)bis(azanediyl))bis(acetyl))bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-((tert-butoxycarbonyl)amino)pentanedioic acid) (5)
5 was prepared according to general procedure C and was in situ deprotected by addition of 5 vol% of piperdine to the reaction mixture. The reaction allowed to stir for additional 10 min, before it was quenched by addition of 5 vol% HOAc and 10 vol% water and subjected to RP-HPLC. HRMS (ESI): calc. for C61H79N13O18 [M+2H]2+: 640.7828, found: 640.7835.
(2S,4S)-2-(4-((4-((E)-(4-((R)-1-amino-41-((2-((4-((E)-(4-((5S,7S)-7-((tert-Butoxycarbonyl)amino)-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)carbamoyl)-39,44-dioxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40,45-diazaheptatetracontan-47-amido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)-4-((tert-butoxycarbonyl)amino)pentanedioic acid (6)
6 was prepared according to general procedure B with the first step conducted at 50 °C. HRMS (ESI): calc. for C88H132N14O31 [M+2H]2+: 940.4586, found: 940.4582.
(2S,4S)-2-(4-((4-((E)-(4-((R)-1-(4-(((2-Amino-9H-purin-6-yl)oxy)methyl)phenyl)-49-((2-((4-((E)-(4-((5S,7S)-7-((tert-butoxycarbonyl)amino)-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)carbamoyl)-3,7,47,52-tetraoxo-11,14,17,20,23,26,29,32,35,38,41,44-dodecaoxa-2,8,48,53-tetraazapentapentacontan-55-amido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)-4-((tert-butoxycarbonyl)amino)pentanedioic acid (7)
7 was prepared according to general procedure B, with BG-COOSu prepared according to general procedure A, without the addition of piperidine. HRMS (ESI): calc. for C106H150N20O34 [M+2H]2+: 1124.0321, found: 1124.0327.
(2S,4S)-2-Amino-4-(4-((4-((E)-(4-((R)-49-(3-((2-((4-((E)-(4-((5S,7S)-7-amino-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)amino)-3-oxopropyl)-1-(4-(((2-amino-9H-purin-6-yl)oxy)methyl)phenyl)-3,7,47,50-tetraoxo-11,14,17,20,23,26,29,32,35,38,41,44-dodecaoxa-2,8,48,51-tetraazatripentacontan-53-amido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)pentanedioic acid (2xBGAG12)
2xBGAG12 was prepared according to general procedure D. HRMS (ESI): calc. for C96H134N20O30 [M+2H]2+: 1032.9797, found: 1032.9787.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-(((2,2’-(((R)-2-(5-((4-(((2-Amino-9H-purin-6-yl)oxy)methyl)benzyl)amino)-5-oxopentanamido)pentanedioyl)bis(azanediyl))bis(acetyl))bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-((tert-butoxycarbonyl)amino)pentanedioic acid) (8)
8 was prepared according to general procedure B, with BG-COOSu prepared according to general procedure A, with the first step conducted at 50 °C and without the addition of piperidine.
HRMS (ESI): calc. for C79H95N19O21 [M–2H]2–: 821.8402, found: 821.8413.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-(((2,2’-(((R)-2-(5-((4-(((2-Amino-9H-purin-6-yl)oxy)methyl)benzyl)amino)-5-oxopentanamido)pentanedioyl)bis(azanediyl))bis(acetyl))bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-aminopentanedioic acid) (2xBGAG0)
2xBGAG0 was prepared according to general procedure D. HRMS (ESI): calc. for C69H79N19O17 [M–2H]2–: 721.7878, found: 721.7870.
(2S,4S)-2-(4-((4-((E)-(4-(1-Amino-39-oxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40-azadotetracontan-42-amido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)-4-((tert-butoxycarbonyl)amino)pentanedioic acid (9)
9 was prepared according to general procedure B. HRMS (ESI): calc. for C55H90N7O21 [M+H]+: 1184.6184, found: 1184.6176.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-((((R)-45-Amino-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontanedioyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-((tert-butoxycarbonyl)amino)pentanedioic acid) (10)
10 was prepared according to general procedure C and was in situ deprotected by addition of 5 vol% of piperdine to the reaction mixture. The reaction allowed to stir for additional 10 min, before it was quenched by addition of 5 vol% HOAc and 10 vol% water and subjected to RP-HPLC. HRMS (ESI): calc. for C115H186N15O44 [M+3H]3+: 827.4264, found: 824.4261.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-((((R)-45-(5-((4-(((2-Amino-9H-purin-6-yl)oxy)methyl)benzyl)amino)-5-oxopentanamido)-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontanedioyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-((tert-butoxycarbonyl)amino)pentanedioic acid) (11)
11 was prepared according to general procedure B, with BG-COOSu prepared according to general procedure A, with the first step conducted at 50 °C and without the addition of piperidine. HRMS (ESI): calc. for C133H204N21O47 [M+3H]3+: 949.4744, found: 949.4747.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-((((R)-45-(5-((4-(((2-Amino-9H-purin-6-yl)oxy)methyl)benzyl)amino)-5-oxopentanamido)-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontanedioyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-aminopentanedioic acid) (2xBGAG12,v2)
2xBGAG12,v2 was prepared according to general procedure D. HRMS (ESI): calc. for C123H188N21O43 [M+3H]3+: 882.7728, found: 882.7723.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-((((R)-45-amino-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontanedioyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-((tert-butoxycarbonyl)amino)pentanedioic acid) (12)
12 was prepared according to general procedure C and was in situ deprotected by addition of 5 vol% of piperdine to the reaction mixture. The reaction allowed to stir for additional 10 min, before it was quenched by addition of 5 vol% HOAc and 10 vol% water and subjected to RP-HPLC. HRMS (ESI): calc. for C115H186N15O44 [M+3H]3+: 827.4264, found: 827.4255.
(2S,4S)-2-[3-({4-[(1E)-2-[4-(2-{1-[(4R)-4-[(2R)-2-Amino-4-{[(1R)-1,3-bis[(38-{[({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-butoxy)carbonyl]amino}−5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}carbamoyl)methyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]propyl]carbamoyl}butanamido]-4-[(38-{[({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-butoxy)carbonyl]amino}−5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}carbamoyl)methyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]butanamido]-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-amido}acetamido)phenyl]diazen-1-yl]phenyl}carbamoyl)propyl]-4-{[(tert-butoxy)carbonyl]amino}pentanedioic acid (13)
13 was prepared according to general procedure B with the first step conducted at 50°C. HRMS (ESI): calc. for C235H375N31O90 [M+4H]4+:1286.3940, found:1286.3925.
(2S,4S)-2-[3-({4-[(1E)-2-[4-(2-{1-[(4R)-4-[(2R)-2-(4-{[(4-{[(2-Amino-9H-purin-6-yl)oxy]methyl}phenyl)methyl]carbamoyl}butanamido)-4-{[(1R)-1,3-bis[(38-{[({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-butoxy)carbonyl]amino}−5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}carbamoyl)methyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]propyl]carbamoyl}butanamido]-4-[(38-{[({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-
butoxy)carbonyl]amino}−5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}carbamoyl)methyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]butanamido]-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-amido}acetamido)phenyl]diazen-1-yl]phenyl}carbamoyl)propyl]-4-{[(tert-butoxy)carbonyl]amino}pentanedioic acid (14)
14 was prepared according to general procedure B, with BG-COOSu prepared according to general procedure A, without the addition of piperidine. HRMS (ESI): calc. for C253H1385N37O93 [M+4H]4+: 1360.1807, found: 1360.1804.
(2S,4S)-2-Amino-4-[3-({4-[(1E)-2-[4-(2-{1-[(4R)-4-[(38-{[({4-[(1E)-2-{4-[(5S,7S)-7-amino-5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}carbamoyl)methyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]-4-[(2R)-2-(4-{[(4-{[(2-amino-9H-purin-6-yl)oxy]methyl}phenyl)methyl]carbamoyl}butanamido)-4-{[(1R)-1,3-bis[(38-{[({4-[(1E)-2-{4-[(5S,7S)-7-amino-5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}carbamoyl)methyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]propyl]carbamoyl}butanamido]butanamido]-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-amido}acetamido)phenyl]diazen-1-yl]phenyl}carbamoyl)propyl]pentanedioic acid (4xBGAG12)
4xBGAG12 was prepared according to general procedure D. HRMS (ESI): calc. for C233H363N37O85 [M+6H]6+: 840.2541, found: 840.2533.
(2S,4S)-2-(4-((4-((E)-(4-((R)-1-Azido-41-((2-((4-((E)-(4-((5S,7S)-7-((tert-butoxycarbonyl)amino)-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)carbamoyl)-39,44-dioxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40,45-diazaheptatetracontan-47-amido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)-4-((tert-butoxycarbonyl)amino)pentanedioic acid (15)
15 was prepared according to general procedure B without adding piperidine for deprotection. The crude was subjected to RP-HPLC and azobenzene containing fractions were collected, dried and subjected to the next step without further characterization.
(2S,4S)-2-Amino-4-(4-((4-((E)-(4-((R)-41-(3-((2-((4-((E)-(4-((5S,7S)-7-amino-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)amino)-3-oxopropyl)-1-azido-39,42-dioxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40,43-diazapentatetracontan-45-amido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)pentanedioic acid (16)
16 was prepared according to general procedure D. HRMS (ESI): calc. for C78H115N16O27 [M+3H]3+: 2700, found: 569.2705.
(2S,4S)-2-Amino-4-(4-((4-((E)-(4-((R)-41-(3-((2-((4-((E)-(4-((5S,7S)-7-amino-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)amino)-3-oxopropyl)-1-(8-(4-((4-(((4-aminopyrimidin-2-yl)oxy)methyl)benzyl)amino)-4-oxobutanoyl)-8,9-dihydro-3H-dibenzo[b,f][1,2,3]triazolo[4,5-d]azocin-3-yl)-39,42-dioxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40,43-diazapentatetracontan-45-amido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)pentanedioicacid (2xBCAG12)
A 4 mL dram vial was charged with 16 dissolved in MeOH. BC-DBCO was added in one portion and the reaction mixture was incubated o.n. before all volatiles were removed under a gentle stream of nitrogen. The crude was taken up in DMF and water (9/1) and subjected to RP-HPLC purification to obtain 2xBCAG12 as a yellow powder after lyophilization. HRMS (ESI): calc. for C109H142N21O30 [M+3H]3+: 742.0082, found: 742.0080.
(2S,4S)-2-((tert-Butoxycarbonyl)amino)-4-(4-((4-((E)-(4-(61-chloro-4,44,48-trioxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55-tetradecaoxa-3,43,49-triazahenhexacontanamido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)pentanedioic acid (17)
17 was prepared according to general procedure B, with Halo-COOSu prepared according to general procedure A, without the addition of piperidine. HRMS (ESI): calc. for C70H117ClN8O25 [M+2H]2+: 753.3913, found: 753.3917.
(2S,4S)-2-Amino-4-(4-((4-((E)-(4-(61-chloro-4,44,48-trioxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55-tetradecaoxa-3,43,49-triazahenhexacontanamido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)pentanedioic acid (ClAG12)
ClAG12 was prepared according to general procedure D. HRMS (ESI): calc. for C65H109ClN8O23 [M+2H]2+: 702.3642, found: 702.3642.
(2S,4S)-2-((tert-Butoxycarbonyl)amino)-4-(4-((4-((E)-(4-((R)-5-(3-((2-((4-((E)-(4-((5S,7S)-7-((tert-butoxycarbonyl)amino)-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)amino)-3-oxopropyl)-64-chloro-4,7,47,51-tetraoxo-10,13,16,19,22,25,28,31,34,37,40,43,55,58-tetradecaoxa-3,6,46,52-tetraazatetrahexacontanamido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)pentanedioic acid (18)
18 was prepared according to general procedure B, with Halo-COOSu prepared according to general procedure A, without the addition of piperidine. HRMS (ESI): calc. for C103H158ClN15O35 [M+2H]2+: 1100.5377, found: 1100.5370.
(2S,4S)-2-Amino-4-(4-((4-((E)-(4-((R)-5-(3-((2-((4-((E)-(4-((5S,7S)-7-amino-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)amino)-3-oxopropyl)-64-chloro-4,7,47,51-tetraoxo-10,13,16,19,22,25,28,31,34,37,40,43,55,58-tetradecaoxa-3,6,46,52-tetraazatetrahexacontanamido)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)pentanedioic acid (2xClAG12)
2xClAG12 was prepared according to general procedure D. HRMS (ESI): calc. for C93H142ClN15O31 [M+2H]2+: 1000.4852, found: 1000.4853.
(2S,4S)-2-(4-((4-((E)-(4-((1-Amino-39-oxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40-azadotetracontan-42-yl)amino)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)-4-((tert-butoxycarbonyl)amino)pentanedioic acid (19)
19 was prepared according to general procedure B without the addition of piperidine. Instead, Fmoc-protected compound was obtained after RP-HPLC purification, dried and redissolved in MeCN with the addition of 5% DBU. The reaction mixture was incubated for 1 h, before it was quenched by addition of HOAc and water and subjected to RP-HPLC. HRMS (ESI): calc. for C55H93N7O20 [M+2H]2+: 585.8232, found: 585.8232.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-((((R)-45-Amino-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontane-1,91-diyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-((tert-butoxycarbonyl)amino)pentanedioic acid) (20)
20 was prepared according to general procedure B without the addition of piperidine. Instead, Fmoc-protected compound was obtained after RP-HPLC purification, dried and redissolved in MeCN with the addition of 5% DBU. The reaction mixture was incubated for 1 h, before it was quenched by addition of HOAc and water and subjected to RP-HPLC. HRMS (ESI): calc. for C115H190N15O42 [M+3H]3+: 818.1069, found: 818.1075.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-((((R)-45-(5-((4-(((2-Amino-9H-purin-6-yl)oxy)methyl)benzyl)amino)-5-oxopentanamido)-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontane-1,91-diyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-((tert-butoxycarbonyl)amino)pentanedioic acid) (21)
21 was prepared according to general procedure B, with BG-COOSu prepared according to general procedure A, with the first step conducted at 50 °C and without the addition of piperidine. HRMS (ESI): calc. for C133H208N21O45 [M+3H]3+: 940.1549, found: 940.1546.
(2S,2’S,4S,4’S)-4,4’-(((((1E,1’E)-((((R)-45-(5-((4-(((2-Amino-9H-purin-6-yl)oxy)methyl)benzyl)amino)-5-oxopentanamido)-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontane-1,91-diyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))bis(2-aminopentanedioic acid) (2xBGAG12,460)
2xBGAG12,460 was prepared according to general procedure D. HRMS (ESI): calc. for C123H193N21O41 [M+4H]4+: 655.3418, found: 655.3416.
Dimethyl (2S,4S)-2-(4-((4-((E)-(4-((1-amino-39-oxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40-azadotetracontan-42-yl)amino)phenyl)diazenyl)phenyl)amino)-4-oxobutyl)-4-((tert-butoxycarbonyl)amino)pentanedioate (23)
23 was prepared according to general procedure B without the addition of piperidine. Instead, Fmoc-protected compound was obtained after RP-HPLC purification, dried and redissolved in MeCN with the addition of 5% DBU. The reaction mixture was incubated for 1 h, before it was quenched by addition of HOAc and water and subjected to RP-HPLC. HRMS (ESI): calc. for C57H97N7O20 [M+2H]2+: 599.8389,found: 599.8387.
Tetramethyl 4,4’-(((((1E,1’E)-((((R)-45-amino-4,44,48,88-tetraoxo-7,10,13,16,19,22,25,28,31,34,37,40,52,55,58,61,64,67,70,73,76,79,82,85-tetracosaoxa-3,43,49,89-tetraazahennonacontane-1,91-diyl)bis(azanediyl))bis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4,1-phenylene))bis(azanediyl))bis(4-oxobutane-4,1-diyl))(2S,2’S,4S,4’S)-bis(2-((tert-butoxycarbonyl)amino)pentanedioate) (24)
24 was prepared according to general procedure B without the addition of piperidine. Instead, Fmoc-protected compound was obtained after RP-HPLC purification, dried and redissolved in MeCN with the addition of 5% DBU. The reaction mixture was incubated for 1 h, before it was quenched by addition of HOAc and water and subjected to RP-HPLC. HRMS (ESI): calc. for C119H198N15O42 [M+3H]3+: 836.7945, found: 836.7940.
1,5-Dimethyl (2S,4S)-2-[3-({4-[(1E)-2-{4-[(2-{1-[(4R)-4-[(2R)-2-amino-4-{[(1R)-1,3-bis[(38-{[2-({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-butoxy)carbonyl]amino}−8-methoxy-5-(methoxycarbonyl)-8-oxooctanamido]phenyl}diazen-1-yl]phenyl}amino)ethyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]propyl]carbamoyl}butanamido]-4-[(38-{[2-({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-butoxy)carbonyl]amino}−8-methoxy-5-(methoxycarbonyl)-8-oxooctanamido]phenyl}diazen-1-yl]phenyl}amino)ethyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]butanamido]-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-amido}ethyl)amino]phenyl}diazen-1-yl]phenyl}carbamoyl)propyl]-4-{[(tert-butoxy)carbonyl]amino}pentanedioate (25)
25 was prepared according to general procedure B without the addition of piperidine. Instead, Fmoc-protected compound was obtained after RP-HPLC purification, dried and redissolved in MeCN with the addition of 5% DBU. The reaction mixture was incubated for 1 h, before it was quenched by addition of HOAc and water and subjected to RP-HPLC. HRMS (ESI): calc. for C243H399N31O86 [M+4H]4+: 1282.4460, found: 1282.4447.
1,5-Dimethyl (2S,4S)-2-[3-({4-[(1E)-2-{4-[(2-{1-[(4R)-4-[(2R)-2-(4-{[(4-{[(2-amino-9H-purin-6-yl)oxy]methyl}phenyl)methyl]carbamoyl}butanamido)-4-{[(1R)-1,3-bis[(38-{[2-({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-butoxy)carbonyl]amino}−8-methoxy-5-(methoxycarbonyl)-8-oxooctanamido]phenyl}diazen-1-yl]phenyl}amino)ethyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]propyl]carbamoyl}butanamido]-4-[(38-{[2-({4-[(1E)-2-{4-[(5S,7S)-7-{[(tert-butoxy)carbonyl]amino}−8-methoxy-5-(methoxycarbonyl)-8-oxooctanamido]phenyl}diazen-1-yl]phenyl}amino)ethyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]butanamido]-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-amido}ethyl)amino]phenyl}diazen-1-yl]phenyl}carbamoyl)propyl]-4-{[(tert-butoxy)carbonyl]amino}pentanedioate (26)
A 4 mL dram vial was charged with 25 (1.0 equiv.), BG-COOH (1.1 equiv.) and dissolved in DIPEA (4.0 equiv.) and DMF before HBTU (1.2 equiv.) was added in one portion. The mixture was incubated for 2 h before it was quenched by addition of 5 vol% HOAc and subjected to RP-HPLC. HRMS (ESI): calc. for C261H417N37O89 [M+4H]4+: 1374.2328, found: 1374.2305.
(2S,4S)-2-Amino-4-[3-({4-[(1E)-2-{4-[(2-{1-[(4R)-4-[(38-{[2-({4-[(1E)-2-{4-[(5S,7S)-7-amino-5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}amino)ethyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]-4-[(2R)-2-(4-{[(4-{[(2-amino-9H-purin-6-yl)oxy]methyl}phenyl)methyl]carbamoyl}butanamido)-4-{[(1R)-1,3-bis[(38-{[2-({4-[(1E)-2-{4-[(5S,7S)-7-amino-5,7-dicarboxyheptanamido]phenyl}diazen-1-yl]phenyl}amino)ethyl]carbamoyl}−3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxaoctatriacontan-1-yl)carbamoyl]propyl]carbamoyl}butanamido]butanamido]-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-amido}ethyl)amino]phenyl}diazen-1-yl]phenyl}carbamoyl)propyl]pentanedioic acid (4xBGAG12,460)
A 15 mL Falcon tube was charged with 26 and dissolved in MeOH / 1 M LiOH (1/1) and allowed to stand at r.t. for 1 h it was quenched by addition of 5 vol% HOAc and subjected to RP-HPLC. The azobenzene-containing fractions (27) were pooled and lyophilized, and final deprotection was performed in neat TFA at 0 °C according to procedure 1.6. HRMS (ESI): calc. for C233H372N37O81 [M+7H]7+: 712.3736, found: 712.3733.
1-(6-(((S)-5-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-5-carboxypentyl)amino)-6-oxohexyl)-3,3-dimethyl-2-((1E,3E)-5-((Z)-1,3,3-trimethylindolin-2-ylidene)penta-1,3-dien-1-yl)-3H-indol-1-ium (30) A round bottom flask was charged with 35.0 mg (72.4 μmol, 1.0 equiv.) of 1-(5-carboxypentyl)-3,3-dimethyl-2-((1E,3E)-5-((Z)-1,3,3-trimethylindolin-2-ylidene)penta-1,3-dien-1-yl)-3H-indol-1-ium (29) (Ueno et al., 2011) which was dissolved in 1.5 mL DMSO and 50 μL DIPEA. TSTU (21.8 mg, 72.4μmol, 1.0 equiv.) was added in one portion and the mixture was incubated for 30 min before 32.0 mg (86.8 μmol, 1.2 equiv.) of Fmoc-Lys-OH (28) was added in one portion. The reaction mixture was incubated for another hour before it was quenched by addition of 50 μL HOAc and subjected to RP-HPLC to obtain 26 mg (32.2 μmol) of the desired product as a blue powder after lyophilization in 45% yield. HRMS (ESI): calc. for C53H61N4O5 [M]+: 833.4636, found: 833.4639.
1-(6-(((S)-5-Amino-6-((2-((4-((E)-(4-((5S,7S)-7-((tert-butoxycarbonyl)amino)-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)amino)-6-oxohexyl)amino)-6-oxohexyl)-3,3-dimethyl-2-((1E,3E)-5-((Z)-1,3,3-trimethylindolin-2-ylidene)penta-1,3-dien-1-yl)-3H-indol-1-ium (31)
31 was prepared according to general procedure B. HRMS (ESI): calc. for C66H85N10O10 [M]+: 1177.6445, found: 1177.6452.
1-((S)-1-Amino-41-((2-((4-((E)-(4-((5S,7S)-7-((tert-butoxycarbonyl)amino)-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)carbamoyl)-39,47-dioxo-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxa-40,46-diazadopentacontan-52-yl)-3,3-dimethyl-2-((1E,3E)-5-((Z)-1,3,3-trimethylindolin-2-ylidene)penta-1,3-dien-1-yl)-3H-indol-1-ium (32)
32 was prepared according to general procedure B with the first step conducted at 50 °C. HRMS (ESI): calc. for C93H139N11O23 [M+H]2+: 889.5033, found: 889.5037.
1-((S)-1-(4-(((2-Amino-9H-purin-6-yl)oxy)methyl)phenyl)-49-((2-((4-((E)-(4-((5S,7S)-7-((tert-butoxycarbonyl)amino)-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)carbamoyl)-3,7,47,55-tetraoxo-11,14,17,20,23,26,29,32,35,38,41,44-dodecaoxa-2,8,48,54-tetraazahexacontan-60-yl)-3,3-dimethyl-2-((1E,3E)-5-((Z)-1,3,3-trimethylindolin-2-ylidene)penta-1,3-dien-1-yl)-3H-indol-1-ium (33)
33 was prepared according to general procedure B, with BG-COOSu prepared according to general procedure A, without the addition of piperidine. HRMS (ESI): calc. for C111H158N17O24 [M+2H]3+: 3859, found: 715.3859.
1-((S)-49-((2-((4-((E)-(4-((5S,7S)-7-Amino-5,7-dicarboxyheptanamido)phenyl)diazenyl)phenyl)amino)-2-oxoethyl)carbamoyl)-1-(4-(((2-amino-9H-purin-6-yl)oxy)methyl)phenyl)-3,7,47,55-tetraoxo-11,14,17,20,23,26,29,32,35,38,41,44-dodecaoxa-2,8,48,54-tetraazahexacontan-60-yl)-3,3-dimethyl-2-((1E,3E)-5-((Z)-1,3,3-trimethylindolin-2-ylidene)penta-1,3-dien-1-yl)-3H-indol-1-ium (BGAG12-Cy5)
BGAG12-Cy5 was prepared according to general procedure D. HRMS (ESI): calc. for C106H150N17O24 [M+2H]3+: 682.0351, found: 682.0354.
Molecular Cloning, Cell Culture, and Gene Expression
Experiments were performed using previously described N-terminally SNAP- or CLIP-tagged rat or human mGluRs(Doumazane et al., 2011). Mutations, insertions, and deletions were introduced using standard PCR-based techniques. SNAPfast or Halo were introduced into mGluR2 at the same position as SNAP using Gibson assembly. The chimeric SNAP-mGluR2-mGluR5 was made, also via Gibson assembly, using the extracellular domain of SNAP-tagged mGluR2 (K23 to E559) and rat mGluR5 transmembrane domain and C-terminal domain (Y571 to STOP). For adeno-associated virus production, SNAP-mGluR2 was cloned into a previously described FLEX construct (addgene ID: 26966).
HEK 293T cells were plated at low density on poly-L-lysine coated coverslips (18 mm) and transfected the following day using either Lipofectamine 2000 or 3000 (Thermo Fisher). 24–48 hrs after transfection, cells were used for electrophysiology or imaging experiments. As previously described(Levitz et al., 2017), for electrophysiology experiments each coverslip received 0.7 μg each of the receptor of interest and GIRK1-F137S and 0.1 ug of tdTomato as a transfection marker. The GIRK1-F137S homo-tetramerization mutant (Vivaudou et al., 1997) was used to reduce the number of constructs to simplify transfection and expression. For calcium imaging experiments, each coverslip received 0.7 μg of the receptor of interest plus 0.2–0.3 μg of the relevant calcium indicator.
Neurons were transfected at DIV 7–9 using the calcium phosphate method. Each coverslip received 1.6 μg of SNAP-mGluR2 and 0.2 μg of tdTomato, both under a CMV promoter. Neurons were identified based on morphology and confirmed by their ability to fire action potentials. Glia were transfected at DIV 7–9 using the calcium phosphate method with 2 μg DNA/well for GCaMP6f and/or SNAP-mGluR2-mGluR5, both under a CMV promoter.
HEK Cell and Cultured Neuron Electrophysiology
Whole cell patch clamp recordings from HEK 293T cells were performed 24–48 hours after transfection as previously described (Farrants et al., 2018). Briefly, voltage clamp recordings at −60 mV were performed in a high potassium (120 mM) solution to enable large inward currents upon receptor activation.
Whole cell patch clamp recordings of cultured cortical neurons were performed 4–6 days after transfection (11–15 DIV) in an extracellular solution containing (in mM): 138 NaCl, 1.5 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 glucose and 5 HEPES, pH 7.4. Intracellular solution contained (in mM): 140 potassium gluconate, 10 NaCl, 5 EGTA, 2 MgCl2, 1 CaCl2, 10 HEPES, 2 MgATP and 0.3 Na2GTP, pH 7.2. For hyperpolarization measurements, cells were adjusted to −60 mV with current injection prior to photoswitching. Only cells with a resting potential ≤−40 mV were analyzed.
Unless otherwise noted, cells were incubated with 1–10 μM of PORTL for 45–60 min at 37°C in the appropriate extracellular recording solution. Labeling efficiency was determined as previously described (Levitz et al., 2017). Illumination was applied to the entire field of view using a CoolLED pE-4000 through a 40× objective. Light intensity in the sample plane was 1–2 mW/mm2. pClamp software was used for both data acquisition and control of illumination. All drugs were purchased from Tocris and applied using a gravity-driven perfusion system.
Single Molecule FRET
Single-molecule Förster resonance energy transfer (smFRET) experiments were performed on an inverted microscope (Olympus IX73) in total internal reflection (TIR) mode using a 100x objective (NA=1.49) and a 561 nm laser diode. Movies were recorded simultaneously with two sCMOS ORCA-Flash4 v3.0 cameras (Hamamatsu) separated by a 635 nm long-pass dichroic mirror and with appropriate emission filters for donor (595/50) and acceptor (655LP). A microflow chamber was assembled using a passivated glass coverslip prepared with mPEG-SVA and biotinylated PEG (MW=5000, 50:1 molar ratio, Laysan Bio) as previously described(Vafabakhsh et al., 2015). The microflow channels were first incubated with 0.2 mg/ml of NeutrAvidin (ThermoFisher) for 2 min, followed by 10 nM of biotinylated secondary donkey anti-rabbit, antibody (ThermoFisher, A16039) for 30 min, and 10 nM of anti-Metabotropic Glutamate Receptor 2+3 antibody (abcam, ab6438) for 30 min. Microchannels were washed 5 times between each conjugation step with T50 buffer (10 mM Tris, 50 mM NaCl, pH 8).
Following 48 hr expression of either HA-SNAP-mGluR2 or HA-SNAPf-mGluR2, HEK293T cells were labeled for 1 hour at 37°C using a 1:3 molar ratio of BG-LD555 (1 μM) and BG-LD655 (3 μM) dyes (Lumidyne Technologies) dissolved in EX buffer containing (in mM): 10 HEPES, 135 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2. After labeling, cells were washed 2 times with EX buffer, harvested by incubating the cells in PBS (Ca2+ and Mg2+ free) for 20 min, and lysed for 1 hr at 4 °C using lysis buffer containing (in mM): 150 NaCl, 1 EDTA, protease inhibitor cocktail (Thermo Scientific) and 1.2% IGEPAL detergent (Sigma). Finally, cell lysates were spun down at 16,000 x g for 20 min at 4 °C, supernatant was collected, and maintained on ice prior to imaging. Protein samples were diluted using a dilution buffer (EX containing 0.1% IGEPAL) and added to the microflow chamber until the density reached ~400 molecules per 2,000 μm2. Any unbound proteins were washed using the dilution buffer. Single molecule fluorescence movies were recorded at one frame per 100 ms in the presence of an oxygen scavenging system (1 mg/ml glucose oxidase, 0.04 mg/ml catalase, 0.8% w/v D-glucose) and photostabilizing agents (5 mM cyclooctatetraene).
Calcium Imaging
HEK 293T cells were imaged at 30 °C in extracellular solution containing (in mM): 135 NaCl, 5.4 KCl, 10 HEPES, 2 CaCl2, 1 MgCl2, pH = 7.4 with continuous perfusion on a Zeiss LSM880 scanning confocal microscope using ZEN Black software with a 63x objective, a 488 nm and/or 561 nm laser for imaging and a 405 nm laser for photoactivation. For photoactivation experiments, a brief 10–100 μM glutamate application was used to identify healthy cells, based on calcium responses, which would be suitable for photoactivation experiments. Photoactivation was performed by scanning a 405 nm laser at 100% power in a defined region for 40 iterations between each imaging frame. For drug application experiments, imaging was performed at room temperature on an Olympus IX-73 microscope using a 60× 1.49 NA APO N TIRFM objective (Olympus) and snapshots were taken with a sCMOS ORCA-Flash4 v3.0 camera (Hamamatsu). GFP was excited with a 488 nm laser diode and glutamate was added by a gravity-driven perfusion system. Astrocytes were imaged at 37°C with the same protocol described above in an extracellular solution containing (in mM): 138 NaCl, 1.5 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 glucose and 5 HEPES, pH 7.4. DHPG was purchased from Tocris and applied using a gravity-driven perfusion system. For calcium-free experiments, calcium was exchanged for magnesium to maintain osmolarity and ionic strength.
Viral Expression and in vivo PORTL labeling
For experiments in wild-type mice, Male C57BL/6J mice were injected at p60 with either 1 μL of a 1:1 viral cocktail of AAV9-EF1a-FLEX-SNAP-mGluR2-WPRE-hGH (Penn Vector Core) and pENN-AAV9-CamKII 0.4-Cre-SV40 (Addgene) or, as a control, only AAV9-EF1a-FLEX-SNAPmGluR2-WPRE-hGH. For experiments in Grm2-Cre mice, male heterozygous mice were injected at p60 with AAV9-EF1a-FLEX-SNAP-mGluR2-WPRE-hGH. Mice were injected in the medial prefrontal cortex (AP + 1.85, ML +/− 0.35, DV −2.2, −1.8) with 500 nL per site using a Kopf stereotaxic and World Precision Instruments microinjection syringe pump with a 10 μL syringe and 33g blunt needle. For slice recordings, mice received unilateral infusions (ML + 0.35) and for behavior mice received bilateral infusions (ML +/−0.35). All imaging and slice experiments were performed at least 6 weeks after viral injection. For in vivo labeling for slice experiments, mice received infusion of 500 nL of 10 μM BGAG12-Cy5 targeted to the same site as viral injection. For in vivo labeling with BG-LD555 for visualization, mice received infusion of 1000 nL of 1–3 μM BG-LD555 targeted to the same site as viral injection. For imaging of slices, 4 hours later mice underwent transcardial perfusion and were fresh fixed with 4% paraformaldehyde. Brains were extracted and bathed in 4% paraformaldehyde for 24 hours followed by 72 hours in 30% sucrose PBS solution. Brains were mounted and frozen at −20 °C in OCT block and medial prefrontal cortex was sliced at 40 μM thick on a cryostat at −22 °C. Slices were wet mounted to glass slides and secured with coverslip using VECTASHIELD HardSet Antifade Mounting Medium with DAPI (Vector Laboratories). Glass slides were imaged using an Olympus Confocal FV3000 and images were processed in ImageJ.
For behavioral experiments, custom Dual opto-fluid cannula with interchangeable injectors designed and manufactured by Doric Lenses, Inc were implanted to target bilateral mPFC (cannula: AP +1.85, ML +/−0.35, DV −1.5, fiberoptic: AP +1.85, ML +/−0.35, DV −1.8) and adhered to the scull using C & B Metabond (Parkell). Prior to behavioral tests, both experimental and control mice were anesthetized using isoflurane and dual opto-cannula plugs were replaced with 100 μM inner diameter micro-injectors. 10 μM 2xBGAG12 was infused through micro-injectors at 100 nl per minute using polyethylene tubing connected to a Harvard Apparatus (Harvard Biosciences, Inc.) 11 Elite dual syringe infusion pump and 10 μL Hamilton Company syringes. Micro-injectors were then replaced with optical fibers with 200 μM diameter and 0.22 numerical aperture (NA).
Brain Slice Electrophysiology
14–18 hours following BGAG12-Cy5 injection, coronal slices of the medial prefrontal cortex were prepared at room temperature in an NMDG-HEPES aCSF containing (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 Glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, 0.5 CaCl2. Slices were maintained in this solution for ~10 min at 34 °C, and then allowed to recover for at least 45 minutes at room temperature in a modified aCSF containing (in mM): 92 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 Glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 2 MgSO4, 2 CaCl2. Following recovery, slices were transferred to a modified superfusion chamber (Warner Instruments) and mounted on the stage of an Olympus U-TLUIR microscope. Recording were performed at 30 °C in a standard oxygenated aCSF that continually circulated and bubbled with 95% O2/5% CO2 containing: 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 24 NaHCO3, 5 HEPES, 12.5 Glucose, 2 MgSO4, 2 CaCl2
Whole-cell patch-clamp recordings were performed on a computer-controlled amplifier (MultiClamp 700B Axon Instruments, Foster City, CA) and acquired with an Axoscope 1550B (Axon Instruments) at a sampling rate of 10 kHz in current clamp and 500 Hz for current-evoked firing. For current clamp experiments, pipettes of 2–5 MΩ resistance were filled with a solution containing (in mM): 135 K-Gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 Na2ATP, 0.4 Na2GTP, pH 7.35, 280–290 mOsm. Medial prefrontal cortex pyramidal neurons were identified under visual guidance using infrared-differential interference contrast (IR-DIC) video microscopy through a QImaging optiMOS Camera with a 40× water immersion objective. Illumination for both visualizing BGAG12-Cy5 fluorescence and photoswitching was from a CoolLED pE-4000 coupled to the microscope and filtered through an Olympus U-MF2 filter cube with a 635 BrightLine Beamsplitter and 692/40 filter. Light intensity for photoswitching at the sample was 1–2 mW/mm2. Local application of LY379268 was performed via Perfusion Pencil (AutoMate Scientific, Berkeley, CA). Neurons were recorded at their resting potential following a minimum 2-minute baseline. For current-induced firing, 250 ms depolarizing current steps were injected with 50 pA increments.
For voltage clamp experiments, recording pipettes were filled with a solution containing (in mM): 117 D-gluconic acid, 118 CsOH, 20 HEPES, 0.4 EGTA, 5 TEA, 2 MgCl2, 4 Na2APT, 0.4 Na2GPT, 2 QX-314, pH 7.35, 280–290 mOsm. To isolate action potential-independent miniature excitatory postsynaptic currents (mEPSCs), 25 μM picrotoxin and 1 μM tetrodotoxin (TTX) were included in the recording solution. mEPSCs were recorded at −70 mV from mPFC pyramidal neurons at baseline for at least 5 minutes and following either photoswitch activation (385 nm) or local application of 10 nM LY379268.
Fluorescence in situ RNA Hybridization and Neuroanatomical Analysis
For all experiments, male mice between 8 – 10 weeks of age were used. For fluorescence in situ RNA hybridization (FISH), staining for RNA of interest was accomplished using RNA Scope Fluorescent Multiplex 2.5 labeling kit (ACD Bio). Probes utilized for staining were tdTomato-C1 for tdTomato and Mm-Grm2-C2 for Grm2. Brains were extracted and frozen on dry ice before 10ௗμm sections were taken using a cryostat. RNAscope procedures were completed to manufacturers’ specifications (ACD Bio). For analysis, images from 3 different animals were used, and a minimum of 2 images were used from each animal for each brain region. Analysis of percent positive cells for each probe on each image was performed using HALO software (Indica labs)
For neuroanatomical analysis of Grm2-Cre x FLEX-tdTomato mice, brains were harvested and immediately bathed in 4% paraformaldehyde for 48 hours. 70 μm coronal and 200 μm sagittal slices were prepared using a Leica VT1200 Vibratome and were mounted on glass slides using VECTASHIELD HardSet Antifade Mounting Medium with DAPI (Vector Laboratories). Slices were imaged using an Olympus Confocal FV3000 and images were processed in ImageJ.
Dual Optical Fiber Cannulas and Y-Maze Behavior
All behavior was performed 6 to 10 weeks after virus injection and 12 to 16 hours after 2xBGAG12 injection. All optogenetic equipment was purchased from Doric Lenses. Dual opto-fluid cannulas with interchangeable injectors (part number: DiOFC_S_0.7_320/430_1.7) were customized to target the mPFC. Parts contained the implant (part name: DOFC-SL; 15 degree angle, p=700 μm, h=8mm, guiding tube outer diameter=430 μm, inner diameter=320 μm), guide tube (part name: Plug-SL; polyimide-coated glass, outer diameter = 280 μm), cannula (part name: Micro-injector SL; tubing outer diameter=166 μm, inner diameter=100 μm), and optic fiber (part name: Optical injector SL, part number: OI_DiOFC-S-ZF_200/250_0.66_FLT_2.0; outer diameter = 200 μm, numerical aperture=0.66). Cannula protrusion length into the brain was 1.7 mm and optic fiber depth was 2.0 mm. Optic fiber inserts were connected via a zirconia sleeve to a Branching Fiberoptic Patchcord (part number: BFP(2)_200/220/900–0.53_2.5m_FCM-2xZF1.25) with a 200 μm inner diameter per hemisphere. Patchcord was connected to a Fiberoptic Rotary Joint (part number: FRJ_1×1_FC-FC), which was then connected to a Mono Fiberoptic Patchord (part number: MFP_480/500/1000–0.63_1m_FC-FC) with a 400 μm inner diameter. Patchcord was connected to a Connectorized 4 LED cluster (part number: LEDC4–385/465/515/635_FC), which was connected to a 4 Channel LED Driver (part number: LEDD_4) and a 12V Fan Power Adaptor. LED unit was manipulated via Doric Neuroscience studio software (DORIC STUDIO V5.3.3.6). 385 nm and 515 nm power output was measured with a Thor Lab PM100D Optic Power Meter and was approximately 14 mW/mm2 at the fiberoptic tip. During light stimulation, 3 2-second pulses of 385 nm or 515 nm light were delivered in triplicate with a 2-second interval, followed by a delay interval of 114 seconds and repeated continuously until the paradigm was complete.
For Y-maze behavior, experimental and control mice all received light stimulation. Behavioral testing occurred during the light cycle, approximately 4–8 hours after lights on. For MK-801 experiments, all mice received an intra-peritoneal (IP) injection of 0.01 mg/kg of MK-801 diluted in saline or saline control 45 minutes before testing began. Dual fiberoptic patchcords were attached to dual fiberoptic implants and mice were returned to their home cage. For all experiments with continuous SNAP-mGluR2 activation, the 385 nm light protocol started immediately after mice were returned to their home cage following patch chord attachment, and continued until behavior was complete. Mice remained in their home cage for 2 minutes, and were then transferred to the Y-maze apparatus for a 5-minute test. For the reversibility experiment, after the first 150 s in the Y maze, light protocol was switched from 385 nm to 515 nm for the remaining 150 – 300 s. The apparatus consisted of three equally sized plastic arms (33.0 × 7.6 × 38.1 cm), separated by a 120° angle.
QUANTIFICATION AND STATISTICAL ANALYSIS
Electrophysiology data analysis was performed using Clampfit (Axon), Prism (GraphPad) and Microsoft excel. All conditions in HEK 293T cell and cultured neuron experiments were tested in at least 2 separate transfections and all slice electrophysiology data comes from at least 3 separate mice per conditions. smFRET data analysis was performed using SPARTAN (Juette et al., 2016). FRET histograms (averaged from 3 separate movies per condition) were then plotted and fitted using a single Gaussian function to get a peak position and FWHM. Calcium imaging analysis was performed using ZEN (Zeiss) and ImageJ (Fiji). Intensities were normalized to baseline prior to photoactivation or drug application. Oscillation frequency was measured as the number of events per unit time. Ca2+ wave velocity was calculated by first determining the time at 50% of the peak for both the photoactivation region of interest (ROI) and a secondary ROI of the same size (5 μm2) on the other side of the cell (typically 15–30 μm away from the photoactivation ROI). The wave velocity was then calculated as the distance between the ROIs divided by the difference in time between 50% of the peaks. For astrocyte calcium imaging analysis, processes were defined as subcellular areas at least 15 μm from the nucleus where the thickness of decreased from that of the soma. ROIs for both analysis and photoactivation were maintained at ~60 μm2. ROIs for photoactivation in processes were placed at least 15–20 μm from the soma. Oscillations were defined as more than one increase in intensity during a single application of UV or drug (at least one minute). For localization analysis of cells in the prelimbic cortex (PL), cell counting was performed in ImageJ on a multicolor image of tdTomato labeled neurons with DAPI labeling for 3 mice. Labeled cell bodies were manually counted in 300 × 900 μM regions in PL. Distance from the pial surface was used to sort cells into 50 μM bins. The number of cells per bin was averaged across two slices from each animal, and these average values were used to calculate averages ± SEM across animals. For Y-maze behaviora, mice were scored manually for both number of arm changes and number of spontaneous alterations. A spontaneous alternation occurred whenever a mouse entered all three arms of the maze in succession without repeating entry into any of the arms. Percentage of spontaneous alternations was calculated as [100 * (number of spontaneous alternations/number of total arm entries)]. For all analysis, statistical information is provided in the figure legends including details on error bars and statistical tests.
Supplementary Material
Highlights:
Branched photoswitchable tethered ligands enable near-complete optical GPCR agonism
Efficient optical control of mGluR2, 3 and 5 across labeling and spectral modalities
mGluR5 activation in astrocytic processes leads to local calcium oscillations.
mGluR2 activation in prefrontal cortex rapidly and reversibly modulates working memory.
Acknowledgements:
The authors thank Andreas Reiner for discussion, Bettina Mathes and Fabio Raith for assistance with chemical synthesis, Zhu Fu and Konstantinos Vlachos for assistance with cloning and Anjali Rajadhyashka for assistance with behavioral experiments. The authors acknowledge Scott Blanchard for providing LD fluorophores and Timothy Ryan for providing the ER-GCaMP6 plasmid. JL is supported by an R35 grant (1 R35 GM124731) from NIGMS and the Rohr Family Research Scholar Award. MS is funded by an F32 grant (F32AA025530) from NIAAA. AGO is supported by an R00 grant (R00 AG048222) from the NIA, an Alzheimer’s Association Research Grant, the Leon Levy Fellowship in Neuroscience, and the Kellen Foundation Junior Faculty Fellowship. CL is supported by grants from NIMH, the One Mind Institute, the Rita Allen Foundation, and the Klingenstein-Simons Foundation Fund. KEP is supported by an R00 from NIAAA (R00 AA23559) and a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation.
Footnotes
Declaration of Interests:
The authors declare no competing interests.
DATA AND CODE AVAILABILITY
This study did not generate or analyze large datasets or code. All the datasets are available from the corresponding authors on request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Aultman JM, and Moghaddam B. (2001). Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 153, 353–364. [DOI] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, and Kramer RH (2004). Light-activated ion channels for remote control of neuronal firing. Nat Neurosci 7, 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MH, Holt A, Levitz J, Broichhagen J, Gaub BM, Visel M, Stanley C, Aghi K, Kim YJ, Cao K, et al. (2017). Restoration of patterned vision with an engineered photoactivatable G protein-coupled receptor. Nat Commun 8, 1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, Lukacs IP, Stacey R, Plaha P, Apostolopoulos V, Livermore L, Sen A, Ansorge O, Gillies MJ, Somogyi P, et al. (2018). Group II Metabotropic Glutamate Receptors Mediate Presynaptic Inhibition of Excitatory Transmission in Pyramidal Neurons of the Human Cerebral Cortex. Front Cell Neurosci 12, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broichhagen J, Damijonaitis A, Levitz J, Sokol KR, Leippe P, Konrad D, Isacoff EY, and Trauner D. (2015). Orthogonal Optical Control of a G Protein-Coupled Receptor with a SNAP-Tethered Photochromic Ligand. ACS Cent Sci 1, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Schools GP, and Kimelberg HK (2000). Metabotropic glutamate receptors in acutely isolated hippocampal astrocytes: developmental changes of mGluR5 mRNA and functional expression. Glia 29, 70–80. [DOI] [PubMed] [Google Scholar]
- Codazzi F, Teruel MN, and Meyer T. (2001). Control of astrocyte Ca(2+) oscillations and waves by oscillating translocation and activation of protein kinase C. Curr Biol 11, 1089–1097. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, and Klingberg T. (2016). The neuroscience of working memory capacity and training. Nat Rev Neurosci 17, 438–449. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, and Smith SJ (1990). Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473. [DOI] [PubMed] [Google Scholar]
- De Filippis B, Lyon L, Taylor A, Lane T, Burnet PW, Harrison PJ, and Bannerman DM (2015). The role of group II metabotropic glutamate receptors in cognition and anxiety: comparative studies in GRM2(−/−), GRM3(−/−) and GRM2/3(−/−) knockout mice. Neuropharmacology 89, 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Juan-Sanz J, Holt GT, Schreiter ER, de Juan F, Kim DS, and Ryan TA (2017). Axonal Endoplasmic Reticulum Ca(2+) Content Controls Release Probability in CNS Nerve Terminals. Neuron 93, 867–881 e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donthamsetti PC, Broichhagen J, Vyklicky V, Stanley C, Fu Z, Visel M, Levitz JL, Javitch JA, Trauner D, and Isacoff EY (2019). Genetically Targeted Optical Control of an Endogenous G Protein-Coupled Receptor. J Am Chem Soc 141, 11522–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, and Pin JP (2011). A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J 25, 66–77. [DOI] [PubMed] [Google Scholar]
- Farrants H, Gutzeit VA, Acosta -Ruiz A, Trauner D, Johnsson K, Levitz J, and Broichhagen J. (2018). SNAP-Tagged Nanobodies Enable Reversible Optical Control of a G Protein-Coupled Receptor via a Remotely Tethered Photoswitchable Ligand. ACS Chem Biol 13, 2682–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F. (2018). Metabotropic glutamate receptors as targets for novel anxiolytics. Curr Opin Pharmacol 38, 37–42. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, and Shigemoto R. (2006). Metabotropic glutamate receptors. Cell Tissue Res 326, 483–504. [DOI] [PubMed] [Google Scholar]
- Gao Y, Hisey E, Bradshaw TWA, Erata E, Brown WE, Courtland JL, Uezu A, Xiang Y, Diao Y, and Soderling SH (2019). Plug-and-Play Protein Modification Using Homology-Independent Universal Genome Engineering. Neuron 103, 583–597 e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier A, Juillerat A, Heinis C, Correa IR Jr., Kindermann M, Beaufils F, and Johnsson K. (2008). An engineered protein tag for multiprotein labeling in living cells. Chem Biol 15, 128–136. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, and Heintz N. (2013). GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Miyawaki H, Helmstetter FJ, and Diba K. (2013). Prefrontal activity links nonoverlapping events in memory. J Neurosci 33, 10910–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1995). Cellular basis of working memory. Neuron 14, 477–485. [DOI] [PubMed] [Google Scholar]
- Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, and Isacoff EY (2007). Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci U S A 104, 10865–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Pichat P, Boulay D, Naimoli V, Potestio L, Featherstone R, Sahni S, Defex H, Desvignes C, Slowinski F, et al. (2016). The mGluR2 positive allosteric modulator, SAR218645, improves memory and attention deficits in translational models of cognitive symptoms associated with schizophrenia. Sci Rep 6, 35320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Lorrain DS, Wei H, Cole RL, Zhang X, Daggett LP, Schaffhauser HJ, Bristow LJ, and Lechner SM (2008). Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Res 1197, 47–62. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Kew JN, Richards JG, Kemp JA, Adam G, Woltering T, Nakanishi S, and Mutel V. (2004). Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology 46, 907–917. [DOI] [PubMed] [Google Scholar]
- Homayoun H, and Moghaddam B. (2007). NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27, 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull K, Morstein J, and Trauner D. (2018). In Vivo Photopharmacology. Chem Rev 118, 10710–10747. [DOI] [PubMed] [Google Scholar]
- Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT, and Paspalas CD (2018). mGluR2 versus mGluR3 Metabotropic Glutamate Receptors in Primate Dorsolateral Prefrontal Cortex: Postsynaptic mGluR3 Strengthen Working Memory Networks. Cereb Cortex 28, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LE, Wang M, Yang ST, Yang Y, Galvin VC, Lightbourne TC, Ottenheimer D, Zhong Q, Stein J, Raja A, et al. (2017). mGluR2/3 mechanisms in primate dorsolateral prefrontal cortex: evidence for both presynaptic and postsynaptic actions. Mol Psychiatry 22, 1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juette MF, Terry DS, Wasserman MR, Altman RB, Zhou Z, Zhao H, and Blanchard SC (2016). Single-molecule imaging of non-equilibrium molecular ensembles on the millisecond timescale. Nat Methods 13, 341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung BG (2004). Basic & clinical pharmacology, 9th edn (New York: Lange Medical Books/McGraw Hill; ). [Google Scholar]
- Kawabata S, Kohara A, Tsutsumi R, Itahana H, Hayashibe S, Yamaguchi T, and Okada M. (1998). Diversity of calcium signaling by metabotropic glutamate receptors. J Biol Chem 273, 17381–17385. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, and Okada M. (1996). Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 383, 89–92. [DOI] [PubMed] [Google Scholar]
- Kim CH, Braud S, Isaac JT, and Roche KW (2005). Protein kinase C phosphorylation of the metabotropic glutamate receptor mGluR5 on Serine 839 regulates Ca2+ oscillations. J Biol Chem 280, 25409–25415. [DOI] [PubMed] [Google Scholar]
- Kim D, Jeong H, Lee J, Ghim JW, Her ES, Lee SH, and Jung MW (2016). Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory. Neuron 92, 902–915. [DOI] [PubMed] [Google Scholar]
- Kiritoshi T, and Neugebauer V. (2015). Group II mGluRs modulate baseline and arthritis pain-related synaptic transmission in the rat medial prefrontal cortex. Neuropharmacology 95, 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy VM, Semetey V, Bracher PJ, Shen N, and Whitesides GM (2007). Dependence of effective molarity on linker length for an intramolecular protein-ligand system. J Am Chem Soc 129, 1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, et al. (2005). Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 179, 303–309. [DOI] [PubMed] [Google Scholar]
- Lagerstrom MC, and Schioth HB (2008). Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 7, 339–357. [DOI] [PubMed] [Google Scholar]
- Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, and Derouiche A. (2011). Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A 108, 12915–12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leippe P, Koehler Leman J, and Trauner D. (2017). Specificity and Speed: Tethered Photopharmacology. Biochemistry 56, 5214–5220. [DOI] [PubMed] [Google Scholar]
- Lester HA, Krouse ME, Nass MM, Wassermann NH, and Erlanger BF (1980). A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at Electrophorus electroplaques. J Gen Physiol 75, 207–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Broichhagen J, Leippe P, Konrad D, Trauner D, and Isacoff EY (2017). Dual optical control and mechanistic insights into photoswitchable group II and III metabotropic glutamate receptors. Proc Natl Acad Sci U S A 114, E3546–E3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, and Isacoff EY (2016). Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron 92, 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, et al. (2013). Optical control of metabotropic glutamate receptors. Nat Neurosci 16, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gu X, Zhu J, Zhang X, Han Z, Yan W, Cheng Q, Hao J, Fan H, Hou R, et al. (2014). Medial prefrontal activity during delay period contributes to learning of a working memory task. Science 346, 458–463. [DOI] [PubMed] [Google Scholar]
- Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M. , et al. (2008) . HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3, 373–382. [DOI] [PubMed] [Google Scholar]
- Meyer T. (1991). Cell signaling by second messenger waves. Cell 64, 675–678. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, et al. (2012). Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11, 141–168. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, and Adams BW (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281, 1349–1352. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, and Javitt D. (2012). From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Okada M, and Nakanishi S. (1997). The metabotropic glutamate receptor mGluR5 induces calcium oscillations in cultured astrocytes via protein kinase C phosphorylation. J Neurochem 69, 1467–1475. [DOI] [PubMed] [Google Scholar]
- Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, and Challiss RA (2002). Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J Biol Chem 277, 35947–35960. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, and Pin JP (2011). Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama J, Mikuni T, and Yasuda R. (2017). Virus-Mediated Genome Editing via Homology-Directed Repair in Mitotic and Postmitotic Cells in Mammalian Brain. Neuron 96, 755–768 e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, and Robitaille R. (2016). Astrocytic mGluR5 and the tripartite synapse. Neuroscience 323, 29–34. [DOI] [PubMed] [Google Scholar]
- Petrelli F, and Bezzi P. (2018). mGlu5-mediated signalling in developing astrocyte and the pathogenesis of autism spectrum disorders. Curr Opin Neurobiol 48, 139–145. [DOI] [PubMed] [Google Scholar]
- Podewin T, Ast J, Broichhagen J, Fine NHF, Nasteska D, Leippe P, Gailer M, Buenaventura T, Kanda N, Jones BJ, et al. (2018). Conditional and Reversible Activation of Class A and B G Protein-Coupled Receptors Using Tethered Pharmacology. ACS Cent Sci 4, 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, and Levitz J. (2018). Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 98, 1080–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost BR, Schneider-Warme F, Schmitz D, and Hegemann P. (2017). Optogenetic Tools for Subcellular Applications in Neuroscience. Neuron 96, 572–603. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schafer D, Barberi C, More L, Nagel J, Pietraszek M, Schmidt WJ, and Danysz W. (2009). Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behav Pharmacol 20, 56–66. [DOI] [PubMed] [Google Scholar]
- Shields BC, Kahuno E, Kim C, Apostolides PF, Brown J, Lindo S, Mensh BD, Dudman JT, Lavis LD, and Tadross MR (2017). Deconstructing behavioral neuropharmacology with cellular specificity. Science 356. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Patel S, and Khakh BS (2016). Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol 26, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler SM, and Bruchas MR (2017). Optogenetic approaches for dissecting neuromodulation and GPCR signaling in neural circuits. Curr Opin Pharmacol 32, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley BJ, and Conn PJ (2018). The therapeutic potential of metabotropic glutamate receptor modulation for schizophrenia. Curr Opin Pharmacol 38, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, and Nedergaard M. (2013). Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhang A, Baker B, Sun L, Howard A, Buswell J, Maurel D, Masharina A, Johnsson K, Noren CJ, et al. (2011). Development of SNAP-tag fluorogenic probes for wash-free fluorescence imaging. Chembiochem 12, 2217–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YH, Essig S, James JR, Lang K, and Chin JW (2015). Selective, rapid and optically switchable regulation of protein function in live mammalian cells. Nat Chem 7, 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y, Jose J, Loudet A, Perez-Bolivar C, Anzenbacher P Jr., and Burgess K. (2011). Encapsulated energy-transfer cassettes with extremely well resolved fluorescent outputs. J Am Chem Soc 133, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafabakhsh R, Levitz J, and Isacoff EY (2015). Conformational dynamics of a class C G-protein-coupled receptor. Nature 524, 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M, Chan KW, Sui JL, Jan LY, Reuveny E, and Logothetis DE (1997). Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. J Biol Chem 272, 31553–31560. [DOI] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, and Trauner D. (2006). Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol 2, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Stevens RC, and Roth BL (2017). How Ligands Illuminate GPCR Molecular Pharmacology. Cell 170, 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Karpenko IA, Hiblot J, and Johnsson K. (2015). Imaging and manipulating proteins in live cells through covalent labeling. Nat Chem Biol 11, 917–923. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, et al. (2011). An expanded palette of genetically encoded Ca(2)(+) indicators. Science 333, 1888–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.