Abstract
Background
Data on the incidence, etiology, and prognosis of non–ventilator‐associated pneumonia in hospitalized patients with solid tumors are scarce. We aimed to study the characteristics of non–ventilator‐associated pneumonia in hospitalized patients with solid tumors.
Materials and Methods
This was a prospective noninterventional cohort study of pneumonia in patients hospitalized in an oncology ward in a tertiary teaching hospital. Pneumonia was defined according to the American Thoracic Society criteria. Patients were followed for 1 month after diagnosis or until discharge. Survivors were compared with nonsurvivors.
Results
A total of 132 episodes of pneumonia were diagnosed over 1 year (9.8% of admissions to the oncology ward). They were health care–related (67.4%) or hospital‐acquired pneumonia (31.8%). Lung cancer was the most common malignancy. An etiology was established in 48/132 episodes (36.4%). Knowing the etiology led to changes in antimicrobial therapy in 58.3%. Subsequent intensive care unit admission was required in 10.6% and was linked to inappropriate empirical therapy. Ten‐day mortality was 24.2% and was significantly associated with hypoxia (odds ratio [OR], 2.1). Thirty‐day mortality was 46.2%. The independent risk factors for 30‐day mortality were hypoxia (OR, 3.3), hospital acquisition (OR, 3.1), and a performance status >1 (OR, 2.6). Only 40% of patients who died within 30 days were terminally ill.
Conclusion
Pneumonia is a highly prevalent condition in hospitalized patients with solid tumors, even with nonterminal disease. Etiology is diverse, and poor outcome is linked to inappropriate empirical therapy. Efforts to get the empirical therapy right and reach an etiological diagnosis to subsequently de‐escalate are warranted.
Implications for Practice
The present study shows that pneumonia is a prevalent infectious complication in patients admitted to oncology wards, with a very high mortality, even in non–terminally ill patients. Etiology is diverse, and etiological diagnosis is reached in fewer than 40% of cases in nonintubated patients. Intensive care unit admission, a marker of poor outcome, is associated with inappropriate empirical therapy. These results suggest that, to improve prognosis, a more precise and appropriate antimicrobial empirical therapy for pneumonia in patients with solid tumors is necessary, together with an effort to reach an etiological diagnosis to facilitate subsequent de‐escalation.
Keywords: Pneumonia, Solid tumor, Oncology, Etiology, Prognosis
Short abstract
Pneumonia is a complication in patients admitted to oncology wards, but data on incidence, etiology, and prognosis is lacking. This articles reports on characteristics of nonventilator‐associated pneumonia in hospitalized patients with solid tumors.
Introduction
In nonventilated patients, the etiology and prognosis of hospital‐acquired pneumonia or health care–associated pneumonia are infrequently addressed in the medical literature.
Patients with solid tumors are at risk for unusual etiologies as a result of immunosuppression, and available research in this group focuses specifically on neutropenic patients. Information regarding the etiology and outcome of patients hospitalized with pneumonia in oncology wards is very limited 1, and risk factors for poor outcome have not been specifically addressed. To improve pneumonia prognosis in this setting, there is a need for information to best adjust empirical antibiotics and make an educated guess of which patients will benefit most from aggressive management
We investigated the frequency, etiology, and prognosis of pneumonia in a noninterventional prospective cohort of consecutive patients admitted to the oncology ward of a teaching hospital over 1 year.
Materials and Methods
Setting
Our institution is a 1,550‐bed tertiary teaching hospital in Madrid, Spain. During the study period, its catchment population was 715,000 inhabitants. The Oncology department encompasses a day hospital, multiple specialized external clinics, and a 38‐bed ward for hospitalized patients with solid tumors. There is also a Radiation Oncology department and a Palliative Care department at the institution. Our patients participate in a large number of clinical trials, and ours is a referral center for sarcoma and germinal tumors.
Patients
Consecutive episodes of pneumonia in patients with solid cancer admitted to the oncology ward were prospectively included in a registry between May 2015 and April 2016. A standardized case report form (including epidemiological, clinical, and microbiological data) was completed for each episode. The prescribed daily dose of antibiotic was retrieved from the Pharmacy department. Patients were managed according to usual practice in the Oncology department and followed for 1 month after diagnosis or until discharge. Patients that were discharged before the 30‐day threshold were further followed up to register outcome at day 30.
Clinical Criteria and Definitions
Cancer was stratified by stage at diagnosis of pneumonia. Stage was considered advanced when the tumor was locally advanced or metastatic. Cancer was considered terminal when incurable and reasonably expected to result in the death of the patient within a short period (arbitrarily, an estimated life expectancy of 6 months or less, under the assumption that the disease would run its normal course) 2.
Pneumonia was defined according to the Infectious Diseases Society of America/American Thoracic Society 3. Episodes requiring admission and episodes that presented once the patient was admitted for a different reason were both included. Episodes of pneumonia that developed while on mechanical ventilation (ventilator‐associated pneumonia) were excluded; however, episodes of pneumonia in nonventilated patients were included, regardless of whether they required subsequent mechanical ventilation. An episode was considered a recurrence when it occurred after complete resolution of clinical and microbiological signs of the previous one.
Conventional criteria 4 were used to determine the place of acquisition: community‐acquired pneumonia was defined as that diagnosed within the first 48 hours of admission. After this period, the infection was considered hospital‐acquired pneumonia or nosocomial. Health care–associated pneumonia was diagnosed within 48 hours of admission of an outpatient with any of the following criteria 5: intravenous therapy, wound care, or specialized nursing care at home within the 30 days before the onset of pneumonia; attendance at a hospital or hemodialysis clinic or receipt of intravenous chemotherapy within the 30 days before the onset of pneumonia; hospitalization in an acute care hospital for ≥2 days during the 90 days before the onset of pneumonia; or residence in a nursing home or long‐term care facility.
We used the age‐adjusted Charlson comorbidity index to categorize comorbidities 6. Performance status was assessed according to Eastern Cooperative Oncology Group performance status scale 7.
Chemotherapy was considered recent when administered during the 30 days before diagnosis of pneumonia. Neutropenia was analyzed according to two cutoffs: absolute neutrophil count below 1,000 cells/mm3 and below 500 cells/mm3. We considered that the patient was receiving corticosteroid therapy when the dose was equivalent to more than 20 mg/day of prednisone for more than 7 days.
The patient was considered to have hypoxia when PaO2 was below 60 mmHg or oxygen saturation (measured by pulse oximetry) was below 95% while breathing room air.
Radiologic patterns were registered as per the radiologist's report.
Bronchoscopy, when indicated by the treating physician, was performed using a diagnostic fiberoptic bronchoscope (Olympus Q180; Olympus America, Tokyo, Japan). Procedures were carried out mostly in an operating room, except one in the intensive care unit (ICU) and one in a semiacute medical unit. After intravenous administration of propofol and a routine inspection of the tracheobronchial tree, the bronchoscope was wedged into a segmental or subsegmental bronchus, and bronchoalveolar lavage fluid was obtained by instilling 100–150 mL of saline solution into the bronchus and aspirating. The sample was obtained from a bronchus of the affected area or as close as possible.
Etiologic diagnoses were based on microbiological results compatible with clinical and radiological findings. Etiology was considered proven when the diagnosis was based on sterile fluid cultures (blood culture, pleural fluid culture), low respiratory or surgical sample culture, nasopharyngeal swab viral polymerase chain reaction or culture (as very good correlation has been demonstrated between positivity of viral polymerase chain reaction detection in nasopharyngeal samples and bronchoalveolar lavage (BAL) samples in immunosuppressed patients 8), or antibody seroconversion or immunoglobulin M positivity for atypical bacteria; etiology was considered probable when it was based on urinary antigen positivity (as Streptococcus pneumoniae antigen has the ability to remain positive for periods as long as 1 year, thus potentially producing false positives, etiology based only in this result were not considered proven), fungal biomarkers, or serum cytomegalovirus polymerase chain reaction.
Diagnostic yield of microbiologic tests was defined as the ratio between tests leading to a proven or probable etiologic diagnosis and tests performed, and was expressed as a percentage.
We considered the initial treatment with an effective antibiotic according to in vitro susceptibility testing as appropriate empirical therapy.
Bacteria were classified as multidrug‐resistant microorganisms according to Magiorakos et al 9.
The prescribed daily dose of antibiotics was measured according to de With et al. 10.
Statistical Analysis
Clinical presentation, etiology, diagnostic tests, antimicrobial therapy, management, and outcome were analyzed. Survivors were compared with nonsurvivors.
Quantitative variables were expressed as mean (SD) or as medians with interquartile range (IQR), as appropriate; qualitative variables were expressed as frequency and percentage. Continuous variables were compared using the t test, and categorical variables were compared using the chi‐square test or Fisher's exact test when the chi‐square test was not appropriate.
Adjusted odds ratios (ORs) were computed using logistic regression analysis to determine prognostic factors. Logistic regression analysis for mortality and for ICU admission were performed on per‐episode basis. Stepwise logistic regression analysis was performed including variables with a p value <.1 in the univariate analysis. All statistical analyses were performed using PASW Statistics for Windows, version 18.0 (SPSS Inc, Chicago, IL).
Ethics
The study and the case report form were approved by the local institutional review board and ethics committee (MICRO.HGUGM.2015‐069).
Results
Characteristics of Patients with Cancer with Pneumonia
During the study period, 132 episodes of pneumonia were diagnosed in 117 patients out of a total of 1,354 admissions (9.8%) to the oncology ward.
Most episodes were health care–associated pneumonia and required hospital admission (89, 67.4%), 42 episodes (31.8%) were hospital‐acquired pneumonia, and only 1 case was considered strictly community‐acquired pneumonia. The type of underlying solid tumor is summarized in Figure 1. Lung cancer was the most common type (one third of patients), followed by colon cancer and breast cancer. Most had metastatic disease, although only 32 (24%) were considered to have terminal disease. Performance status (PS) was good (PS 0–1) in about 50% of cases at diagnosis of pneumonia. Pulmonary involvement was recorded in more than half of the episodes.
Figure 1.
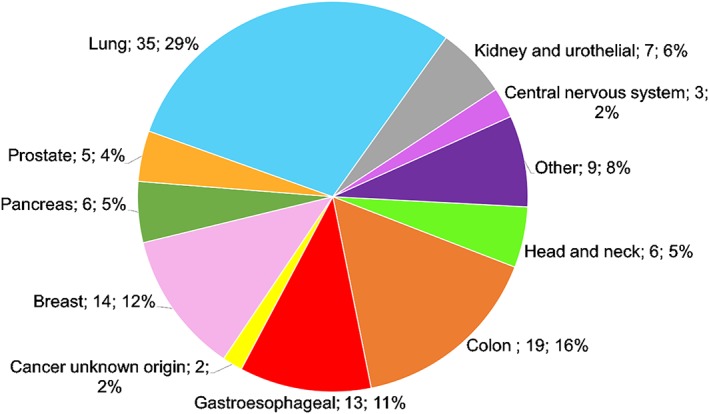
Underlying cancer in pneumonia episodes.
Only one third of the cases were vaccinated against both influenza and S. pneumoniae, 8% against influenza only (current season), and 5% against S. pneumoniae only.
Clinical Characteristics
Fever and hypoxia were the most common clinical presentations. Almost 60% of episodes of pneumonia presented with acute respiratory failure. Only nine episodes (6.8%) were considered obstructive pneumonia. Overall, only 13 (9%) were neutropenic (9 [6.8%] below 500 neutrophils/mm3), and 90 (68.2%) had received chemotherapy during the previous month. Other characteristics of pneumonia episodes in patients with cancer are displayed in Table 1.
Table 1.
Characteristics of pneumonia in hospitalized patients with cancer and risk factors for 10‐day and 30‐day mortality
| Characteristics | Total, n (%) | Survivors, 10‐day (100, 75.8%), n (%) | Nonsurvivors, 10‐day (32, 24.2%), n (%) | p value | Survivors, 30‐day (71, 53.8%), n (%) | Nonsurvivors, 30‐day (61, 46.2%), n (%) | p value |
|---|---|---|---|---|---|---|---|
| Characteristics of cancer | |||||||
| Type of tumor (lung vs. other) | 42 (31.8) | 31 (31.0) | 11 (34.4) | .828 | 25 (35.2) | 17 (27.9) | .454 |
| Tumor stage (metastatic vs. other) | 102 (77.3) | 74 (74.0) | 28 (87.5) | .148 | 52 (73.2) | 50 (82) | .299 |
| Terminal | 31 (23.5) | 15 (15) | 16 (50) | .0001 | 6 (8.5) | 25 (41) | .0001 |
| Presence of lung involvement | 73 (55.3) | 53 (53.0) | 20 (62.5) | .416 | 41 (57.7) | 32 (52.5) | .600 |
| Recent chemotherapy (previous month) | 89 (67.9) | 67 (67.0) | 22 (68.8) | 1 NS | 53 (74.6) | 36 (60) | .092 |
| Therapy (cytostatics vs. other) | 84 (63.3) | 63 (63.0) | 21 (32) | .836 | 47 (66.2) | 37 (60.7) | .587 |
| Clinical characteristics | |||||||
| Sex (men) | 97 (73.5) | 76 (76.8) | 21 (63.3) | .258 | 55 (77.5) | 42 (68.9) | .324 |
| Age, mean (SD), years | 65.7 (12.9) | 66.0 (13.6) | 64.8 (10.5) | .603 | 67.8 (13.2) | 63.2 (12.2) | .044 |
| Age‐adjusted Charlson (mean, SD) | 8.5 (2.3) | 8.4 (2.5) | 8.5 (1.7) | .857 | 8.5 (2.4) | 8.4 (2.3) | .984 |
| PS status (0–1) | 63 (50.8) | 49 (52.1) | 14 (45.2) | .677 | 40 (59.7) | 23 (40.4) | .047 |
| Place of acquisition (hospital vs. other) | 42 (31.8) | 30 (30.0) | 12 (37.5) | .514 | 16 (22.5) | 26 (42.6) | .016 |
| Vaccinated against influenza | 53 (40.5) | 44 (44) | 9 (29) | .150 | 34 (47.9) | 19 (31.7.) | .074 |
| Vaccinated against Streptococcus pneumoniae | 48 (36.9) | 41 (41) | 7 (23.3) | .088 | 32 (45.1) | 16 (27.1) | .045 |
| Hypoxemia at presentation | 75 (56.8) | 50 (50.0) | 25 (78.1) | .007 | 33 (46.5) | 42 (68.9) | .013 |
| Fever at presentation | 76 (57.6) | 62 (62.0) | 14 (43.8) | .099 | 44 (629 | 32 (52.5) | .293 |
| BP, mean (SD) | 90.4 (16.9) | 90.9 (17.2) | 89 (16.4) | .610 | 92.3 (17.1) | 88.0 (16.6) | .202 |
| Creatinine, mean (SD) | 0.8 (0.5) | 0.8 (0.5) | 0.8 (0.4) | .353 | 0.9 (0.5) | 0.7 (0.4) | .065 |
| Bilirubin, mean (SD) | 0.67 (0.8) | 0.6 (0.8) | 0.8 (0.7) | .335 | 0.6 (0.9) | 0.7 (0.6) | .594 |
| Neutropenia | 13 (9.8) | 9 (9.0) | 4 (12.5) | .515 | 7 (9.9) | 6 (9.8) | 1 NS |
| Neutropenia <500 ANC | 9 (6.8) | 5 (5) | 4 (12.5) | .219 | 4 (5.6) | 5 (8.2) | .732 |
| Corticosteroids | 42 (31.8) | 29 (29.0) | 13 (40.6) | .276 | 16 (22.5) | 26 (42.6) | .016 |
| Influenza | 5 (3.8) | 4 (4.0) | 1 (3.1) | 1 NS | 4 (5.7) | 1 (1.6) | .371 |
| Recurrent episode | 15 (11.4) | 9 (9.0) | 6 (18.8) | .196 | 4 (5.6) | 11 (18.0) | .003 |
| Radiology | |||||||
| Interstitial infiltrate | 12 (20.3) | 10 (10.0) | 2 (6.3) | .730 | 6 (8.5) | 6 (9.8) | 1 NS |
| Lobar infiltrate | 110 (90.2) | 81 (81.0) | 29 (90.6) | .279 | 56 (78.9) | 54 (88.5) | .164 |
| Pleural effusion | 40 (55.6) | 29 (29.0) | 11 (34.4) | .785 | 20 (28.2) | 20 (32.8) | .575 |
| Nodules | 8 (14) | 5 (5.0) | 3 (9.4) | .401 | 5 (7.0) | 3 (4.9) | .725 |
| Obstructive pneumonia | 9 (6.8) | 8 (8.1) | 1 (3) | .687 | 6 (8.5) | 3 (4.9) | .504 |
| Diagnosis | |||||||
| Bronchoscopy | 7 (5.3) | 6 (6.1) | 1 (3) | 1 NS | 5 (7) | 2 (3.3) | .450 |
| Etiological diagnosis | 48 (36.6) | 37 (37.0) | 11 (33.3) | .836 | 28 (39.4) | 20 (32.8) | .471 |
| Management and outcome | |||||||
| Antimicrobial PDD, mean (SD) | 20.3 (52.1) | 24.4 (59.5) | 8.2 (8.8) | .135 | 26.2 (69.9) | 13.5 (11.6) | .165 |
| Appropriate empirical antimicrobials according to etiologya | 35 (77.8) | 28 (80) | 7 (70) | .668 | 19 (76) | 16 (80) | .999 |
| Appropriate empirical antimicrobials according to local guideline (all) | 60 (45.8) | 49 (81.7) | 11 (18.3) | .157 | 33 (55) | 27 (45) | .861 |
| Appropriate empirical antimicrobials according to local guideline if no etiologic diagnosis | 34 (41.0) | 30 (88.2) | 4 (11.8) | .022 | 18 (52.9) | 16 (47.1) | .824 |
| ID consultation | 34 (25.8) | 24 (24) | 10 (31.3) | .487 | 15 (21.1) | 19 (31.1) | .232 |
| Vasoactive drugs | 5 (3.8) | 2 (2) | 3 (9.4) | .092 | 2 (2.8) | 3 (4.9) | .662 |
| ICU admission | 17 (12.9) | 6 (6) | 11 (34.4) | .000 | 3 (4.2) | 14 (22.9) | .006 |
| MV | 3 (2.3) | 1 (1) | 2 (6.3) | .146 | 0 (0) | 3 (4.9) | .096 |
| NIMV | 14 (10.6) | 3 (2) | 11 (27.3) | .000 | 0 (0) | 14 (22.9) | .0001 |
Bolded p values are statistically significant.
Evaluated only in 48 episodes with etiological diagnosis.
Abbreviations: ANC, absolute neutrophil count; BAL, bronchoalveolar lavage; ICU, intensive care unit; ID, infectious diseases; MDR, multidrug‐resistant; MV, mechanical ventilation; NIMV, noninvasive mechanical ventilation; NS, nonsignificant; PDD, prescribed daily dose; PS, performance status.
Etiology
An etiological diagnosis was reached in 48/132 cases (36.4%; Fig. 2; Table 2) and was considered proven in 22 (45.8 %) and probable in 26 (54.2%). There were no differences in etiology according to place of acquisition. S. pneumoniae and Staphylococcus aureus were the most common bacterial pathogens. Even though in 10 cases patients (7.6%) were previously colonized by multidrug‐resistant microorganisms, none developed pneumonia caused by those microorganisms. We recorded only one episode of multidrug‐resistant Pseudomonas aeruginosa and one episode of methicillin‐resistant S. aureus pneumonia. Fungi such as Pneumocystis jiroveci (three cases) and Aspergillus fumigatus were occasionally identified. Viruses other than influenza (five episodes) included respiratory syncytial virus (one episode), herpes zoster (one episode), and cytomegalovirus (one episode). The median time to the etiological diagnosis was 1 day (IQR 1–4.25).
Figure 2.
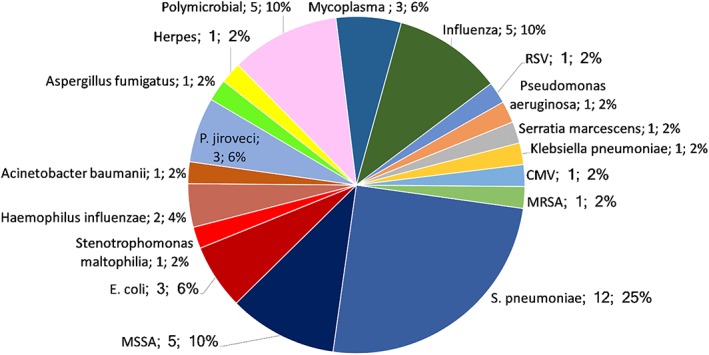
Etiology of pneumonia in 48 episodes with an etiological diagnosis.Abbreviations: CMV, cytomegalovirus; MRSA, methicillin‐resistant Staphylococcus aureus; RSV, respiratory syncytial virus.
Table 2.
Etiology
| Microorganism | Total, n (%) | Proven etiology, n (%) | Probable etiology, n (%) | Non–hospital‐acquired, n (%) | Hospital‐acquired, n (%) | p value |
|---|---|---|---|---|---|---|
| Number of cases with known etiology | 48 (36.4) | 22 (45.8) | 26 (54.2) | .134 | ||
| 33 (68.8) | 15 (31.3) | .154 | ||||
| Streptococcus pneumoniae | 12 (25) | 3 (13.6) | 9 (34.6) | 10 (30.3) | 2 (13.3) | |
| MS Staphylococcus aureus | 5 (10.4) | 1 (4.5) | 4 (15.4) | 5 (15.2) | 0 (0.0) | |
| Influenza | 5 (10.4) | 5 (22.7) | 0 (0) | 3 (9.1.) | 2 (13.3) | |
| Polymicrobial | 5 (10.4) | 2 (9.1) | 3 (11.5) | 2 (6.1) | 3 (20.0) | |
| Pneumocystis jiroveci | 3 (6.3) | 2 (9.1) | 1 (3.8) | 2 (6.1) | 1 (6.7) | |
| Escherichia coli | 3 (6.3) | 2 (9.1) | 1 (3.8) | 1 (3.0) | 2 (13.3) | |
| Mycoplasma sp. | 3 (6.3) | 0 80) | 3 (13.6) | 3 (9.1.) | 0 (0.0) | |
| Haemophilus influenzae | 2 (4.2) | 1 (4.5) | 1 (3.8) | 2 (6.1) | 0 (0.0) | |
| Aspergillus fumigatus | 1 (2.1) | 0 (0) | 1 (3.8) | 1 (3.0) | 0 (0.0) | |
| MR Staphylococcus aureus | 1 (2.1) | 0 (0) | 1 (3.8) | 1 (3.0) | 0 (0.0) | |
| Acinetobacter sp. | 1 (2.1) | 0 (0) | 1 (3.8) | 0 (0.0) | 1 (6.7) | |
| Klebsiella pneumoniae | 1 (2.1) | 1 (4.5) | 0 (0) | 1 (3.0) | 0 (0.0) | |
| Serratia marcescens | 1 (2.1) | 0 (0) | 1 (3.8) | 1 (3.0) | 0 (0.0) | |
| Pseudomonas aeruginosa | 1 (2.1) | 1 (4.5) | 0 (0) | 0 (0.0) | 1 (6.7) | |
| Stenotrophomonas maltophilia | 1 (2.1) | 0 (0) | 1 (3.8) | 0 (0.0) | 1 (6.7) | |
| RSV | 1 (2.1) | 1 (4.5) | 0 (0) | 0 (0.0) | 1 (6.7) | |
| Herpes zoster | 1 (2.1) | 0 (0) | 1 (3.8) | 0 (0.0) | 1 (6.7) | |
| CMV | 1 (2.1) | 0 (0) | 1 (3.8) | 1 (3.0) | 0 (0.0) |
Abbreviations: CMV, cytomegalovirus; MR, methicillin‐resistant; MS, methicillin‐susceptible; RSV, respiratory syncytial virus.
Microbiological diagnostic tests and their diagnostic yield are summarized in Figure 3 and supplemental online Table 1. No microbiological diagnostic test was performed in 13 episodes (9.8%). The sample with the best diagnostic yield was bronchoalveolar lavage (28.6%). Median time from suspicion of pneumonia to bronchoalveolar lavage was 6 days (IQR 3–6). Only one of the bronchoalveolar lavages was performed in the ICU, and no procedure‐related complications were recorded.
Figure 3.
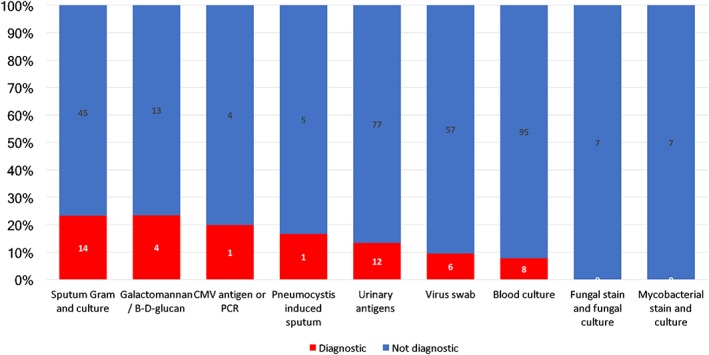
Microbiological test.Abbreviations: CMV, cytomegalovirus; PCR, polymerase chain reaction.
Antimicrobial Therapy
Empirical therapy was chosen by the treating oncologist and consisted mainly of meropenem (27.7%), piperacillin‐tazobactam or levofloxacin (22.7% each), amoxicillin‐clavulanate (7.5%), or a combination of antibiotics (12.1%). Appropriateness of the antimicrobial treatment could only be evaluated in cases in which an etiological diagnosis had been established, with 64.6% of episodes receiving adequate empirical therapy in the first 24 hours. Only 34 episodes (26%) were evaluated by an infectious diseases specialist.
Knowledge of the etiology of pneumonia led to changes in antimicrobial therapy in 28 out of 48 episodes (58.3%), namely, de‐escalation in 20/48 cases (41.6 %) and broader coverage in 8/48 (16.6%).
In patients without an etiologic diagnosis, compliance with the local guidelines for empirical antimicrobial therapy was analyzed to assess adequacy. Only in 41% of episodes empirical antimicrobial therapy followed the local guidelines.
Outcome and Risk Factors for Mortality
ICU Admission
Only 10.6% of episodes of pneumonia in patients with cancer required subsequent ICU admission and mechanical ventilation. Median time from diagnosis to mechanical ventilation was 1.5 days (IQR 0.75–4). Only 3 out of 17 episodes (17.6%) requiring ICU admission were discharged.
Inappropriate empirical therapy in the first 24 hours in episodes with an etiologic diagnosis was the only independent variable associated with ICU admission (40% vs. 11.4%; 95% confidence interval [CI], 0.011–0.0929; p = .043; supplemental online Tables 2, 3).
Mortality
Median time from diagnosis of pneumonia to death was 19 days (IQR 5–32.5). Only two patients that were discharged before day 30 and transferred to other facilities were lost to follow‐up.
Ten‐day mortality, which we consider as a surrogate marker of attributable mortality, was 24.2%. The only independent risk factor for 10‐day mortality was hypoxia (OR, 2.1; 95% CI, 1.3–4.5; p = .043). In the subset of patients lacking an etiologic diagnosis, administering empirical antimicrobials following the local guidelines was a protective factor for 10‐day mortality (OR, 0.3; 95% CI, 0.08–0.83; p = .024). When analyzing episodes with a known etiology, this association disappeared. Among patients who died within 10 days of the diagnosis of pneumonia, 50% were considered terminally ill.
Thirty‐day mortality was 46.2%. The only independent risk factors for 30‐day mortality were hypoxia at presentation, hospital acquisition of pneumonia, and a PS >1 (Tables 1, 4). No cancer‐related factors other than PS were associated with 30‐day prognosis. Of the patients who died within 30 days of the diagnosis of pneumonia, only 41% were considered terminally ill.
Table 4.
Independent risk factors for 30‐day mortality
| Multivariate 30‐day mortality | OR | 95% CI | p value |
|---|---|---|---|
| Hospital acquisition | 3.1 | 1.3–7.2 | .009 |
| PS >1 | 2.6 | 1.2–5.7 | .021 |
| Hypoxia | 3.3 | 1.4–7.4 | .007 |
| Vasoactive drugs | 1.7 | 0.2–11.7 | .617 |
| Corticosteroids | 1.5 | 0.9–2.2 | .085 |
Bolded p values are statistically significant.
Abbreviations: CI, confidence interval; OR, odds ratio; PS, performance status.
Table 3.
Independent risk factors for intensive care unit admission
| Multivariate ICU admission | OR | 95% CI | p value |
|---|---|---|---|
| Hypoxia | 5.2 | 0.5–58.3 | .182 |
| Terminal | 0.0 | 0 | .999 |
| Appropriate empirical antimicrobials | 0.1 | 0.01–0.9 | .045 |
| Vasoactive drugs | 5.7 | 0.2–143.9 | .288 |
| Neutropenia | 6.2 | 0.4–92.9 | .186 |
Bolded p values are statistically significant.
Abbreviations: CI, confidence Interval; ICU, intensive care unit; OR, odds ratio.
Analysis per patient (only the first episode of pneumonia; supplemental online Table 3) yielded similar multivariable results.
Recurrence
Recurrence was recorded in 15 patients (11.4%) during the 12‐month period. The characteristics of cases with more than one episode during the study period were comparable to those of nonrecurrent episodes, except for a higher 30‐day mortality (73.3% vs. 42.7%; p = .03).
Discussion
The present study shows that pneumonia is a prevalent infectious complication in patients admitted to oncology wards. Most cases were acquired outside the hospital (although they were health care–associated pneumonia), and a large proportion of the patients were not terminally ill. Etiology was diverse and included bacteria, viruses, and fungi. Mortality was very high, even in non–terminally ill patients, and poor outcome was linked to inappropriate empirical therapy.
In the present series, pneumonia was mostly health care–associated pneumonia and non–ventilator‐associated hospital‐acquired pneumonia. Little is known about the etiology of health care–associated pneumonia and hospital‐acquired pneumonia in nonintubated patients 11, 12, and even less information is available for patients with cancer 13. In a study by our group (ENEMI study) 14 on patients with pneumonia admitted to internal medicine wards, a high proportion had health care–associated pneumonia in which etiology differed from that of community‐acquired pneumonia and prognosis was worse.
Etiologic agents such as S. aureus, Pseudomonas species, P. jiroveci, and Aspergillus are distinctly uncommon in community‐acquired pneumonia but relatively present in health care–associated pneumonia 15. Furthermore, management of hospital‐acquired pneumonia in nonintubated patients in terms of etiologic evaluation is not adequately addressed in treatment guidelines for nonintubated patients 16, 17, which are unclear and variable with respect to recommendations for diagnostic evaluation and empirical antimicrobial therapy.
In the present study, which was based on routine clinical practice, an etiological diagnosis was reached in only 36.4% of episodes; therefore, empirical therapy could be adjusted in a relatively small percentage of patients. This percentage is lower than that reported in other studies, in which an etiologic diagnosis was reached in 34.9%–67.5% of health care–associated pneumonia and hospital‐acquired pneumonia cases 11, 12, 15, 18. When the etiology was available, however, treatment had to be de‐escalated or escalated in a significant number of cases.
In view of the diverse etiologic results obtained in cases with known etiology, and considering that in many occasions those altered management, an attempt to obtain lower respiratory samples for examination is warranted in patients with cancer with pneumonia who require admission or are already admitted at diagnosis. New diagnostic tools not based on cultures, such as molecular tests 19, would potentially add value in the subset of patients with cancer where the diversity of etiologies hampers an appropriate early antimicrobial therapy.
To know the etiology potentially could correct the effect of an inappropriate empirical therapy. When analyzing compliance with local guidelines for empirical therapy in cases without an etiological diagnosis, an association with 10‐day mortality was found for noncompliants, that was not detected when considering episodes with a known etiology. One could hypothesize that optimizing antimicrobial therapy to a known pathogen overcame potential errors in empirical therapy that otherwise would have led to an increased 10‐day mortality.
The contradictory data reported in the literature for performance of bronchoalveolar lavage may be the result of differences in technique, although they are more likely due to delays in execution 20, 21, 22, 23, 24. As for safety, bronchoalveolar lavage is safe when performed in the ICU with close monitoring and noninvasive mechanical ventilation when necessary 22, 25. We did not detect any procedure‐related complication in the study population.
The mortality of hospital‐acquired pneumonia and health care–associated pneumonia in nonintubated patients is estimated to be between 10.3% and 51% 11, 12, 15, 18, 26, 27 and depends on the underlying conditions of the study population. In our series, 10‐day mortality was 24% and 30‐day mortality was 46%. We were not able to find similar reported data for patients with cancer, other than those referring to ventilated patients.
In our series, ICU admission, which is a marker of poor outcome, was associated with inappropriate empirical therapy, thus necessitating more precise and possibly broader antimicrobial empirical therapy for pneumonia in this population than that recommended by current guidelines 28, together with an effort to reach an etiological diagnosis to facilitate subsequent de‐escalation.
Excess mortality in patients with health care–associated pneumonia has been thought to be due to a lower frequency of ICU admission and use of aggressive therapies in severely ill patients 29. Intensive management is frequently not offered to patients with cancer, at least not in a timely manner, because it is thought to be futile. However, the characteristics of cancer, except performance status, were not among the additional risk factors for mortality in the present series. Performance status is linked to cancer stage but also depends on other variables such as comorbidity. More than half of the patients who eventually died of pneumonia in the present study were not terminally ill; therefore, pneumonia should not be routinely regarded as a terminal event in patients with cancer, and when evaluating intensive management, performance status should be taken into consideration ahead of cancer stage.
Recent studies in patients with cancer and acute respiratory failure report an improvement in survival 30, particularly in patients admitted to specific ICUs for cancer and in episodes with a known etiology 31. Improvements in cancer care, supportive therapies, and critical care make it necessary to reassess the effectiveness of offering intensive management to selected patients with cancer 32.
Our study is subject to a series of limitations. As it was performed in a single center, our results might not necessarily be extrapolated to other institutions. We cannot rule out the possibility that noninfectious cases were included, as microbiological samples were not obtained for every patient, although all cases fulfilled the American Thoracic Society criteria for pneumonia. Our strengths are that our study reflects real practice. In addition, we analyze a whole year, thus avoiding seasonality, and our population comprises a specific and homogeneous subset of health care–associated pneumonia and nosocomial pneumonia in nonventilated patients.
Larger multicenter studies are necessary to establish specific prognostic scores for patients with cancer admitted with pneumonia. It is also necessary to determine the efficiency of tailored diagnostic and management strategies in this population (“Pneumonia bundle”) to implement new diagnostic techniques in a timely manner in patients stratified according to specific risk factors. These strategies should also include measures aimed at prevention and control of infection.
Conclusion
Pneumonia is a highly prevalent condition in hospitalized patients with solid tumors, even with nonterminal disease. Etiology is diverse, and poor outcome is linked to inappropriate empirical therapy. Efforts to get the empirical therapy right and reach an etiological diagnosis to subsequently de‐escalate are warranted.
Author Contributions
Conception/design: Ana Fernández‐Cruz, Emilio Bouza
Provision of study material or patients: Laura Ortega, Gonzalo García, Iria Gallego, Esther Chamorro‐de‐Vega, José Javier García‐López, Ricardo González‐del‐Val, Pablo Martín‐Rabadán, Carmen Rodríguez
Collection and/or assembly of data: Laura Ortega, Gonzalo García, Iria Gallego, Ana Álvarez‐Uría
Data analysis and interpretation: Ana Fernández‐Cruz, Laura Ortega, Gonzalo García, Iria Gallego, Ana Álvarez‐Uría, Emilio Bouza
Manuscript writing: Ana Fernández‐Cruz, José Javier García‐López, Pablo Martín‐Rabadán, Emilio Bouza
Final approval of manuscript: Ana Fernández‐Cruz, Laura Ortega, Gonzalo García, Iria Gallego, Ana Álvarez‐Uría, Esther Chamorro‐de‐Vega, José Javier García‐López, Ricardo González‐del‐Val, Pablo Martín‐Rabadán, Carmen Rodríguez, María Luisa Pedro‐Botet, Miguel Martín, Emilio Bouza
Disclosures
Miguel Martín: AstraZeneca, Novartis, Roche‐Genentech, Pfizer Glaxo, PharmaMar, Taiho Oncology, Lilly (H, SAB), Novartis, Roche (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Acknowledgments
We thank Thomas O'Boyle for his help in the preparation of the manuscript. We thank the members of the PRION study group for their contribution: Ricardo González del Val, Concepción García Aroca, Miguel Martín, Carmen Rodríguez, Ma Dolores Vigil, Marta Grande, Patricia Muñoz, Cristina Rincón, Emilio Bouza, Ana Fernández‐Cruz. This study was previously presented in part at the 26th ECCMID as a poster (P1784); April 9–12, 2016; Amsterdam, The Netherlands.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Leoni D, Encina B, Rello J. Managing the oncologic patient with suspected pneumonia in the intensive care unit. Expert Rev Anti Infect Ther 2016;14:943–960. [DOI] [PubMed] [Google Scholar]
- 2. Aabom B, Kragstrup J, Vondeling H et al. Defining cancer patients as being in the terminal phase: Who receives a formal diagnosis, and what are the effects? J Clin Oncol 2005;23:7411–7416. [DOI] [PubMed] [Google Scholar]
- 3. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis 2007;44(suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garner JS, Jarvis WR, Emori TG et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128–140. [DOI] [PubMed] [Google Scholar]
- 5. American Thoracic Society , Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 6. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 7. Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. [PubMed] [Google Scholar]
- 8. Lachant DJ, Croft DP, McGrane Minton H et al. Nasopharyngeal viral PCR in immunosuppressed patients and its association with virus detection in bronchoalveolar lavage by PCR. Respirology 2017;22:1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magiorakos AP, Srinivasan A, Carey RB et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–281. [DOI] [PubMed] [Google Scholar]
- 10. de With K, Maier L, Steib‐Bauert M et al. Trends in antibiotic use at a university hospital: Defined or prescribed daily doses? Patient days or admissions as denominator? Infection 2006;34:91–94. [DOI] [PubMed] [Google Scholar]
- 11. Sopena N, Sabria M, Neunos 2000 Study Group . Multicenter study of hospital‐acquired pneumonia in non‐ICU patients. Chest 2005;127:213–219. [DOI] [PubMed] [Google Scholar]
- 12. Polverino E, Torres A, Menendez R et al. Microbial aetiology of healthcare associated pneumonia in Spain: A prospective, multicentre, case‐control study. Thorax 2013;68:1007–1014. [DOI] [PubMed] [Google Scholar]
- 13. Schnell D, Mayaux J, Lambert J et al. Clinical assessment for identifying causes of acute respiratory failure in cancer patients. Eur Respir J 2013;42:435–443. [DOI] [PubMed] [Google Scholar]
- 14. Giannella M, Pinilla B, Capdevila JA et al. Pneumonia treated in the internal medicine department: Focus on healthcare‐associated pneumonia. Clin Microbiol Infect 2012;18:786–794. [DOI] [PubMed] [Google Scholar]
- 15. Carratala J, Mykietiuk A, Fernandez‐Sabe N et al. Health care‐associated pneumonia requiring hospital admission: Epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med 2007;167:1393–1399. [DOI] [PubMed] [Google Scholar]
- 16. Kalil AC, Metersky ML, Klompas M et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torres A, Niederman MS, Chastre J et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital‐acquired pneumonia and ventilator‐associated pneumonia: Guidelines for the management of hospital‐acquired pneumonia (HAP)/ventilator‐associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J 2017;50. [DOI] [PubMed] [Google Scholar]
- 18. Cakir Edis E, Hatipoglu ON, Yilmam I et al. Hospital‐acquired pneumonia developed in non‐intensive care units. Respiration 2009;78:416–422. [DOI] [PubMed] [Google Scholar]
- 19. Murdoch DR. How recent advances in molecular tests could impact the diagnosis of pneumonia. Expert Rev Mol Diagn 2016;16:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray PV, O'Brien ME, Padhani AR et al. Use of first line bronchoalveolar lavage in the immunosuppressed oncology patient. Bone Marrow Transplant 2001;27:967–971. [DOI] [PubMed] [Google Scholar]
- 21. Azoulay E, Mokart D, Rabbat A et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: Prospective multicenter data. Crit Care Med 2008;36:100–107. [DOI] [PubMed] [Google Scholar]
- 22. Azoulay E, Mokart D, Lambert J et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: Randomized controlled trial. Am J Respir Crit Care Med 2010;182:1038–1046. [DOI] [PubMed] [Google Scholar]
- 23. Shannon VR, Andersson BS, Lei X et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647–655. [DOI] [PubMed] [Google Scholar]
- 24. Sampsonas F, Kontoyiannis DP, Dickey BF et al. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: A prospective 2‐year study. Cancer 2011;117:3424–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saillard C, Mokart D, Lemiale V et al. Mechanical ventilation in cancer patients. Minerva Anestesiol 2014;80:712–725. [PubMed] [Google Scholar]
- 26. Barreiro‐Lopez B, Tricas JM, Mauri E et al. Risk factors and prognostic factors in nosocomial pneumonia outside the intensive care units setting [in Spanish]. Enferm Infecc Microbiol Clin 2005;23:519–524. [DOI] [PubMed] [Google Scholar]
- 27. Sopena N, Heras E, Casas I et al. Risk factors for hospital‐acquired pneumonia outside the intensive care unit: A case‐control study. Am J Infect Control 2014;42:38–42. [DOI] [PubMed] [Google Scholar]
- 28. Rabello LS, Lisboa T, Soares M et al. Personalized treatment of severe pneumonia in cancer patients. Expert Rev Anti Infect Ther 2015;13:1319–1324. [DOI] [PubMed] [Google Scholar]
- 29. Rello J, Lujan M, Gallego M et al. Why mortality is increased in health‐care‐associated pneumonia: Lessons from pneumococcal bacteremic pneumonia. Chest 2010;137:1138–1144. [DOI] [PubMed] [Google Scholar]
- 30. Saillard C, Darmon M, Mokart D. Acute kidney injury in patients with cancer. N Engl J Med 2017;377:499. [DOI] [PubMed] [Google Scholar]
- 31. Azoulay E, Schellongowski P, Darmon M et al. The Intensive Care Medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med 2017;43:1366–1382. [DOI] [PubMed] [Google Scholar]
- 32. Shimabukuro‐Vornhagen A, Boll B, Kochanek M et al. Critical care of patients with cancer. CA Cancer J Clin 2016;66:496–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables


