Abstract
Purpose
This article reports on the long‐term impact of radiotherapy adapted to stage, histology, and previous resection in a large cohort of patients with intestinal lymphoma (iL) treated with definitive or adjuvant curative‐intent radiation therapy (RT) ± chemotherapy (CHOP, MCP, or COP).
Patients and Methods
In two consecutive prospective study designs, 134 patients with indolent (stage IE–IIE) or aggressive (stage IE–IVE) iL were referred to 61 radiotherapeutic institutions between 1992 and 2003. Patients with indolent iL received extended field (EF) 30 Gy (+10 Gy boost in definitive treatment); patients with aggressive iL received involved field (IF) (EF) 40 Gy by means of stage‐, histology‐, and operation‐adapted radiation fields.
Results
The patients had median age 58 years and were predominantly male (2:1). Histology showed aggressive prevalence (1.6:1), stage IE–to–stage IIE ratio of iL 1.04:1, and localized stages–to–advanced stages ratio of aggressive lymphoma 23:1. Median follow‐up was in total 11.7 years: 10.0 years in the first study, GIT (GastroIntestinal‐Tract) 1992, and 11.8 years in the second study, GIT 1996. Lymphoma involvement was predominantly a single intestinal lesion (82.1%). Decrease of radiation field size from EF to IF in stage I aggressive iL from GIT 1992 to GIT 1996 resulted in a nonsignificant partial reduction of chronic toxicity while maintaining comparable survival rates (5‐year overall survival 87.9 vs. 86.7%, 10‐year overall survival 77.4 vs. 71.5%) with nonsignificant difference in event‐free survival (5‐year event‐free survival 82.6 vs. 86.7%, 10‐year event‐free survival 69.7 vs. 71.5%) and lymphoma‐specific survival (5‐year lymphoma‐specific survival 90.1 vs. 91.9%, 10‐year lymphoma‐specific survival 87.6% vs. 91.9%). Comparative dose calculation of two still available indolent duodenal lymphoma computed tomography scans revealed lower radiation exposure to normal tissues from applying current standard involved site RT (ISRT) 30 Gy in both cases.
Conclusion
RT adapted to stage, histology, and resection in multimodal treatment of iL, despite partially decreasing field size (EF to IF), achieves excellent local tumor control and survival rates. The use of modern RT technique and target volume with ISRT offers the option of further reduction of normal tissue complication probability.
Implications for Practice
Although patients with intestinal lymphoma (iL) are heterogeneous according to histology and subtype, they benefit from radiotherapy. Prospective study data from 134 patients with indolent iL (stage IE–IIE) or aggressive iL (stage IE–IVE) show 100% tumor control after definitive or adjuvant curative‐intent radiation therapy ± chemotherapy. Radiation treatment was applied between 1992 and 2003. Median follow‐up in total was 11.7 years. No radiotherapy‐associated death occurred. Relapse developed in 15.7% of the entire cohort; distant failure was more frequent than local (4:1). Normal tissue complication probability can be further improved using modern involved site radiation therapy techniques.
Keywords: Intestinal lymphoma, Extended field, Involved field, Involved site radiation therapy, Normal tissue complication probability
Short abstract
This article reports the details of radiation therapy in the therapeutic multimodality approach for treatment of patients with intestinal lymphoma.
Introduction
For primary intestinal lymphoma (iL), prospective studies are rare and contain only a few patients. A multidisciplinary therapy concept of surgery, radiation, and chemotherapy is widely recognized, depending on localization, histology, and stage of intestinal lymphoma 1, 2. In contrast to primary gastric lymphoma, patients with iL, particularly with multiple organ involvement, have a poorer prognosis 3. Lymphoma localized in the small bowel is often only diagnosed late, namely, when complications occur, leading to surgical procedure. Organ‐preserving treatment can be provided for lymphoma of the duodenum or colon; surgical procedure for the ileocecal region is not clearly defined. Patients with aggressive intestinal lymphoma undergo systemic chemotherapy followed by radiation therapy 2. The use of chemotherapy in indolent intestinal lymphoma strongly depends on the stage. The field size and radiation dosage in iL depend on histologic subtype and potential previous resection 1. The development of modern radiation techniques throughout the past 25 years 4, 5, 6, 7, 8 makes radiotherapy a central, well‐tolerated treatment option with curative potential in gastrointestinal lymphoma.
In these multi‐institution cohorts we describe the details of radiation therapy in a therapeutic multimodality approach for patients with iL treated between 1992 and 2003 within two consecutive prospective study designs, GIT 1992 9 and GIT 1996 10. The tolerability and efficacy of stage‐, histology‐, and resection‐adapted radiation therapy volume is shown with reference to tumor control, acute and chronic adverse effects, survival results, and patterns of relapse regarding long‐term follow‐up. Dose calculation in two technically available cases of indolent duodenal iL compares normal tissue complication probability (NTCP) of primary radiotherapy with current standard involved site radiation therapy (RT) 30 Gy.
Subjects, Materials, and Methods
Patients
We prospectively analyzed the medical paper records of 134 patients who had biopsy‐proven intestinal lymphoma and either indolent lymphoma at stage IE or IIE or aggressive lymphoma at any stage. Radiation therapy was applied at 61 radiotherapeutic institutions between 1992 and 2003; the follow‐up data closure date was March 29, 2019. The patients received staging and follow‐up based on conventional computer tomography because positron emission tomography–computed tomography (CT) had not yet been implemented in clinical routine in the 1990s and furthermore is not recommended for small bowel lymphoma diagnostics. All patients received RT with curative intent. In aggressive and simultaneous aggressive‐indolent (mixed) lymphoma, RT was applied after four or six cycles of CHOP (cyclophosphamide, adriamycin, vincristine, prednisolone) chemotherapy. In indolent unresected lymphoma at stage IIE, RT was applied after six cycles of COP (cyclophosphamide, vincristine, prednisolone) in the first study or after six cycles of MCP (mitoxantrone, chlorambucil, prednisolone) in the second study 9, 10. In all cases the primary pathological diagnosis of intestinal lymphoma was confirmed by an expert consultant of an institute of lymphoma pathology. Each patient gave written informed consent for the study. We compiled the data about clinical characteristics, Eastern Cooperative Oncology Group (ECOG) performance status, lymphoma stage, treatment, adverse effects, follow‐up examinations, and relapse characteristics. Follow‐up assessment was completed through additional review of all patients’ charts.
Compliance with Ethical Guidelines
The trials were conducted in accordance with the Declaration of Helsinki on Ethical Principles for Medical Research after approval of the Ethical Board of the Physicians chamber of Westfalia‐Lippe and the Westfalian Wilhelms‐University of Muenster. The studies were reviewed by the local Ethical Committees (institutional review boards) of the involved trials sites.
Registration
Registration was not applicable. The trials were initiated in 1992 and 1996, before the setting up of internationally accepted databases like http://clinicaltrials.gov (2000) or German Clinical Trials Register (2008).
Treatment Strategy
Pathologic confirmation of primary intestinal lymphoma was obtained by biopsy or optional surgery in resectable lymphoma using standardized processes. After application of chemotherapy in aggressive lymphoma or in stage IIE unresected indolent lymphoma, all patients of the cohort were referred to the radiation oncologist for curative‐intent RT. Protocol RT was stratified according to histologic subtype, resection status, and stage of disease 9, 10 containing either wide extended field including the mediastinum (EF‐supradiaphragmatic), extended field limited to the abdomen (EF), or involved field limited to the organ and lymph nodes affected by lymphoma (IF). The field size in aggressive stage I lymphoma decreased between the two study designs from EF (abdomen) to IF (Fig. 1B, C). In the second study all stages of aggressive lymphomas received IF radiotherapy after six cycles of CHOP. The extended field borders were determined as follows: the EF comprised the whole abdomen from the diaphragm to the lower border of the foramina obturatoria by omitting the right hepatic lobe; the EF‐supradiaphragmatic additionally included the mediastinal and supraclavicular lymph nodes. The IF comprised the involved organ and lymph nodes. The typical field borders are illustrated in Figure 1A–C. Patients were treated with initially anterior‐posterior opposing fields or increasingly with three‐dimensional (3D) conformal RT (CRT) (Table 1). In the case of the two opposing fields technique, the treatment started with anterior‐posterior fields blocking out the right hepatic lobe; after application of 18 Gy the fields were split and caudal maintained, whereas in the cranial part of the abdomen the RT was continued by lateral fields omitting the kidneys. The later 3D CRT was a typical four‐field arrangement with anterior‐posterior and two lateral beams. A central review was performed of all RT plans (Department of Radiation Oncology, University Hospital of Muenster, Germany).
Figure 1.
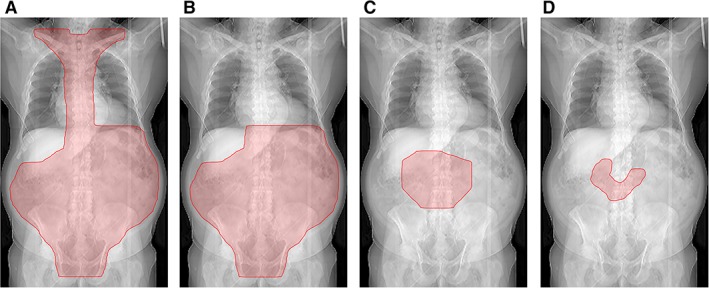
Radiation fields. (A): Abdomen with mediastinal and supraclavicular lymph nodes. (B): Extended field. (C): Involved field. (D): Involved site.
Table 1.
Study design and radiation technique in intestinal lymphoma
| Stage | Chemotherapy | Radiotherapy |
|---|---|---|
| GIT 1992a | ||
| Indolent, resected intestinal NHL (n = 6) | ||
| IE, II1E, II2E (n = 6) | Violation 6× COP (n = 1) | EF 30 Gy (n = 6), boost to 40 Gy (n = 3/6) |
| Indolent, unresected intestinal NHL (n = 1) | ||
| IE (n = 0) | — | EF 30 Gy + boost to 40 Gy (n = 0) |
| II1E, II2E (n = 1) |
6× COP‐21 (n = 0) Violation no COP (n = 1) |
EF‐supradiaphragmaticb 30 Gy + boost to 40 Gy (n = 0) Violation EF 30 Gy + boost to 40 Gy (n = 1) |
| Aggressive,c resected intestinal NHL (n = 26) | ||
| IE (n = 8) |
4× CHOP‐14 (n = 7) Violation 6× CHOP (n = 1) |
EF 30 Gy (n = 7), boost to 40 Gy (n = 2/7) Violation IF 40 Gy (n = 1) |
| IIE–IV (n = 18) |
6× CHOP‐14 (n = 17) Violation 5× CHOEP (n = 1) |
IF 40 Gy (n = 12) Violation EF 30 Gy + boost to 40 Gy (n = 5), EF 30 Gy (n = 1) |
| Aggressive,c unresected intestinal NHL (n = 5) | ||
| IE (n = 1) |
4× CHOP‐14 (n = 0) Violation 6× CHOP (n = 1) |
EF 30 Gy + boost to 40 Gy (n = 0) Violation IF 40 Gy (n = 1) |
| IIE–IV (n = 4) |
6× CHOP‐14 (n = 3) Violation no CHOP (n = 1) |
IF 40 Gy (n = 1) Violation EF 29.4 Gy (n = 1), EF 30 Gy + boost to 37.2 Gy (n = 1), IF 46 Gy (n = 1) |
| GIT 1996d | ||
| Indolent, resected intestinal NHL (n = 18) | ||
| IE, II1E, II2E (n = 18) | Violation 2× CHOP (n = 1), 4× (n = 1), 6× (n = 1) |
EF 30 Gy (n = 16), boost to 40 Gy (n = 9/16) Violation EF to 25.5 Gy (n = 1), IF to 40 Gy (n = 1) |
| Indolent, unresected intestinal NHL (n = 26) | ||
| IE (n = 17) | Violation 4× CHOP (n = 1) |
EF 30 Gy + boost to 40 Gy (n = 16) Violation no boost (n = 1) |
| II1E, II2E (n = 9) |
6× MCP‐28 (n = 2) Violation no MCP (n = 7) |
EF‐supradiaphragmaticb 30 Gy + boost to 40 Gy (n = 5) Violation EF + boost to 35.4 Gy (n = 1), EF 24 Gy + boost to 40 Gy (n = 1), EF to 40 Gy (n = 1), IF to 40 Gy (n = 1) |
| Aggressive, resected, and unresected intestinal NHL (n = 52) | ||
| IE–IV (n = 52) |
6× CHOP‐14 (n = 45) Violation 2× CHOP (n = 1), 4× (n = 3), 5× (n = 1), 8× (n = 1), 3× CHOP +3× IMEP (n = 1) |
IF 40 Gy (n = 40) Violation EF to 18, 19.5, or 45 Gy (n = 3), EF + boost to 40 Gy (n = 6), EF + boost to 35.7 Gy (n = 1), IF to 21.6 or 30 Gy (n = 2) |
Radiation techniques for GIT 1992 (n = 38 patients) included AP/PA opposing fields for 33 (86.8%) patients and three‐dimensional conformal radiation therapy for 5 (13.2%) patients.
EF‐supradiaphragmatic includes mediastinum and supraclavicular lymph nodes.
Aggressive includes mixed‐histology NHL.
Radiation techniques for GIT 1996 (n = 96 patients) included one field for one (1.0%) patient (this patient, with multilocular intestinal and testis involvement of lymphoma, received one field radiotherapy of the testis), AP/PA opposing fields for 26 (27.1%) patients, and three‐dimensional conformal radiation therapy for 69 (71.9%) patients.
Abbreviations: AP/PA, anterior‐posterior/posterior‐anterior; CHOEP, CHOP + etoposide; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; COP, cyclophosphamide, vincristine, prednisone; EF, extended field; IF, involved field; IMEP, ifosfamide, methotrexate, etoposide, prednisone; MCP, mitoxantrone, chlorambucil, prednisone; NHL, non‐Hodgkin lymphoma.
In technically available CT scans of two patients with indolent duodenal lymphoma, the dose calculation for normal tissues of original 3D CRT was compared with current standard involved site radiation therapy (ISRT) 30 Gy for estimation of NTCP in both cases. The planning target volume definition for ISRT (Fig. 1D) was conducted according to current guidelines of International Radiation Oncology Group; thus Clinical Target Volume (CTV) for the two cases was defined as the entire duodenum 8.
Follow‐Up
Patients were routinely seen 8 weeks after finishing treatment for evaluation of potential radiogenic toxicity and initial response by use of esophago‐gastro‐duodenoscopy (EGD) 11. EGD with topographic mapping biopsy, abdominal ultrasound, hematology laboratory results, and monitoring of liver and renal function was performed in standard follow‐up intervals. Response was categorized as one of the following: complete response (CR), complete response uncertain (CRu), partial response, no change, and progressive disease. Relapse was classified as new manifestation of lymphoma after a time interval of at least 1 month after the first follow‐up–proven CR/CRu. Failure in the primary site of the bowel or regional lymph nodes was categorized as locoregional failure or relapse and relapse at other sites as distant failure. Early relapses occurred within the first year after treatment, late relapses thereafter 12. Transformation to aggressive lymphoma was also registered as recurrent disease.
Statistical Analysis
Baseline patient characteristics and radiotherapy treatment were outlined. Absolute and relative frequency in contingency tables described the categorical data. Acute and chronic adverse effects were evaluated by use of contingency tables; the chi‐square test was used for comparison of varying severity of adverse effects between the two study designs. Endpoints of the studies were event‐free survival (EFS), overall survival (OS), and lymphoma‐specific survival (LSS). The log‐rank test was used to compare survival curves for assessing the influence of treatment design and disease factors. We examined the number and the type of relapses after primary treatment. All statistical analyses were performed in IBM SPSS Statistics (version 25.0). The p values were two‐sided, descriptive measures. Values of p ≤ .05 were considered to indicate statistically significant differences.
Results
Patient Characteristics
Clinical patient and lymphoma disease data are shown in Table 2A and 2B. The majority of all patients had stage IIE disease (57%). ECOG performance status was ≤1 in 98.5% of patients. Primary site of disease was predominantly the ileocecal region in 34.3%. For staging examinations all patients had CT scans, EGD, and colonoscopy. The histopathologic subtypes were indolent lymphoma in 38.0% (18 follicular, 26 marginal zone, 5 mantle cell, 2 immunocytoma), aggressive lymphoma in 53.0% (68 diffuse large B‐cell lymphoma [DLBCL], 3 T‐cell lymphoma), and a mixed lymphoma in 9.0% (as aggressive component a DLBCL in all 12 patients and as indolent component in 4 patients with follicular lymphoma, 7 with marginal zone lymphoma, and 1 with immunocytoma). Multimodal treatment in both studies is displayed in Table 1.
Table 2A.
Patient characteristics and acute toxicity (CTCAE)
| Characteristics and toxicity | GIT 1992, n (%) | GIT 1996, n (%) | Total, n (%) |
|---|---|---|---|
| Patients with intestinal lymphoma | 38 (28.4) | 96 (71.6) | 134 (100) |
| Age, median (range), years | 53 (19–81) | 60 (18–72) | 58 (18–81) |
| Gender | |||
| Female | 13 (34.2) | 30 (31.3) | 43/134 (32.1) |
| Male | 25 (65.8) | 66 (68.8) | 91/134 (67.9) |
| Histology | |||
| Indolent | 7 (18.4) | 44 (45.8) | 51/134 (38.1) |
| Aggressive | 26 (68.4) | 45 (46.9) | 71/134 (53.0) |
| Aggressive/indolent | 5 (13.2) | 7 (7.3) | 12/134 (9.0) |
| Primary localization | |||
| Duodenal | 1/38 (2.6) | 12/96 (12.5) | 13/134 (9.7) |
| Small bowel | 10/38 (26.3) | 23/96 (24.0) | 33/134 (24.6) |
| Ileocecal | 16/38 (42.1) | 30/96 (31.3) | 46/134 (34.3) |
| Colonic | 1/38 (2.6) | 8/96 (8.3) | 9/134 (6.7) |
| Rectal | 3/38 (7.9) | 6/96 (6.3) | 9/134 (6.7) |
| Multilocular intestinal | 7/38 (18.4) | 17/96 (17.7) | 24/134 (17.9) |
| Ann Arbor stage | |||
| IE | 11 (28.9) | 42 (43.8) | 53/134 (39.6) |
| II1E | 14 (36.8) | 30 (31.3) | 44/134 (32.8) |
| II2E | 13 (43.2) | 20 (20.8) | 33/134 (24.6) |
| III | — | 2 (2.1) | 2/134 (1.5) |
| IV | — | 2 (2.1) | 2/134 (1.5) |
| Resection | |||
| Total | 32/38 (84.2) | 58/96 (60.4) | 90/134 (67.2) |
| Indolent | 6/38 (15.8) | 18/96 (18.8) | 34/134 (25.4) |
| Aggressive | 23/38 (60.5) | 35/96 (36.5) | 58/134 (43.3) |
| Aggressive/indolent | 3/38 (7.9) | 5/96 (5.2) | 8/134 (6.0) |
| Chemotherapy | |||
| Total | 31/38 (81.6) | 58/96 (60.4) | 89/134 (66.4) |
| Indolent | 1/38 (2.6) | 6/96 (6.3) | 7/134 (5.2) |
| Aggressive | 26/38 (68.4) | 45/96 (46.9) | 71/134 (53.0) |
| Aggressive/indolent | 4/38 (10.5) | 7/96 (7.3) | 11/134 (8.2) |
| Complete remission | 38 (100) | 96 (100) | 134 (100) |
| ECOG score | |||
| ECOG 0 | 32/38 (84.2) | 88/96 (91.7) | 120/134 (89.6) |
| ECOG 1 | 4/38 (10.5) | 8/96 (8.3) | 12/134 (8.9) |
| ECOG 2 | 1/38 (2.6) | — | 1/134 (0.7) |
| ECOG 3 | 1/38 (2.6) | — | 1/134 (0.7) |
| Acute toxicity, CTCAE | |||
| Drop in hemoglobin | |||
| 0 | 27/38 (71.1) | 72/96 (75.0) | 99/134 (73.9) |
| 1–2 | 11/38 (28.9) | 21/96 (21.9) | 32/134 (23.9) |
| 3–4 | — | 3/96 (3.1) | 3/134 (2.2) |
| Leukocytopenia | |||
| 0 | 19/38 (50.0) | 34/96 (35.4) | 53/134 (39.6) |
| 1–2 | 15/38 (39.5) | 58/96 (60.4) | 73/134 (54.5) |
| 3–4 | 4/38 (10.5) | 4/96 (4.2) | 8/134 (6.0) |
| Thrombocytopenia | |||
| 0 | 28/38 (73.7) | 67/96 (69.8) | 95/134 (70.9) |
| 1–2 | 8/38 (21.1) | 27/96 (28.1) | 35/134 (26.1) |
| 3–4 | 2/38 (5.3) | 2/96 (2.1) | 4/134 (3.0) |
| Elevated bilirubina | |||
| 0 | 36/38 (94.7) | 88/96 (91.7) | 124/134 (92.5) |
| 1–2 | 2/38 (5.3) | 4/96 (4.2) | 6/134 (4.5) |
| 3–4 | — | — | — |
| Elevated transaminasesa | |||
| 0 | 38/38 (100.0) | 82/96 (85.4) | 120/134 (89.6) |
| 1–2 | — | 9/96 (9.4) | 9/134 (6.7) |
| 3–4 | — | 1/96 (1.0) | 1/134 (0.7) |
| Loss of appetiteb | |||
| 0 | 22/38 (57.9) | 48/96 (50.0) | 70/134 (52.2) |
| 1–2 | 15/38 (39.5) | 42/96 (43.8) | 57/134 (42.5) |
| 3–4 | 1/38 (2.6) | 5/96 (5.2) | 6/134 (4.5) |
| Weight lossb | |||
| 0 | 26/38 (68.4) | 57/96 (59.4) | 83/134 (61.9) |
| 1–2 | 12/38 (31.6) | 38/96 (39.6) | 50/134 (37.3) |
| 3–4 | — | — | — |
| Nauseab | |||
| 0 | 16/38 (42.1) | 42/96 (43.8) | 58/134 (43.3) |
| 1–2 | 14/38 (36.8) | 28/96 (29.2) | 42/134 (31.3) |
| 3–4 | 8/38 (21.1) | 25/96 (26.0) | 33/134 (24.6) |
| Diarrheab | |||
| 0 | 17/38 (44.7) | 35/96 (36.5) | 52/134 (38.8) |
| 1–2 | 7/38 (18.4) | 37/96 (38.5) | 44/134 (32.8) |
| 3–4 | 14/38 (36.8) | 23/96 (24.0) | 37/134 (27.6) |
| Constipationb | |||
| 0 | 37/38 (97.4) | 90/96 (93.8) | 127/134 (94.8) |
| 1–2 | — | 5/96 (5.2) | 5/134 (3.7) |
| 3–4 | 1/38 (2.6) | — | 1/134 (0.7) |
The available numbers are given, with missing data being the difference between total numbers and given numbers.
Acute toxicity (CTCAE) missing four patients (4.2%) from GIT 1996 in total 4 of 134 patients (3.0%) because of missing evaluation of the parameters “bilirubin” and “elevated transaminases.”
Acute toxicity (CTCAE) missing one patient (1.0%) from GIT 1996, in total 1 of134 patients (0.7%) because of missing evaluation of the parameters “clinical symptoms.”
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events (version 4.03); ECOG Eastern Cooperative Oncology Group.
Table 2B.
Patient characteristics with primary localization according to histological subtype
| Localization | Histology, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| Indolent | Aggressive | Mixeda | ||
| Duodenal | 12/51 (23.5) | — | 1/12 (8.3) | 13/134 (9.7) |
| Small bowel | 12/51 (23.5) | 19/71 (26.8) | 2/12 (16.7) | 33/134 (24.6) |
| Ileocecal | 8/51 (15.7) | 34/71 (47.9) | 4/12 (33.3) | 46/134 (34.3) |
| Colonic | 3/51 (5.9) | 6/71 (8.5) | — | 9/134 (6.7) |
| Rectal | 3/51 (5.9) | 5/71 (7.0) | 1/12 (8.3) | 9/134 (6.7) |
| Multilocular intestinal | 13/51 (25.5) | 7/71 (9.9) | 4/12 (33.3) | 24/134 (17.9) |
Mixed indicates aggressive + indolent histology.
RT Dose, Technique, and Response
The applied radiation techniques in both studies are an expression of the historical development from opposing fields to 3D conformal radiotherapy (Table 1). Radiation therapy dose was 30 Gy ± 10% (n = 15) or 40 Gy ± 10% (n = 107) per protocol, in all but 12 patients, of whom 10 received an underdosage of >10% and 2 received an overdosage of >10%. In the second study, only two of the nine patients with unresected stage II indolent non‐Hodgkin lymphoma received six cycles of MCP before RT. Of the patients with aggressive lymphoma, 38 of 45 patients at any stage received RT after six cycles of CHOP (Table 1). The response to radiotherapy in the context of multimodal treatment was CR in 134 (100%) patients (Table 2A).
OS, EFS, and LSS
During the follow‐up period 46 patients died; of these 10 deaths were related to second malignancies and 1 was related to recurrent previous gastric cancer (Fig. 2). The 15 lymphoma‐related deaths were caused mostly by relapses (n = 13/15; Table 3); only 2 were caused by lymphoma‐related ileus or cachexia. These 15 patients had primary stage I (n = 3), stage II (n = 11), or stage IV (n = 1) iL; of them 10 died from distant recurrence, 2 died from local recurrence, and 1 died from both distant and local recurrence. The remaining 20 of 46 patients died from nononcological causes.
Figure 2.
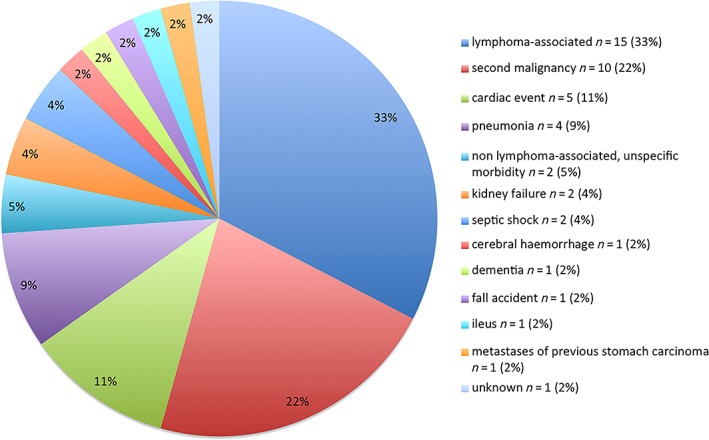
Causes of death in 46 events of death.
Table 3.
Relapse characteristics (n = 21)
| Characteristic | GIT 1992 | GIT 1996 | Total |
|---|---|---|---|
| Follow‐up patients with relapses, n (%) | 3 (14.3) | 18 (85.7) | 21 (15.7) |
| Total relapse events, n | 3 | 25 | 28 |
| 1 relapse | 3 | 12 | 15 |
| 2 relapses | 0 | 5 | 5 |
| 3 relapses | 0 | 1 | 1 |
| Sex, n | |||
| Female | 0 | 6 | 6 |
| Male | 3 | 12 | 15 |
| Age, years | 44, 65, 69 | 18, 30, 44, 49, 58, 60, 60, 60, 60, 61, 61, 63, 64, 64, 65, 65, 69, 69 |
Median, 61 Range, 18–69 |
| Ann Arbor Stage, n | |||
| I | 0 | 4 | 4 |
| II | |||
| II1 | 1 | 6 | 7 |
| II2 | 2 | 7 | 9 |
| IV | 0 | 1 | 1 |
| ECOG, n | |||
| 0 | 2 | 16 | 18 |
| 1 | 1 | 2 | 3 |
| 2 | 0 | 0 | 0 |
| Primary histology, n | |||
| Indolent | 1 | 8 | 9 |
| FL | 1 | 5 | 6 |
| MZL | 0 | 3 | 3 |
| Aggressive | 2 | 9 | 11 |
| T‐cell lymphoma | 0 | 1 | 1 |
| DLBCL | 2 | 8 | 10 |
| Indolent‐aggressive | 0 | 1 | 1 |
| Primary localization, n | |||
| Small bowel | 1 | 6 | 7 |
| Ileocecal region | 2 | 5 | 7 |
| Duodenum | 0 | 1 | 1 |
| Colon | 0 | 1 | 1 |
| Rectum | 0 | 1 | 1 |
| Ileocecal region + lung | 0 | 1 | 1 |
| Multilocular intestinal | 0 | 3 | 3 |
| Protocol‐deviation,a n | |||
| No | 2 | 7 | 9 |
| Yes | 0 | 0 | 0 |
| Protocol‐violation,b n | |||
| Yes | 1 | 11 | 12 |
| Chemotherapy | 0 | 8 | 8 |
| RT | 1 | 2 | 3 |
| RT + chemotherapy | 0 | 1 | 1 |
| First relapse histology, n | |||
| Indolent | 1 | 7 | 8 |
| Presumed indolent | 0 | 2 | 2 |
| Aggressive | 2 | 9 | 11 |
| Indolent‐aggressive | 0 | 0 | 0 |
| First relapse localization, n | |||
| Local | 1 | 3 | 4 |
| Distant | 2 | 14 | 16 |
| Local + distantc | 0 | 1 | 1 |
| First relapse interval,d months | 4, 5, 163 | 2, 4, 4, 5, 5, 6, 15, 16, 20, 24, 32, 46, 48, 61, 88, 97, 101, 172 |
Median, 20 Range, 2–172 |
| First relapse timing,e n | |||
| Early | 2 | 6 | 8 |
| Late | 1 | 12 | 13 |
| First salvage therapy, n | |||
| Surgery | 0 | 0 | 0 |
| Chemotherapy | 1 | 11 | 12 |
| Radiotherapy | 1 | 1 | 2 |
| Radiochemotherapy | 0 | 3 | 3 |
| Radiochemotherapy + surgery | 0 | 1 | 1 |
| Watch and wait | 1 | 2 | 3 |
| First salvage CR,f n | |||
| Persistent | 0 | 4 | 4 |
| Temporary | 0 | 6 | 6 |
| No | 3 | 8 | 11 |
| Re‐relapse interval,g months | 19, 27, 53, 75, 66, 170, 195 |
Median, 75 Range, 19–195 |
|
| Re‐salvage therapy, n | |||
| Chemotherapy | 3 | 3 | |
| Radiotherapy | 1 | 1 | |
| Radiochemotherapy | 1 | 1 | |
| Cytoreductive therapy | 1 | 1 | |
| No therapy | 1 | 1 | |
| Second salvage CR,h n | |||
| Persistent | 2 | 2 | |
| Temporary | 1 | 1 | |
| No | 4 | 4 | |
| Death, n | |||
| No | 1 | 7 | 8 |
| Yes | 2 | 11 | 13 |
| DOD, n | |||
| No | 0 | 0 | 0 |
| Yes | 2 | 11 | 13 |
Protocol deviation means dose deviation >5% and < 10%.
Protocol violation means dose deviation ≥10% or false radiation field size.
Local at first relapse and distant at second/third relapse.
Relapse interval between start of RT and first relapse.
Relapse timing is early within the first year after start of treatment and late after the first year after start of treatment.
First salvage CR means de novo complete remission after first salvage therapy.
Re‐relapse interval between start of RT and second or third relapse.
Second salvage CR means de novo complete remission after second or third salvage therapy.
Abbreviations: CR, complete response; DLBCL, diffuse large B‐cell lymphoma; DOD, died of disease; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; MZL, marginal zone lymphoma; RT, radiation therapy.
No radiotherapy‐associated death is shown for the entire cohort (Table 2A). Overall survival was higher in the second study with 77.4% after 10 years, respectively, without significant difference (p = .280; Fig. 3A; Table 2C). The different histologic subtypes differed by their OS rates in favor of indolent lymphoma with 84.7% after 10 years, in contrast to mixed or aggressive lymphoma, not statistically significant (p = .095; Fig. 3B; Table 2C).
Figure 3.
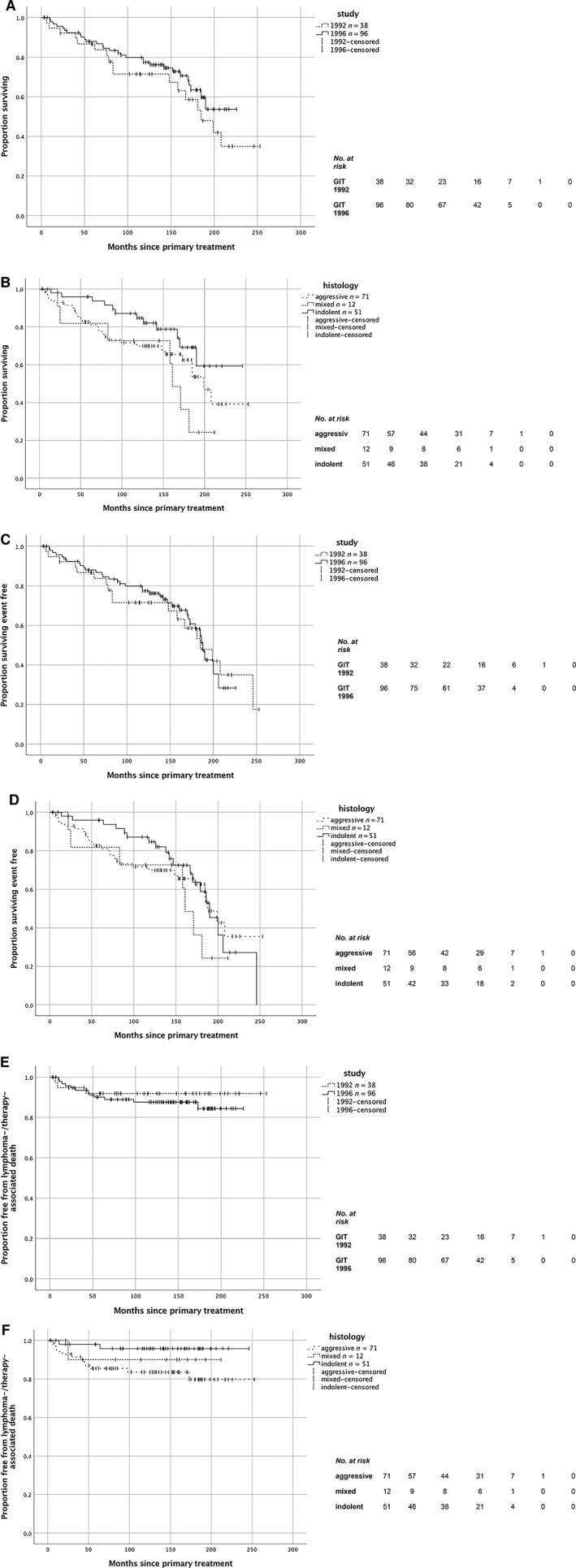
Survival curves. (A): Overall survival, p = .280. Number of deaths: GIT 1992, 17; GIT 1996, 29. Median follow‐up time: GIT 1992, 10.0 years (120 months); GIT 1996, 11.8 years (141.5 months). 10‐year overall survival: GIT 1992, 71.5% (95% confidence interval [CI], 56.4–86.6); GIT 1996, 77.4% (95% CI, 68.6–86.2). (B): Overall survival related to histologic subtype, p = .095. Number of deaths: aggressive, 27; mixed, 7; indolent, 12. Median follow‐up time: in total 11.7 years (140 months). 10‐year overall survival related to histologic subtype of lymphoma: indolent, 84.7% (95% CI, 74.3–95.1); mixed, 72.7% (95% CI, 46.4–99.0); aggressive, 69.6% (95% CI, 58.7–81.1). (C): Event‐free survival, p = .712. Number of events: GIT 1992, 18; GIT 1996, 36. Median follow‐up time: GIT 1992, 10.0 years (120 months); GIT 1996, 11.8 years (141.5 months). 10‐year event‐free survival: GIT 1992, 71.5% (95% CI, 56.4–86.6); GIT 1996, 69.7% (95% CI, 60.1–79.3). (D): Event‐free survival related to histologic subtype, p = .665. Number of events: aggressive, 28; mixed, 7; indolent, 19. Median follow‐up time: in total 11.7 years (140 months). 10‐year event‐free survival related to histologic subtype of lymphoma: indolent, 74.1% (95% CI, 61.4–86.8); mixed, 73.3% (95% CI, 47.4–99.2); aggressive, 66.9% (95% CI, 55.5–78.3). (E): Lymphoma‐specific survival, p = .481. Number of events: GIT 1992, 3; GIT 1996, 12. Median follow‐up time: GIT 1992, 10.0 years (120 months); GIT 1996, 11.8 years (141.5 months). 10‐year lymphoma‐specific survival: GIT 1992, 91.9% (95% CI, 83.1–100.7); GIT 1996, 87.6% (95% CI, 80.7–94.5). (F): Lymphoma‐specific survival related to histologic subtype, p = .093. Number of events: aggressive, 12; mixed, 1; indolent, 2. Median follow‐up time: in total 11.7 years (140 months). 10‐year lymphoma‐specific survival related to histologic subtype of lymphoma: indolent, 95.7% (95% CI, 89.8–101.6); mixed, 90.0% (95% CI, 71.4–108.6); aggressive, 83.5% (95% CI, 74.5–92.5).
Table 2C.
Patient characteristics with follow‐up results and chronic toxicity (LENT SOMA)
| Characteristics | GIT 1992, n (%) | GIT 1996, n (%) | Total, n (%) |
|---|---|---|---|
| Patients with intestinal lymphoma, n | 38 | 96 | 134 |
| Median observation time in years (months) | 10.0 (120) | 11.8 (141.5) | 11.7 (140) |
| Local control | 38 (100)a | 96 (100)b | 134 (100) |
| Events (progression, relapse, death) | 18 (47.4)a | 36 (37.5)b | 54 (40.3) |
| Progression | 0 (0) | 0 (0) | 0 (0) |
| Relapse in total | 3 (7.9) | 18 (18.8) | 21 (15.7) |
| Local | 1 (33.3) | 3 (16.7) | 4 (19.0) |
| Distant | 2 (66.7) | 14 (77.8) | 16 (76.2) |
| Local and distant | 0 (0) | 1 (5.6) | 1 (4.8) |
| Early relapsec | 2 (66.7) | 6 (33.3) | 8 (38.1) |
| Late relapsed | 1 (33.3) | 12 (66.7) | 13 (61.9) |
| Death in total | 17 (44.7) | 29 (30.2) | 46 (34.3) |
| Related to treatment | 0 (0) | 0 (0) | 0 (0) |
| DOD | 3 (17.6) | 12 (41.4) | 15 (32.6) |
| Second malignancy | 4 (23.5) | 6 (20.7) | 10 (21.7) |
| Other diseases | 9 (52.9) | 11(37.9) | 20 (43.5) |
| Unknown | 1 (5.9) | 0 (0) | 1 (2.2) |
| Overall survival (with 95% CI) | |||
| 5‐year | 86.7 (75.9–97.5) | 87.9 (81.2–94.6) | — |
| 10‐year | 71.5 (56.4–86.6) | 77.4 (68.6–86.2) | — |
| 15‐year | 58.6 (40.6–76.6) | 63.4 (51.4–75.4) | — |
| Event‐free survival (with 95% CI) | |||
| 5‐year | 86.7 (75.9–97.5) | 82.6 (74.8–90.4) | — |
| 10‐year | 71.5 (56.4–86.6) | 69.7 (60.1–79.3) | — |
| 15‐year | 54.1 (35.3–72.9) | 55.0 (42.5–67.5) | — |
| Lymphoma‐specific survival (with 95% CI) | |||
| 5‐year | 91.9 (83.1–100.7) | 90.1 (84.0–96.2) | — |
| 10‐year | 91.9 (83.1–100.7) | 87.6 (80.7–94.5) | — |
| 15‐year | 91.9 (83.1–100.7) | 84.4 (75.4–93.4) | — |
| Overall survival of histologic subtypes (with 95% CI) | |||
| Indolent lymphoma | |||
| 5‐year | — | — | 95.9 (90.2–101.6) |
| 10‐year | — | — | 84.7 (74.3–95.1) |
| 15‐year | — | — | 69.2 (52.5–85.9) |
| Mixed lymphoma | |||
| 5‐year | — | — | 81.8 (59.1–104.5) |
| 10‐year | — | — | 72.7 (46.4–99.0) |
| 15‐year | — | — | 36.4 (4.5–68.3) |
| Aggressive lymphoma | |||
| 5‐year | — | — | 82.6 (73.6–91.6) |
| 10‐year | — | — | 69.6 (58.7–81.1) |
| 15‐year | — | — | 62.6 (49.9–75.3) |
| Event‐free survival of histologic subtypes (with 95% CI) | |||
| Indolent lymphoma | |||
| 5‐year | — | — | 87.7 (78.5–96.9) |
| 10‐year | — | — | 74.1 (61.4–86.8) |
| 15‐year | — | — | 47.9 (28.5–67.3) |
| Mixed lymphoma | |||
| 5‐year | — | — | 82.5 (60.4–104.6) |
| 10‐year | — | — | 73.3 (47.4–99.2) |
| 15‐year | — | — | 36.7 (4.6–68.8) |
| Aggressive lymphoma | |||
| 5‐year | — | — | 81.4 (72.2–90.6) |
| 10‐year | — | — | 66.9 (55.5–78.3) |
| 15‐year | — | — | 62.4 (50.2–74.6) |
| Lymphoma‐specific survival of histologic subtypes (with 95% CI) | |||
| Indolent lymphoma | |||
| 5‐year | — | — | 98.0 (94.8–101.9) |
| 10‐year | — | — | 95.7 (89.8–101.6) |
| 15‐year | — | — | 95.7 (89.8–101.6) |
| Mixed lymphoma | |||
| 5‐year | — | — | 90.0 (71.4–108.6) |
| 10‐year | — | — | 90.0 (71.4–108.6) |
| 15‐year | — | — | 90.0 (71.4–108.6) |
| Aggressive lymphoma | |||
| 5‐year | — | — | 85.4 (77.0–93.8) |
| 10‐year | — | — | 83.5 (74.5–92.5) |
| 15‐year | — | — | 79.7 (68.5–90.9) |
| Impaired organ function (LENT SOMA)e | |||
| Liver (p = .089) | |||
| 0 | 34 (89.5) | 89 (92.7) | 123 (91.8) |
| 1–2 | 0 (0) | 4 (4.2) | 4 (3.0) |
| Kidney (p = .178) | |||
| 0 | 28 (73.7) | 81 (84.4) | 109 (81.3) |
| 1–2 | 6 (15.8) | 12 (12.5) | 18 (13.4) |
| Bladder (p = .107) | |||
| 0 | 34 (89.5) | 89 (92.7) | 123 (91.8) |
| 1–2 | 0 (0) | 4 (4.2) | 4 (3.0) |
| Stomach and bowel (p = .128) | |||
| 0 | 24 (63.2) | 74 (77.1) | 98 (73.1) |
| 1–2 | 10 (26.3) | 19 (19.8) | 29 (21.6) |
Eighteen patients developed events in the first study, of whom 3 patients died from relapse.
Thirty‐six patients developed events in the second study, of whom 12 patients died from relapse.
Early relapse indicates relapse within the first year after start of treatment.
Late relapse indicates relapse after the first year of treatment.
Impaired organ function (LENT SOMA; grade 0, grade 1–2), missing patients: GIT 1992, 4 of 38 (10.5%); GIT 1996, 3 of 96 (3.1%); in total 7 of 134 (5.2%).
Abbreviations: CI, confidence interval; DOD, died of disease; LENT SOMA, Late Effects in Normal Tissue Subjective, Objective, Management, Analytic.
The EFS was stable between the two studies despite decreasing radiation field size in stage I aggressive iL: 10‐year EFS in the second study was 69.7%, not differing significantly between the two studies (p = .712; Fig. 3C; Table 2C). The histologic subtypes differed by their EFS rates in favor of indolent lymphoma with 74.1% after 10 years, also not statistically significant (p = .665; Fig. 3D).
Only 3 of 15 lymphoma‐related causes of death occurred in the first study. Because they occurred in less than 5 years, the LSS rate remained constantly 91.9% in the first study. In contrast, in the second study the 10‐year LSS rate amounted to 87.6%. Of the 12 lymphoma‐related deaths in the second study, 9 occurred in less than 5 years. LSS differed not significantly between the two studies (p = .481; Fig. 3E). Most of the lymphoma‐related deaths developed in aggressive lymphoma (12/15); only one was in mixed lymphoma and two in indolent lymphoma. The different histologic subtypes differed by their LSS rates in favor of indolent lymphoma with 10‐year LSS of 95.7% without significant difference (p = .093; Fig. 3F).
Patterns of Relapse and Infield Failure
A limited number of 21 patients experienced relapse, mainly coming from initial stages II1/II2 and original tumor localization in the small bowel or ileocecal region (Table 3). Histopathological subtype remained the same in all 21 relapses: 11 aggressive, 9 indolent, and 1 mixed lymphoma (relapsing first as indolent and second as aggressive lymphoma). Localizations of the 21 relapses were mainly distant in 76% (16/21). On further follow‐up six patients developed a second relapse (five distant, one local), and one of these developed a third relapse (distant).
Analysis of the primary radiotherapy of the 21 recurrences revealed a protocol deviation in 12 patients: 8 incomplete chemotherapy applications, 3 protocol violations relating to radiotherapy (underdosage of the target >10% ± inadequate field size); the remaining patient showed a combination of incomplete chemotherapy, inadequate field size, and underdosage of the target >10% (Table 3). The first relapse occurred at median after 20 months. Salvage therapy of first relapse was successful in 10 of 21 patients. Thirteen patients with recurrent disease died related with lymphoma, 9 with distant and 4 with local (ileocecal region) relapses.
Toxicities and Second Malignancies
Acute Toxicity
Of the 134 patients treated with RT, acute radiotherapy‐related toxicities were analyzed using the Common Terminology Criteria for Adverse Events version 4.03 reporting system 13. In total, 4 of 134 (3.0%) patients could not be evaluated regarding the blood values of bilirubin and transaminases, and for 1 of 134 (0.7%) patients, the clinical symptoms could not be evaluated because data on these variables were not documented by the treating physicians (Table 2A).
Regarding grade 3 or 4 hematotoxicity, the tendency toward reduction in relative frequency of leukocytopenia from 10.5% to 4.2% (p = .161) and thrombocytopenia from 5.3% to 2.1% (p = .330) between the two studies is not statistically significant.
Acute grade 3 or 4 hepatotoxicity related to transaminases and bilirubin occurred in only 0.7% of patients.
In the entire cohort, the most frequent grade 3 or 4 gastrointestinal side effects were related to diarrhea, followed by nausea and loss of appetite, rare constipation, and no weight loss of higher grade. The reduction of relative frequency of grade 3/4 diarrhea from 36.8% to 24.2% (p = .142), the slight increase in relative frequency of grade 3/4 nausea from 21.1% to 26.3% (p = .526), and the slight rise in relative frequency of loss of grade 3/4 appetite from 2.6% to 5.3% (p = .509) between the two studies are all not statistically significant (Table 2A).
Chronic Toxicity
Of the 134 patients followed up, chronic toxicities could be analyzed in 127 (94.8%) patients according to the Late Effects in Normal Tissue Subjective, Objective, Management, Analytic (LENT SOMA) scoring system 14. For the remaining 7 of 134 (5.2%) patients, follow‐up data was not obtainable (Table 2A).
The trend toward lower relative frequencies of grade 1 or 2 chronic organ function impairment of the gastrointestinal tract from 26.3% to 19.8% and of kidneys from 15.8% to 12.5%, and toward higher relative frequencies of impaired organ function of the liver from 0.0% to 4.2% and urinary bladder from 0.0% to 4.2% between the two studies are all not statistically significant.
In 1 of the 55 patients with chronic toxicity, a potential causal relationship between radiotherapy could not be ruled out because of inadequate field size (EF instead of IF). Among the other 54 patients with chronic toxicity, no causal relationship with the primary radiotherapy (or previous chemotherapy) was demonstrable.
A total of 10 deaths resulted from second malignancies in the entire cohort. Of these patients who died, three were treated primarily with radiotherapy and seven with chemotherapy and radiotherapy.
The dose calculation in technically available CT scans of two patients with indolent duodenal lymphoma displays examples showing obviously lower radiation exposure of bilateral kidneys, liver, and duodenum/small bowel from current standard involved site RT 30 Gy compared with original extended field (EF 30 Gy + boost to 40 Gy) treatment. Also compared with involved field RT 40 Gy, ISRT 30 Gy causes lower radiation exposure of the organs at risk (Table 4).
Table 4.
Normal tissue complication probability (NTCP) in two patients with duodenal lymphoma, comparison with current standards (ISRT 30 Gy)
| Normal tissue | Dose constraint | Patient 1 Field size and dose, Gy | Patient 2 Field size and dose, Gy | Risk | ||||
|---|---|---|---|---|---|---|---|---|
| EF 30 + boost to 40 | IFRT 40 | ISRT 30 | EF 30 + boost to 40 | IFRT 40 | ISRT 30 | |||
| Right kidney |
Dmeana <15 Gy, Gy |
11.4 | 12.4 | 4.4 | 18.7 | 18.0 | 8.8 | Clinical dysfunction <5% |
| Left kidney |
Dmeana <15 Gy, Gy |
21.3 | 17.9 | 5.1 | 17.7 | 7.9 | 4.5 | Clinical dysfunction <5% |
| Liver |
Dmeanb 21.4 Gy vs. 17.5 Gy, Gy |
15.6 | 15.0 | 5.0 | 21.7 | 11.6 | 3.0 |
RILD yes or no |
|
V30b 34.6% vs. 26.6%, % |
16.6 | 13.6 | 0.1 | 28.4 | 9.1 | 0.7 |
RILD yes or no |
|
| Duodenum |
V35c <5.4%, % |
100.0 | 100.0 | 0.0 | 86.5 | 100.0 | 0.0 |
Grade ≥2 gastroduodenal toxicity 9% vs. 46% |
| Small bowel |
V35c <5.4%, % |
71.7 | 24.5 | 0.0 | 25.8 | 15.8 | 0.0 |
Grade ≥2 gastroduodenal toxicity 9% vs. 46% |
Kidney Dmean constraint according to quantitative analyses of normal tissue effects in the clinic (QUANTEC), Bentzen et al., 2010 26.
Liver Dmean, V30 constraints according to NTCP for liver disease, Cheng et al., 2005 27.
Duodenum/small bowel V35 constraint according to NTCP for duodenum toxicity, Holyoake et al., 2017 28.
Abbreviations: EF, extended field (abdomen); IFRT, involved field radiation therapy; ISRT, involved site radiation therapy; RILD, radiation‐induced liver disease.
Discussion
We present, to our knowledge, the largest cohort of intestinal lymphoma treated with curative‐intent radiation therapy as part of multimodal treatment. The two subgroups, reported with a median follow‐up of 10.0 and 11.8 years, respectively, present an extensive analysis of the course of this rare disease. Radiation therapy of intestinal lymphoma adapted to stage, histology, and surgical resection has been established as curative approach in primary therapy because of the option for organ maintenance and success in causing lymphoma regression 1, 15.
The outcome in our cohort of patients after locoregional radiation therapy in the context of multimodal treatment (67.2% resected, 66.4% received chemotherapy previously) is excellent with an exceptionally high complete response rate of 100% and low disease‐specific death in 11.2% (15/134) of the patients. Relapse occurred in 15.7% (21/134) of the entire cohort; in 12 of 21 relapsing patients a possible causal relationship between primary therapy and development of relapse cannot be ruled out, in view of protocol violations.
Distant relapse was more frequent than local (4:1), differing from the results of a retrospective analysis of 37 patients showing predominant locoregional relapse 16. The different histological subtypes of the gastrointestinal lymphomas show different specific spread of disease 17.
Local and distant relapses were always in histological accordance with the primary lymphoma. First recurrences could be salvaged successfully with at least stable disease in 12 of 21 patients, most commonly resulting in continuing de novo complete remission and second relapse occurring in only six patients after renewed disease‐free intervals between 15 and 69 months. A third relapse occurred in only one patient after another 120 months. Disease‐specific survival remains high by reason of effective salvage therapies, and we observed a small proportion of progression to further advanced disease.
We identified the histologic subtype of iL as a relevant but nonsignificant factor associated with lymphoma‐specific survival, observing more patients with aggressive histologic subtype who died of disease 17, 18.
The proportion of surgical procedure has decreased over the two studies (84.2 vs. 60.4%), partly triggered by the good outcome of the patients without surgery in gastric lymphoma 1, 15. Radiation therapy as definitive treatment approach in iL was well tolerated; no radiotherapy‐associated death was shown in the entire cohort. Acute toxicity particularly was related to leukocytopenia and diarrhea of low grades, without significant difference between the two studies.
Of the 10 patients who died from second malignancies, 7 patients received chemotherapy before radiation during primary treatment, as potential additional trigger for secondary tumors.
The composition of this cohort and the acute and chronic side effects that have arisen refer to a larger collective of patients than published by other groups 16, 19, 20. Our results confirm the excellent outcome of early‐stage iL when treated with additional RT in multimodal treatment, as reported in a retrospective analysis, which demonstrated the important role of radiotherapy for local control and survival especially for patients with unresected lymphoma disease 16. In this cohort the survival rates are comparable to or even more favorable than those of studies on intestinal lymphoma with small sample size 16, 19, 20.
No transformation to aggressive lymphoma was shown in our follow‐up subgroup, compatible with the general low rate in other studies 21, 22. The follow‐up results show that patents with iL can have late relapse until 14.3 years after initial diagnosis, emphasizing the importance of lifelong follow‐up 22, 23. The reduction of prescribed RT dose in indolent non‐Hodgkin lymphomas is an object of study in recent trials 24, 25. Considering combined underdosage and inadequate field size among four relapsing study patients with infield failure, the significance of adequate target volume coverage must be emphasized.
The different follow‐up periods of the two study designs need to be considered in comparing the results. The strengths of our cohort include the large number of patients treated with definitive radiation therapy as part of multimodal treatment, the confirmed diagnoses by experienced hematopathologists, and the standardized radiation therapy approach with follow‐up examinations. The large sample size and the length of follow‐up supply detailed information about the efficacy and long‐term outcome of stage‐ and histology‐adapted RT fields in intestinal lymphoma.
The comparative dose results in technically available CT scans of two patients with indolent duodenal lymphoma demonstrate exemplary lower radiation exposure to adjacent organs at risk from current standard ISRT 30 Gy. Reduced dose values for current ISRT 30 Gy in contrast to the past concepts are associated with significant lower NTCP for kidney dysfunction, radiation‐induced liver disease and higher‐grade gastroduodenal toxicity 26, 27, 28.
Conclusion
Intestinal lymphoma can be successfully treated with RT as component of multimodal treatment, resulting in minimal toxicity and excellent long‐term lymphoma control rates. The natural course of disease depends on indolent or aggressive histology, and the overall survival of intestinal lymphoma remains high after salvage therapy. The present long‐term results are encouraging for prospective radiation treatment in intestinal lymphoma. Current standard target volume ISRT and modern radiation techniques, intensity modulated radiation therapy and image‐guided radiation therapy, potentially allow for further improvement of patient outcomes as well as sparing of normal tissue and optional increase of tumor control. The established biophysical models for determination of NTCP offer the possibility of anticipating the apparently good (normal tissue) compatibility of modern target volume definition and radiation technique in intestinal lymphoma. The results of these large prospective studies can be a valuable contribution to the renaissance of radiation therapy using current concepts in intestinal lymphoma.
Author Contributions
Conception/design: Gabriele Reinartz, Caroline Molavi Tabrizi, Ruediger Liersch, Dominik Hering, Juergen Schultze, Oliver Micke, Severin Daum, Georg Lenz, Wolfgang Berdel, Normann Willich, Hans T. Eich
Provision of study material or patients: Gabriele Reinartz, Caroline Molavi Tabrizi, Ruediger Liersch, Hansjoerg Ullerich, Dominik Hering, Kay Willborn, Juergen Schultze, Oliver Micke, Christian Ruebe, Wolfgang Fischbach, Martin Bentz, Severin Daum, Markus Tiemann, Peter Moeller, Andreas Neubauer, Martin Wilhelm, Georg Lenz, Wolfgang E. Berdel, Normann Willich, Hans T. Eich
Collection and/or assembly of data: Gabriele Reinartz, Caroline Molavi Tabrizi, Ruediger Liersch, Hansjoerg Ullerich, Dominik Hering, Kay Willborn, Juergen Schultze, Oliver Micke, Christian Ruebe, Wolfgang Fischbach, Martin Bentz, Severin Daum, Markus Tiemann, Peter Moeller, Andreas Neubauer, Martin Wilhelm, Georg Lenz, Wolfgang E. Berdel, Normann Willich, Hans T. Eich
Data analysis and interpretation: Gabriele Reinartz, Caroline Molavi Tabrizi, Ruediger Liersch, Hansjoerg Ullerich, Dominik Hering, Kay Willborn, Juergen Schultze, Oliver Micke, Christian Ruebe, Wolfgang Fischbach, Martin Bentz, Severin Daum, Christiane Pott, Markus Tiemann, Peter Moeller, Andreas Neubauer, Martin Wilhelm, Georg Lenz, Wolfgang E. Berdel, Normann Willich, Hans T. Eich
Manuscript writing: Gabriele Reinartz, Caroline Molavi Tabrizi, Dominik Hering, Juergen Schultze, Oliver Micke, Martin Bentz, Severin Daum, Andreas Neubauer, Martin Wilhelm, Georg Lenz, Wolfgang E. Berdel, Normann Willich, Hans T. Eich
Final approval of manuscript: Gabriele Reinartz, Caroline Molavi Tabrizi, Ruediger Liersch, Hansjoerg Ullerich, Dominik Hering, Kay Willborn, Juergen Schultze, Oliver Micke, Christian Ruebe, Wolfgang Fischbach, Martin Bentz, Severin Daum, Christiane Pott, Markus Tiemann, Peter Moeller, Andreas Neubauer, Martin Wilhelm, Georg Lenz, Wolfgang E. Berdel, Normann Willich, Hans T. Eich
Disclosures
The authors indicated no financial relationships.
Acknowledgments
We thank all patients, treating physicians, investigators, and participants who provided ongoing support for these trials. The biostatistical assistance of Dr. Rene Schmidt, Institute of Biostatistics and Clinical Research University of Muenster, is gratefully acknowledged. We acknowledge support from the Open Access Publication Fund of the University of Muenster. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. In addition to the listed authors, this work was supported by 61 radiotherapeutic institutions in Germany, as follows: Aachen University Hospital, Aurich Norden Medical Care Center, Radiotherapy Practice, Berlin Robert‐Rössle‐Hospital, Bielefeld Municipal Central Hospital, Bocholt St. Agnes Hospital, Bonn Protestant Hospital and Practice Bad Godesberg, Bonn University Hospital, Bremen Hospital and Practice for Radiotherapy, Celle General Hospital, Dortmund Knappschafts‐Hospital, Dortmund Radiotherapy Practice, Düsseldorf University Hospital, Essen Alfried Krupp Hospital, Flensburg St. Franziskus Hospital, Fulda Hospital, Greifswald University Hospital, Halle/Saale University Hospital, Hamm St. Marien Hospital, Hannover Radiotherapy Practice, Hannover Medical University, Hannover Radiotherapy Practice, Heidelberg University Hospital, Herford Hospital, Hildesheim Radiotherapy Practice, Hof Hospital, Kassel Radiotherapy Practice, Koblenz Municipal Hospital Kemperhof, Köln University Hospital, Krefeld Hospital, Landshut Hospital, Lüdenscheid Hospital, Ludwigshafen Hospital, Magdeburg University Hospital, Marburg University Hospital, Minden Radiotherapy Practice, München Ludwig Maximilian University Hospital, Neubrandenburg Hospital, Neuss Lukas‐Hospital, Osnabrück Paracelsus Hospital, Ostfildern Medius Hospital, Paderborn Brüder St. Josef Hospital, Ravensburg St. Elisabeth Hospital, Rendsburg Hospital, Rostock University Hospital, Saarbrücken Winterberg‐Hospital, Schwerin Helios Hospital, Siegen St. Marien Hospital, Stade Dr. Hancken Hospital, Stendal Johanniter Hospital, Trier Mutterhaus der Borromäerinnen Hospital, Vechta Medical Care Center, Wernigerode Harz‐Hospital, Wetzlar Hospital, Wilhelmshaven Reinhard‐Nieter Hospital, and Zwickau Heinrich‐Braun Hospital.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Koch P, del Valle F, Berdel WE et al.; German Multicenter Study Group. Primary gastrointestinal non‐Hodgkin's lymphoma: II. Combined surgical and conservative or conservative management only in localized gastric lymphoma. Results of the prospective German multicenter study GIT NHL 01/92. J Clin Oncol 2001;19:3874–3883. [DOI] [PubMed] [Google Scholar]
- 2. Koch P, Willich N, Berdel WE. Primäre gastrointestinale non‐Hodgkin‐Lymphome In: Schmoll HJ, Höffken K, Possinger K, eds. Kompendium Internistsche Onkologie. Berlin: Springer‐Verlag, 2006:3066–3085. [Google Scholar]
- 3. Gobbi PG, Ghirardelli ML, Cavalli C et al. The role of surgery in the treatment of gastrointestinal lymphomas other than low‐grade MALT lymphomas. Haematologica 2000;85:372–380. [PubMed] [Google Scholar]
- 4. Aebersold DM, Vetterli D, Greiner RH. Therapieplanung: 3D‐konformierende Bestrahlung und intensitätsmodulierte Strahlentherapie In: Krukemeyer MG, Wagner W, eds. Strahlenmedizin: Ein Leitfaden für Praktiker. Berlin: De Gruyter, 2004:175–189. [Google Scholar]
- 5. Hodapp N. Der ICRU‐Report 83: Verordnung, Dokumentation und Kommunikation der fluenzmodulierten Photonenstrahlentherapie (IMRT). Strahlenther Onkol 2012;188:97–99. [DOI] [PubMed] [Google Scholar]
- 6. Reinartz G, Kardels B, Koch P et al. Analysis of failures after whole abdominal irradiation in gastrointestinal lymphomas. Is prophylactic irradiation of inguinal lymph nodes required? German Multicenter Study Group on GI‐NHL, University of Muenster. Strahlenther Onkol 1999;175:601–605. [DOI] [PubMed] [Google Scholar]
- 7. Reinartz G, Pyra RP, Lenz G et al. Favorable radiation field decrease in gastric marginal zone lymphoma: Experience of the German Study Group on Gastrointestinal Lymphoma (DSGL). Strahlenther Onkol 2019;195:544–557. [DOI] [PubMed] [Google Scholar]
- 8. Yahalom J, Illidge T, Specht L et al.; International Lymphoma Radiation Oncology Group. Modern radiation therapy for extranodal lymphomas: Field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:11–31. [DOI] [PubMed] [Google Scholar]
- 9. Koch P, Hiddemann W, Willich N. Therapiestudie maligner Non‐Hodgkin‐Lymphome des Magen‐Darm‐Traktes. Prospektive Untersuchung zur Analyse prognostischer Faktoren unter standardisierter Behandlung bei primären Lymphomen des Gastrointestinaltraktes. 1992;Stand 01.02.1993.
- 10. Koch P. Deutsche Studiengruppe Gastrointestinale Lymphome DSGL. Prospektive Untersuchung zur Optimierung der Behandlung primärer Lymphome des Magen‐Darm‐Traktes. Protokoll GIT NHL STG 02/1996. 1996;Stand 01.02.1997.
- 11. Zucca E, Copie‐Bergman C, Ricardi U et al.; ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24(suppl 6):vi144–vi148. [DOI] [PubMed] [Google Scholar]
- 12. Hübel K, Thomas RK, Diehl V. Morbus Hodgkin In: Hiddemann W, Dreyling M, Stein H, eds. Lymphome: Neue Erkenntnisse und Therapiestrategien. 1st ed. Stuttgart: Thieme, 2005:52–69. [Google Scholar]
- 13. Trotti A, Colevas AD, Setser A et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181. [DOI] [PubMed] [Google Scholar]
- 14. Rubin P, Constine LS, Fajardo LF et al. RTOG Late Effects Working Group. Overview. Late effects of normal tissues (LENT) scoring system. Int J Radiat Oncol Biol Phys 1995;31:1041–1042. [DOI] [PubMed] [Google Scholar]
- 15. Koch P, del Valle F, Berdel WE et al.; German Multicenter Study Group. Primary gastrointestinal non‐Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German multicenter study GIT NHL 01/92. J Clin Oncol 2001;19:3861–3873. [DOI] [PubMed] [Google Scholar]
- 16. Wang SL, Liao ZX, Liu XF et al. Primary early‐stage intestinal and colonic non‐Hodgkin's lymphoma: Clinical features, management, and outcome of 37 patients. World J Gastroenterol 2005;11: 5905–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matysiak‐Budnik T, Fabiani B, Hennequin C et al. Gastrointestinal lymphomas: French Intergroup clinical practice recommendations for diagnosis, treatment and follow‐up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFH). Dig Liver Dis 2018;50:124–131. [DOI] [PubMed] [Google Scholar]
- 18. Zhang S, Wang L, Yu D et al. Localized primary gastrointestinal diffuse large B cell lymphoma received a surgical approach: an analysis of prognostic factors and comparison of staging systems in 101 patients from a single institution. World J Surg Oncol 2015;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ding D, Pei W, Chen W et al. Analysis of clinical characteristics, diagnosis, treatment and prognosis of 46 patients with primary gastrointestinal non‐Hodgkin lymphoma. Mol Clin Oncol 2014;2:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daum S, Ullrich R, Heise W et al. Intestinal non‐Hodgkin's lymphoma: A multicenter prospective clinical study from the German Study Group on Intestinal non‐Hodgkin's lymphoma. J Clin Oncol 2003;21:2740–2746. [DOI] [PubMed] [Google Scholar]
- 21. Kim SW, Lim DH, Ahn YC et al. Clinical outcomes of radiation therapy for early‐stage gastric mucosa‐associated lymphoid tissue lymphoma. World J Gastroenterol 2013;19:6062–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teckie S, Qi S, Lovie S et al. Long‐term outcomes and patterns of relapse of early‐stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int J Radiat Oncol Biol Phys 2015;92:130–137. [DOI] [PubMed] [Google Scholar]
- 23. Raderer M, Streubel B, Woehrer S et al. High relapse rate in patients with MALT lymphoma warrants lifelong follow‐up. Clin Cancer Res 2005;11:3349–3352. [DOI] [PubMed] [Google Scholar]
- 24. Hoskin PJ, Kirkwood AA, Popova B et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): A randomized phase 3 non‐inferiority trial. Lancet Oncol 2014;15:457–463. [DOI] [PubMed] [Google Scholar]
- 25. Lowry L, Smith P, Qian W et al. Reduced dose radiotherapy for local control in non‐Hodgkin lymphoma: A randomised phase III trial. Radiother Oncol 2011;100:86–92. [DOI] [PubMed] [Google Scholar]
- 26. Bentzen SM, Constine LS, Deasy JO et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): An introduction to the scientific issues. Int J Radiat Oncol Biol Phys 2010;76(suppl 3):S3–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng JC, Liu HS, Wu JK et al. Inclusion of biological factors in parallel‐architecture normal‐tissue complication probability model for radiation‐induced liver disease. Int J Radiat Oncol Biol Phys 2005;62:1150–1156. [DOI] [PubMed] [Google Scholar]
- 28. Holyoake DLP, Aznar M, Mukherjee S et al. Modelling duodenum radiotherapy toxicity using cohort dose‐volume‐histogram data. Radiother Oncol 2017;123:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]


