Abstract
Introduction
Objective response rates (ORR) appear to be higher in melanoma patients who develop immune‐related adverse events (irAEs), but whether there is a similar association between irAEs and survival remains unknown.
Materials and Methods
Patients with advanced melanoma treated with single‐agent pembrolizumab or nivolumab in the province of Alberta from June 2014 to May 2017 were identified through the provincial pharmacy database. Chart review identified and categorized all irAEs that occurred while on anti–programmed cell death protein 1 (PD‐1) checkpoint inhibitors. The primary objective was to compare overall survival (OS) with patients who developed any irAEs versus those who did not. Secondary outcomes included progression‐free survival (PFS) and ORR.
Results
Among 186 patients, any‐grade and grade ≥3 irAEs occurred in 88 (47%) and 27 (15%) patients, respectively; one patient died of pneumonitis. In a landmark analysis excluding patients who died within the first 12 weeks, the median follow‐up was 24 months, 20 months in patients without any irAEs and 26 months in patients with irAEs (p = .006). Median OS was 39 versus 23 months (hazard ratio [HR], 0.46; p = .001) for any irAE and no irAE, respectively, and median OS not reached versus 29 months for grade ≥3 irAEs and no grade ≥3 irAEs, respectively. In multivariate analysis, elevated lactate dehydrogenase correlated with reduced OS (HR, 2.34; p = .001), whereas each additional cycle of treatment received (HR, 0.94; p < .001) and development of grade ≥3 irAEs (HR, 0.29, p = .024) were significantly associated with longer OS.
Conclusion
Anti‐PD‐1–associated grade ≥3 irAEs in patients with advanced melanoma is associated with better patient outcomes, including overall survival.
Implications for Practice
Previous prospective randomized clinical trials demonstrate improved response rates in patients with melanoma who develop select adverse events. The current population‐based real‐world study in advanced melanoma reports an association with anti–programmed cell death protein 1 (PD‐1)–induced grade ≥3 immune‐related adverse events (irAEs) and better patient outcomes, including overall survival. These results suggest that irAEs may be a manifestation of a patient's ability to mount a systemic immune response from PD‐1–directed therapies, which may be associated with therapeutic benefit. The finding of irAEs coinciding with clinical benefit from these therapies supposes that these events are, by and large, unavoidable, and the critical management of irAEs remains essential for optimizing patient outcomes.
Keywords: Anti‐PD‐1, Checkpoint blockade, Immune‐related adverse events, Immunotherapy, Melanoma
Short abstract
Real world patient data on efficacy, side‐effects, and clinical predictors of outcomes are lacking for immune checkpoint inhibitors. This multicenter population‐based study investigated the incidence of immune‐related adverse events and how the adverse events related to patient outcomes.
Introduction
Immune checkpoint inhibitors have revolutionized the treatment for metastatic melanoma. The programmed cell death protein 1 (PD‐1) monoclonal antibodies, pembrolizumab and nivolumab, as single agents have demonstrated superior survival and tolerability compared with ipilimumab, with median overall survivals (OS) approaching 3 years, and are now standard first‐line treatment options for patients with advanced melanoma 1, 2, 3, 4. Improved objective response rates (ORR), progression‐free survival (PFS), and OS in patients with advanced melanoma with high programmed cell death ligand 1 (PD‐L1)–expressing tumors have been demonstrated, yet patients with low expression still derive clinical benefit, and no clinical or biological marker to date has been implemented into practice 5.
The inhibitory checkpoint protein, PD‐1, functions as a cellular “rheostat” to regulate the threshold of T cell immune response against infections and cancer, while preventing autoimmune disease toward self‐antigens 6, 7. Consequently, PD‐1 axis checkpoint blockade results in distinct toxicity profiles that include autoimmune side effects frequently termed immune‐related adverse events (irAEs) that can affect any organ or system 8, 9. The diverse roles of the PD‐1 inhibitory pathway and detailed mechanisms involved in regulating the cellular and humoral immune response are still being characterized, and much remains unknown as to why some patients develop irAEs whereas others do not 7, 10.
In a large single‐center retrospective study of 298 patients with melanoma, the development of irAEs from the anti–cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) antibody, ipilimumab, did not correlate with any differences in patient outcomes 11. In contrast, a combined analysis of 5,737 patients receiving various forms of immunotherapy, including interferon alfa, interleukin‐2, vaccines, adoptive transfer of tumor‐infiltrating lymphocytes, CTLA4, and PD‐1 blockade found a survival benefit in those who developed vitiligo 12. Data relating to irAEs from anti‐PD‐1 monoclonal antibodies and associated outcomes in melanoma patients are emerging, with some studies suggesting irAEs are predictive of patient outcomes 8, 12, 13, 14, 15, 16.
The treatment landscape for melanoma has changed with the development of PD‐1–directed checkpoint blockade immunotherapies that have proven survival benefits with favorable side‐effect profiles when compared with ipilimumab alone. However, real‐world unselected patient data on efficacy, side‐effect profiles, and clinical predictors of outcomes are lacking for PD‐1 checkpoint inhibitors. In this article, we report a multicenter population‐based study for the province of Alberta of patients with advanced melanoma treated with anti‐PD‐1 checkpoint blockade with either pembrolizumab or nivolumab and investigate the incidence of irAEs and how these relate to patient outcomes.
Subjects, Materials, and Methods
Patients
CancerControl Alberta coordinates all cancer care within the province of Alberta and patients with melanoma are primarily treated at two academic cancer centers, The Cross Cancer Institute in Edmonton and the Tom Baker Cancer Centre in Calgary. We conducted a 3‐year retrospective analysis (June 2014 to May 2017) of all adult patients with unresectable stage III or IV melanoma treated with pembrolizumab (2 mg/kg intravenous every 3 weeks) or nivolumab (3 mg/kg intravenous every 2 weeks) since their introductions into the province of Alberta. The Health Research Ethics Board of Alberta Cancer Committee approved this study. A pharmacy database was used to identify patients who were treated with pembrolizumab or nivolumab for advanced melanoma during the study dates. Baseline patient characteristics, investigations, number of doses of anti‐PD‐1, occurrence of irAEs (see below), and clinical outcomes were obtained from electronic medical health records. Treatment response was defined by a radiologist as per RECIST version 1.1 17. The primary objective was to compare OS between patients who developed any irAE versus those who did not, and secondary outcomes included ORR and PFS. Associations of relevant clinical factors including age at initiation of anti‐PD‐1 therapy, sex, baseline lactate dehydrogenase (LDH), BRAF mutation status, Eastern Cooperative Oncology Group (ECOG) performance status, M stage (American Joint Committee on Cancer 2017 melanoma staging classification), use of immunomodulatory agents to treat irAEs, line of PD‐1 therapy, and number of cycles received with patient outcomes was also conducted.
Immune‐Related Adverse Events
PD‐1–associated irAEs were graded as per the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. For statistical analysis, the following categorizations of irAEs and definitions were used (Table 2): vitiligo and poliosis were graded as skin hypopigmentation; diarrhea and enterocolitis were combined; hepatotoxicity was defined as a rise in aspartate aminotransferase, alanine aminotransferase, gamma‐glutamyl transpeptidase, alkaline phosphatase (within clinical context), or bilirubin; hypothyroid and hyperthyroid were combined; hypophysitis was included with adrenal insufficiency; pneumonitis included any patient with radiographic evidence of pneumonitis as a differential diagnosis in the absence of support for an alternative etiology; and arthritis and arthralgia were combined.
Table 2.
irAE by organ system, type, and grade
| irAE by system | All patients (n = 186) | |
|---|---|---|
| Any grade, n (%) | Grade ≥3, n (%) | |
| No. of patients with ≥1 irAEa | 88 (47.3) | 27 (14.5) |
| Skin | ||
| Maculopapular rash | 29 (15.6) | 5 (2.7) |
| Hypopigmentation or vitiligo | 17 (9.1) | 0 (0) |
| Poliosis | 1 (0.5) | 0 (0) |
| Gastrointestinal | ||
| Diarrhea or enterocolitis | 27 (14.5) | 7 (3.8) |
| Hepatotoxicity | 12 (6.5) | 2 (1.1) |
| Pancreatitis | 11 (5.9) | 2 (1.1) |
| Endocrine | ||
| Hypothyroid or hyperthyroid | 21 (11.3) | 0 (0) |
| Hypophysitis or adrenal insufficiency | 6 (3.2) | 3 (1.6) |
| Hyperglycemia | 1 (0.5) | 1 (0.5) |
| Pulmonary | ||
| Pneumonitis | 11 (5.9) | 8 (4.3) |
| Rheumatological | ||
| Arthritis or arthralgia | 11 (5.9) | 3 (1.6) |
| Myositis | 3 (1.6) | 1 (0.5) |
| Renal | ||
| Acute kidney injury | 4 (2.2) | 1 (0.5) |
| Ocular | ||
| Uveitis | 2 (1.1) | 0 (0) |
Many patients had more than one irAE. Recurrence of the same irAE on subsequent cycles were not included.
Abbreviation: irAE, immune‐related adverse event.
Statistical Analysis
A landmark analysis excluded patients who died within 12 weeks of initiating anti‐PD‐1 was used to compare outcomes to eliminate bias of poor prognosis patients. OS was defined as the time from initiation of anti‐PD‐1 to death, and PFS was calculated from time of first dose of anti‐PD‐1 to progression by RECIST 1.1 or death from any cause. Comparison of subject characteristics between groups with or without irAEs were made using Wilcoxon tests for continuous variables and chi‐square or Fisher exact tests for categorical variables. Kaplan‐Meier survival analysis of OS and PFS was undertaken and compared across groups using log‐rank tests. The Bonferroni correction was applied to adjust for multiple comparisons. A multivariable Cox proportional‐hazards regression model of PFS and OS adjusted for underlying differences in subject characteristics.
Results
Patient Characteristics
One hundred eighty‐six patients were identified from the pharmacy database over the study period and 2,195 cycles (median, 11; range, 1–60) of anti‐PD‐1 checkpoint inhibitors with either pembrolizumab or nivolumab were delivered in total. Median age at initiation of anti‐PD‐1 therapy was 64 years, 109 (59%) patients were male, and 51 (27%) were BRAF mutation positive (Table 1). The majority were cutaneous primaries (n = 153; 82%), including 2 patients with ungual melanoma and 19 with unknown primaries (supplemental online Table 1). Only 79 (43%) patients had single‐agent nivolumab or pembrolizumab as first‐line therapy for advanced melanoma, with 92 (49%) patients receiving prior ipilimumab, and 43 (23%) had a previous BRAF inhibitor‐containing regimen. There were no differences in ECOG performance scores, BRAF mutational status, M stage or baseline LDH levels in patients who developed irAEs versus those who did not (Table 1). Patients who developed any irAEs received on average more cycles of anti‐PD‐1 (median, 13; interquartile range [IQR], 8–25 vs. median, 8; IQR, 4–14; p < .001).
Table 1.
Patient characteristics by the development of any irAE
| Characteristic | All patients (n = 186), n (%) | No irAE, (n = 98), n (%) | Any irAE, (n = 88), n (%) | p value |
|---|---|---|---|---|
| Age, median (range) | 63.5 (55–74) | 62 (54–74) | 67 (57–75) | .078 |
| Sex, male, n (%) | 109 (58.6) | 62 (63.3) | 47 (53.4) | .173 |
| BRAFa mutation positive, n (%) | 51 (27.4) | 31 (31.6) | 20 (22.7) | .174 |
| ECOG, n (%) | .254 | |||
| 0 | 46 (24.7) | 19 (19.4) | 27 (30.7) | |
| 1 | 109 (58.6) | 61 (62.2) | 48 (54.5) | |
| 2+ | 26 (14) | 16 (16.3) | 10 (11.4) | |
| Unknown | 5 (2.7) | 2 (2) | 3 (3.4) | |
| M stage,b n (%) | .098 | |||
| 0/1a | 44 (23.7) | 22 (22.4) | 22 (25) | |
| 1b | 39 (21) | 15 (15.3) | 24 (27.3) | |
| 1c | 67 (36) | 37 (37.8) | 30 (34.1) | |
| 1d | 36 (19.4) | 24 (24.5) | 12 (13.6) | |
| LDH, n (%) | .251 | |||
| ≤ULN | 110 (59.1) | 53 (54.1) | 57 (64.8) | |
| >ULN | 74 (39.8) | 44 (44.9) | 30 (34.1) | |
| Unknown | 2 (1.1) | 1 (1) | 1 (1.1) | |
| Line of anti‐PD‐1, n (%) | .019 | |||
| 1 | 79 (42.5) | 33 (33.7) | 46 (52.3) | |
| 2 | 40 (21.5) | 26 (26.5) | 14 (15.9) | |
| 3 | 56 (30.1) | 30 (30.6) | 26 (29.5) | |
| ≥4 | 11 (5.9) | 9 (9.2) | 2 (2.3) | |
| Median no. of cycles (IQR) | 11 (5–20) | 8 (4–14) | 13 (8–25) | <.001 |
BRAF mutations include V600E/Ec/D/K/R.
American Joint Committee on Cancer 2017 melanoma staging classification. Patients treated for unresectable stage III (M0) were included with M1a for statistical analysis.
Abbreviations: ECOG, Eastern Cooperative Group; IQR, interquartile range; irAE, immune‐related adverse event; LDH, lactate dehydrogenase; PD‐1, programmed cell death protein 1; ULN, upper limit of normal.
Distribution of irAEs
Any‐grade irAEs occurred in 88 (47%) patients and grade ≥3 irAEs occurred in 27 (15%) patients on anti‐PD‐1 checkpoint blockade (Table 2).
Skin was the most frequently affected organ, with the development of a maculopapular rash occurring in 29 (16%) and hypopigmentation or vitiligo occurring in 17 (9%) patients. Two patients developed a maculopapular rash resembling psoriasis, and one patient had severe worsening of pre‐existing psoriasis requiring temporary discontinuation without exacerbation upon reinitiation of anti‐PD‐1 treatment.
Twenty (11%) patients had grade 1–2 diarrhea or enterocolitis and another seven (4%) had grade ≥3, including one case of small bowel enteritis with upper gastrointestinal bleed, duodenal ulcers, and both large and small bowel lymphocytic infiltrate on endoscopic biopsy without any prior anti‐CTLA4 therapy. The same patient also developed progressive vitiligo over the entire body, erythema marginatum on the lower torso, and grade 1 arthritis with a complete response (CR) of small‐volume visceral metastases following 12 cycles of anti‐PD‐1 checkpoint blockade and is now on surveillance after cycle 18. Any‐grade and grade ≥3 hepatotoxicity occurred in 7% and 1% of patients, respectively. Asymptomatic pancreatic enzyme elevation was seen in nine (5%) patients, and only two developed symptomatic pancreatitis.
Changes in thyroid function were common, occurring in 21 (11%) patients, and consistently required levothyroxine replacement at some point during the course of treatment. Grade 1–2 hypophysitis or adrenal insufficiency necessitating steroid supplementation alone occurred in three (2%) patients, and three (2%) others had severe symptoms requiring hospitalization and treatment delays (grade ≥3) in addition to hormone replacement. One patient developed insulin‐dependent diabetes resulting in grade 4 hyperglycemia, requiring hospitalization.
The occurrence of pneumonitis was high, with any grade occurring in 11 (6%) patients and grade ≥3 occurring in 8 (4%) patients, including one death only 25 days after the first dose of anti‐PD‐1. Cultures did not reveal an infectious source in this case, and antibiotics were ineffective; intravenous methylprednisolone and infliximab briefly stabilized the patients’ condition before the patient and family withdrew care.
Arthritis or arthralgias of any grade were seen in 11 (6%) patients on treatment and grade ≥3 in 3 (2%) patients. Any‐grade myositis, acute kidney injury, and uveitis occurred in 1%–2% of patients, whereas grade ≥3 were rare events.
Forty‐eight (26%) patients had a total of 65 objective laboratory and radiographic irAEs (hepatotoxicity, pancreatitis, hypo‐ and hyperthyroid, hypophysitis or adrenal insufficiency, hyperglycemia, pneumonitis, and myositis).
Treatment Outcomes
Using a 12‐week landmark analysis to eliminate poor prognosis bias, 43 patients who died within 12 weeks of initiating anti‐PD‐1 therapy were excluded from the subgroup survival comparison. Median follow‐up was 24 months in the landmark patients, 20 months in patients without any irAEs, and 26 months in patients with irAEs (p = .006). The development of any‐grade irAEs from anti‐PD‐1 treatment was associated with an improved median OS of 39 versus 23 months (hazard ratio [HR], 0.46; p = .001), and grade ≥3 irAEs with an improved median OS not reached versus 29 months (HR, 0.35; p = .015; Fig. 1; Table 3). Elevated LDH was associated with a median OS of 22 months versus 38 months for patients with normal LDH levels (HR, 2.25; p < .001). Earlier M stage and objective irAEs were also associated with an improvement in OS. There were no significant differences in survival for age, gender, BRAF mutation status, primary disease site, or line of anti‐PD‐1 therapy. ORR for the entire cohort was 36%, 17% in patients without any irAEs and 57% and 70% in patients with any‐grade and ≥3 irAEs, respectively (Table 4; supplemental online Table 1), whereas PFS had no association with irAEs.
Figure 1.
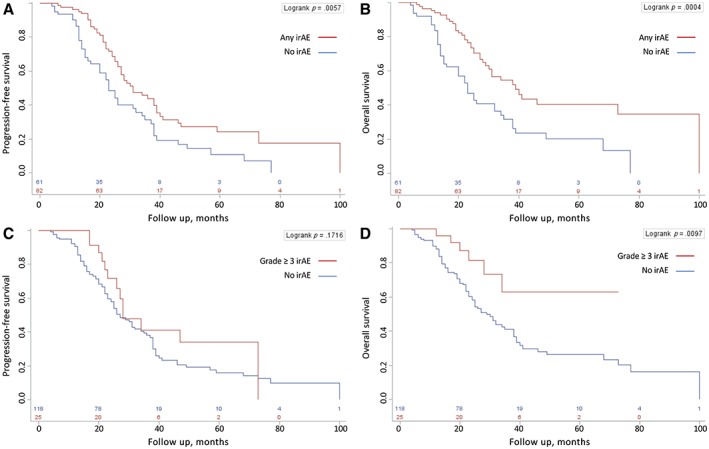
Twelve‐week landmark progression‐free survival and overall survival Kaplan‐Meier survival curves. Any‐grade irAEs (A, B) and grade ≥3 irAEs (C, D).Abbreviation: irAE, immune‐related adverse event.
Table 3.
Median overall survival in 12‐week landmark analysisa subgroups
| Characteristic | Mo (95% CI) | HR (95% CI) | p value |
|---|---|---|---|
| Age | |||
| <60 yr | 32 (25–49) | Ref | |
| ≥60 yr | 31 (24–39) | 1.07 (0.68 – 1.68) | .765 |
| Gender | |||
| Male | 32 (24–41) | Ref | |
| Female | 35 (25–40) | 0.81 (0.51–1.27) | .356 |
| BRAF statusb | |||
| Wild‐type | 29 (23–38) | Ref | |
| Mutant | 41 (28–77) | 0.63 (0.37–1.08) | .095 |
| LDH | |||
| Normal | 38 (31–73) | Ref | |
| Elevated | 22 (17–28) | 2.25 (1.43–3.54) | <.001 |
| Primary sitec | |||
| Cutaneous | 34 (25–40) | Ref | |
| Noncutaneous | 27 (22–39) | 1.12 (0.68–1.83) | .663 |
| M staged | |||
| 0/1a | Not reached | Ref | |
| 1b | 30 (20–32) | 2.82 (1.4–5.67) | .004 |
| 1c | 23 (19–39) | 2.49 (1.27–4.88) | .008 |
| 1de | 39 (25–68) | 1.72 (0.81–3.69) | .161 |
| Line of anti‐PD‐1 | |||
| 1 | 27 (23–NR) | Ref | |
| 2 | 27 (20–41) | 0.89 (0.49–1.64) | .710 |
| 3 | 38 (26–73) | 0.65 (0.37–1.12) | .122 |
| ≥4 | 68 (23–NR) | 0.51 (0.19–1.37) | .180 |
| Any irAE | |||
| No | 23 (16–32) | Ref | |
| Yes | 39 (30–100) | 0.46 (0.3–0.72) | .001 |
| Grade ≥3 irAE | |||
| No | 29 (24–38) | Ref | |
| Yes | Not reached | 0.35 (0.15–0.81) | .015 |
| Objective irAE onlyf | |||
| No | 25 (22–34) | Ref | |
| Yes | 46 (30–100) | 0.45 (0.26–0.77) | .003 |
A total of 43 patients were excluded from the landmark analysis for dying within 12 weeks of anti‐PD‐1 checkpoint blockade to eliminate poor prognosis bias.
BRAF mutations include V600E/Ec/D/K/R.
See supplemental online Table 1 for primary site subcategories.
American Joint Committee on Cancer 2017 melanoma staging classification. Five patients had unresectable stage III (M0) and were included with M1a for statistical analysis.
A total of 12 (33%) of patients with brain metastases died before the 12‐week landmark and were thus excluded, the most of any M stage group (see supplemental online Table S2).
Includes hepatotoxicity, pancreatitis, hypo‐ and hyperthyroid, hypophysitis or adrenal insufficiency, hyperglycemia, pneumonitis, and myositis.
Abbreviations: CI, confidence interval; HR, hazard ratio; irAE, immune‐related adverse event; LDH, lactate dehydrogenase; NR, not reached; PD‐1, programmed cell death protein 1.
Table 4.
Twelve‐week landmark multivariable Cox proportional‐hazards regression model for survival
| Variable | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | ||||
| <60 yr | Reference | Reference | ||
| ≥60 yr | 1.2 (0.74–1.93) | .465 | 1.29 (0.76–2.17) | .340 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.08 (0.68–1.71) | .746 | 0.92 (0.55–1.51) | .731 |
| LDH | ||||
| Normal | Reference | Reference | ||
| Elevated | 1.75 (1.1–2.79) | .019 | 2.34 (1.4–3.92) | .001 |
| BRAF status | ||||
| Normal | Reference | Reference | ||
| Mutant | 0.89 (0.51–1.56) | .690 | 0.53 (0.27–1.03) | .060 |
| ECOG | ||||
| 0 | Reference | Reference | ||
| 1 | 0.77 (0.45–1.3) | .324 | 0.84 (0.45–1.57) | .592 |
| 2+ | 0.85 (0.34–2.14) | .727 | 0.54 (0.16–1.84) | .323 |
| M stage | ||||
| 0/1a | Reference | Reference | ||
| 1b | 1.15 (0.59–2.25) | .674 | 2.38 (1.09–5.18) | .029 |
| 1c | 1.23 (0.68–2.21) | .501 | 1.71 (0.81–3.59) | .160 |
| 1d | 0.9 (0.45–1.8) | .773 | 1.15 (0.49–2.73) | .747 |
| Any irAE | ||||
| No | Reference | Reference | ||
| Yes | 0.75 (0.41–1.37) | .349 | 0.81 (0.42–1.56) | .524 |
| Grade ≥3 irAE | ||||
| No | Reference | Reference | ||
| Yes | 0.73 (0.31–1.74) | .477 | 0.29 (0.1–0.85) | .024 |
| Objective irAE only | ||||
| No | Reference | Reference | ||
| Yes | 0.75 (0.4–1.42) | .378 | 0.58 (0.27–1.23) | .153 |
| Immunomodulatory agent(s) to treat irAE | ||||
| No | Reference | Reference | ||
| Yes | 0.95 (0.46–1.98) | .899 | 1.5 (0.67–3.37) | .324 |
| Line of anti‐PD‐1 | ||||
| 1 | Reference | Reference | ||
| 2 | 0.62 (0.33–1.19) | .150 | 0.79 (0.4–1.55) | .487 |
| 3 | 0.45 (0.25–0.8) | .007 | 0.62 (0.34–1.14) | .125 |
| ≥4 | 0.3 (0.1–0.94) | .039 | 0.69 (0.19–2.47) | .566 |
| Each additional cycle | 0.96 (0.94–0.98) | <.001 | 0.94 (0.91–0.97) | <.001 |
aIncludes TNF‐a inhibitors, mycophenolate mofetil, azathioprine, interleukin‐6 inhibitors, methotrexate, or leflunomide.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; irAE, immune‐related adverse event; LDH, lactate dehydrogenase; OS, overall survival; PD‐1, programmed cell death protein 1; PFS, progression‐free survival.
A multivariable Cox proportional‐hazards regression model was used to correct for age, sex, LDH, BRAF mutation status, ECOG, M stage, any irAEs, grade ≥3 irAEs, objective irAEs, use of immunomodulatory agents to treat irAEs, line of anti‐PD‐1, and number of cycles received (Table 4). The development of grade ≥3 irAEs from anti‐PD‐1 treatment remained significantly associated with an improved overall survival (HR, 0.29; p = .024) in the regression analysis, as did normal LDH, earlier M stage, and the number of anti‐PD‐1 cycles received.
irAEs by Treatment Cycle
A total of 179 irAEs occurred in 88 patients, the majority early in the treatment course, as displayed in Figure 2. Forty‐nine percent transpired within the first six cycles, 70% within 12 cycles, and approximately 15% of irAEs still emerged beyond 1 year of treatment. Half of patients with an irAE had more than one event (supplemental online Table 1); however, after complete resolution of an irAE, the same irAE on subsequent cycles occurred only 15 times in the entire cohort. The incidence of irAEs decreases with treatment duration over time where the exposure‐adjustment irAE event rate is highest from cycles 4–6 for grade ≥3 irAEs and decreases significantly in later treatment cycles (supplemental online Fig. 2; supplemental online Table 5). The use of immunomodulatory agents for treatment of PD‐1–induced irAEs did not affect PFS or OS.
Figure 2.
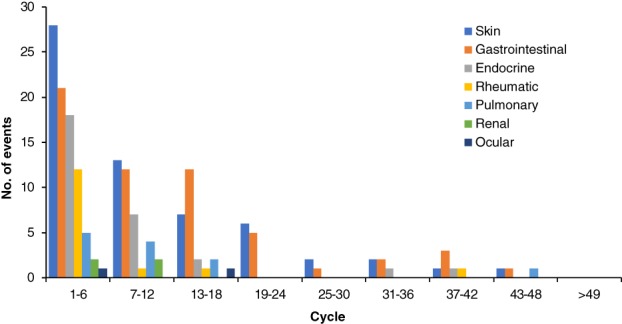
Onset of all immune‐related adverse events (irAEs) by treatment cycle. Eighty‐eight patients had a total of 179 irAEs of any grade; 16 were recurrences of the same irAE on subsequent cycles after complete resolution.
Discussion
We report the first multicenter population‐based data outside of a clinical trial of irAEs and survival outcomes from anti‐PD‐1 checkpoint blockade in advanced melanoma. The landmark KEYNOTE‐006 and CHECKMATE‐067 studies established improved survival of anti‐PD‐1 over CTLA‐4 checkpoint blockade, with long‐term median OS of approximately 3 years with single‐agent nivolumab or pembrolizumab 1, 2, 3, 4.
The association of better patient outcomes with the development of PD‐1–induced irAEs found in our 12‐week landmark analysis is substantial, with a median OS of 39 versus 23 months with and without any irAEs, respectively. The median OS for development of grade ≥3 irAEs was not reached and maintained statistical significance in the Cox regression analysis verifying this clinical benefit associated with irAEs. Previous reports support our findings including a recent study of 80 patients who experienced clinically significant irAEs on combined CTLA4 and anti‐PD‐1 checkpoint blockade in which 70% had either partial or complete responses, similar to 57% in our cohort (supplemental online Table 1) 18. Weber et al. recently reported a nivolumab‐treated pooled analysis of four trials demonstrating an increased ORR with treatment‐related select adverse event 13, and Sznol et al. reported on a pooled analysis of ipilimumab with nivolumab combination–treated patients in which an increased ORR was seen in patients who developed irAEs beyond 12 weeks while on single‐agent PD‐1 compared with patients who developed irAEs less than 12 weeks on combination 14. A single‐center analysis from two phase I cohorts treated with nivolumab and a peptide vaccine showed a survival benefit with cutaneous irAEs 16, and one observational study of 67 patients treated with pembrolizumab for advanced melanoma showed higher ORR with vitiligo 15. Finally, related retrospective analyses in non‐small cell lung cancer have found that patients treated with nivolumab had improved survival with the development of irAEs 19, 20.
Groundwork discovery of high PD‐L1 expression on solid tumors correlating with worse prognosis suggested that tumors escape antitumor immunity through engagement of the expressed ligand to PD‐1 on effector T cells in the tumor microenvironment (TME) 6, 21, 22. Successful antitumor activity through PD‐1 blockade was thought to require activation and expansion of T cells within the TME, which has been the basis for the anatomic site‐of‐action for anti‐PD‐1 antibodies 22, 23, 24, 25, 26. However, Spitzer et al. recently challenged this notion by demonstrating in a mice model that secondary lymphoid organs were critical sites for T cell generation in PD‐1–directed antitumor immune responses, and an expanded population of peripheral CD4 T cells conferred protection to new tumors in responding CTLA‐4‐treated humans 27. Moreover, the efficacy of adjuvant anti‐PD‐1–directed immunotherapy in which the TME is essentially absent provides clinical evidence supporting the systemic immunity hypothesis 28. The association of the development of irAEs with response and survival does not preclude that PD‐1 blockade acts primarily in the TME to stimulate antitumor CD8‐positive T cell responses, as a pre‐existing T cell response within the TME is likely necessary in mounting an adequate systemic immune response from PD‐1 checkpoint inhibitors. Regardless, irAEs from PD‐1 checkpoint blockade are manifestations of systemic immune activation that not all patients acquire and are associated with response and survival benefit.
Immune‐related AE occurred more frequently in patients with normal LDH than those with elevated LDH levels (65% vs. 34%), although this difference was not statistically significant. Elevated LDH was also independently associated with worse survival as seen on the multivariate analysis, consistent with previous studies. A recent analysis in patients from KEYNOTE‐001 also found that elevated LDH correlated with increased tumor size and lower rates of CR 29. When all considered together, this supports aspects of the “cancer immunogram” framework that a patient's immune system may be overwhelmed or perhaps even suppressed by a larger tumor burden, possibly from inhibitory tumor metabolism 30. The independent associations for poorer outcomes in patients with high LDH levels and without the development of any irAEs may also indicate that inhibitory tumor metabolism itself, or some other unknown marker of cellular interference, could have the ability to impact the general immune status on a more systemic level.
It is noteworthy that rates of pneumonitis in the current study are high in comparison with previous clinical trial reports 1, 2, 23, although comparable to other institutional reported outcomes 31. The diagnosis of pneumonitis remains one of exclusion with variable clinical presentations, radiographic changes, and pathologic findings, whereas confirmatory bronchoscopy and tissue biopsies are not always practical, especially for asymptomatic patients. Naidoo et al. demonstrated that a simple treatment delay often results in radiographic resolution of grade 1 pneumonitis with the risk of recurrent pneumonitis in future cycles. Nevertheless, grade ≥3 pneumonitis can progress despite immunosuppressive therapy and fatalities do occur, reflecting the clinical significance of this irAE that requires judicious management.
It is worth noting that there are no clinical data in melanoma to suggest that longer duration of therapy is superior in responding patients. Treatment length is a reflection of physician practice styles, patient preferences, patient tolerance to treatment, and clinical benefit. More therapy is not necessarily better for every patient despite the multivariable analysis suggesting that more cycles result in improved outcomes. Additionally, there are strong data to suggest that stopping treatment after a complete response is safe 29.
Finally, this study carries limitations, including the retrospective nature, which cannot exclude selection bias. It is limited by patient reporting and physician documentation of adverse events, and the low rates of grade 1–2 irAEs in our study may represent underreporting. This low rate of grade 1–2 irAEs reporting may have also contributed to the lack of statistical significance of all irAEs and objective irAEs after multivariate analyses. This limitation of potential underreporting and misattribution of grade 1–2 irAEs is inherent to all real‐world evidence or observational studies. The potential for lead time bias is also noted, in which patients with longer survival are more likely to receive longer treatment exposure and thus more likely to experience irAEs. IrAEs are also more common in early treatment cycles and decrease with exposure over time (supplemental online Fig. 2; supplemental online Table 5). Moreover, a recent study in patients with urothelial cancer treated with immunotherapy has shown an association of irAEs with improved outcomes that was not attributed to increased drug exposure 32. Although, PD‐L1 expression status is a known predictive biomarker for anti‐PD‐1 checkpoint inhibitors, particularly in non‐small cell lung cancer, this was not evaluated in comparison to irAEs in our study. PD‐L1 expression is not required for drug eligibility in advanced melanoma and thus was not completed on our patient population. However, this should be sought in future studies, particularly in other tumor types in which the predictive and prognostic role of PD‐L1 expression impacts management decisions.
Conclusion
These results suggest that host‐specific factors may impact a patient's ability to mount a systemic immune response from PD‐1–directed therapies, perhaps more significantly than previously considered. Further research identifying strategies aimed at monitoring, and conceivably augmenting, the systemic immune response may improve outcomes for nonresponding patients. Nevertheless, the finding of irAEs coinciding with clinical benefit from these therapies supposes that these events are, by and large, unavoidable, at least for the time being, and the critical management of irAEs remains essential for optimizing patient outcomes.
Author Contributions
Conception/design: Aleksi Suo, Jose G. Monzon, Don Morris, Tina Cheng
Provision of study material or patients: Aleksi Suo, Jose G. Monzon, Michael Smylie, John Walker, Tina Cheng
Collection and/or assembly of data: Aleksi Suo, Yin Chan, Carissa Beaulieu
Data analysis and interpretation: Shiying Kong, Winson Y. Cheung
Manuscript writing: Aleksi Suo, Yin Chan, Jose G. Monzon, Don Morris, Tina Cheng
Final approval of manuscript: Aleksi Suo, Yin Chan, Carissa Beaulieu, Shiying Kong, Winson Y. Cheung, Jose G. Monzon, Michael Smylie, John Walker, Don Morris, Tina Cheng
Disclosures
Jose G. Monzon: Amgen, Bristol‐Myers Squibb, Merck, Novartis, Celgene, Taiho (C/A), Merck (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Information
Acknowledgments
The Health Research Ethics Board of Alberta Cancer Committee approved this study. This was a retrospective chart review; individual patient consent was not required. This study did not involve animal or human tissue.
Funding was provided by Alberta Cancer Foundation, The Dr. Robert C. Westbury Melanoma Research Fund, and Arnie Charbonneau Cancer Institute.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Adil Daud, Katy Tsai. Management of Treatment‐Related Adverse Events with Agents Targeting the MAPK Pathway in Patients with Metastatic Melanoma. The Oncologist 2017:22;823–833.
Implications for Practice: Targeted therapy with BRAF plus MEK inhibitors has become the standard of care for patients with advanced‐stage BRAF V600‐mutant metastatic melanoma. To provide optimal therapeutic benefit to patients, clinicians need a keen understanding of the toxicity profiles of these drugs. Prompt identification and an understanding of which adverse events are most likely BRAF or MEK inhibitor associated provide a rationale for appropriate therapy adjustments. Practical recommendations derived from clinical experience are provided for management of key drug‐related toxicities.
References
- 1. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 2. Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodi FS, Chiarion‐Sileni V, Gonazalez R et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (Checkmate 067): 4‐year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–1492. [DOI] [PubMed] [Google Scholar]
- 4. Robert C, Ribas A, Schachter J et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE‐006): Post‐hoc 5‐year results from an open‐label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239–1251. [DOI] [PubMed] [Google Scholar]
- 5. Daud AI, Wolchok JD, Robert C et al. Programmed death‐ligand 1 expression and response to the anti‐programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 2016;34:4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okazaki T, Chikuma S, Iwai Y et al. A rheostat for immune responses: The unique properties of PD‐1 and their advantages for clinical application. Nat Immunol 2013;14:1212–1218. [DOI] [PubMed] [Google Scholar]
- 7. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018;18:153–167. [DOI] [PubMed] [Google Scholar]
- 8. Postow MA, Sidlow R, Hellmann M. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Zhou S, Yang F et al. Treatment‐related adverse events of PD‐1 and PD‐L1 inhibitors in clinical trials: A systematic review and meta‐analysis. JAMA Oncol 2019;5:1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev 2016;271:246–259. [DOI] [PubMed] [Google Scholar]
- 11. Horvat TZ, Adel NG, Dang TO et al. Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teulings HE, Limpens J, Jansen SN et al. Vitiligo‐like depigmentations in patients with stage III‐IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta‐analysis. J Clin Oncol 2015;33:773–784. [DOI] [PubMed] [Google Scholar]
- 13. Weber JS, Hodi FS, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–792. [DOI] [PubMed] [Google Scholar]
- 14. Sznol M, Ferrucci PF, Hogg D et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017;35:3815–3822. [DOI] [PubMed] [Google Scholar]
- 15. Hua C, Boussemart L, Mateus C et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016;152:45–51. [DOI] [PubMed] [Google Scholar]
- 16. Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 18. Pollack MH, Betof A, Dearden H et al. Safety of resuming anti‐PD‐1 in patients with immune‐related adverse events (irAEs) during combined anti‐CLTA‐4 and anti‐PD1 in metastatic melanoma. Ann Oncol 2018;29:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haratani K, Hayashi H, Chiba Y et al. Association of immune‐related adverse events with nivolumab efficacy in non‐small‐cell lung cancer. JAMA Oncol 2018;4:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ricciuti B, Genova C, De Giglio A et al. Impact of immune‐related adverse events on survival in patients with advanced non‐small cell lung cancer treated with nivolumab: Long‐term outcomes from a multi‐institutional analysis. J Cancer Res Clin Oncol 2019;145:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwai Y, Ishida M, Tanaka Y et al. Involvement of PD‐L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD‐L1 blockade. Proc Natl Acad Sci USA 2002;99:12293–12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson RH, Gillet MD, Cheville JC et al. Costimulatory B7‐H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA 2004;101:17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taube JM, Klein A, Brahmer JR et al. Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti‐PD‐1 therapy. Clin Cancer Res 2014;20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma P, Hu‐Lieskovan S, Wargo JA et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Donnell JS, Long GV, Scoyler RA et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev 2017;52:71–81. [DOI] [PubMed] [Google Scholar]
- 27. Spitzer MH, Carmi Y, Reticker‐Flynn NE et al. Systemic immunity is required for effective cancer immunotherapy. Cell 2017;168:487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weber JW, Mandala M, Del Vecchio M et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 29. Robert C, Ribas A, Hamid O et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol 2018;36:1668–1674. [DOI] [PubMed] [Google Scholar]
- 30. Blank CU, Haanen JB, Ribas A et al. Cancer Immunology. The “cancer immunogram”. Science 2016;352:658–660. [DOI] [PubMed] [Google Scholar]
- 31. Naidoo J, Wang X, Woo KM et al. Pneumonitis in patients treated with anti‐programmed death‐1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maher VE, Fernandes LL, Weinstock C et al. Analysis of the association between adverse events and outcomes in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol 2019;37:2730–2737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Information


