Abstract
A breakthrough in oncology over the last 5 years, immunotherapy has proved its salutary effects in a wide range of solid tumors. The targeting of the programmed cell death protein 1 (PD‐1)/programmed death‐ligand 1 (PD‐L1) pathway can restore a competent antitumor T‐cell response by addressing key tumor immune evasion mechanisms. This novel mechanism of action is associated with new patterns of responses that were not observed with conventional treatments such as chemotherapy or targeted therapies. Thus, hyperprogressive disease (HPD), an unexpected acceleration of cancer evolution after starting immunotherapy, has been reported by several groups with a PD‐1/PD‐L1 blockade. This tumor flare‐up phenomenon is associated with a poorer outcome and is suspected to be an immune‐related adverse event. Despite been highly debated, the issue of HPD is currently a real challenge for oncologists’ practice in terms of patients’ information, diagnosis, and management. Herein, we describe the case of a 57‐year‐old man diagnosed with metastatic urothelial carcinoma who developed a rapid tumor growth after an anti‐PD‐L1+ IO combination. This case illustrates how current practice should evolve to address the HPD reality in the anticheckpoint era.
Key Points
Hyperprogressive disease (HPD) is an unexpected acceleration of cancer growth after starting immunotherapy that is associated with a poor outcome. Definition of HPD is based on comparing kinetics of tumor growth before and after starting immunotherapy.
No predictive biomarker has been homogenously identified in the reported studies.
Suspected pathophysiology includes expansion of programmed cell death protein 1 (PD‐1) + regulatory T cells, exhaustion of compensatory T cells, modulation of pro‐tumorigenic immune cell subsets, activation of aberrant inflammation, or activation of oncogenic signaling.
HPD is one of the most controversial immune‐related adverse events, as the liability of immunotherapy in this tumor deleterious flare‐up phenomenon has not been proved yet.
The reported incidence of HPD in retrospective studies varies across different solid tumor types from 6% to 29%. This phenomenon has been mainly suspected in non‐small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), and in urothelial carcinomas, where several randomized phase III trials have shown early crossing over of survival curves.
In the context of anti‐PD‐1/programmed death‐ligand 1 therapy, in particular for NSCLC, HNSCC, or urothelial carcinoma, the authors recommend performing an early computed tomography (CT) assessment at week 3–4. In the case of an early progression, tumor molecular characterization by tumor biopsy or circulating tumor DNA could be urged. Immunotherapy discontinuation should be discussed. Performing a confirmatory CT scan 4 weeks later to exclude pseudoprogression should not be the rule. Early switch to cytotoxic therapy may counteract the deleterious flare‐up.
Patients should be informed of the risk of developing HPD. Health authorities and trial sponsors could monitor and report the rates of tumor flares in trials in order to help oncologists to properly inform their patients about the expected rates of HPD.
Short abstract
Hyperprogressive disease, an unexpected acceleration of cancer evolution after starting immunotherapy, has been reported with PD‐1/PD‐L1 blockade treatment. This case report describes a patient diagnosed with metastatic urothelial carcinoma who developed a rapid tumor growth after anti‐PD‐L1 combination treatment.
Case Report
A 57‐year‐old man with personal history of hypertension and type 2 diabetes and family history of hemochromatosis was diagnosed with urothelial carcinoma of the bladder. After multiple local resections including right nephroureterectomy, BCG therapy, and adjuvant mitomycin, he received multiple lines in metastatic setting (MVAC, GEMOX‐carboplatin, FEC, vinflunine, gemcitabine, paclitaxel, carboplatin, and vinorelbine). As this patient remained with a good performance status (PS = 1), a phase I trial evaluating the association of an anti‐programmed death‐ligand 1 (PD‐L1) immunotherapy combined with another immune checkpoint modulator was proposed.
Three weeks after starting treatment, the patient reported increased hepatic tumor pain without clinical or biological deterioration. Immunotherapy was continued and analgesic therapy adapted. The first imaging evaluation performed at week 6 showed progressive disease by RECIST 1.1 with a 42.5% target lesions progression (Fig. 1) as well as the appearance of new lesions. The patient was maintained on immunotherapy to perform a second computed tomography (CT) scan at week 9 in order to exclude the possibility of pseudoprogressive disease. Unfortunately, while waiting for the confirmatory CT scan, the patient's condition deteriorated. This second CT confirmed progression with a 46% increase of target lesions compared with baseline.
Figure 1.
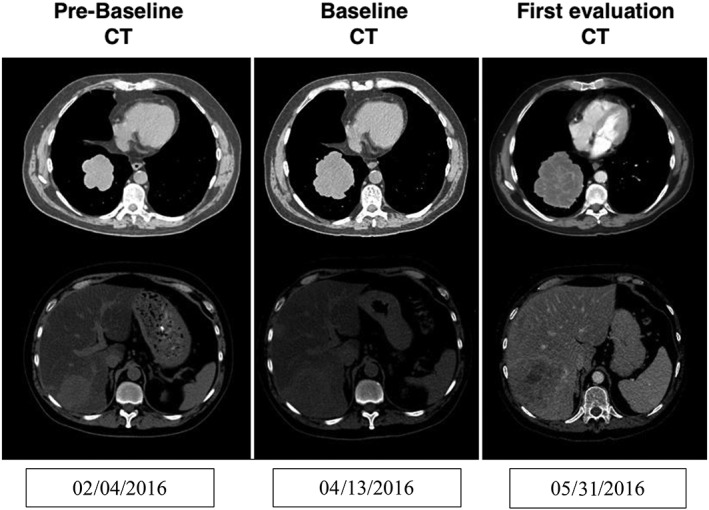
CT imaging evaluations of a 57‐year‐old male treated by an anti‐programmed death‐ligand 1 immunotherapy combined with another immune checkpoint modulator for metastatic urothelial carcinoma. First imaging evaluation performed at week 6 showed progressive disease by RECIST 1.1 with a 42.5% target lesions progression and appearance of new lesions. Abbreviation: CT, computed tomography.
The patient underwent a tumor biopsy for molecular characterization and was immediately rechallenged by vinorelbine monotherapy for a 3‐week period. A BRAFV600E mutation was identified and treatment was switched to vemurafenib. Unfortunately, 1 week after starting targeted therapy, the patient was hospitalized for neurological alteration and cerebral metastases were discovered at magnetic resonance imaging. He was maintained on vemurafenib for another 10 days and deceased consequently to progressive disease just before starting in toto cerebral radiation therapy.
What Is Hyperprogressive Disease? How Can We Identify Hyperprogression?
Hyperprogressive disease (HPD) is clinically defined by the unexpected acceleration of the tumor evolution when patients are starting immunotherapy 1. It is a paradoxical phenomenon of treatment‐induced tumor flare‐up that has been reported in different tumor types and with diverse cancer therapies 1. It is suspected when the patient's clinical status rapidly deteriorates upon treatment initiation and/or when tumor imaging evaluation is showing an important increase in tumor size and/or appearance of multiple new lesions. This phenomenon has been proposed to account for the early crossing over of survival curves in multiple randomized phase III trials such as IMvigor211 and Keynote 045 in urothelial carcinoma 2, 3, CheckMate 057 in non‐small cell lung cancer (NSCLC) 4, or CheckMate 141 in head and neck squamous cell carcinoma (HNSCC) 5.
The formal diagnosis of HPD can be made by measuring the kinetics of tumor growth before and after starting immunotherapy 1. Such diagnosis requires implementing the time in between the different CT evaluations in order to calculate the variations of tumor growth speed (Fig. 2). Thus, the first radiological definition of HPD was evaluating the tumor growth rate (TGR) by comparing tumor lesion sizes on CT scans prior to and upon anti‐PD‐(L)1 therapy 1, 6. Multiple studies using kinetics of tumor growth have reported this phenomenon in patients treated with anticheckpoint therapies with a prevalence between 6% and 29% 1, 6, 7, 8, 9, 10 (Table 1). Retrospectively, this method could demonstrate that the incidence of HPD in NSCLC was higher with anti‐PD(L)1 therapy (14%) than with chemotherapy (5%) 9.
Figure 2.
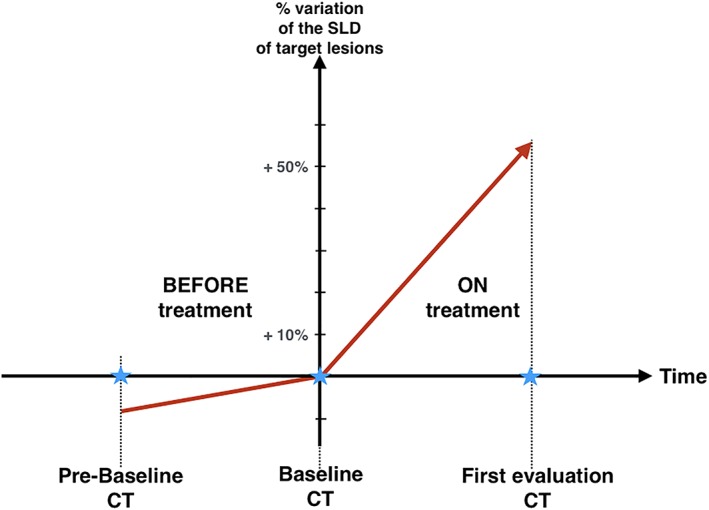
Diagnosis of HPD requires measuring the kinetics of tumor growth before and after starting immunotherapy. Three time points are therefore needed: the baseline CT and the first‐evaluation CT allow to measure the kinetics ON immunotherapy. A prebaseline CT is necessary to measure the kinetics BEFORE starting immunotherapy. Thus, an acceleration of tumor growth speed can be diagnosed. Abbreviations: CT, computed tomography; SLD, sum of the longest diameters.
Table 1.
Reported studies using tumor kinetics to identify hyperprogressive disease
| Study characteristics | Champiat et al. 6 | Kato et al. 7 | Saâda‐Bouzid 8 | Ferrara et al. 9 | Kim et al. 10 |
|---|---|---|---|---|---|
| HPD definition | RECIST PD at first evaluation and TGR ratio ≥2 | TTF <2 months >50% increase in tumor burden compared with preimmunotherapy imaging >2‐fold increase in “progression pace” | TGK ratio ≥2 | RECIST PD at first evaluation and Delta TGR >1.5 | TGK ratio ≥2 and TGR ratio ≥2 and TTF <2 months |
| Patients | n = 131 Metastatic cancers phase I trials Anti‐PD(L)1 monotherapy | n = 155 Metastatic cancers with molecular profiling Anti‐CTLA‐4, PD‐1/PD‐L1, or other investigational agents | n = 34 Recurrent and/or metastatic HNSCC Anti‐PD(L)1 monotherapy | n = 406 Advanced NSCLC Anti‐PD(L)1 ± IO combo | n = 263 Recurrent and/or metastatic NSCLC Anti‐PD(L)1 monotherapy |
| Reported frequency of HPD | 9% (12/131) | 6% (6/102) | 29% (10/34) | 14% (56/406) | 19% (45/237) |
Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte‐associated protein 4; HNSCC, head and neck squamous cell carcinoma; HPD, hyperprogressive disease; NSCLC, non‐small cell lung cancer; PD, progressive disease; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; TGK, tumor growth speed; TGR, tumor growth rate; TTF, time to treatment failure.
In the presented case, the first CT scan reports an increase by 42.5% of target lesions (RECIST 1.1) and the appearance of new lesions. The observed progression could be the natural course of tumor growth. However, by comparing the kinetics of tumor growth before and after starting immunotherapy, we can identify the fact that the patient's tumor actually presented a significant acceleration with a tumor growth rate multiplied by 3.2 (TGR ratio; Fig. 3).
Figure 3.
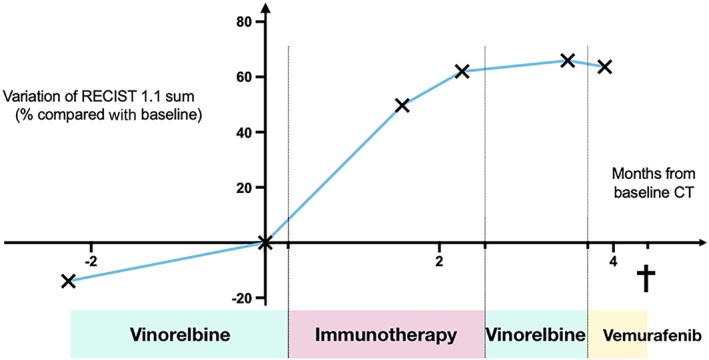
Variations of tumor growth in percentage of target lesions sum compared with baseline (determined by RECIST 1.1) in a 57‐year‐old male treated by an anti‐programmed death‐ligand 1 immunotherapy combined with another immune checkpoint modulator for metastatic urothelial carcinoma. Each cross indicates a CT evaluation. Abbreviation: CT, computed tomography.
Why Is HPD Challenging Our Current Practice?
The current modality of tumor evaluation during immunotherapy is based on a first CT scan performed around 8 weeks after treatment initiation 11. In case of tumor progression at this first imaging, it is recommended to maintain therapy until a confirmatory CT is performed 4 weeks later. In fact, these specific modalities for tumor evaluation during immunotherapy are due to the potential observation of unconventional patterns of responses such as pseudoprogression 12, 13. This new pattern of response has been initially described in patients with advanced melanoma receiving ipilimumab, with patients presenting an objective response after an initial disease progression 12. However, retrospective studies are now showing that pseudoprogression only affects 1%–9% of patients treated with anti‐PD‐(L)1 13. Thus, despite the lack of benefit, a majority of progressive patients are prolonged on immunotherapy.
In the case of HPD, the current method of evaluation is pushing in maintaining patients on therapy while they are experiencing a deleterious effect. Indeed, HPD has been associated with a poorer patient outcome 1. For example, Ferrara et al. report that patients with NSCLC experiencing HPD within the first 6 weeks of PD‐(L)1 inhibitor treatment had significantly lower overall survival (OS) compared with patients with progressive disease (median OS, 3.4 months vs 6.2 months; p = .003) 9. Moreover, in a recent study by Kim et al., 70% of patients undergoing hyperprogression did not receive a subsequent treatment compared with 39% of the patients experiencing a “conventional” progression.
In our case report, immunotherapy was prolonged despite lack of benefit at the first CT scan evaluation. This delay affected not only the timing for therapeutic switch but also the tumor molecular characterization.
Why Is HPD Highly Debated?
The attribution of this tumor deleterious flare‐up phenomenon to immunotherapy remains controversial 14. Some suspect that hyperprogressive disease might just be the natural behavior of an untreated tumor. Thus, the report of an increase of kinetics of tumor growth at the onset of immunotherapy in a subset of patients could be fortuitously concurrent with the natural evolution of disease. In order to suppress this possible bias, kinetics of tumor growth should be analyzed in randomized trials. Unfortunately, reported trials currently lack this information.
Oncologists may want to use classical anti‐PD‐(L)1 predictive biomarkers such as tumor PD‐L1 positivity or tumor mutational burden to select their patients. However, retrospective studies that analyzed kinetics of tumor growth did not identify these markers as predictive of HPD 6, 8, 9, 10. In fact, reported studies have been unable to identify a common predictive factor of HPD. An older age (>65 years of age) 6, a higher number of metastatic lesions at baseline (>2) 9, the occurrence of loco‐regional disease recurrence 8, MDM2 amplifications, or epidermal growth factor receptor alterations 7 have been suspected in separate studies, but overall these predictive factors are contradictory between the different studies.
In the present case, the patient had been heavily pretreated with more than five therapeutic lines in the metastatic setting. This may suggest that HPD in his case may in fact have been related to the natural course of a tumor in a very late stage. However, studies evaluating kinetics of tumor growth did not find an association between HPD and number and types of previous therapies, advanced disease stage, or a poor performance status at baseline 1, 6, 9. No significant differences were detected in terms of blood features at baseline, including serum levels of albumin, lactate dehydrogenase, or lymphocyte counts 1, 6, 9.
Despite lacking clear predictive biomarkers, several biological mechanisms have been proposed to support the HPD phenomenon including expansion of PD‐1+ regulatory T cells, exhaustion of compensatory T cells, modulation of pro‐tumorigenic immune cell subsets, activation of aberrant inflammation or activation of oncogenic signaling 1. Thus, PD‐1 blockade may facilitate the proliferation of highly suppressive intratumoral PD‐1+ effector Treg cells, with a FoxP3high CD45RA− CD4+ phenotype, resulting in HPD 15. Also, analysis of circulating CD8+ lymphocytes at baseline shows that lower frequency of effector/memory subsets (CD45RA−CCR7−) and higher frequency of exhausted TIGIT+ in PD1+ cells were associated with HPD 10. Other reports suggested the role of therapeutic antibody activation of M2‐like CD163+ CD33+ PD‐L1+ macrophages through the Fc γ receptor 16. Finally, Shisuo et al. have reported that PD‐1 may be intrinsically expressed on NSCLC tumor cells and its blockade can promote cancer growth 17.
Adapting Our Practice to the HPD Reality
The evaluation of tumors under immunotherapy currently requires a CT evaluation between weeks 6 and 12. At this first evaluation, the key issue is to differentiate HPD from pseudoprogression. Indeed, pseudoprogression can sometimes be associated with an initial tumor growth acceleration and be confounded with HPD 9. Despite lacking biomarkers for HPD or pseudoprogression, we can easily implement simple practice changes to address this potential major issue (Fig. 4).
Figure 4.
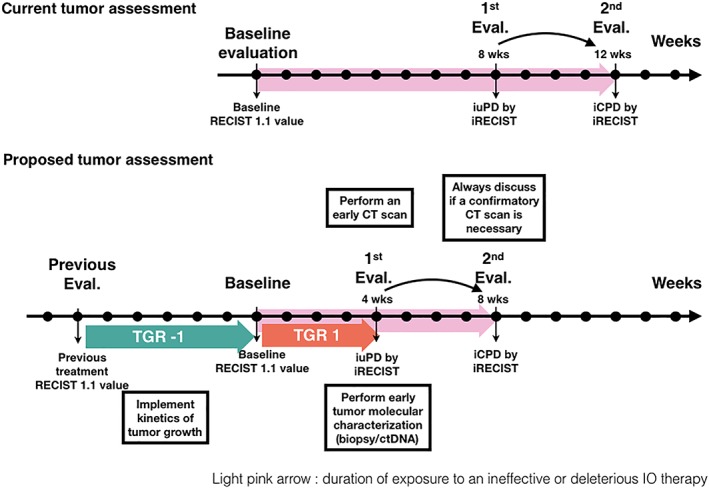
Proposed assessment methods of progressive tumors under immune checkpoint blockade. Abbreviations: CT, computed tomography; ctDNA, circulating tumor DNA; iCPD, immune confirmed progressive disease; iRECIST, immune RECIST criteria; iuPD, immune unconfirmed progressive disease; TGR, tumor growth rate.
First, no matter how much we or the patient expect immunotherapy to work, we should always remember that the diagnosis of pseudoprogression should not be considered in the case of clinical alteration or tumor symptoms worsening.
Second, to limit the duration of exposure to an inefficient or potentially deleterious immunotherapy, we can plan for a systematic early CT scan assessment, that is, around week 3–4 after starting anti‐PD(L)1. Thus, in case of mild or nonthreatening PD at this early time point, patients could have a confirmatory CT scan 1 month later, reducing time for decision of treatment discontinuation. Consequently, a simple change in practice should be able to save precious time and assure a salvage therapy.
We believe that such early CT scans should be performed particularly for patients with NSCLC, HNSCC, and urothelial carcinoma, where several randomized phase III trials have shown early crossing over of survival curves. However, HPD has been observed in other tumor types, so they should probably by monitored with caution as well.
The specific calculation of the kinetics of tumor growth can be implemented in our decision by simply integrating the time in between the different CT evaluations in order to calculate the variations of tumor growth speed 8. However, prebaseline CT scans can be difficult to collect and may be lacking in the context of first‐line therapy. Thus, “fast progression” has been proposed as a surrogate definition of HPD with a ≥50% increase in the sum of long diameters of target lesions within 6 weeks or death due to PD within 12 weeks 18. It has to be noted that “fast progression” may not necessarily overlap with HPD: some patients with HPD could also miss responding to the fast progression criteria if the tumor growth accelerates significantly but without a ≥50% increase in the sum of long diameters of target lesions 14.
In case of an early progression at the first CT scan, tumor molecular characterization by tumor biopsy or circulating tumor DNA should be privileged 19. The 3‐week duration of the molecular analysis allows performing a confirmatory CT at the time of the results and supports (and sometimes guides) the choice of the next therapeutic proposal. Early switch to targeted or cytotoxic therapy may counteract the deleterious flare‐up, even though specific experience in patients with HPD is low.
Finally, the integration of patients into translational programs with comprehensive tumor characterization will be the key for improving the knowledge of the molecular and immunological bases of HPD in order to design the best therapeutic interventions for this subset of patients.
How to Inform Patients About HPD
Whether immunotherapy is directly responsible for the HPD phenomenon is still controversial. However, HPD is a clinical reality that should be shared with our patients. Patients should be informed that hyperprogressive disease is a paradoxical pattern of progression associated with a poor outcome that has been observed in the first month after starting anti‐PD‐(L)1 monotherapy. The reported prevalence of hyperprogressive disease observed in retrospective studies ranges from 6% to 29%. However, data from randomized trials are currently lacking. As suggested by patients’ advocacy representatives during the 2019 HPD session at the American Association for Cancer Research Annual Meeting in Atlanta, GA, the oncologists’ community and their patients need that health authorities and trial sponsors monitor and report the rates of tumor flares in randomized trials.
Patients should be informed that none of the clinical or biological characteristics can help to predict HPD. Even classical predictive biomarkers of response to immunotherapy such as high tumor mutational load or strong PD‐L1 tumor expression cannot exclude a risk of developing HPD. Like the management of any immune‐related adverse events, patients should report any significant worsening of symptoms. Indeed, current management is based on a close and attentive monitoring to allow rapid switch to a different therapeutic line. When possible, the incorporation of liquid biopsies could be complementary to imaging‐based monitoring.
Conclusion
With the development of immune checkpoint blockade therapies, HPD has been reported as a new pattern of progression observed with anti‐PD‐(L)1. Despite the fact that the cause of this tumor flare‐up has been highly debated, a significant proportion of patients seem to be affected. Thus, until rates of tumor flares are reported in randomized trials, we believe that the oncologists’ community should inform patients and adopt simple practice change in tumor monitoring to face this adverse event.
Author Contributions
Conception/design: Miruna Grecea, Aurélien Marabelle, Samy Ammari, Christophe Massard, Stéphane Champiat
Manuscript writing: Miruna Grecea, Aurélien Marabelle, Samy Ammari, Christophe Massard, Stéphane Champiat
Final approval of manuscript: Miruna Grecea, Aurélien Marabelle, Samy Ammari, Christophe Massard, Stéphane Champiat
Disclosures
Stéphane Champiat: Amgen, AstraZeneca, Bristol‐Myers Squibb, Janssen, Merck Sharp & Dohme, Novartis, Roche (H); Aurélien Marabelle, Christophe Massard, Stéphane Champiat: as part of the Drug Development Department (DITEP), are Principal or Sub‐Investigator of Clinical Trials for Abbvie, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen‐X Bvba, Arno Therapeutics, Astex Pharmaceuticals, AstraZeneca, AstraZeneca Ab, Aveo, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Bioalliance Pharma, Biontech Ag, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Inc, Bristol‐Myers Squibb, Bristol‐Myers Squibb International Corporation, Ca, Celgene Corporation, Cephalon, Chugai Pharmaceutical Co, Clovis Oncology, Daiichi Sankyo, Debiopharm S.A., Eisai, Eli Lilly, Exelixis, Forma, Gamamabs, Genentech, Inc, Gilead Sciences, Inc, GlaxoSmithKline, Glenmark Pharmaceuticals, H3 Biomedicine, Inc., Hoffmann La Roche Ag, Incyte Corporation, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Cilag, Janssen Research Foundation, Kura Oncology, Kyowa Kirin Pharm. Dev., Inc, Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Kgaa, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Nanobiotix, Nektar Therapeutics, Nerviano Medical Sciences, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncomed, Oncopeptides, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Pfizer, Pharma Mar, Pierre Fabre Medicament, Plexxikon, Rigontec Gmbh, Roche, Sanofi Aventis, Sierra Oncology, Taiho Pharma, Tesaro, Tioma Therapeutics, Wyeth Pharmaceuticals France, Xencor, Y's Therapeutics, and have also received research grants from AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi, as well as non‐financial support (drug supplied) from AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Johnson & Johnson, Lilly, Medimmune, Merck, NH TherAGuiX, Pfizer, and Roche. The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Champiat S, Ferrara R, Massard C et al. Hyperprogressive disease: Recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018;15:748–762. [DOI] [PubMed] [Google Scholar]
- 2. Powles T, Durán I, van der Heijden MS et al. Atezolizumab versus chemotherapy in patients with platinum‐treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open‐label, phase 3 randomised controlled trial. Lancet 2018;391:748–757. [DOI] [PubMed] [Google Scholar]
- 3. Bellmunt J, de Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferris RL, Blumenschein G, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Champiat S, Dercle L, Ammari S et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti‐PD‐1/PD‐L1. Clin Cancer Res 2017;23:1920–1928. [DOI] [PubMed] [Google Scholar]
- 7. Kato S, Goodman A, Walavalkar V et al. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saâda‐Bouzid E, Defaucheux C, Karabajakian A et al. Hyperprogression during anti‐PD‐1/PD‐L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605–1611. [DOI] [PubMed] [Google Scholar]
- 9. Ferrara R, Mezquita L, Texier M et al. Hyperprogressive disease in patients with advanced non‐small cell lung cancer treated with PD‐1/PD‐L1 inhibitors or with single‐agent chemotherapy. JAMA Oncol 2018;4:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim C, Kim K, Pyo K‐H et al. Hyperprogressive disease during PD‐1/PD‐L1 blockade in patients with non‐small‐cell lung cancer. Ann Oncol 2019;30:1104–1113. [DOI] [PubMed] [Google Scholar]
- 11. Seymour L, Bogaerts J, Perrone A et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolchok JD, Hoos A, O'Day S et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune‐related response criteria. Clin Cancer Res 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 13. Borcoman E, Kanjanapan Y, Champiat S et al. Novel patterns of response under immunotherapy. Ann Oncol 2019;30:385–396. [DOI] [PubMed] [Google Scholar]
- 14. Champiat S, Besse B, Marabelle A. Hyperprogression during immunotherapy: Do we really want to know? Ann Oncol 2019;30:1028–1031. [DOI] [PubMed] [Google Scholar]
- 15. Kamada T, Togashi Y, Tay C et al. PD‐1+ regulatory T cells amplified by PD‐1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci 2019;116:201822001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lo Russo G, Moro M, Sommariva M et al. Antibody‐Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non‐small cell lung cancer subsequent to PD‐1/PD‐L1 blockade. Clin Cancer Res 2019;25:989–999. [DOI] [PubMed] [Google Scholar]
- 17. Du S, McCall N, Park K et al. Blockade of tumor‐expressed PD‐1 promotes lung cancer growth. Oncoimmunology 2018;7:e1408747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gandara D, Reck M, Morris S et al. LBA1Fast progression in patients treated with a checkpoint inhibitor (cpi) vs chemotherapy in OAK, a phase III trial of atezolizumab (atezo) vs docetaxel (doc) in 2L+ NSCLC. Ann Oncol 2018;29(suppl 10). [Google Scholar]
- 19. Weiss GJ, Beck J, Braun DP et al. Tumor cell‐free DNA copy number instability predicts therapeutic response to immunotherapy. Clin Cancer Res 2017;23:5074–5081. [DOI] [PubMed] [Google Scholar]


