Abstract
Immune checkpoint inhibitor (ICI)‐induced immune‐related adverse events (irAEs) may affect almost any organ system and occur at any point during therapy. Autoantibody analysis may provide insight into the mechanism, nature, and timing of these events. We report a case of ICI‐induced late‐onset Raynaud's‐like phenomenon in a patient receiving combination immunotherapy. A 53‐year‐old woman with advanced non‐small lung cancer received combination anti‐cytotoxic T‐lymphocyte antigen 4 and anti‐programmed death 1 ICI therapy. She developed early (hypophysitis at 4 months) and late (Raynaud's at >20 months) irAEs. Longitudinal assessment of 124 autoantibodies was correlated with toxicity. Although autoantibody levels were generally stable for the first 18 months of therapy, shortly before the development of Raynaud's, a marked increase in multiple autoantibodies was observed. This case highlights the potential for delayed autoimmune toxicities and provides potential biologic insights into the dynamic nature of these events.
Key Points
A patient treated with dual anti‐PD1 and anti‐CTLA4 therapy developed Raynaud's‐like signs and symptoms more than 18 months after starting therapy.
In this case, autoantibody changes became apparent shortly before onset of clinical toxicity.
This case highlights the potential for late‐onset immune‐related adverse events checkpoint inhibitors, requiring continuous clinical vigilance.
The optimal duration of checkpoint inhibitor therapy in patients with profound and prolonged responses remains unclear.
Short abstract
Autoantibody analysis may provide insight into the mechanism, nature, and timing of immune‐related adverse events. This case report describes a case of immune checkpoint inhibitor‐induced late‐onset Raynaud's‐like phenomenon in a patient receiving combination immunotherapy.
Background
Immune‐related adverse events (irAEs) represent a unique class of toxicity associated with immune checkpoint inhibitor (ICI) targeting of anti‐programmed death 1 (PD1), PD1 ligand (PDL1), or cytotoxic T‐lymphocyte antigen 4 (CTLA4). These autoimmune toxicities can potentially affect almost every organ system. With combination regimens, up to 85% of patients may develop irAEs, and more than 40% of patients may discontinue treatment because of these toxicities 1.
Adding to this challenge, the occurrence, type, severity, and timing of irAEs remain unpredictable. There are no established clinical or serologic biomarkers for the identification of patients at high risk for toxicity. Biomarkers under investigation include pre‐existing systemic and organ‐specific autoantibodies, including antithyroidal antibodies 2, as well as peripheral blood cellular ratios 3.
We present a case of a patient with advanced non‐small cell lung cancer treated with combined anti‐CTLA4 and anti‐PD1 checkpoint blockade who derived long‐term benefit. The patient developed a late‐onset rheumatologic irAE after more than 20 months. The presented serial autoantibody levels may provide insight into the humoral immunity underlying this delayed toxicity.
Case Presentation
A 53‐year‐old woman with a 35 pack‐year smoking history developed progressive mid‐back pain. She was found to have a paraspinal mass, multiple bilateral pulmonary nodules, and a dominant 1.7‐cm right lower lobe mass. Biopsy revealed adenocarcinoma consistent with lung primary, harboring a KRAS G12C mutation. The patient had no history of autoimmune disease. She initiated combination ipilimumab 1 mg/kg intravenously (IV) every 6 weeks and nivolumab 3 mg/kg IV every 2 weeks and was transitioned to nivolumab 480 mg IV every 4 weeks as consolidation therapy after 28 months.
After 4 months of therapy, she developed weakness, fatigue, and orthostatic hypotension. Laboratory assessment revealed low serum concentrations of adrenocorticotropic hormone (<5 pg/mL) and cortisol (0.8 μ/dL). She was diagnosed with hypophysitis featuring secondary adrenal insufficiency. She improved with hydrocortisone.
After 22 months, she noted an abnormal, painful cold sensation in her fingers and toes, which turned white upon cold exposure and then red upon rewarming (Fig. 1). She was diagnosed with Raynaud's‐like phenomenon, which improved with behavior modification and treatment with calcium channel blockers.
Figure 1.
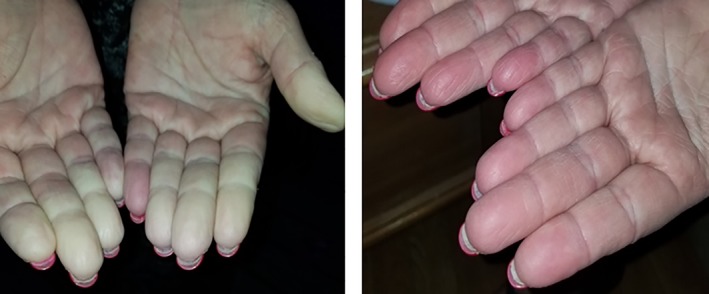
Images of patient's Raynaud's episode during cold and rewarming.
Immunotherapy was continued without interruption throughout this period. Regarding efficacy, the patient achieved a deep partial (near complete) radiographic response, which had been sustained for more than 38 months after treatment initiation at the time of this report.
Autoantibody Analysis
Prior to therapy initiation, the patient was enrolled in a prospective biospecimen collection protocol (Institutional Review Board STU 082015‐053). Peripheral blood samples were collected from the patient at pretreatment baseline and throughout therapy. Unfortunately, samples were not collected beyond the onset of Raynaud's‐like symptoms.
Autoantigen Array Analysis
As described previously 4, we measured autoantibody reactivates against a panel of 124 autoantigens using a microarray platform developed by the Microarray Core at UT Southwestern. The panel, designed by Q.‐Z.L. and E.K.W., includes autoantibodies associated in the literature with a broad range of autoimmune and inflammatory conditions. Briefly, diluted serum samples were incubated with the autoantigen arrays, and autoantibodies were detected with cy3‐conjugated anti‐mouse immunoglobulin (Ig)G and cy5‐conjugated anti‐mouse IgM (1:2,000, Jackson ImmunoResearch Laboratories, West Grove, PA), using a Genepix 4200A scanner (Molecular Devices, San Jose, CA) with laser wavelength of 532 nm and 635 nm. The resulting images were analyzed using Genepix Pro 7.0 software (Molecular Devices). The net fluorescence intensity of each autoantibody was used to generate heatmaps using Cluster and Treeview software 5.
Additionally, in this case, serum antinuclear antibody (ANA) titers were assessed at each time point in duplicates across 96‐microwell ANA enzyme‐linked immunosorbent assay (ELISA) plates using Quanta Lite ANA ELISA kits by Inova Diagnostics (San Diego, CA).
Results
Hierarchical clustering of 124 autoantibodies demonstrated dynamic changes in serum levels of multiple autoantibodies throughout immunotherapy (Fig. 2A). Notably, meaningful increases in antibody levels did not occur until more than 1 year after treatment initiation, with the greatest changes noted after month 18. After 21 months, weeks before the emergence of Raynaud's phenomenon, we observed a rise in multiple autoantibody levels, including antivitronectin, anti‐β2 glycoprotein, antiprothrombin, antiangiotensin receptor 2 type 1, and anti‐double‐stranded DNA (Fig. 2B). ANA levels were not elevated at baseline or at any time point after treatment initiation (data not shown). Anticentromere antibodies had a modest and sustained increase throughout therapy (CENPB in Fig. 2A).
Figure 2.
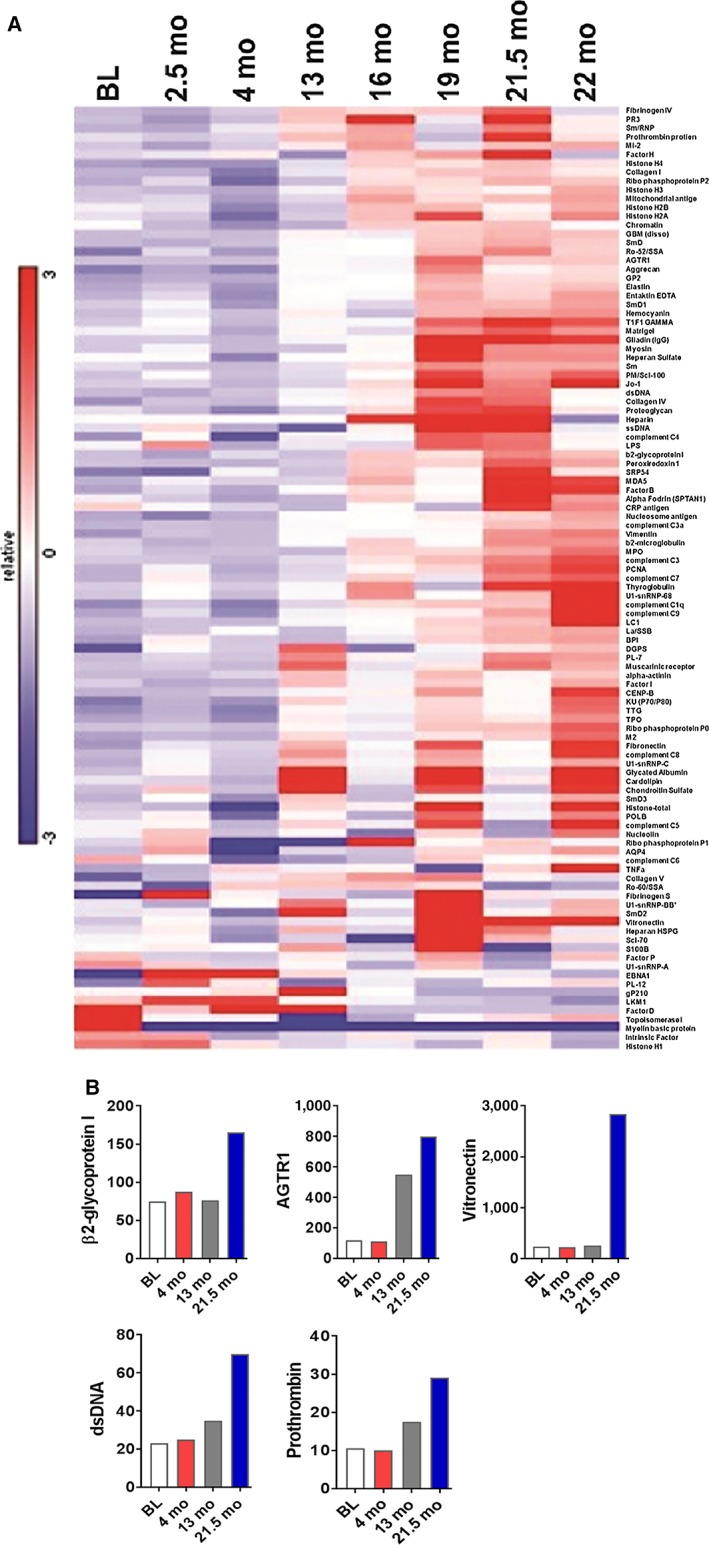
Longitudinal autoantibody analysis. (A): Heatmap of 124 autoantibodies at eight time points. (B): Serum levels of selected autoantibodies associated with Raynaud's phenomenon over time. At BL, 4 months, 13 months, and 21.5 months (shortly before development of clinical features of Raynaud's). Values on y‐axis represent net fluorescence intensity as described in methods. Abbreviations: BL, baseline; dsDNA, double‐stranded DNA; mo, months.
Discussion
Unlike characteristic toxicities of conventional chemotherapy and molecularly targeted therapies, which have relatively predictable onset and duration, irAEs may occur at any point throughout treatment. Serious irAEs have been reported as early as after a single dose of ICI and as late as several months after treatment discontinuation 6. For rheumatic irAEs—a category that would encompass this patient's Raynaud's‐like symptoms—reported onset ranges from 2 to 13 months after initiating checkpoint inhibitor therapy 7.
Although cases of digital ischemia have been reported in the setting of immunotherapy 8, to our knowledge this is the first description of ICI‐induced Raynaud's‐like symptoms. The occurrence of Raynaud's almost 1 year later than the latest‐onset rheumatic irAE described previously 7 highlights the truly unpredictable nature of these events and the importance of not relying exclusively on earlier published experience to guide suspicion for and evaluation of potential irAEs. Although Raynaud's symptoms are far preferable to progressive, advanced malignancy, this case does raise theoretical questions about the optimal duration of immunotherapy in individuals with prolonged clinical benefit.
The delayed Raynaud's symptoms may reflect humoral stimulation, which in turn leads to increased autoantibody production. The temporal relationship between autoantibody changes and symptom onset suggests the possibility that these autoantibodies may be involved in the Raynaud's pathophysiology. For instance, vitronectin regulates cell adhesion and proteolysis in the vascular extracellular matrix 9. Antivitronectin antibodies may prevent smooth muscle migration, leading to dysfunctional vascular tone, immune‐mediated vasculitis, and vasoconstriction 10, 11. We did note a particularly profound increase in antivitronectin antibodies prior to toxicity, which target endothelial cell function. One might hypothesize a potential mechanistic link between these antibodies and the clinical vasoconstriction experienced by the patient in this case report. However, antivitronectin antibodies have not been associated with Raynaud's phenomenon historically, and their elevation in this particular case does not necessarily indicate mechanistic causality. Indeed, Raynaud's phenomenon lacks a well‐defined serologic profile. ANA—negative throughout the clinical course in this patient—is positive in only 50% of cases 12. Anticentromere antibodies—elevated and increasing over time in this case—are positive in approximately one third of cases and are associated with greater clinical severity 13. This leads us the following conclusions: either (a) the antivitronectin antibodies are coincidental and unrelated or, (b) as has been suggested of multiple irAEs, these events resemble the associated autoimmune disease but may not have the same pathophysiology 14.
Notably, we observed no substantial autoantibody changes at the time of hypophysitis, suggesting either that this event did not involve humoral immune changes or that our panel did not capture the relevant autoantibodies. Alternatively, it has been reported that hypophysitis may arise from the direct interaction between anti‐CTLA4 agents and CTLA4 expressed by pituitary cells 15.
We recognize the lack of antibody data after the development of Raynaud's like symptoms in this case as a key limitation. Immune profiling during the post‐toxicity time period hypothetically could have relevance to key clinical questions, including ICI treatment decisions (continue, withhold, or discontinue) and the dose and duration of steroids or other immunosuppressive agents.
Conclusion
The development of a late‐onset irAE in this case reinforces the importance of continuous and close monitoring of patients receiving immunotherapy. Although pretreatment autoantibody profiles have been associated with subsequent development of certain irAEs, the delayed emergence of potentially relevant autoantibodies presented here suggests that baseline assessments may not fully capture predisposition to autoimmune toxicity. As clinicians and investigators seek to determine the optimal duration of ICI therapy, consideration of the timing of antitumor and anti‐self‐immune responses will be critical.
Author Contributions
Conception/design: Shaheen Khan, Mitchell S. von Itzstein, David E. Gerber
Provision of study material or patients: Shaheen Khan, Farjana J. Fattah, Quan‐Zhen Li, Edward K. Wakeland, David E. Gerber
Collection and/or assembly of data: Shaheen Khan, Mitchell S. von Itzstein, Saad A. Khan, Farjana J. Fattah, Jason Y. Park, Jessica M. Saltarski, Yvonne Gloria‐McCutchen, Quan‐Zhen Li, Edward K. Wakeland, David E. Gerber
Data analysis and interpretation: Shaheen Khan, Mitchell S. von Itzstein, Rong Lu, Yang Xie, David E. Gerber
Manuscript writing: Shaheen Khan, Mitchell S. von Itzstein, Bonnie L. Bermas, David R. Karp, David E. Gerber
Final approval of manuscript: Shaheen Khan, Mitchell S. von Itzstein, Rong Lu, Bonnie L. Bermas, David R. Karp, Saad A. Khan, Farjana J. Fattah, Jason Y. Park, Jessica M. Saltarski, Yvonne Gloria‐McCutchen, Yang Xie, Quan‐Zhen Li, Edward K. Wakeland, David E. Gerber
Disclosures
Jason Y. Park: Miraca Holdings (subsidiaries include Baylor Genetics and Fujirebio Inc) (SAB), Guanylyl Cyclase C biomarker; inventor with patents assigned to Thomas Jefferson University; licensed to Takeda Pharmaceutical (IP); David E. Gerber: U.S. and international patent pending (IP), Bristol‐Myers Squibb (CA). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Helen Mayo, MLS, from the UT Southwestern Medical Library, for assistance with literature searches. We thank Dru Gray for assistance with manuscript preparation. The patient was enrolled in a prospective biospecimen collection protocol, which was approved by the UT Southwestern Institutional Review Board (IRB STU 082015‐053). The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request. This work was supported in part by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient‐Oriented Research (K24 CA201543‐01, to D.E.G.), the David M. Crowley Foundation, the Peter Bradley Carlson Trust, a V Foundation Robin Roberts Cancer Survivorship Award (DT2019‐007, to D.E.G.), the University of Texas Lung Cancer Specialized Program in Research Excellence (SPORE, P50‐CA‐070907‐08S1, to D.E.G.), and the Cancer Prevention and Research Institute of Texas (CPRIT, RP15096), a Melanoma Research Alliance‐Society for Immunotherapy of Cancer Young Investigator Award in Immune‐related Adverse Events (Award number 619351, to S.K.), the Human Genomics/Microarray Core, and the Biomarker Research Core and Biostatistics Shared Resources at the Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, Texas, which is supported in part by NCI Cancer Center Support Grant 1P30 CA142543‐01.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Hassel JC, Heinzerling L, Aberle J et al. Combined immune checkpoint blockade (anti‐PD‐1/anti‐CTLA‐4): Evaluation and management of adverse drug reactions. Cancer Treat Rev 2017;57:36–49. [DOI] [PubMed] [Google Scholar]
- 2. Osorio JC, Ni A, Chaft JE et al. Antibody‐mediated thyroid dysfunction during T‐cell checkpoint blockade in patients with non‐small‐cell lung cancer. Ann Oncol 2017;28:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pavan A, Calvetti L, Dal Maso A et al. Peripheral blood markers identify risk of immune‐related toxicity in advanced non‐small cell lung cancer treated with immune‐checkpoint inhibitors. The Oncologist 2019;24:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li QZ, Zhou J, Wandstrat AE et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol 2007;147:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisen MB, Spellman PT, Brown PO et al. Cluster analysis and display of genome‐wide expression patterns. Proc Natl Acad Sci U S A 1998;95:14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mandala M, Merelli B, Indriolo A et al. Late‐occurring toxicity induced by an immune checkpoint blockade in adjuvant treatment of a stage III melanoma patient. Eur J Cancer 2018;95:130–132. [DOI] [PubMed] [Google Scholar]
- 7. Cappelli LC, Gutierrez AK, Baer AN et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padda A, Schiopu E, Sovich J et al. Ipilimumab induced digital vasculitis. J Immunother Cancer 2018;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dufourcq P, Louis H, Moreau C et al. Vitronectin expression and interaction with receptors in smooth muscle cells from human atheromatous plaque. Arterioscler Thromb Vasc Biol 1998;18:168–176. [DOI] [PubMed] [Google Scholar]
- 10. Dufourcq P, Couffinhal T, Alzieu P et al. Vitronectin is up‐regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res 2002;53:952–962. [DOI] [PubMed] [Google Scholar]
- 11. Ekmekci OB, Ekmekci H. Vitronectin in atherosclerotic disease. Clin Chim Acta 2006;368:77–83. [DOI] [PubMed] [Google Scholar]
- 12. Kallenberg CG, Pastoor GW, Wouda AA et al. Antinuclear antibodies in patients with Raynaud's phenomenon: Clinical significance of anticentromere antibodies. Ann Rheum Dis 1982;41:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarkozi J, Bookman AA, Lee P et al. Significance of anticentromere antibody in idiopathic Raynaud's syndrome. Am J Med 1987;83:893–898. [DOI] [PubMed] [Google Scholar]
- 14. Young A, Quandt Z, Bluestone JA. The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol Res 2018;6:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwama S, De Remigis A, Callahan MK et al. Pituitary expression of CTLA‐4 mediates hypophysitis secondary to administration of CTLA‐4 blocking antibody. Sci Transl Med 2014;6:230ra45. [DOI] [PubMed] [Google Scholar]


