Abstract
Every year millions of pulmonary nodules are discovered incidentally and through lung cancer screening programs. Management of these nodules is often suboptimal, with low follow‐up rates and poor provider understanding of management approaches. There is an emerging body of literature about how to optimize management of pulmonary nodules. The Pulmonary Nodule and Lung Cancer Screening Clinic (PNLCSC) at Massachusetts General Hospital was founded in 2012 to manage pulmonary nodules via a multidisciplinary approach with optimized support staff. Recommendations from clinic providers and treatment details were recorded for all patients seen at the PNLCSC. Adherence to recommendations and outcomes were also tracked and reviewed. From October 2012 to September 2019, 1,136 patients were seen at the PNLCSC, each for a mean of 1.8 appointments (range, 1–10). A total of 356 procedures were recommended by the clinic and 271 patients were referred for surgery and/or radiation. The majority of interventions (74%) were recommended at the initial PNLCSC appointment. In total, 211 patients (19%) evaluated at the PNLCSC had pathologically confirmed pulmonary malignancies or were treated empirically with radiation. Among patients followed by the clinic, the adherence rate to clinic recommendations was 95%. This study shows how a multidisciplinary approach to pulmonary nodule management can streamline care and optimize follow‐up. The PNLCSC provides a template that can be replicated in other health systems. It also provides an example of how multidisciplinary approaches can be applied to other complex conditions.
Implications for Practice
This work demonstrates how an integrated, multidisciplinary approach to management of pulmonary nodules can streamline patient care and improve adherence to provider recommendations. This approach has the potential to improve patient outcomes and reduce health care costs.
Keywords: Lung cancer, Low‐dose CT, Cancer screening, Pulmonary nodules, Multidisciplinary care
Short abstract
This descriptive study summarizes the experiences during the first 6 years of the Pulmonary Nodule and Lung Cancer Screening Clinic at Massachusetts General Hospital and describes the effect of an integrated, multidisciplinary approach on patient care.
Introduction
A recent study estimated 1.6 million pulmonary nodules were incidentally discovered on computed tomography (CT) scans in 2012 1. Given trends in imaging use and demographics, this number could rise to nearly 2 million incidentally discovered pulmonary nodules by 2022 2. Additionally, despite low adherence rates with U.S. Preventive Services Task Force (USPSTF) lung cancer screening recommendations, it is estimated that between 250,000 and 2.2 million patients received low‐dose chest CTs (LDCTs) in 2015 3, 4, 5. Based on the rate of positive screening seen in the National Lung Cancer Screening Trial, these LDCTs could discover several hundred thousand additional pulmonary nodules requiring follow‐up 6.
Frequently, chest CT scans are interpreted by general radiologists who may lack specialized training in the interpretation and management of pulmonary nodules, and physicians ordering the radiology studies similarly feel they do not have adequate information to appropriately manage follow‐up for pulmonary nodules 7. Different types of nodules may require follow‐up from different specialists—pulmonologists, medical oncologists, surgical oncologists, radiation oncologists, infectious disease experts—and many physicians do not know how to appropriately direct referrals and coordinate follow‐up care. These challenges are some of the reasons as many as 35% of radiology studies showing pulmonary nodules do not receive appropriate follow‐up 8. Suboptimal follow‐up recommendations, delays accessing the appropriate specialists, and unnecessary procedures can lead to delayed lung cancer diagnoses 9, 10, more advanced tumor stage at the time of diagnosis, worse prognoses, and higher health care costs 11, 12. As health systems seek to increase value and improve outcomes, they must develop resources to facilitate timely interpretation and management of pulmonary nodules.
Several organizations, including the National Comprehensive Cancer Network (NCCN), the Fleischner Society, and the International Association for the Study of Lung Cancer, have recommended approaches that include multidisciplinary evaluation of lung nodules to ensure timely, appropriate management 13, 14, 15, 16. Multiple programs related to lung cancer screening have been described in the literature. However, these programs focused on increasing use of screening services and developing systems to flag high‐risk nodules for follow‐up rather than optimizing the follow‐up 17, 18. There are no published data on how integrated, multidisciplinary programs may affect follow‐up care and management of pulmonary nodules. This descriptive study summarizes data from the first 6 years of the Pulmonary Nodule and Lung Cancer Screening Clinic (PNLCSC) at Massachusetts General Hospital, and describes how integrated, multidisciplinary management may impact patient care.
Materials and Methods
The Pulmonary Nodule and Lung Cancer Screening Clinic (PNLCSC) at Massachusetts General Hospital was founded in 2012 with the goal of improving the management of pulmonary nodules. The clinic accepts patients with imaging findings that meet the following criteria: a lung cancer screening CT of the chest with a finding of Lung‐RADS 4, any pulmonary nodule (subsolid, mixed, or solid) 6 mm in diameter or greater found incidentally on a CT scan of the chest or a screen‐detected nodule that warrants further evaluation in the opinion of the referring provider and a member of the PNLCSC staff. The clinic is staffed by specialists from interventional radiology, medical oncology, radiation oncology, surgical oncology, and pulmonology, as well as nurse practitioners (NPs), an access nurse navigator, and a tobacco treatment specialist.
Figure 1 illustrates the general structure of the PNLCSC. Upon referral to the clinic, patients are scheduled for an initial appointment. Prior to the initial appointment, the nurse navigator obtains any pertinent radiology images and collates the patients’ medical records. This information is reviewed in a multidisciplinary meeting held prior to the clinic where providers collectively determine a preliminary recommendation for management of the lung nodule and which specialist should evaluate each patient during the appointment. Patients are seen at initial visits by one or more of the team physicians based on the preliminary recommendations. Follow‐up visits in the clinic are discussed in multidisciplinary preclinic conferences and then seen by NPs if follow‐up surveillance is recommended or by the specialist physicians if intervention is warranted. All patients with a history of smoking are offered smoking cessation counseling with on‐site and virtual smoking cessation services available. Additional details about the clinic workflow and structure have been previously reported 19.
Figure 1.
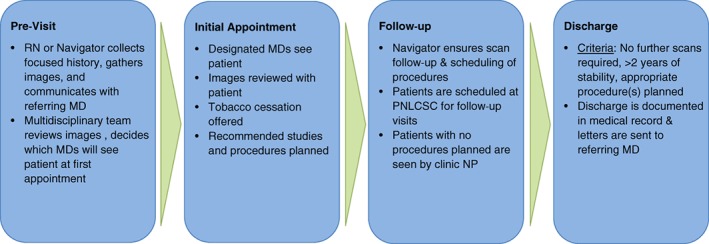
Overview of PNLCSC workflow.Abbreviations: MD, doctor of medicine; PNLCSC, Pulmonary Nodule and Lung Cancer Screening Clinic; RN, registered nurse.
All patients are followed within the clinic until a final treatment plan is recommended (additional imaging, biopsy, surgery, radiation, or ablation) or it is determined that their imaging findings are stable and the patient can return to routine follow‐up under the guidance of the referring physician. Clinic providers follow NCCN and Fleischner Society guidelines to guide management 13, 16. Candidates for empiric radiation are selected using established guidelines 20. Throughout the time patients are followed by the PNLCSC, the nurse navigator regularly communicates with patients in person and via phone to improve patient comprehension of provider recommendations, ensure adherence to these recommendations, and facilitate scheduling of imaging and procedures.
The clinic has collected data from all patients seen in the PNLCSC since 2012. This analysis includes data through September 30, 2019. When patients come to their initial PNLCSC consultation, they complete a brief background survey that includes demographic information and details about medical care received prior to referral to the PNLCSC. Clinic staff record the treatment and monitoring plan recommended for each patient by the PNLCSC team and perform chart reviews to record treatment details and diagnoses. Clinical staff also collect rates of adherence to clinic recommendations while patients are being actively followed by the PNLCSC. Institutional review board approval was received from the Partners Human Research Committee, and data were stored and managed using a secure online database. All statistical analyses were done using Stata version 15.1.
Results
During the period of analysis, 1,136 patients were evaluated at the PNLCSC. The characteristics of these patients are described in Table 1. Patient age was normally distributed with a mean of 67.2 years (SD, 11.1; range, 20–101). PNLCSC patients were 57.8% female, 89.2% white, and 53.4% married, and 29.2% were college graduates (although 29.0% of patients declined to provide their highest level of education). The majority (72.4%) of patients were current or former smokers and 11.8% had known prior asbestos exposure. Among patients with a history of smoking, mean pack‐year history was 40.3 (SD, 26.0), with a range of 0.1–180. Consistent with the high rate of health insurance coverage in Massachusetts, approximately 99% of patients were medically insured. Primary care providers made 63.6% of the referrals to the PNLCSC, specialists made 27.8% of the referrals, and 8.5% of patients were self‐referred. The median time interval between the diagnostic study prompting referral and the initial appointment was 29 days (range, 1–148).
Table 1.
Demographics of the 1,136 patients seen at the PNLCSC from October 2012–September 2019
| Variable | n (%), unless otherwise noted |
|---|---|
| Age, mean (SD) | 67.2 (11.1) |
| Gender | |
| Female | 657 (57.8) |
| Male | 479 (42.2) |
| Race | |
| White | 1,013 (89.2) |
| Asian | 44 (3.9) |
| Black/African‐American | 37 (3.3) |
| American Indian/Alaska Native | 2 (0.2) |
| Hispanic | 9 (0.9) |
| Other | 31 (2.7) |
| Education | |
| Less than High School/GED | 53 (4.7) |
| High School/GED | 255 (22.5) |
| Some College/Bachelors | 55 (4.8) |
| College graduate | 332 (29.2) |
| Postgraduate/professional | 112 (9.9) |
| Declined to answer | 329 (29.0) |
| Marital status | |
| Married/living with partner | 607 (53.4) |
| Single, never married | 263 (23.2) |
| Divorced | 148 (13.0) |
| Widowed | 90 (7.9) |
| Declined | 28 (2.5) |
| Health insurance | |
| Medicare/Medicaid | 494 (43.5) |
| Private | 630 (55.5) |
| None/unknown | 12 (1.1) |
| Smoking status | |
| Current | 297 (26.1) |
| Former | 526 (46.3) |
| Never | 313 (27.6) |
| Asbestos exposure | |
| Yes | 134 (11.8) |
| No | 842 (74.1) |
| Unknown | 134 (11.8) |
| TB exposure | |
| Yes | 53 (4.7) |
| No | 933 (82.1) |
| Unknown | 150 (13.2) |
Abbreviations: GED, General Educational Development; TB, tuberculosis.
Most nodules were found incidentally (n = 905, 79.7%), and 20.3% were found during routine lung cancer screening (n = 231). The number of referrals to the PNLCSC program increased annually. The proportion of referrals for screening‐detected nodules also increased each year since the USPSTF recommendations were first issued in 2013 (Fig. 2). In 2018, the last full year of analysis, 67 nodules (25.4%) evaluated at the PNLCSC were discovered through LDCT screening scans. Lung‐RADS categorizations were available for 228 patients at the time of their initial appointment. Consistent with the referral criteria for the clinic, Lung‐RADS scores for referred patients were skewed toward the higher scores, with a corresponding higher probability of malignancy (Table 2). Among patients referred to the clinic from screening LDCTs, 26 patients had fewer than 30 pack‐years of smoking and 18 patients quit smoking more than 15 years ago. In total, 17.4% of patients referred from screening LDCTs (n = 40) did not meet USPSTF criteria for lung cancer screening 21.
Figure 2.
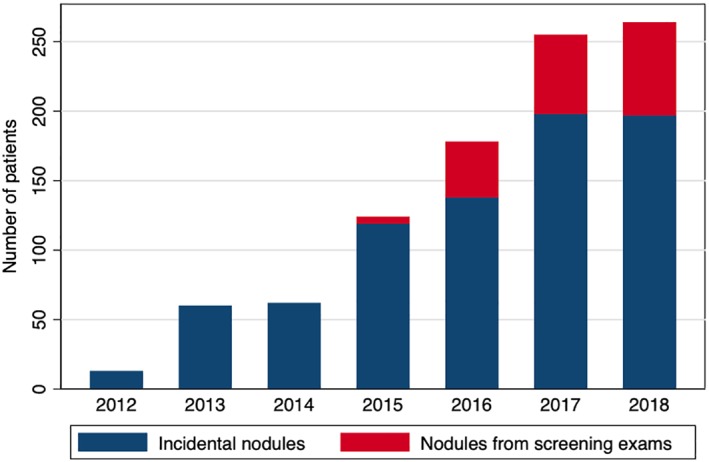
Number of new patients seen at PNLCSC by year (2012–2018).Abbreviation: PNLCSC, Pulmonary Nodule and Lung Cancer Screening Clinic.
Table 2.
Lung‐RADS categorizations for patients who had undergone a lung screening computed tomography scan at the time of initial evaluation
| Lung‐RADS category | n (%) |
|---|---|
| 2 | 10 (4.4) |
| 2S | 5 (2.2) |
| 3 | 10 (4.4) |
| 3 S | 8 (3.5) |
| 4 | 1 (0.4) |
| 4A | 72 (31.6) |
| 4A S | 59 (25.9) |
| 4B | 25 (11.0) |
| 4B S | 15 (6.6) |
| 4X | 11 (4.8) |
| 4X S | 11 (4.8) |
| S | 1 (0.4) |
| Total | 228 (100) |
Percent refers to percent of pulmonary nodules that had Lung‐RADS categorization assigned at time of initial evaluation. Most patients did not have Lung‐RADS categorization as this is only assigned on computed tomography (CT) scans ordered as a screening CT scan.
Over half the patients (55.0%) were seen at the PNLCSC clinic just once. The mean number of appointments per patient was 1.8 (median, 1; range, 1–10). Figure 3 shows the number of visits per patient and the total time they were followed by the clinic, categorized by the type of intervention (biopsy, surgery, radiation therapy, or no intervention) ultimately recommended. Interventions were recommended to 23.1% (n = 262) of patients at the first visit, and 73.6% of all interventions recommended by the PNLCSC were recommended at the initial appointment. PNLCSC providers recommended further imaging to 81.3% of patients (n = 922), and 18.5% of patients (n = 209) were discharged from the clinic after the first visit to follow‐up with the referring provider. Supplemental online Table 1 summarizes recommendations made at initial visits. While patients were followed by the PNLCSC, the adherence rate to clinic recommendations was 95.0%.
Figure 3.
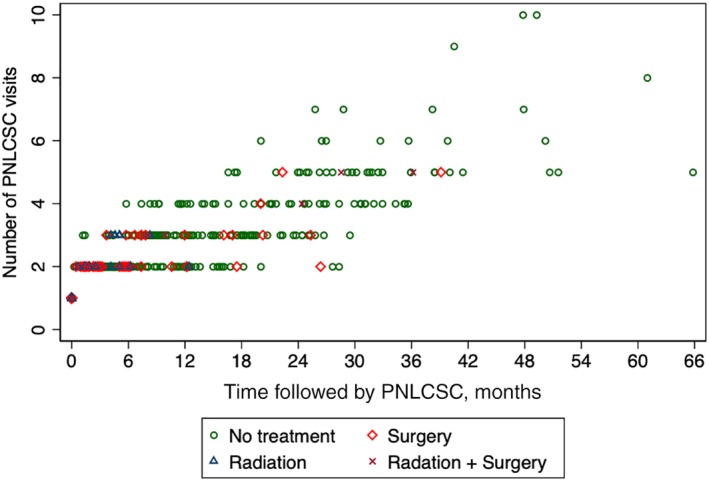
Scatter plot of number of visits vs time followed by the PNLCSC for each patient evaluated at the clinic, grouped by type of final PNLCSC recommendation.Abbreviation: PNLCSC, Pulmonary Nodule and Lung Cancer Screening Clinic.
In total, PNLCSC physicians recommended interventions (biopsy, surgery, or radiation) to 356 patients (Table 3). Approximately one‐quarter (23.8%, n = 271) of patients evaluated at the clinic were recommended to undergo definitive procedures (surgery or radiation). Of the patients who were recommended surgery or radiation, 203 (87.1%) underwent surgery and 46 (85.2%) underwent radiation treatment without having a biopsy before the intervention. When surgical management was recommended, the median interval between the initial appointment and surgery was 47 days (range, 4–1238). Twelve patients were followed by the clinic for more than 1 year before undergoing surgery. In each of these cases, surveillance was recommended at the first PNLCSC visit and then interval changes led to the decision to pursue surgery at a later date.
Table 3.
Final intervention recommendations made by PNLCSC providers
| Recommendations | Number | Percent of PNLCSC patients | Percent of interventions |
|---|---|---|---|
| Any Intervention | 356 | 31.3 | 100 |
| Biopsy | 122 | 10.7 | 34.3 |
| Surgery | 233 | 20.5 | 65.4 |
| Bronchoscopy | 89 | 7.8 | 25.0 |
| Mediastinoscopy | 15 | 1.3 | 4.2 |
| Wedge resection | 105 | 9.2 | 29.5 |
| Lobectomy | 58 | 5.1 | 16.3 |
| VATS | 100 | 8.8 | 28.1 |
| Segmentectomy | 15 | 1.3 | 4.2 |
| Radiation | 54 | 4.8 | 15.2 |
| No Intervention | 780 | 68.7 | ‐ |
Multiple interventions were recommended for some patients. Radiation + surgery was recommended to 16 patients.
Abbreviations: PNLCSC, Pulmonary Nodule and Lung Cancer Screening Clinic; VATS, video‐assisted thoracoscopic surgery.
Pathologic diagnoses were recorded for 256 patients (22.5%) seen at the PNLCSC (Table 4). Non‐small cell lung cancer (NSCLC) was diagnosed in 170 patients. Just three patients were diagnosed with small cell lung cancer (SCLC), and 12 nodules were diagnosed as nonpulmonary malignancies. Of patients diagnosed with NSCLC after evaluation at PNLCSC, 94.3% had stage I or stage II NSCLC at the time of diagnosis. When accounting for the additional 42 patients who were treated empirically with radiation therapy, 215 patients (18.9%) evaluated at the PNLCSC were diagnosed with or treated empirically for primary lung malignancies.
Table 4.
Diagnosis and staging information from PNLCSC patients who underwent procedures that allowed for pathologic diagnosis
| Diagnostic procedure | Biopsy | Surgery | Totala |
|---|---|---|---|
| NSCLC | 39 | 131 | 170 |
| Adenocarcinoma | 28 | 113 | 141 |
| AIS/MIA | 2 | 4 | 6 |
| Stage IA | 13 | 88 | 101 |
| Stage IB | 1 | 8 | 9 |
| Stage IC | 2 | 0 | 2 |
| Stage IIA | 1 | 5 | 6 |
| Stage IIB | 0 | 2 | 2 |
| Stage IIIA | 2 | 2 | 4 |
| Stage IIIB | 2 | 0 | 2 |
| Stage IV | 0 | 2 | 2 |
| Unknown | 5 | 2 | 7 |
| Squamous cell carcinoma | 6 | 11 | 17 |
| Stage IA | 4 | 7 | 11 |
| Stage IB | 0 | 3 | 3 |
| Stage IIA | 0 | 1 | 1 |
| Stage IIIA | 1 | 0 | 1 |
| Unknown | 1 | 0 | 1 |
| Lung carcinoid | 4 | 6 | 10 |
| Stage IA | 3 | 2 | 5 |
| Stage IB | 0 | 2 | 2 |
| Stage IIB | 0 | 1 | 1 |
| Unknown | 1 | 1 | 2 |
| Other NSCLC | 1 | 1 | 2 |
| SCLC | 2 | 1 | 3 |
| LS‐SCLC | 2 | 1 | 3 |
| Other malignancyb | 6 | 6 | 12 |
| No malignancyc | 35 | 29 | 64 |
| Nondiagnostic | 6 | 1 | 7 |
| Total | 82 | 167 | 256 |
Bolded rows represent pathologic diagnoses. Additional rows represent staging information.
Some patients underwent recommended procedures in different health systems, so information on diagnosis and staging could not be recorded.
Other malignancies included breast adenocarcinoma, hepatocellular carcinoma, Langerhans cell histiocytosis, melanoma, Müllerian adenocarcinoma, pancreatic adenocarcinoma, renal cell carcinoma, squamous cell carcinoma of unknown primary.
Benign diagnoses included hamartomas, Cryptococcus, granulomas, intraparenchymal lymph nodes, lymphoid hyperplasia.
Abbreviations: AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; LS‐SCLC, limited‐stage small cell lung cancer; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer.
Pathology demonstrated no evidence of malignancy in 29 (17.4%) patients who underwent surgical procedures and 35 (42.7%) of patients who underwent biopsies. Of the 29 patients who underwent surgical procedures and were found to have no evidence of malignancy, 5 underwent only video‐assisted thoracoscopic surgery (VATS) or mediastinoscopy and 19 underwent wedge resections. Five patients who underwent lobectomies had no evidence of malignancy on pathology.
Discussion
This study describes the experience of over 1,000 patients seen at one of the first multidisciplinary clinics for the management of pulmonary nodules. It demonstrates process outcomes that suggest this approach leads to streamlined assessment and improved adherence. Patients seen at the PNLCSC represent a high‐risk population, with nearly 20% of patients ultimately diagnosed with malignancy. Nearly 95% of PNLCSC patients diagnosed with NSCLC had stage I or stage II disease at the time of diagnosis, and the median time interval before intervention for patients was less than 50 days. Most significantly, the adherence rate to clinic recommendations was approximately 95% across all patients seen at the clinic. These numbers compare favorably to previously reported data 22, 23, 24. Given the high rates of early stage diagnoses, high adherence rate, and reduced intervals to initiation of treatment recommended by a multidisciplinary team, few PNLCSC patients are lost to follow‐up or experience unnecessary treatment delays. These features could potentially improve outcomes, improve patient satisfaction, reduce costs and reduce the frequency of malpractice suits 25, 26.
As with all screening programs, these potential benefits need to be carefully weighed against the potential harms of unnecessary invasive procedures 13. Twenty‐nine individuals recommended for surgery, 17.4% of patients who underwent surgical procedures, were found to have no evidence of malignancy on pathology. Five of these patients (86.2%) underwent only VATS or mediastinoscopy, the least invasive approach to obtain tissue from the concerning lesions. The acceptable rate of benign nodules is not firmly established, with previously reported rates ranging from 10% to 30% 17, 27. In many PNLCSC patients, the decision to pursue upfront resection is influenced by the risk of biopsy, severity of the imaging findings, and the ability to do an intraoperative wedge resection or segmentectomy for pathology confirmation before lobectomy. However, the potential harms of unnecessary interventions must continue to be carefully considered in treatment decisions.
This multidisciplinary approach may help avoid some unnecessary procedures. Eighty‐five percent of patients who underwent radiation therapy (mostly stereotactic body radiation therapy [SBRT]) after evaluation at the PNLCSC did so without a preceding biopsy. SBRT without biopsy is often appropriate if the result of a biopsy is unlikely to change the management plan, obtaining a biopsy represents significant risk, and prior testing raises high suspicion for malignancy 20. Multidisciplinary evaluation helps identify the patients who can proceed directly to treatment and not incur the risk of an unnecessary biopsy. Similarly, patients with multiple comorbidities or attributes that make surgery challenging can be simultaneously evaluated for SBRT without waiting for separate appointments.
The presence of a nurse navigator within the clinic likely contributed to the high adherence rates. Several studies have shown the benefits of nurse navigators in cancer care, particularly in processes in which patients can easily feel lost and become lost to follow‐up 28, 29, 30. Pulmonary nodule management is a complex process that can be confusing for patients. The nurse navigator provides education, ensures adherence to recommendations, and facilitates patients’ transitions from the multidisciplinary clinic to individual specialists’ care. We believe this role was one of the primary drivers of the high adherence rates seen at the PNLCSC.
The effects of the PNLCSC on outcomes, costs, patient satisfaction, and malpractice claims could not be assessed with the available data. Future efforts to collect outcomes, cost, and patient satisfaction data for PLNCSC patients will improve understanding of the full breadth of the clinic's effects. Additionally, developing comparison groups of similar patients treated in other systems or using other approaches will create opportunities to more rigorously evaluate how the PNLCSC affects outcomes, treatment costs, and patient experience.
The low proportion of minority patients in the study population limits this analysis. Only 10.8% of the patients evaluated by the PLNCSC over the period of analysis identified as minorities. This pattern is consistent with demographic data from similar institutions providing oncology care in the region 31. Prior research has also demonstrated issues with LDCT screening guidelines and lower screening rates among minorities that could have reduced the proportion of minority patients evaluated at this clinic 32, 33, 34. Regardless of the reason, these data again highlight the imperative to improve access to high‐quality oncology care in minority populations. The lack of racial diversity in this patient population prevents assessment of how similar programs may impact care in diverse communities and whether similar programs could target specific populations to address health disparities. Given the improved metrics seen in the clinic population, the lack of minority patients seen at the PNLCSC could lead to increased racial disparities if it is not addressed in the future. Recent outreach efforts by the PNLCSC have resulted in an increased proportion of minority patients. However, these data do not contain sufficient information to further analyze the reasons minorities are underrepresented in this patient population.
This retrospective review only includes patients that were evaluated at the PNLCSC. It does not include all pulmonary nodules found within this health system. Automated referrals to PNLCSC would capture a more complete panel of patients with high‐risk pulmonary nodules. It also does not assess the appropriateness of lung cancer screening across the system. The high percentage of patients referred to the clinic based on LDCT scans who did not meet criteria for lung cancer screening suggests more outreach is needed to educate providers on lung cancer screening criteria. Future efforts will focus on automating referrals, improving communication among providers and developing infrastructure to manage all patients with pulmonary nodules.
These results also raise questions about what additional health conditions may benefit from similar multidisciplinary care models. The PNLCSC's multidisciplinary evaluation and intensive care coordination allows patients to receive recommendations faster and increases compliance with recommendations. Other medical conditions such as chronic back pain, morbid obesity, and other malignancies that require input from multiple specialists and integrated treatment approaches may benefit from similar approaches. Future efforts should focus on ways this model of care can be applied to other complex medical conditions.
Author Contributions
Conception/design: Thomas J. Roberts, Inga T. Lennes, Lecia V. Sequist, Elyse R. Park, Henning Willers, Angela Frank, Henning Gaissert, Jo‐Anne Shepard, David Ryan
Provision of study material or patients: Inga T. Lennes, Elyse R. Park, Henning Willers, Angela Frank, Henning Gaissert, Lecia V. Sequist, Jo‐Anne Shepard, David Ryan
Collection and/or assembly of data: Thomas J. Roberts, Inga T. Lennes, Saif Hawari
Data analysis and interpretation: Thomas J. Roberts, Inga T. Lennes
Manuscript writing: Thomas Roberts, Inga T. Lennes, Saif Hawari, Lecia V. Sequist, Elyse R. Park, Henning Willers, Angela Frank, Henning Gaissert, Jo‐Anne Shepard, David Ryan
Final approval of manuscript: Thomas Roberts, Inga T. Lennes, Saif Hawari, Lecia V. Sequist, Elyse R. Park, Henning Willers, Angela Frank, Henning Gaissert, Jo‐Anne Shepard, David Ryan
Disclosures
Lecia V. Sequist: AstraZeneca, Janssen (C/A) Novartis, AstraZeneca, Boehringer Ingelheim, Genentech, Blueprint, LOXO (RF); David Ryan: MPM Capital (C/A, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgements
We acknowledge the contributions of the Pulmonary Nodule Research Group at Massachusetts General Hospital.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Editor's Note: See the related commentary, “Exploring Ways to Improve Access to and Minimize Risk from Lung Cancer Screening,” by Humberto Choi and Nathan A. Pennell on page https://doi.org/10.1634/theoncologist.2020-0149 of this issue.
References
- 1. Gould MK, Tang T, I‐LA Liu et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med 2015;192:1208–1214. [DOI] [PubMed] [Google Scholar]
- 2. Computed tomography (CT) exams . OECD iLibrary website. Available at https://www.oecd-ilibrary.org/social-issues-migration-health/computed-tomography-ct-exams/indicator/english_3c994537-en. Accessed March 12, 2019.
- 3. Nishi S, Zhou J, Kuo YF et al. Use of lung cancer screening with low‐dose computed tomography in the medicare population. Mayo Clin Proc Innov Qual Outcomes 2019;3:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richards TB, Doria‐Rose VP, Soman A et al. Lung cancer screening inconsistent with U.S. Preventive Services Task Force recommendations. Am J Prev Med 2019;56:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jemal A, Fedewa SA. Lung cancer screening with low‐dose computed tomography in the United States‐2010 to 2015. JAMA Oncol 2017;3:1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Lung Screening Trial Research Team ; Aberle DR, Adams AM et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med 2011;365:395‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golden SE, Wiener RS, Sullivan D et al. Primary care providers and a system problem: A qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest 2015;148:1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Callen JL, Westbrook JI, Georgiou A et al. Failure to follow‐up test results for ambulatory patients: A systematic review. J Gen Intern Med 2012;27:1334–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koyi H, Hillerdal G, Brandén E. Patients’ and doctors’ delays in the diagnosis of chest tumors. Lung Cancer 2002;35:53–57. [DOI] [PubMed] [Google Scholar]
- 10. Salomaa ER, Sällinen S, Hiekkanen H et al. Delays in the diagnosis and treatment of lung cancer. Chest 2005;128:2282–2288. [DOI] [PubMed] [Google Scholar]
- 11. Gildea TR, DaCosta Byfield S, Hogarth DK et al. A retrospective analysis of delays in the diagnosis of lung cancer and associated costs. Clin Outcomes Res 2017;9:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cipriano LE, Romanus D, Earle CC et al. Lung cancer treatment costs, including patient responsibility, by stage of disease and treatment modality, 1992–2003. Value Health 2011;14:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wood DE, Kazerooni EA, Baum SL et al. Lung cancer screening, version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fintelmann FJ, Bernheim A, Digumarthy SR et al. The 10 pillars of lung cancer screening: Rationale and logistics of a lung cancer screening program. Radiographics 2015;35:1893–1908. [DOI] [PubMed] [Google Scholar]
- 15. Ito M, Miyata Y, Okada M. Management pathways for solitary pulmonary nodules. J Thorac Dis 2018;10(Suppl 7):S860–S866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacMahon H, Naidich DP, Goo JM et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology 2017;284:228–243. [DOI] [PubMed] [Google Scholar]
- 17. McKee BJ, McKee AB, Flacke S et al. Initial experience with a free, high‐volume, low‐dose CT lung cancer screening program. J Am Coll Radiol 2013;10:586–592. [DOI] [PubMed] [Google Scholar]
- 18. Wrightson WR, Gauhar U, Hendler F et al. Centralized lung nodule management at a veterans hospital using a multidisciplinary lung nodule evaluation team (LNET). Zhongguo Fei Ai Za Zhi 2018;21:828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campo MJ, Lennes IT. Managing patients with screen‐detected nodules: The Nodule Clinic. Semin Roentgenol 2017;52:161–165. [DOI] [PubMed] [Google Scholar]
- 20. Berman AT, Jabbour SK, Vachani A et al. Empiric Radiotherapy for Lung Cancer Collaborative Group multi‐institutional evidence‐based guidelines for the use of empiric stereotactic body radiation therapy for non‐small cell lung cancer without pathologic confirmation. Transl Lung Cancer Res 2019;8:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Final recommendation statement: Lung cancer: Screening. U.S . Preventive Services Task Force website. Available at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening. Accessed October 29, 2019.
- 22. Vidaver RM, Shershneva MB, Hetzel SJ et al. Typical time to treatment of patients with lung cancer in a multisite, US‐based study. J Oncol Pract 2016;12:e643–e653. [DOI] [PubMed] [Google Scholar]
- 23. Myrdal G, Lambe M, Hillerdal G et al. Effect of delays on prognosis in patients with non‐small cell lung cancer. Thorax 2004;59:45–49. [PMC free article] [PubMed] [Google Scholar]
- 24. O'Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141–144. [DOI] [PubMed] [Google Scholar]
- 25. Noone A, Holander N, Krapcho M et al. SEER Cancer Statistics Review, 1975‐2015. Bethesda, MD: National Cancer Institute; Available at https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_15_lung_bronchus.pdf. Accessed June 1, 2019. [Google Scholar]
- 26. Baker SR, Patel RH, Yang L et al. Malpractice suits in chest radiology: An evaluation of the histories of 8265 radiologists. J Thorac Imaging 2013;28:388–391. [DOI] [PubMed] [Google Scholar]
- 27. Grogan EL, Weinstein JJ, Deppen SA et al. Thoracic operations for pulmonary nodules are frequently not futile in patients with benign disease. J Thorac Oncol 2011;6:1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagner EH, Ludman EJ, Aiello Bowles EJ et al. Nurse navigators in early cancer care: A randomized, controlled trial. J Clin Oncol 2014;32:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vargas RB, Ryan GW, Jackson CA et al. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer 2008;113:426–433. [DOI] [PubMed] [Google Scholar]
- 30. Hendren S, Fiscella K. Patient navigation improves the care experience for patients with newly diagnosed cancer J Clin Oncol 2014;32:3–4. [DOI] [PubMed] [Google Scholar]
- 31. Kowalczyk L, Wallack T, Dungca N et al. Color line persists, in sickness as in health. http://bostonglobe.com. Available at https://apps.bostonglobe.com/spotlight/boston-racism-image-reality/series/hospitals. Published December 12, 2017. Accessed March 12, 2019.
- 32. Annangi S, Nutalapati S, Foreman MG et al. Potential racial disparities using current lung cancer screening guidelines. J Racial Ethn Health Disparities 2019;6:22–26. [DOI] [PubMed] [Google Scholar]
- 33. Japuntich SJ, Krieger NH, Salvas AL et al. Racial disparities in lung cancer screening: An exploratory investigation. J Natl Med Assoc 2018;110:424–427. [DOI] [PubMed] [Google Scholar]
- 34. Li CC, Matthews AK, Rywant MM et al. Racial disparities in eligibility for low‐dose computed tomography lung cancer screening among older adults with a history of smoking. Cancer Causes Control 2019;30:235–240. [DOI] [PubMed] [Google Scholar]


