Abstract
Background
Patients with pituitary metastasis (PM) have a relatively poor prognosis. We describe the presentation, management, and outcomes of patients with PM.
Subjects, Materials, and Methods
We performed a retrospective review of patients diagnosed with PM at a single institution from 1996 to 2015. Eighty‐five patients diagnosed with metastasis to the pituitary or sella turcica by pathology or based on a combination of neuroimaging and clinical findings were included. Univariate and multivariable Cox regressions evaluated associations between clinical factors and overall survival.
Results
The most frequent sites of primary malignancies resulting in PM were lung (26%) and breast (26%). Median age at diagnosis was 60 years (range, 18–95). The most common complaints at diagnosis included visual deficits (62%), headache (47%), and cranial nerve palsy (31%). Seventy percent of patients had pituitary insufficiency—adrenal insufficiency (59%), hypothyroidism (59%), or diabetes insipidus (28%). Management of PM included radiation therapy (76%), chemotherapy (68%), surgical resection (21%), or combination therapy (71%). Fifty percent and 52% of patients who received surgical treatment and irradiation, respectively, reported symptomatic improvement. Median overall survival (OS) was 16.5 months (95% confidence interval: 10.7–25.4). On multivariable analysis, a primary cancer site other than lung or breast (p = .020), age <60 years (p = .030), and surgical resection (p = .016) were associated with longer OS.
Conclusion
Patients <60 years of age, those with primary tumor sites other than lung or breast, and those who undergo surgical resection of the pituitary lesion may have prolonged survival. Surgical resection and radiation treatment resulted in symptomatic improvement in ~50% of patients.
Implications for Practice
This study is the largest original series of patients with metastatic disease to the sella. In patients with pituitary metastasis, younger age, primary site other than lung or breast, and metastatic resection may prolong survival. Resection and radiation led to symptomatic improvement in ∼50% of patients. Seventy percent of patients had hypopituitarism. These hormonal deficiencies can be life threatening and can result in substantial morbidity if left untreated. Patients should be treated using a multimodality approach—including a potential role for surgery, radiation, chemotherapy, and hormone replacement—with the goal of improving survival and quality of life.
Keywords: Pituitary, Sella turcica, Metastasis, Prognosis
Short abstract
With advancements in neuroimaging, transsphenoidal resection approaches, and stereotactic radiosurgery, the landscape for the diagnosis and management of pituitary metastasis has evolved over the past decade. This article reports the largest study of pituitary metastasis diagnosed and treated at a single institution, retrospectively assessing the clinical features and the effect of various treatment approaches on survival outcomes.
Introduction
Although a rare complication of malignancy, pituitary metastasis (PM) has been increasingly reported in the literature during the past decade 1, 2, 3, 4, 5, 6, 7, 8, 9. Advancements in neuroimaging modalities, increased sensitivity of endocrine testing, and improved survival of patients with cancer with systemic disease are likely facilitating earlier detection and contributing to a potentially rising incidence of PM. Metastasis from breast and lung primary tumors consist of more than half of all reported cases of PM 3, 10, 11, 12. Other primary carcinomas known to metastasize to the pituitary include renal 13, melanoma 10, 11, 12, gastrointestinal 9, leukemia 14, lymphoma 10, prostate 12, bladder 1, thyroid 2, 15, pancreatic 10, and hepatocellular cancers 4, 16.
The management of patients with PM requires a multidisciplinary team approach and necessitates complex decision‐making regarding the potential roles of surgery and chemoradiation. In addition, sellar metastases are distinct from other central nervous system metastases as they are outside the blood–brain barrier. Several authors have published their own institutional experience with the diagnosis and management of PM 3, 5, 7, 9, 11, 12, 17. Prognosis among patients with PM is generally poor, with a reported median survival time as low as 4 months 7 to 13 months 3, 5, 9, 17. Historically, patients with PM have been treated with palliative intent. However, a recent Japanese review 3 reported a rising percentage of patients undergoing surgical resection of PM as well as a relatively longer median survival time of 12.9 months 3 in comparison with previous studies 5, 7, 9, 17. With advancements in neuroimaging, transsphenoidal resection approaches, and stereotactic radiosurgery, the landscape for the diagnosis and management of PM has likely evolved significantly over the past decade. Therefore, we designed the largest study of PM diagnosed and treated at a single institution to retrospectively assess the clinical features and the impact of various treatment approaches on survival outcomes.
Subjects, Materials, and Methods
We performed a retrospective chart review of patients diagnosed with metastasis to the pituitary gland or sella turcica by histological studies or based on a combination of neuroimaging and clinical findings between 1996 and 2015 at Memorial Sloan Kettering Cancer Center (MSK). For patients who did not undergo biopsy or resection of their pituitary lesions, PM was diagnosed based on magnetic resonance imaging (MRI) or computed tomography (CT) reports using terminology including “consistent with,” “suspicious for,” “probable,” and “probably” to describe the likelihood of PM. Radiologic features indicative of PM included rapid increase in volume of the sellar lesion, which was based on the rate of interval growth of the sellar lesion between serial imaging and often noted in the final radiographic report as a clinical indicator for the likelihood of metastatic disease in conjunction with other characteristics. These characteristics included invasive features (extrasellar extension and cavernous sinus invasion); presence of additional intracranial metastatic lesions; and edema in the surrounding tissue, such as the optic tract. Surgical patients were those who had operative reports indicating that a transsphenoidal resection, hypophysectomy, subtotal resection, or partial excision of the metastatic lesion was performed. Patients who only underwent a biopsy were not included in the surgical group. Additionally, patients with PM diagnosed by radiology or surgery were also analyzed for clinical findings frequently associated with PM, such as visual deficits and hormonal studies indicating pituitary insufficiency, including the following: secondary adrenal insufficiency determined by abnormally low serum adrenocorticotropic and cortisol levels secondary hypothyroidism judged by inappropriately low thyroid stimulating hormone and thyroxine levels; hyperprolactinemia judged by elevated serum prolactin levels; and secondary hypogonadism determined by abnormally low luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) levels with low testosterone (males) or low estrogen levels (females).
This retrospective assessment is considered minimal‐risk research; therefore, this study was approved by the MSK Institutional Review Board (IRB) and consent was waived. All data were obtained as per the IRB protocol and analyzed using Microsoft Excel for Mac 2011 software and Statistical Analysis System 9.4 (SAS Institute Inc., Cary, NC). Overall survival (OS) was defined as the interval from the date of diagnosis of PM to the date of death or of last follow‐up. The Kaplan‐Meier method and Cox regression model were used to evaluate association between clinical factors and OS univariately. Multivariable Cox regressions were further applied to associate univariately significant factors with OS, and the final model included only significant factors. Time to progression was defined from the date of diagnosis of PM to the date of progression. Death was treated as a competing risk. One‐year cumulative incidence of progression was estimated. Fisher's exact test was used to examine whether patients who had undergone surgery had different clinical characteristics than those of other patients. A statistical test was considered significant at p < .05. The denominators used for calculating percentages were the total number of patients with known data for the particular criterion being evaluated.
Results
Patient and Primary Tumor Characteristics
Between 1996 and 2015, 85 patients with metastases to the pituitary or sella turcica were diagnosed and treated at MSK. Fifty patients (59%) were female; 35 (41%) were male. The most common site of primary tumor in women was the breast (22/50, 44%), and the most frequent site in men was the lung (12/35, 34%; supplemental online Fig. 1). Of the 67 patients who had staging information at the time of primary cancer diagnosis, 35 (52%) had metastatic disease and 47 (70%) had stage IIIa disease or greater. For 37 (44%) of the 85 total patients, the pituitary was among the first sites of metastatic spread. The median interval between diagnosis of primary cancer and diagnosis of PM was 3.4 years (range, 1.9 months to 21 years). Fifteen patients (18%) lacked a prior diagnosis of malignancy at the time of presentation with PM. Among these 15 patients, the most common presenting symptom was a visual defect (12/15, 80% of patients) and the most common pathology was non‐small cell lung carcinoma (4/15) and neuroendocrine tumor of the lung (2/15).
The median age at diagnosis of PM was 60.8 (range, 18–95) years. Patients had PM originating from 15 different primary malignancies (Table 1). The most common sites of primary tumor were the lung (26%) and breast (26%) followed by the thyroid (9.4%) and kidney (8.2%). The most frequent lung malignancy was non‐small cell adenocarcinoma (14/22), and the most common histology of breast cancer was invasive ductal carcinoma (17/22). Among eight patients with thyroid carcinoma, there were five cases of papillary thyroid carcinoma, two of follicular thyroid carcinoma, and one of medullary thyroid carcinoma.
Table 1.
Primary malignancies metastatic to the pituitary and sella turcica (N = 85)
| Site of primary tumor | n | % | Histological types (%) |
|---|---|---|---|
| Breast | 22 | 25.9 |
Ductal carcinoma (77.3) Lobular carcinoma (13.6) |
| Lung | 22 | 25.9 |
Non‐small cell (63.6) Neuroendocrine (27.2) Small cell (9.1) |
| Thyroid | 8 | 9.4 |
Papillary thyroid carcinoma (62.5) Follicular thyroid carcinoma (25.0) Medullary thyroid carcinoma (12.5) |
| Kidney | 7 | 8.2 |
Clear cell carcinoma (85.7) Chromophobe renal cell carcinoma (14.3) |
| Sarcoma | 4 | 4.7 |
Myxoid liposarcoma (50.0) Myxoid chondrosarcoma (25.0) Ewing's sarcoma (25.0) |
| Unknown | 4 | 4.7 |
Atypical carcinoid (25.0) Squamous cell carcinoma (25.0) Myeloid sarcoma (25.0) Serous carcinoma of uterine adnexal origin (25.0) |
| Lymphoma | 3 | 3.5 |
Diffuse large B‐cell lymphoma (66.7) Follicular lymphoma (33.3) |
| Skin | 3 | 3.5 | Malignant melanoma (100.0) |
| Colon | 2 | 2.4 |
Invasive mucinous adenocarcinoma (50.0) Infiltrating sigmoid adenocarcinoma (50.0) |
| Pancreas | 2 | 2.4 |
Pancreatic neuroendocrine carcinoma (50.0) Pancreatic islet cell tumor (50.0) |
| Prostate | 2 | 2.4 | Adenocarcinoma (100.0) |
| Uterus/cervix | 2 | 2.4 |
Leiomyosarcoma (50.0) Unknown (50.0) |
| Brain | 1 | 1.2 | Medulloblastoma (100.0) |
| Leukemia | 1 | 1.2 | Acute myeloid leukemia (100.0) |
| Liver | 1 | 1.2 | Hepatocellular carcinoma (100.0) |
| Salivary gland | 1 | 1.2 | Salivary duct carcinoma (100.0) |
Clinical Features and Endocrinopathies
Patients had a variety of presenting symptoms at the time of PM diagnosis; the most common was visual deficits (62%), including complaints of double vision, blurry vision, visual disturbances, tunnel vision, changes in peripheral vision, decreased visual acuity, and visual hallucinations (supplemental online Table 1). Other common chief complaints at the time of PM diagnosis included headache (47%), cranial nerve palsy (31%) presenting as diplopia or ptosis, and polyuria and polydipsia (28%), suggesting diabetes insipidus (DI). Additional symptoms at the time of PM diagnosis included nausea and vomiting, retroorbital pain, epistaxis, infertility, erectile dysfunction, facial numbness, weight loss, confusion, and fatigue.
Overall, 42 (70%) of 60 patients with at least a partial endocrinopathy workup had laboratory studies indicating pituitary insufficiency (supplemental online Table 1). Twenty‐three (28%) of 82 patients evaluated presented with DI. Twenty‐seven (59%) of 46 patients evaluated presented with secondary adrenal insufficiency (AI); 27 (59%) of 46 patients presented with hypothyroidism (excluding 10 patients who had previously underwent thyroidectomy); 25 (66%) of 38 patients presented with hypogonadism; and 5 (16%) of 32 patients presented with hyperprolactinemia. Nineteen (45%) of 42 patients had anterior pituitary dysfunction only without posterior lobe insufficiency. Twenty‐six patients had a detailed pituitary hormone evaluation, including serum adrenocorticotropic hormone and cortisol levels, thyroid function tests, prolactin, LH, FSH, testosterone, and/or estradiol levels. Among these 26 patients, 4 (15%) lacked evidence of endocrinopathy at the time of PM diagnosis and 6 (23%) presented with panhypopituitarism (DI, AI, hypothyroidism, hypogonadism, hyperprolactinemia), which was the most common set of lab results for this subgroup. Three patients (12%) had only DI and 7 (27%) had anterior insufficiency only. Given the retrospective nature of this study, some patients may have had laboratory data from an outside hospital that was unavailable for review or did not have a full hormone panel performed; therefore, these patients were not included in our evaluation for the presence of endocrinopathy at the time of PM diagnosis.
Neuroradiological Findings
Twenty‐nine patients (34%) had a diagnosis of PM confirmed by histological studies, and 56 (66%) were diagnosed with PM based on neuroimaging and correlation with clinical manifestations. For patients lacking a tissue diagnosis, 53 patients and 3 patients were diagnosed based on MRI and CT reports, respectively, favoring a diagnosis of PM in conjunction with clinical findings suggestive of PM, including pituitary dysfunction and visual deficits. The average size of the lesion within the pituitary or sella was 2.3 ± 1.1 cm (n = 47). Regarding neuroimaging findings, 31% of cases demonstrated suprasellar extension and another 31% of cases displayed cavernous sinus extension or invasion. Twenty (24%) imaging reports revealed thickening or enhancement of the infundibulum or pituitary stalk, and 16 cases (19%) demonstrated compression, displacement, or invasion of the optic chiasm or hypothalamus. Five patients (5.9%) had MRI or CT reports that presumed a diagnosis of a pituitary adenoma, and an incidental diagnosis of PM was later made by biopsy or surgical resection.
Therapeutic Management
For therapeutic management after diagnosis of PM, 65 patients (76%) received whole‐brain radiation therapy (WBRT, n = 28) or focal radiation therapy (RT, n = 35) or focal RT followed by WBRT (n = 1), and in one patient, the type of RT was unknown; 18 (21%) underwent surgical resection of the pituitary lesion, and 58 (68%) continued to receive or began systemic chemotherapy (Fig. 1; Table 2). Among 18 patients who underwent surgical resection of PM, 17 underwent transsphenoidal resection and 1 patient had a pterional craniotomy for resection of a parasellar lesion. Five patients (28%) experienced postoperative complications, including pituitary insufficiency (n = 3; hypothyroidism and DI (n = 1), DI only (n = 1), unspecified pituitary hormone insufficiency (n = 1), hypernatremia (n = 1), and partial cranial nerve III paralysis (n = 1). Fourteen patients (78%) who underwent surgical resection also received neoadjuvant or adjuvant RT. Nine patients (50%) experienced symptomatic improvement after surgical resection consisting of improvement in or resolution of headaches (n = 4) and/or visual defects (n = 7).
Figure 1.
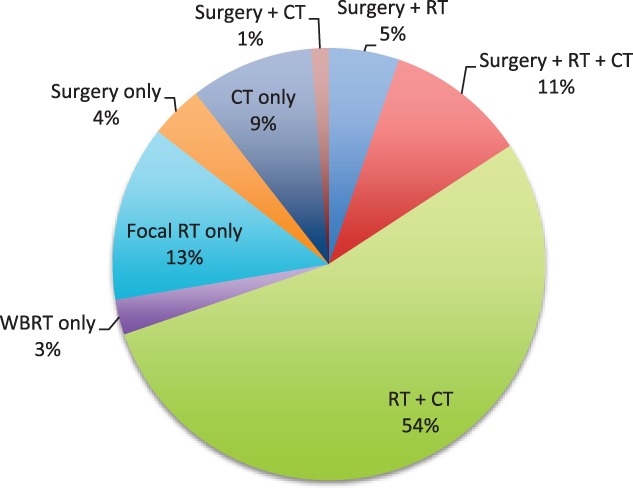
Treatment of pituitary metastasis.
Abbreviations: CT, chemotherapy; RT, radiation therapy; WBRT, whole‐brain radiation therapy.
Table 2.
Post‐treatment symptomatic improvement
| Treatment modality | Total no. | No. with symptomatic improvement (%)a |
|---|---|---|
| Surgery | 18 | 9 (50) |
| WBRTb | 29 | 12 (44)c |
| Focal RTb | 36 | 21 (60)d |
| CT | 58 | 9 (16) |
Documentation of symptomatic improvement following treatment initiation.
One patient received focal RT followed by WBRT, and in one patient, type of RT was unknown.
The percentage was calculated based on available follow‐up data regarding whether or not symptomatic improvement with WBRT was documented (12/27; two of 29 patients had inadequate follow‐up data).
The percentage was calculated based on available follow‐up data regarding whether or not symptomatic improvement with focal RT was documented (21/35; one of 36 patients had inadequate follow‐up data).
Abbreviations: CT, chemotherapy; RT, radiation therapy; WBRT, whole‐brain radiation therapy.
Among 65 patients who received RT, 35 (54%) received focal irradiation alone delivered to the clivus, skull base, pituitary gland, or sella turcica, 28 (43%) received WBRT alone, 18 (64%) of whom had evidence of other brain lesions, and 1 (1.5%) received focal RT followed by WBRT. For one patient (1.5%), information regarding the type of RT received was unavailable. Patients who underwent WBRT received a mean dose of 3,051 cGy (range, 2,000–4,500), with a mean of 10 (range, 3–20) fractions delivered. For patients who underwent focal irradiation, the mean dose was 3,091 cGy (range, 1,500–5,580), with a mean of 10 (range, 1–30) fractions delivered. Among the four patients who were treated with stereotactic radiosurgery, the mean dose was 2,283 cGy (range, 1,950–2,500), with a mean of 6.5 (range, 3–14) fractions delivered.
The most common adverse effects from RT included fatigue (n = 12), headache (n = 5), and dermatitis (n = 4). Thirty‐two patients (52%) subsequently reported symptomatic improvement after RT consisting of improvements in or resolution of headaches, visual deficits, or endocrinopathies (three patients noted decreased or resolution of polyuria and polydipsia following RT). Forty‐six patients (54%) received both RT and chemotherapy after PM diagnosis. Among 58 patients who continued to receive or began a new chemotherapeutic agent after the diagnosis of PM, only 9 (16%) had documented symptomatic improvement or resolution of systemic symptoms or activity/energy level (n = 7), improved vision (n = 1), or resolution of headaches (n = 1) following treatment initiation. Among patients with available follow‐up neuroimaging (n = 67), 15 experienced progression of PM after treatment with all regimens (surgical resection, RT, and/or chemotherapy) whereas 52 patients had stable PM.
Survival Outcomes and Prognostic Factors
Fifty‐four patients (64%) died during the study period. The median follow‐up time for the 31 survivors (36%) was 6.5 months (range, 0–154). All patients with a known cause of death died from cancer‐related complications. Two patients died less than 10 days after the diagnosis of PM. The median follow‐up time from date of primary cancer diagnosis was 7.3 years (95% confidence interval [CI]: 5.9–13.8). Median patient OS from date of PM diagnosis until date of death or of last follow‐up was 16.5 months (95% CI: 10.7–25.4).
There was no significant difference in OS based on univariate analysis of gender, presence of other brain metastases, presence of endocrinopathy, and treatment with RT (Table 3). Systemic chemotherapy had a statistically significant impact on OS univariately (Fig. 2), but the association was not significant in multivariable analysis.
Table 3.
Univariate overall survival analysis (N = 85)
| Clinical characteristic | n (%)a | HR (95% CI) | p value |
|---|---|---|---|
| Gender | .640 | ||
| Female | 50 (58.8) | 1 | |
| Male | 35 (41.2) | 1.14 (0.66–1.98) | |
| Age at PM diagnosis, years | .003 | ||
| ≤60 | 41 (48.2) | 1 | |
| >60 | 44 (51.8) | 2.49 (1.38–4.5) | |
| Site of primary tumor | .010 | ||
| Lung | 22 (25.9) | 1 | |
| Breast | 22 (25.9) | 0.49 (0.23–1.03) | |
| Thyroid | 8 (9.4) | 0.24 (0.09–0.68) | |
| Others | 33 (38.8) | 0.33 (0.16–0.68) | |
| Presence of other brain metastases | .118 | ||
| Yes | 29 (34.1) | 1 | |
| No | 56 (65.9) | 0.63 (0.36–1.11) | |
| Endocrinopathy | .263 | ||
| No | 43 (50.6) | 1 | |
| Yes | 42 (49.4) | 1.37 (0.79–2.38) | |
| Chemotherapy | .015 | ||
| No | 27 (31.8) | 1 | |
| Yes | 58 (68.2) | 0.45 (0.24–0.83) | |
| Surgery | .022 | ||
| No | 67 (78.8) | 1 | |
| Yes | 18 (21.2) | 0.35 (0.14–0.86) | |
| RT | .581 | ||
| No | 20 (23.5) | 1 | |
| Yes | 65 (76.5) | 1.24 (0.57–2.69) | |
| WBRT | .103 | ||
| No | 56 (65.9) | 1 | |
| Yes | 29 (34.1) | 1.59 (0.91–2.78) | |
| Local radiation | .165 | ||
| No | 49 (57.6) | 1 | |
| Yes | 36 (42.4) | 0.66 (0.37–1.18) | |
| Months between primary cancer diagnosis and PM, median (range), n | 29.9 (0–254.8), 81 | 1.00 (0.99–1.01) | .882 |
| Pituitary lesion size, cm, median (range), n | 2.3 (0.4–5.9), 46 | 1.01 (0.71–1.43) | .962 |
Data presented as n (%) except as otherwise indicated in the last two rows.
Abbreviations: CI, confidence interval; HR, hazard ratio; PM, pituitary metastasis; RT, radiation therapy; WBRT, whole‐brain radiation therapy.
Figure 2.
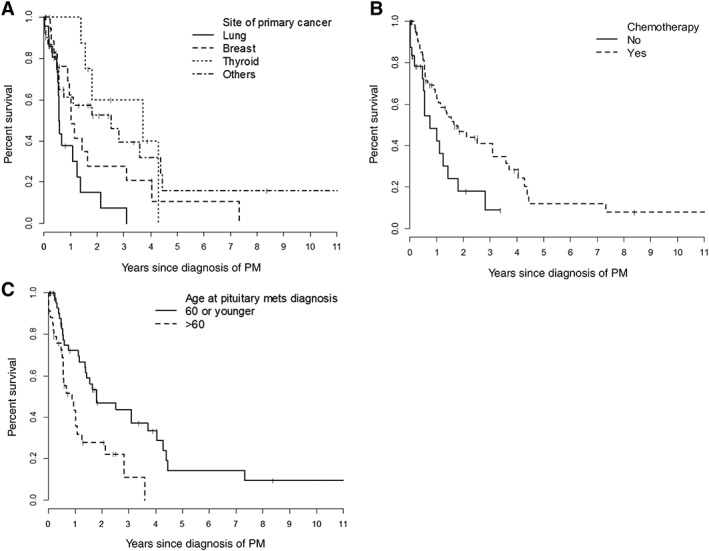
Overall survival analysis. Overall survival by site of primary cancer (A), chemotherapy (B), and age at PM (C).
Abbreviation: PM, pituitary metastasis.
Multivariable analysis of potential prognostic factors revealed that patients with a primary cancer site other than the lung or the breast had improved OS after PM diagnosis (lung, hazard ratio [HR] = 1.0, p = .020; breast, HR = 0.73, 95% CI: 0.34–1.57; thyroid, HR = 0.28, 95% CI: 0.09–0.83; other sites, HR = 0.38, 95% CI: 0.18–0.77; Table 4; Fig. 2). Patients older than 60 years of age at the time of PM diagnosis also had decreased OS (HR = 2.0, 95% CI: 1.07–3.73, p = .030).
Table 4.
Multivariable overall survival analysis
| Clinical characteristic | HR in OS (95% CI) | p value |
|---|---|---|
| Primary cancer site | .020 | |
| Lung | 1.0 | |
| Breast | 0.73 (0.34–1.57) | |
| Thyroid | 0.28 (0.09–0.83) | |
| Others | 0.38 (0.18–0.77) | |
| Age at PM, years | .030 | |
| ≤60 | 1.0 | |
| >60 | 2.0 (1.07–3.73) | |
| Surgical resection | .016 | |
| Yes | 0.31 (0.12–0.81) | |
| No | 1.0 |
Abbreviations: HR, hazard ratio; OS, overall survival; PM, pituitary metastasis.
Surgical resection of the pituitary lesion was associated with improved OS (HR = 0.31, 95% CI: 0.12–0.81, p = .016). Median survival after date of surgical resection for patients who underwent surgery was 48.6 months (95% CI: 6.2–84.7) in comparison with a median survival of 13.3 months after date of PM diagnosis for patients who did not undergo surgery (95% CI: 7.0–18.6). Clinical characteristics of the surgical and nonsurgical subgroups were compared in order to elucidate potential confounding factors impacting OS. Although no statistically significant difference was found, the nonsurgical subgroup had a higher rate of lung cancer as the primary pathology (30% vs. 17%, respectively, p = .313; Table 5) and the surgical subgroup had a younger median age at PM diagnosis (56 vs. 61 years, respectively; Table 5). Patients who underwent surgery also had a lower rate of major comorbidities (heart disease, chronic obstructive pulmonary disease [COPD], hypertension [HTN], diabetes mellitus [DM], chronic kidney disease [CKD], or prior cancers; 39% vs. 48%, respectively, p = .596) and a lower rate of multiple brain metastases (28% vs. 36% for the nonsurgical subgroup, p = .587). The surgical and nonsurgical subgroups had relatively equal rates of cavernous sinus invasion demonstrated on imaging (33% vs. 34%, respectively). With regard to initial clinical presentation, the surgical subgroup had a higher rate of visual defects (78% vs. 58% among the nonsurgical subgroup, p = .173).
Table 5.
Comparison of surgical and nonsurgical subgroups
| Characteristic | All patients (N = 85) | Surgery subgroup (N = 18) | Nonsurgical subgroup (n = 67) |
|---|---|---|---|
| Primary site |
Breast (26%) Lung (26%) |
Breast (28%) Lung (17%) Kidney (11%) |
Lung (30%) Breast (27%) Thyroid (11%) |
| Cavernous sinus invasion | 29 (34%) | 6 (33%) | 23 (34%) |
| Cranial nerve palsy | 26 (31%) | 5 (28%) | 21 (31%) |
| Vision defects | 53 (62%) | 14 (78%) | 39 (58%) |
| No. of patients with major comorbidities (heart disease/COPD/HTN/DM/CKD/ or prior cancers) | 39 (46%) | 7 (39%) | 32 (48%) |
| Median age at primary cancer diagnosis (range), years | 56 (13–95) | 47 (18–77) | 56 (13–95) |
| Median age at PM diagnosis (range), years | 60 (18–95) | 56 (18–80) | 61 (23–95) |
| Presence of multiple brain metastases | 29 (34%) | 5 (28%) | 24 (36%) |
| Average no. of metastatic sites (range)a | 3 (1–5) | 2 (1–4) | 3 (1–5) |
| Treatment with RT | 76% (n = 65) | 78% (n = 14) | 76% (n = 51) |
| Treatment with CT | 68% (n = 58) | 56% (n = 10) | 72% (n = 48) |
| Median survival after PM diagnosis (95% CI) | 16.5 months (10.7–25.4) |
48.6 months (6.2–84.7) |
13.3 months (7.0–18.6) |
A metastatic site was defined as a distant organ demonstrating at least one metastatic lesion on imaging; leptomeningeal disease was considered a separate site from parenchymal brain involvement; all bony sites were considered as one site; lymph nodes were not considered a metastatic site.
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CT, chemotherapy; DM, diabetes mellitus; HTN, hypertension; PM, pituitary metastasis; RT, radiation therapy.
Discussion
Several mechanisms for metastatic spread to the pituitary have been postulated 7, 9, 10, 11, 18: (a) direct blood‐borne metastasis to the posterior lobe; (b) blood‐borne metastasis via the hypothalamus‐hypophyseal portal system; (c) metastatic extension from the skull‐base or juxtasellar region (clivus, dorsum sellae, or cavernous sinus); and (d) leptomeningeal spread to the pituitary gland. The tendency for metastasis to the posterior lobe can be ascribed to the lack of direct blood supply to the anterior lobe, which is fed by portal vessels, and the larger area of contact between the posterior lobe and the dura 10, 12, 19, 20, 21. PM is a clinically challenging complication of malignancy, with a poor prognosis and the potential to dramatically impact patient quality of life secondary to visual deficits, endocrinopathies, and headaches. We conducted a retrospective assessment of 85 patients diagnosed and treated for PM at a single institution and were able to elucidate clinical features, treatment approaches, and prognostic factors for this rare and devastating disease entity.
The most frequent primary tumors resulting in PM have previously been reported to be breast 10, 12 and lung cancers 3, 11. Our institutional series confirms these findings, also showing that lung (26%) and breast (26%) primary tumors are the most frequent malignancies to result in PM. The predilection for breast cancer cells to metastasize to the pituitary may be attributable to the prolactin‐rich environment of the gland 9, 12. The main reasons for breast and lung cancers to commonly result in PM are perhaps the overall high incidence of these tumors in the general population as well as their systemic invasiveness 9. Our finding that thyroid (9%) and renal (8%) carcinomas are the next most common tumors to result in PM also confirms previous results 3, 9. Notably, 15 patients (18%) in our study had PM diagnosed as the first sign of occult malignancy, and for 37 patients (44%), the pituitary was among the first sites of metastatic spread. Morita et al. reported a higher rate of 56% (20 patients) for PM as the first manifestation of occult malignancy, and a Japanese study by Habu et al. reported a 10.8% rate of PM detected prior to the primary tumor 3. Other case reports 8, 14, 22 have also noted that pituitary dysfunction may be the first presentation of an occult malignancy as well as the only site of metastasis.
Differentiation of PM from benign adenomas, which represent 76% of pituitary tumors 10, is often difficult, as most patients do not undergo surgical resection or biopsy and radiological findings can be unreliable 19, 23, 24, 25, 26. Nevertheless, certain clinical findings based on the predilection for metastasis to the posterior lobe and rapid lesion growth have been postulated to be highly suggestive of the presence of PM, including central DI, abnormal eye motility, or a visual deficit 3, 7. A literature review of 190 symptomatic PM cases found 45% of patients presented with DI, 28% with cranial nerve palsy, and 23% with anterior pituitary insufficiency 10. All patients who underwent a biopsy and/or surgical resection of their pituitary lesion with final pathology reports that confirmed metastatic disease were included in our study. For those who did not undergo a biopsy and/or surgical resection of their pituitary lesion, clinical data from these patients were carefully analyzed to ensure that the diagnosis was most consistent with PM rather than a benign lesion. The clinical presentations of patients in our series were similar, although with a lower rate of DI; 62% of patients presented with visual deficits, 31% with cranial nerve palsy, and 28% with symptoms suggestive of DI. Notably, these symptoms are rarely seen in the context of initial manifestation of pituitary adenomas 3; DI has been reported to occur in only 1% of pituitary adenomas 11, 19.
Diagnosis of PM is most often based on a combination of clinical features and neuroimaging reports. In a Japanese review by Habu et al., the most common radiological features associated with PM included a mass lesion in the pituitary stalk, constriction at the diaphragm sellae, a hypothalamic mass lesion, and hyperintensity in the optic tract 3. Erosion of surrounding bony structures and sellar enlargement are also common findings 10. CT often demonstrates a hyperdense or isodense enhancing mass, at times heterogeneously enhancing in contrast images if necrosis, hemorrhage, or cystic degeneration is present 10. Cases of PM on MRI have been reported to demonstrate an isointense or hypointense mass on T1W1, homogeneously enhancing after gadolinium 10, 11, 18, 19, 26, 27. A dumbbell‐shaped intra‐ and suprasellar tumor, with a clear indentation at the level of the diaphragm sellae, best seen on sagittal images, has also been postulated to be highly suggestive of PM 10, 21, 27. In our study, only three cases presented as a dumbbell‐shaped mass, whereas 31% of cases demonstrated suprasellar extension, 31% invaded or extended into the cavernous sinuses, and 24% demonstrated infundibulum enhancement or thickening. Our institutional series demonstrates that for patients presenting with a new, sudden‐onset visual deficit, eye motility defect, or polyuria and polydipsia and neuroimaging findings of a sellar lesion with suprasellar or cavernous sinus extension or involvement of the infundibulum, even in the absence of a history of malignancy, clinicians should have a high suspicion for PM.
Prior studies regarding therapeutic management of patients with PM have published varying outcomes. Surgical resection of PM is often difficult and total resection improbable as lesions are often diffuse, invasive, and hemorrhagic 10, 17, 21, 24, 25. However, studies by Morita et al. and Zoli et al. reported that surgical resection results in improvement in pain and ophthalmological symptoms, although endocrinologic dysfunction did not improve. The authors concluded that aggressive treatment with surgical decompression and irradiation may improve the quality of life for symptomatic patients despite the lack of a clear survival benefit 5, 9, 10, 11, 28. Other case series report that stereotactic radiosurgery is a minimally invasive outpatient management strategy for treatment 5, 7, 9, 11, 29 of PM resulting in effective local control 5.
In our series, 76% of patients received WBRT (because of the presence of multiple intracranial metastases [n = 18] or leptomeningeal disease [n = 1]) or focal irradiation, whereas 21% of patients underwent surgical resection. Notably, our study is the largest series to date to report an association between surgical resection of PM and prolonged OS. Patients who underwent surgery in our cohort (n = 18) had a median OS after resection of 48.6 months (95% CI: 6.2–84.7) compared with an OS of 13.3 months after date of PM diagnosis for patients who did not undergo surgery (95% CI: 7.0–18.6). Because the treatment approach selected for each patient depends on the severity of the patient's condition and functional status, this correlation between surgical resection and improved OS is subject to susceptibility bias (confounding by indication). Potential confounding factors for this association include the higher rate of lung cancer as the primary pathology in the nonsurgical subgroup, their relatively older age at PM diagnosis, their higher rate of major comorbidities (heart disease, COPD, HTN, DM, CKD, or prior cancers), and the higher rate of diffuse brain metastases in patients treated with a nonsurgical approach (Table 5). Given that prolonged survival was observed in patients who underwent surgical resection of their pituitary lesion and had final pathology reports that confirmed PM, it is unlikely that these patients had benign lesions rather than true PM.
At our institution, the most common reasons for surgical resection of PM included large tumor size or rapid interval growth, profound symptom burden affecting quality of life (significant displacement of the optic chiasm resulting in visual field deficits), failure to respond to RT, or the pituitary lesion was presumed to be an adenoma and surgical resection subsequently revealed a pathology of metastatic cancer. The most common reasons patients were not treated with surgical resection included significant medical comorbidities, expected poor overall survival from primary disease, the presence of diffuse brain metastases and/or leptomeningeal disease, proximity to vital structures, in which case physician preference was for image‐guided RT over surgery, and breast cancer metastatic to the brain was viewed as more RT sensitive by the treatment team.
Among surgical patients, complications (pituitary insufficiency [n = 3], hypernatremia, and partial cranial nerve III paralysis) were minimal, and 50% and 52% of patients who received surgical treatment and irradiation, respectively, reported symptomatic improvement of headaches, visual deficits, or endocrinopathy symptoms. Prior studies have noted that surgery and whole‐brain or focal irradiation are well tolerated in noncompromised patients and associated with low overall morbidity but have failed to detect a clear association between OS and surgical resection, perhaps as a result of low statistical power 7, 10, 11, 19.
Notably, the median OS of patients in our series was 16.5 months (range, 3 days to 12.8 years), which is relatively prolonged in comparison with previously reported median OS for patients with PM. Prior studies have reported a median OS of 12.9 months or less 3, 5, 7, 9, 17. Ntyonga‐Pono et al. reviewed 72 patients with PM from the literature and found that only 10% of patients survived longer than 1 year after diagnosis, the longest survival being 3 years 30. The relatively improved survival of our cohort in comparison with prior studies may in fact be due to improvements in the management of advanced cancers, as most patients die of cancer‐related causes rather than as a direct result of their pituitary lesion. Habu et al. found that younger age, late metastasis to the pituitary, smaller PM size, and irradiation were associated with a better overall prognosis, and patients with breast and renal carcinomas had improved survival in comparison with patients with lung cancer 3. Morita et al. demonstrated an improvement in survival along with amelioration of disabling symptoms and local tumor control using a multimodality approach including surgery, radiation, and chemotherapy 11.
Our study demonstrates that surgical resection of PM may provide a survival benefit. The relatively prolonged survival of our cohort in comparison with those reported in prior studies and the symptomatic improvement of approximately half of patients who underwent surgical resection or irradiation supports the direct treatment of PM lesions, especially for the improvement and maintenance of patient quality of life. Our study also highlights the critical importance of assessing for and treating hormonal dysfunction with steroid replacement, levothyroxine, and desmopressin in patients with PM. Based on our retrospective review, many patients did not undergo a comprehensive pituitary hormone evaluation during their workup or after the diagnosis of a pituitary metastasis. However, based on our findings, a significant portion of patients who were evaluated had pituitary hormonal insufficiency. Overall, 42 patients (70%) had symptoms or laboratory studies indicating pituitary insufficiency, including hypothyroidism (59%), adrenal insufficiency (59%), and DI (28%). These hormonal deficiencies can be life threatening and can result in substantial morbidity if left untreated. Symptoms and signs of pituitary insufficiency are typically reversed with hormonal replacement and can result in significant improvement in patients’ quality of life.
This study is the largest series of PM diagnosed and treated at a single institution. However, the retrospective nature of the report and rarity of the disease entity lends itself to several limitations. Most patients in the nonsurgical group did not undergo a biopsy to confirm PM and were instead diagnosed based on neuroradiology and clinical features. For this subgroup of patients, it is possible that some cases of benign pituitary lesions were inadvertently included in the study. Our evaluation of symptomatic improvement after treatment for PM consisted of a review of follow‐up visit notes, and it is possible that such improvements were underreported during charting. In the future, a large, prospective study is necessary to further elucidate the ideal diagnostic method and management approach for patients with PM.
Conclusion
The most frequent primary malignancies resulting in PM are lung non‐small cell adenocarcinoma and invasive ductal carcinoma of the breast. The most common patient chief complaint at the time of PM diagnosis was a visual deficit. Despite the relatively poor prognosis associated with PM, patients diagnosed with PM at less than 60 years of age, those with a primary cancer site other than the lung or breast, and those treated with surgical resection of the pituitary lesion appear to have a longer OS. Seventy percent of patients had symptoms or laboratory studies indicating pituitary insufficiency, which can lead to significant morbidity if left untreated. Additionally, half of patients who undergo surgical resection or receive RT report symptomatic improvement. Patients with metastatic disease to the pituitary or sella turcica should therefore be treated using a multimodality approach—including a potential role for surgical resection, irradiation, chemotherapy, and hormonal replacement—with the goal of improving OS and optimizing patient quality of life.
Author Contributions
Conception/design: Krupa R. Patel, Monica Girotra
Provision of study material or patients: Krupa R. Patel, Monica Girotra
Collection and/or assembly of data: Krupa R. Patel, Monica Girotra
Data analysis and interpretation: Krupa R. Patel, Junting Zheng, Viviane Tabar, Marc A. Cohen, Monica Girotra
Manuscript writing: Krupa R. Patel, Junting Zheng, Viviane Tabar, Marc A. Cohen, Monica Girotra
Final approval of manuscript: Krupa R. Patel, Junting Zheng, Viviane Tabar, Marc A. Cohen, Monica Girotra
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1
Supplemental Table S1
Acknowledgments
This study was supported in part by the National Institutes of Health/National Cancer Institute Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Al‐Aridi R, El Sibai K, Fu P et al. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica: An analytical review. Pituitary 2014;17:575–587. [DOI] [PubMed] [Google Scholar]
- 2. Barbaro D, Desogus N, Boni G. Pituitary metastasis of thyroid cancer. Endocrine 2013;43:485–493. [DOI] [PubMed] [Google Scholar]
- 3. Habu M, Tokimura H, Hirano H et al. Pituitary metastases: Current practice in Japan. J Neurosurg 2015;123:998–1007. [DOI] [PubMed] [Google Scholar]
- 4. He W, Chen F, Dalm B et al. Metastatic involvement of the pituitary gland: A systematic review with pooled individual patient data analysis. Pituitary 2015;18:159–168. [DOI] [PubMed] [Google Scholar]
- 5. Kano H, Niranjan A, Kondziolka D et al. Stereotactic radiosurgery for pituitary metastases. Surg Neurol 2009;72:248–255; discussion 255–246. [DOI] [PubMed] [Google Scholar]
- 6. Kim YH, Lee BJ, Lee KJ et al. A case of pituitary metastasis from breast cancer that presented as left visual disturbance. J Korean Neurosurg Soc 2012;51:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin CY, Huang WK, Chung FT et al. Prognostic factors in cancer patients with symptomatic pituitary metastasis: A clinical case study. Anticancer Res 2015;35:983–987. [PubMed] [Google Scholar]
- 8. Mao JF, Zhang JL, Nie M et al. Diabetes insipidus as the first symptom caused by lung cancer metastasis to the pituitary glands: Clinical presentations, diagnosis, and management. J Postgrad Med 2011;57:302–306. [DOI] [PubMed] [Google Scholar]
- 9. Zoli M, Mazzatenta D, Faustini‐Fustini M et al. Pituitary metastases: Role of surgery. World Neurosurg 2013;79:327–330. [DOI] [PubMed] [Google Scholar]
- 10. Komninos J, Vlassopoulou V, Protopapa D et al. Tumors metastatic to the pituitary gland: Case report and literature review. J Clin Endocrinol Metab 2004;89:574–580. [DOI] [PubMed] [Google Scholar]
- 11. Morita A, Meyer FB, Laws ER Jr. Symptomatic pituitary metastases. J Neurosurg 1998;89:69–73. [DOI] [PubMed] [Google Scholar]
- 12. Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the putuitary gland. Cancer 1975;36:216–220. [DOI] [PubMed] [Google Scholar]
- 13. Gopan T, Toms SA, Prayson RA et al. Symptomatic pituitary metastases from renal cell carcinoma. Pituitary 2007;10:251–259. [DOI] [PubMed] [Google Scholar]
- 14. Kimmel DW, O'Neill BP. Systemic cancer presenting as diabetes insipidus. Clinical and radiographic features of 11 patients with a review of metastatic‐induced diabetes insipidus. Cancer 1983;52:2355–2358. [DOI] [PubMed] [Google Scholar]
- 15. Williams MD, Asa SL, Fuller GN. Medullary thyroid carcinoma metastatic to the pituitary gland: An unusual site of metastasis. Ann Diagn Pathol 2008;12:199–203. [DOI] [PubMed] [Google Scholar]
- 16. Moreno‐Perez O, Peiro FM, Lopez P et al. An isolated pituitary metastasis as presentation of a differentiated hepatocellular carcinoma mimicking a nonfunctioning macroadenoma. J Endocrinol Invest 2007;30:428–433. [DOI] [PubMed] [Google Scholar]
- 17. Houck WA, Olson KB, Horton J. Clinical features of tumor metastasis to the pituitary. Cancer 1970;26:656–659. [DOI] [PubMed] [Google Scholar]
- 18. Max MB, Deck MD, Rottenberg DA. Pituitary metastasis: Incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 1981;31:998–1002. [DOI] [PubMed] [Google Scholar]
- 19. Branch CL Jr, Laws ER Jr. Metastatic tumors of the sella turcica masquerading as primary pituitary tumors. J Clin Endocrinol Metab 1987;65:469–474. [DOI] [PubMed] [Google Scholar]
- 20. McCormick PC, Post KD, Kandji AD et al. Metastatic carcinoma to the pituitary gland. Br J Neurosurg 1989;3:71–79. [DOI] [PubMed] [Google Scholar]
- 21. Sioutos P, Yen V, Arbit E. Pituitary gland metastases. Ann Surg Oncol 1996;3:94–99. [DOI] [PubMed] [Google Scholar]
- 22. Granata A, Figura M, Gulisano S et al. Central diabetes insipidus as a first manifestation of lung adenocarcinoma [in Italian]. Clin Ter 2007;158:519–522. [PubMed] [Google Scholar]
- 23. Chiang MF, Brock M, Patt S. Pituitary metastases. Neurochirurgia (Stuttg) 1990;33:127–131. [DOI] [PubMed] [Google Scholar]
- 24. Nelson PB, Robinson AG, Martinez AJ. Metastatic tumor of the pituitary gland. Neurosurgery 1987;21:941–944. [DOI] [PubMed] [Google Scholar]
- 25. Ruelle A, Palladino M, Andrioli GC. Pituitary metastases as presenting lesions of malignancy. J Neurosurg Sci 1992;36:51–54. [PubMed] [Google Scholar]
- 26. van Seters AP, Bots GT, van Dulken H et al. Metastasis of an occult gastric carcinoma suggesting growth of a prolactinoma during bromocriptine therapy: A case report with a review of the literature. Neurosurgery 1985;16:813–817. [DOI] [PubMed] [Google Scholar]
- 27. Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am 1999;28:81–117, vi. [DOI] [PubMed] [Google Scholar]
- 28. Piedra MP, Brown PD, Carpenter PC et al. Resolution of diabetes insipidus following gamma knife surgery for a solitary metastasis to the pituitary stalk. Case report. J Neurosurg 2004;101:1053–1056. [DOI] [PubMed] [Google Scholar]
- 29. Iwai Y, Yamanaka K, Honda Y et al. Radiosurgery for pituitary metastases. Neurol Med Chir (Tokyo) 2004;44:112–116; discussion 117. [DOI] [PubMed] [Google Scholar]
- 30. Ntyonga‐Pono MP, Thomopoulos P, Luton JP. Pituitary metastases. 3 cases [in French]. Presse Med 1999;28:1567–1571. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1
Supplemental Table S1


