Abstract
Background
International Metastatic Renal Cell Carcinoma (mRCC) Database Consortium (IMDC) risk groups are important when considering therapeutic options for first‐line treatment.
Materials and Methods
Adult patients with clear cell mRCC initiating first‐line sunitinib between 2010 and 2018 were included in this retrospective database study. Median time to treatment discontinuation (TTD) and overall survival (OS) were estimated using Kaplan‐Meier analysis. Outcomes were stratified by IMDC risk groups and evaluated for those in the combined intermediate and poor risk group and separately for those in the intermediate risk group with one versus two risk factors.
Results
Among 1,769 patients treated with first‐line sunitinib, 318 (18%) had favorable, 1,031 (58%) had intermediate, and 420 (24%) had poor IMDC risk. Across the three risk groups, patients had similar age, gender, and sunitinib initiation year. Median TTD was 15.0, 8.5, and 4.2 months in the favorable, intermediate, and poor risk groups, respectively, and 7.1 months in the combined intermediate and poor risk group. Median OS was 52.1, 31.5, and 9.8 months in the favorable, intermediate, and poor risk groups, respectively, and 23.2 months in the combined intermediate and poor risk group. Median OS (35.1 vs. 21.9 months) and TTD (10.3 vs. 6.6 months) were significantly different between intermediate risk patients with one versus two risk factors.
Conclusion
This real‐world study found a median OS of 52 months for patients with favorable IMDC risk treated with first‐line sunitinib, setting a new benchmark on clinical outcomes of clear cell mRCC. Analysis of intermediate risk group by one or two risk factors demonstrated distinct clinical outcomes.
Implications for Practice
This analysis offers a contemporary benchmark for overall survival (median, 52.1 months; 95% confidence interval, 43.4–61.2) among patients with clear cell metastatic renal cell carcinoma who were treated with sunitinib as first‐line therapy in a real‐world setting and classified as favorable risk according to International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group classification. This study demonstrates that clinical outcomes differ between IMDC risk groups as well as within the intermediate risk group based on the number of risk factors, thus warranting further consideration of risk group when counseling patients about therapeutic options and designing clinical trials.
Keywords: International Metastatic Renal Cell Carcinoma Database Consortium, Metastatic renal cell carcinoma, Sunitinib, Real‐world clinical outcome, Overall survival
Short abstract
This article reports real‐world data on patients with metastatic renal cell carcinoma treated with first‐line sunitinib to provide benchmarks on clinical outcomes by IMDC prognostic risk group.
Introduction
Sunitinib is a standard first‐line treatment for metastatic renal cell carcinoma (mRCC) 1. Clinical trials have reported that clinical outcomes of patients with mRCC treated with first‐line sunitinib may vary across prognostic risk groups defined by International mRCC Database Consortium (IMDC) criteria 2, 3, 4, 5. Based on six risk factors (i.e., <1 year from time of renal cell carcinoma [RCC] diagnosis to first‐line treatment initiation, Karnofsky performance status [KPS] <80%, serum hemoglobin less than the lower limit of normal [LLN], corrected calcium more than the upper limit of normal [ULN], neutrophil count >ULN, platelet count >ULN), the IMDC risk group categorizes patients as having favorable risk (no factors), intermediate risk (one or two factors), or poor risk (at least three factors). The IMDC risk model is a well‐established prognostic model for mRCC that provides crucial information for guiding treatment decisions and trial design and predicting drug effectiveness 5, 6.
In previous studies of clinical trials, outcomes according to the different IMDC risk groups of patients treated with first‐line sunitinib have varied substantially, limiting their application to patients seen in routine clinical practice. A retrospective analysis of the phase III sunitinib versus interferon alfa trial demonstrated that, of the 375 patients treated with sunitinib, there were 38% in the favorable risk group, 55% in the intermediate risk group, and 11% in the poor risk group based on the IMDC prognostic risk group. The median progression‐free survival (PFS) for patients treated with sunitinib was 16.0 months (95% confidence interval [CI], 13.6–17.3), 10.7 months (95% CI, 8.6–12.5), and 2.5 months (95% CI, 2.3–6.5) for favorable, intermediate, and poor risk groups, respectively. When intermediate and poor IMDC risk groups were combined, the median PFS was 10.6 months (95% CI, 8.1–10.9). On the other hand, in the phase III CheckMate 214 clinical trial that compared sunitinib with nivolumab plus ipilimumab, median PFS for patients treated with sunitinib was 25.1 months (95% CI, 20.6–not estimable) and 8.4 months (95% CI, 7.0–10.8) for favorable and combined intermediate and poor risk groups, respectively. Based on these results, the effect of sunitinib on clinical outcomes may vary by patients’ IMDC prognostic risk group 3, 5.
The effectiveness of first‐line sunitinib by IMDC prognostic risk group in contemporary real‐world settings has not been widely reported in the literature. Furthermore, limited studies have examined heterogeneity in clinical outcomes among the intermediate risk group. Prior studies on heterogeneity in the intermediate risk group have focused on data collected in trials 5. To address this gap in knowledge, the objective of this study was to assess real‐world data on patients with mRCC treated with first‐line sunitinib to provide contemporary benchmarks on clinical outcomes by IMDC prognostic risk group. Also, this study assessed heterogeneity in patient characteristics and clinical outcomes among patients with mRCC in the IMDC intermediate risk group who received first‐line sunitinib in real‐world settings.
Materials and Methods
Study Design and Study Population
A retrospective, longitudinal cohort study was conducted using data from select IMDC clinical sites. Demographic, clinical, laboratory, and outcome data on patients with mRCC were collected retrospectively from medical charts using uniform database templates and standardized definitions to ensure data were collected consistently. Consecutive patient cohorts were identified from pharmacy databases, registries, or clinic lists.
For this study, eligible patients were diagnosed with mRCC when aged at least 18 years and initiated sunitinib after mRCC diagnosis as the first‐line of targeted treatment between 2010 and 2018. Patients with non‐clear cell mRCC and those who could not be classified into an IMDC risk group were excluded. The index date was defined as the date of first‐line sunitinib treatment initiation, and the baseline period was defined as the time from mRCC diagnosis to the index date. The follow‐up period spanned from the time from the index date to the date of last contact or death.
Study Variables and Outcomes
Patient demographic and clinical characteristics during the baseline period or at index date were assessed. The IMDC prognostic risk group was computed at index date based on the presence of six individual risk factors (i.e., <1 year from time of RCC diagnosis to first‐line treatment initiation, KPS <80%, serum hemoglobin <LLN, corrected calcium >ULN, neutrophil count >ULN, platelet count >ULN). Those with no risk factors had favorable risk, those with one or two risk factors had intermediate risk, and those with or more than three risk factors had poor risk disease 6. For the analysis of individual risk factors, patients classified as intermediate risk in the main analysis for having one risk factor and one missing risk factor were excluded from this subgroup analysis, as their number of risk factors could not be determined.
Clinical outcomes following initiation of first‐line sunitinib initiation were assessed in the follow‐up period, including time to treatment discontinuation (TTD), overall survival (OS), reasons for sunitinib treatment discontinuation, physician‐assessed best response, and distribution of second‐line treatment. TTD was defined as the time from initiation to discontinuation of sunitinib for any reason, including progression, death, or toxicity, and was used as a proxy for PFS, similar to other studies 7. OS was defined as the time from initiation of sunitinib to death. Real‐world physician‐assessed best response was based on clinical criteria or radiographic criteria using the RECIST guidelines with imaging assessments occurring at clinically variable time points. Best response included partial response or complete response, stable disease, and progressive disease. Objective response rate (ORR) was reported as the proportion of patients with partial or complete response.
Statistical Analysis
Patients were classified in the favorable, intermediate, or poor IMDC risk group as described above. For the overall cohort and stratified by each risk group, baseline demographic and clinical characteristics were described using frequencies and proportions for categorical variables, and means, SDs, and medians for continuous variables. For comparisons between risk groups, a global chi‐square test (or Fisher's exact test as appropriate) for categorical variables and a Wilcoxon rank‐sum test for continuous variables were used.
TTD and OS were estimated using Kaplan‐Meier analysis, and log‐rank tests were performed for statistical comparison across risk groups. Cox proportional hazards models were also used to estimate hazard ratios (HRs) and 95% CIs between favorable versus nonfavorable IMDC groups for TTD and OS. Models were adjusted for potential baseline confounders including age, gender, year of sunitinib initiation, number of metastases, and prior nephrectomy. Reasons for first‐line sunitinib treatment discontinuation, physician‐assessed best response, and type of second‐line treatment were described with frequencies and proportions. Similar analyses were conducted among patients in the combined intermediate and poor risk group and among the subgroup of patients in the intermediate risk group stratified by patients having one versus two risk factors. All p values were two‐sided, and a threshold of p < .05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Demographic and Clinical Characteristics
Patient demographic and clinical characteristics are reported in Table 1. Among the 1,769 patients included in the study, 318 (18.0%) had favorable risk, 1,031 (58.3%) had intermediate risk, and 420 (23.7%) had poor risk. Across the favorable, intermediate, and poor IMDC risk groups, patients had similar age, gender distribution, and year of sunitinib initiation. The proportion of patients who received nephrectomy was highest in the favorable risk group (99.1%), followed by intermediate (88.1%) and poor (66.3%) risk groups.
Table 1.
Baseline demographics and clinical characteristics among patients with clear cell metastatic renal cell carcinoma who received first‐line sunitinib since 2010, stratified by IMDC prognostic risk groups
| Characteristics | Overall (n = 1,769), n (%) | IMDC prognostic risk group, n (%) | ||
|---|---|---|---|---|
| Favorable (n = 318, 18.0%) | Intermediate (n = 1,031, 58.3%) | Poor (n = 420, 23.7%) | ||
| Demographic characteristics | ||||
| Age, mean ± SD [median], years | 63.0 ± 9.9 [63.7] | 63.8 ± 9.6 [64.6] | 62.9 ± 10.2 [63.6] | 62.6 ± 9.6 [63.5] |
| Race | 1,096 | 217 | 624 | 255 |
| White | 897 (81.8) | 183 (84.3) | 505 (80.9) | 209 (82.0) |
| Nonwhite | 199 (18.2) | 34 (15.7) | 119 (19.1) | 46 (18.0) |
| Gender | 1,769 | 318 | 1,031 | 420 |
| Male | 1,309 (74.0) | 234 (73.6) | 772 (74.9) | 303 (72.1) |
| Female | 460 (26.0) | 84 (26.4) | 259 (25.1) | 117 (27.9) |
| Tumor characteristics | ||||
| Number of metastases | 1,636 | 290 | 948 | 398 |
| 1 | 1,303 (79.6) | 230 (79.3) | 729 (76.9) | 344 (86.4) |
| >1 | 333 (20.4) | 60 (20.7) | 219 (23.1) | 54 (13.6) |
| Brain metastases | 1,364 | 225 | 788 | 351 |
| Yes | 151 (11.1) | 20 (8.9) | 85 (10.8) | 46 (13.1) |
| No | 1,213 (88.9) | 205 (91.1) | 703 (89.2) | 305 (86.9) |
| Bone metastases | 1,457 | 243 | 845 | 369 |
| Yes | 536 (36.8) | 73 (30.0) | 300 (35.5) | 163 (44.2) |
| No | 921 (63.2) | 170 (70.0) | 545 (64.5) | 206 (55.8) |
| Prior treatment | ||||
| Prior nephrectomy | 1,768 | 318 | 1,031 | 419 |
| Yes | 1,501 (84.9) | 315 (99.1) | 908 (88.1) | 278 (66.3) |
| No | 267 (15.1) | 3 (0.9) | 123 (11.9) | 141 (33.7) |
| Prior IL‐2 or IFN therapy | 1,765 | 318 | 1,027 | 420 |
| Yes | 65 (3.7) | 21 (6.6) | 35 (3.4) | 9 (2.1) |
| No | 1,700 (96.3) | 297 (93.4) | 992 (96.6) | 411 (97.9) |
| Year of therapy initiation | 1,769 | 318 | 1,031 | 420 |
| 2010–2013 | 1,256 (71.0) | 226 (71.1) | 730 (70.8) | 300 (71.4) |
| 2014–2018 | 513 (29.0) | 92 (28.9) | 301 (29.2) | 120 (28.6) |
The italicized numbers represent the number of patients that had information available for each characteristic.
Abbreviations: IFN, interferon; IL‐2, interleukin 2; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
Clinical Outcomes
The most common reason for discontinuing first‐line sunitinib treatment across all risk groups was disease progression (supplemental online Table 1). Of 1,521 patients who discontinued first‐line treatment, 915 patients subsequently received second‐line treatment. The distribution of second‐line treatment is shown in supplemental online Table 2. Everolimus was the most common second‐line treatment across all IMDC risk groups, accounting for approximately 40% of all second‐line treatments. Other common second‐line treatments included pazopanib (15.3%), axitinib (14.5%), and nivolumab (11.1%). The second‐line treatment distribution was similar in the favorable and intermediate risk groups, but in the poor risk group, the proportion of patients who used axitinib (19.8%) was higher than that of pazopanib (14.2%).
The median TTD was 8.1 months (95% CI, 7.7–8.6) for the overall population, 15.0 months (95% CI, 12.4–17.3) for patients in the favorable risk group, 8.5 months (95% CI, 8.0–9.5) for those in the intermediate risk group, 4.2 months (95% CI, 3.4–4.9) for those in the poor risk group, and 7.1 months (95% CI, 6.5–7.9) in the combined intermediate and poor risk group (Fig. 1). After adjusting for baseline demographic and clinical characteristics, patients in the favorable IMDC risk group had a 37% reduction in the hazard of TTD (adjusted HR, 0.63; 95% CI, 0.54–0.72; p < .01) compared with patients in nonfavorable IMDC risk groups (i.e., those in the intermediate and poor risk groups).
Figure 1.
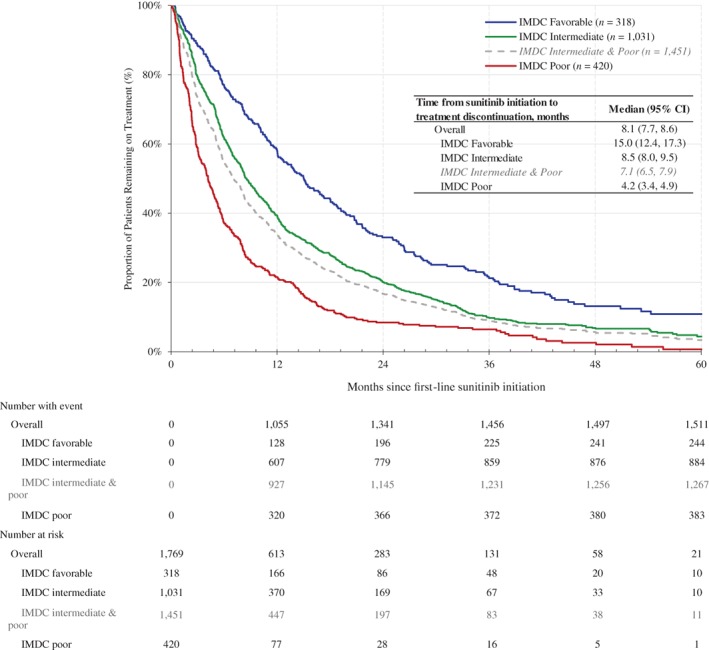
Kaplan‐Meier analysis of time to treatment discontinuation for patients with clear cell metastatic renal cell carcinoma who received first‐line sunitinib since 2010, stratified by IMDC prognostic risk groups. Abbreviations: CI, confidence interval; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
The median OS was 28.6 months (95% CI, 25.9–31.0); the median OS was 52.1 months (95% CI, 43.4–61.2) in the favorable risk group, 31.5 months (95% CI, 28.9–33.9) in the intermediate risk group, 9.8 months (95% CI, 8.3–11.4) months in the poor risk group, and 23.2 months (95% CI, 21.0–25.8) in the combined intermediate and poor risk groups (Fig. 2). After adjusting for baseline demographic and clinical characteristics, patients in the favorable IMDC risk group had a significant lower hazard of death (adjusted HR, 0.47; 95% CI, 0.39–0.57; p < .01) compared with patients in nonfavorable IMDC risk groups (i.e., those in the intermediate and poor risk groups).
Figure 2.
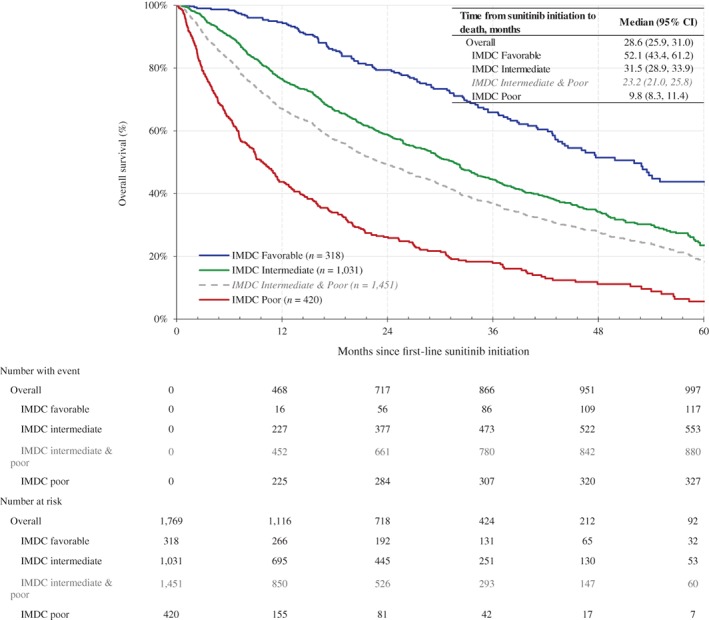
Kaplan‐Meier analysis of overall survival for patients with clear cell metastatic renal cell carcinoma who received first‐line sunitinib since 2010, stratified by IMDC prognostic risk groups. Abbreviations: CI, confidence interval; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
The ORR was 38.5%, 34.6%, and 21.7% in the favorable, intermediate, and poor risk groups, respectively; the proportion of patients with response of stable disease was 45.1%, 38.4%, and 32.3% in the favorable, intermediate, and poor risk groups, respectively (Table 2).
Table 2.
Physician‐assessed best response among patients with clear cell metastatic renal cell carcinoma who received first‐line sunitinib since 2010, stratified by IMDC prognostic risk groups
| Physician‐assessed best response | Overall (n = 1,540), n (%) | IMDC prognostic risk group, n (%) | ||
|---|---|---|---|---|
| Favorable (n = 288, 18.7%) | Intermediate (n = 902, 58.6%) | Poor (n = 350, 22.7%) | ||
| Complete or partial response | 499 (32.4) | 111 (38.5) | 312 (34.6) | 76 (21.7) |
| Complete response | 57 (3.7) | 17 (5.9) | 35 (3.9) | 5 (1.4) |
| Partial response | 442 (28.7) | 94 (32.6) | 277 (30.7) | 71 (20.3) |
| Stable disease | 589 (38.2) | 130 (45.1) | 346 (38.4) | 113 (32.3) |
| Progressive disease | 452 (29.4) | 47 (16.3) | 244 (27.1) | 161 (46.0) |
Only patients with physician‐assessed best response information were included.
Abbreviation: IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
Subgroup Analysis of Patients with Intermediate IMDC Risk
Within the subgroup of patients classified as intermediate risk, 458 and 427 patients had one and two risk factors, respectively. For this subgroup, the most common IMDC risk factors were less than 1 year from RCC diagnosis to first‐line treatment initiation (65%) and anemia (50%). Patients with one versus two risk factors had a significantly lower proportion of brain metastases (8.3% vs. 13.5%, p = .03) and bone metastases (32.3% vs. 41.9%, p < .01) and a higher proportion of prior nephrectomy (91.7% vs. 81.5%, p < .01) and prior interleukin‐2 (IL‐2)/interferon (IFN) therapy (5.7% vs. 2.1%, p < .01). For patients with one versus two risk factors, both the median OS and TTD were significantly greater (p < .01) in patients with one risk factor; the median OS was 35.1 months (95% CI, 31.7–39.6) versus 21.9 months (95% CI, 18.5–25.8; Fig. 3), and the median TTD was 10.3 months (95% CI, 8.7–12.0) versus 6.6 months (95% CI, 5.6–7.9). For patients with one risk factor, median OS ranged from 31.9 months (95% CI, 24.9–44.8) in patients with anemia to 38.1 months (95% CI, 25.8–not reached) in patients with hypercalcemia; median TTD ranged from 7.5 months (95% CI, 3.1–12.9) in patients with neutrophilia to 22.6 months (95% CI, 9.8–28.3) in patients with hypercalcemia (supplemental online Table 3). After adjusting for baseline characteristics and significantly different clinical characteristics (i.e., age, gender, year of sunitinib initiation, number of metastases, brain and bone metastases, prior nephrectomy, and prior IL‐2/IFN therapy), patients with one risk factor had a significantly lower hazard of death (adjusted HR, 0.70; 95% CI, 0.57–0.85; p < .01) and had significantly lower hazard of discontinuing sunitinib treatment (adjusted HR, 0.81; 95% CI, 0.69–0.96; p = .01) compared with those with two risk factors.
Figure 3.
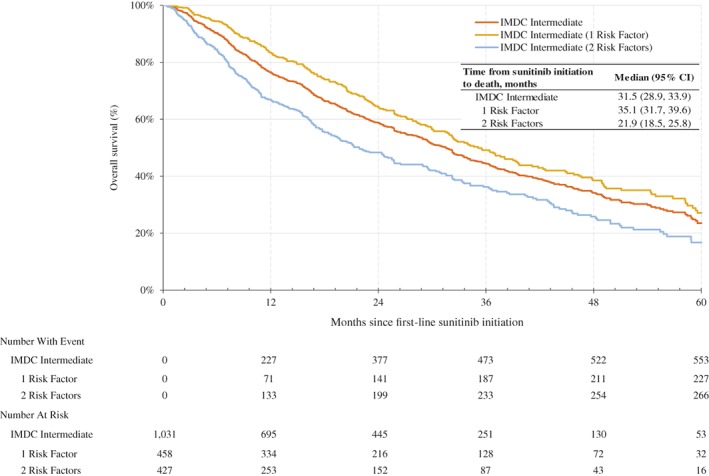
Kaplan‐Meier analysis of overall survival for IMDC intermediate risk patients with clear cell metastatic renal cell carcinoma who received first‐line sunitinib since 2010. Abbreviations: CI, confidence interval; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
Discussion
The IMDC risk model has been validated multiple times for patients treated with first‐line through fourth‐line targeted therapy 8, 9, 10, 11 and has been used in clinical practice for treatment decision making as well as in trial design and data interpretation. In this study, patients in the favorable IMDC risk group had significantly higher median OS, TTD, and ORR compared with patients in the intermediate, poor, or combined intermediate and poor risk groups. In addition, heterogeneity was observed within the intermediate risk group as indicated by more favorable OS, TTD, and ORR in patients with one versus two risk factors.
An OS of 52.1 months in the favorable risk group found in this study improved compared with the 43.2 months found in a previous real‐world study by Heng et al. 6, 12, which may be attributed to a number of reasons. First, this study included only patients with clear cell histology who may have better clinical outcomes 8. In addition, there has been better access to second‐ and third‐line therapies that are more contemporary and effective, as well as better optimization of sunitinib use by physicians over time (i.e., refined dosing strategies). In comparison with previous clinical trial results using IMDC risk group classification, findings from the current study are generally consistent. Table 3 examines clinical outcomes of two phase III clinical trials containing a first‐line sunitinib arm, stratified by IMDC risk group. CheckMate 214, a phase III trial comparing nivolumab plus ipilimumab versus sunitinib for previously untreated clear cell advanced RCC, reported that the median OS of the sunitinib arm was not reached at 30 months for the favorable risk group and 26.6 months (95% CI, 22.1–33.4) for the combined intermediate and poor risk group 13. In another phase III trial comparing sunitinib versus IFN‐α as first‐line treatment for patients with mRCC, the median OS in the sunitinib arm was not reached for those in the favorable risk group and was 20.3 months (95% CI, 16.8–23.0) for the combined intermediate and poor risk group 3, 5. These results from clinical trial data are consistent with the results of this real‐world study.
Table 3.
Select characteristics of first‐line sunitinib patients in the IMDC database and first‐line sunitinib patients in phase III clinical trials
| Characteristics and outcomes | IMDC real‐world databasea | CheckMate 214 trialb | Sunitinib vs. IFN‐α trialc Overall | KEYNOTE‐426 triald Overall | JAVELIN Renal 101 triale Overall | ||
|---|---|---|---|---|---|---|---|
| Overall | Intermediate & poor | Overall | Intermediate & poor | ||||
| First‐line sunitinib treatment period | January 2010 to February 2018 | October 2014 to February 2016 | August 2004 to October 2005 | October 2016 January 2018 | March 2016 December 2017 | ||
| Number of patients, n (%) | |||||||
| Overall | 1,769 | 1,451 | 546 | 422 | 375 | 429 | 444 |
| IMDC favorable | 318 (18.0) | — | 124 (22.7) | — | 134 (35.7) | 131 (30.5) | 96 (21.6) |
| IMDC intermediate | 1,031 (58.3) | 1,031 (71.1) | 333 (61.0) | 333 (78.9) | 205 (54.7) | 246 (57.3) | 276 (62.2) |
| IMDC poor | 420 (23.7) | 420 (28.9) | 89 (16.3) | 89 (21.1) | 34 (9.1) | 52 (12.1) | 71 (16.0) |
| Prior nephrectomy, n (%) | |||||||
| Yes | 1,501 (84.9) | 1,186 (81.8) | 437 (80.0) | 319 (76.0) | 340 (90.7) | 358 (83.4) | 355 (80.0) |
| No | 267 (15.1) | 264 (18.2) | 109 (20.0) | 103 (24.0) | 35 (9.3) | 71 (16.6) | 89 (20.0) |
| Median OS, months | |||||||
| Overall | 28.6 | NR | — | — | — | ||
| IMDC favorable | 52.1 | 32.9 | NR | — | — | ||
| IMDC intermediate & poor | 23.2 | 26.0 | 20.3 | — | — | ||
| IMDC intermediate | 25.8 | — | 23.0 | — | — | ||
| IMDC poor | 9.8 | — | 5.1 | — | — | ||
| Median TTD or PFS,f months | |||||||
| Overall | 8.1 | 12.3 | — | 11.1 | 8.4 | ||
| IMDC favorable | 15.0 | 25.1 | 16.0 | 12.7 | — | ||
| IMDC intermediate & poor | 7.1 | 8.4 | 9.7 | — | — | ||
| IMDC intermediate | 8.5 | — | 10.7 | 9.5 | — | ||
| IMDC poor | 4.2 | — | 2.5 | 2.9 | — | ||
| ORR, % | |||||||
| Overall | 32.4 | 32.0 | 35.7 | 25.7 | |||
| IMDC favorable | 38.5 | 52.0 | 58.2 | — | 37.5 | ||
| IMDC intermediate & poor | — | 27.0 | 38.9 | — | — | ||
| IMDC intermediate | 34.6 | — | 42.4 | — | 25.4 | ||
| IMDC poor | 21.7 | — | 17.6 | — | 11.3 | ||
IMDC data for first‐line sunitinib was received on September 1, 2018.
Motzer et al. 4.
Rini et al. 5.
Rini et al. 23.
Motzer et al. 19.
TTD served as a proxy for PFS in the IMDC database.
Abbreviations: —, not available; IFN, interferon; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; NR, not reached; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TTD, time to treatment discontinuation.
Similar results were also observed in the current study compared with previous clinical trial results, which assessed the intermediate risk group specifically. The retrospective analysis of the phase III trial of sunitinib versus IFN‐α assessed clinical heterogeneity within the intermediate risk group and reported that for patients with one and two risk factors, the median OS was 28.2 months (95% CI, 23.0–not estimable) and 16.3 months (95% CI, 13.2–19.4), respectively 5. With intermediate risk patients constituting the largest risk category among patients with mRCC, heterogeneity in clinical outcomes in this group should be considered when counseling and treating patients with mRCC 4, 5.
The treatment landscape for mRCC has transformed and will continue to do so rapidly given the ongoing trials for first‐line treatment and the integration of immune checkpoint inhibitors 14. The IMDC risk group classification is relevant for clinicians, as treatments are often approved for patients in a particular risk group 15, 16. For patients classified as intermediate or poor risk, nivolumab plus ipilimumab was approved for first‐line treatment. Axitinib, although it is not an approved first‐line treatment option, is recommended by the National Cancer Center Network (NCCN) as a treatment option (category 2A) 14. For patients in all risk groups, the U.S. Food and Drug Administration recently approved two combinations, pembrolizumab plus axitinib and avelumab plus axitinib, as first‐line treatment based on improved OS, PFS, and ORR relative to patients treated with sunitinib in the KEYNOTE‐426 and JAVELIN Renal 101 trials, respectively 17, 18, 19, 20. Findings from this study show that approximately 60% of patients were treated with a second‐line after discontinuing first‐line sunitinib, and 11% of which received nivolumab as their second‐line. Nivolumab was first approved in November 2015 for advanced RCC, and 2019 NCCN guidelines recommend nivolumab as second‐line treatment (Category 1) 21. As patients in this study initiated first‐line sunitinib in 2010–2018, we expect that some patients in this study may be treated differently today, when a larger proportion of patients would receive second‐line nivolumab treatment.
Despite the introduction of new first‐line therapies, sunitinib may remain a cornerstone for treatment for many patients. Certain patient populations, such those after organ transplant, with autoimmune disorder, or with ongoing immunosuppressant, were underrepresented in immune checkpoint inhibitor studies, and this class of drugs might present substantial risks 22. In addition, many countries outside the U.S. (especially in developing countries) may have very limited access to up‐front combination therapies. Further analyses in the age of immunotherapy will be needed to observe the exact magnitude of cost differences.
The results of this analysis should be interpreted with caution in light of several limitations. Clinical outcomes were reported for patients in different risk groups, but unmeasured confounding and potential bias (e.g., selection bias) could account for some observed differences. We attempted to reduce bias by including consecutive unselected series and adjusting for potential confounders including age, gender, year of sunitinib initiation, number of metastases, and prior nephrectomy. Incomplete data exist in this IMDC data set; 419 out of 2,190 (19%) patients had missing data for IMDC risk group. If patients who were excluded from the analyses because of a missing IMDC risk factor had different outcomes from those with no missing IMDC risk factor, this would affect generalizability of the results to patients with a missing IMDC risk factor. In addition, in contrast to clinical trials with protocol‐specified definitions of clinical events, assessments of progression and clinical response in retrospective studies of real‐world clinical practice may not be made consistently across patients and across physician practices.
Conclusion
This analysis offers a contemporary benchmark for OS for patients with clear cell mRCC treated with sunitinib as first‐line therapy in a real‐world setting. This real‐world study corroborates findings from clinical trial studies in the context of modern treatment landscape and demonstrates that differences in clinical outcomes between IMDC risk groups warrant considering risk group when counseling patients about therapeutic options and designing clinical trials. In particular, differences in whether patients have one versus two risk factors among patients categorized as IMDC intermediate risk should be considered.
Author Contributions
Conception/design: Marie‐France Savard, Lynn Huynh, Rose Chang, Mei S. Duh, Daniel Y.C. Heng
Provision of study material or patients: Marie‐France Savard, J. Connor Wells, Jeffrey Graham, Shaan Dudani, John A. Steinharter, Bradley A. McGregor, Frede Donskov, Georg A. Bjarnason, Ulka N. Vaishampayan, Aaron R. Hansen, Marco A.J. Iafolla, Daniel Y.C. Heng
Collection and/or assembly of data: Marie‐France Savard, J. Connor Wells, Jeffrey Graham, Shaan Dudani, John A. Steinharter, Bradley A. McGregor, Frede Donskov, Georg A. Bjarnason, Ulka N. Vaishampayan, Aaron R. Hansen, Marco A.J. Iafolla, Daniel Y.C. Heng
Data analysis and interpretation: Marie‐France Savard, J. Connor Wells, Jeffrey Graham, Shaan Dudani, John A. Steinharter, Bradley A. McGregor, Frede Donskov, Georg A. Bjarnason, Ulka N. Vaishampayan, Aaron R. Hansen, Marco A.J. Iafolla, Giovanni Zanotti, Lynn Huynh, Rose Chang, Mei S. Duh, Daniel Y.C. Heng
Manuscript writing: Marie‐France Savard, Lynn Huynh, Rose Chang, Mei S. Duh, Daniel Y.C. Heng
Final approval of manuscript: Marie‐France Savard, J. Connor Wells, Jeffrey Graham, Shaan Dudani, John A. Steinharter, Bradley A. McGregor, Frede Donskov, Georg A. Bjarnason, Ulka N. Vaishampayan, Aaron R. Hansen, Marco A.J. Iafolla, Giovanni Zanotti, Lynn Huynh, Rose Chang, Mei S. Duh, Daniel Y.C. Heng
Disclosures
Marie‐France Savard: Amgen (H); J. Connor Wells: Pfizer (other—travel); Bradley A. McGregor: Bayer, Seattle Genetics/Astellas, Exelixis, AstraZeneca, Astellas Pharma, Genentech/Roche, Nextar, Janssen Oncology, Pfizer (C/A), Bristol‐Myers Squibb, Exelixis, Calithera Biosciences, Seattle Genetics/Astellas (RF); Frede Donskov: Pfizer, Novartis, Ipsen (RF); Georg A. Bjarnason: Pfizer, Novartis (C/A), Pfizer, Novartis, Bristol‐Myers Squibb, Eisai, Ipsen (C/A), Pfizer, Novartis, Bristol‐Myers Squibb, Eisai, Ipsen (H), Pfizer, Merck (other—travel); Ulka N. Vaishampayan: Pfizer, Bristol‐Myers Squibb, Exelixis, Bayer (C/A), Pfizer, Bristol‐Myers Squibb, Exelixis, Bayer (C/A), Pfizer, Bristol‐Myers Squibb, Exelixis, Bayer (H); Aaron R. Hansen: Pfizier, Roche, Merck, AstraZeneca, Ipsen, Bristol‐Myers Squibb (C/A), Genentech, Roche, Merck, GlaxoSmithKline, Bristol‐Myers Squibb, Novartis (RF); Giovanni Zanotti: Pfizer (E); Lynn Huynh: Pfizer (RF); Rose Chang: Pfizer (RF); Mei S. Duh: Pfizer (RF); Daniel Y.C. Heng: Pfizer, Novartis, Bristol‐Myers Squibb (C/A), Pfizer, Novartis, Bristol‐Myers Squibb (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Acknowledgments
The authors would like to thank Caroline Korves, Sc.D., Catherine Nguyen, M.P.H., and Suna Park, M.S., of Analysis Group, Inc., for their assistance with developing this manuscript. This study was sponsored by Pfizer, Inc., New York, NY.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Kidney Cancer. Version 2.2017. Plymouth Meeting, PA: National Comprehensive Cancer Center, 2017. Available at https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. Accessed February 6, 2019.
- 2. Choueiri TK, Halabi S, Sanford BL et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN trial. J Clin Oncol 2017;35:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Motzer RJ, Hutson TE, Tomczak P et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Motzer RJ, Tannir NM, McDermott DF et al.; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378(14):1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rini BI, Hutson TE, Figlin RA et al. Sunitinib in patients with metastatic renal cell carcinoma: Clinical outcome according to International Metastatic Renal Cell Carcinoma Database Consortium risk group. Clin Genitourin Cancer 2018;16:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heng DY, Xie W, Regan MM et al. External validation and comparison with other models of the International Metastatic Renal‐Cell Carcinoma Database Consortium prognostic model: A population‐based study. Lancet Oncol 2013;14:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johal S, Santi I, Doan J et al. Is RECIST‐defined progression free‐survival a meaningful endpoint in the era of immunotherapy? J Clin Oncol 2017;35(suppl 6)488A. [Google Scholar]
- 8. Kroeger N, Xie W, Lee JL et al. Metastatic non‐clear cell renal cell carcinoma treated with targeted therapy agents: Characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer 2013;119:2999–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanaka N, Mizuno R, Ito K et al. External validation of the MSKCC and IMDC risk models in patients treated with targeted therapy as a first‐line and subsequent second‐line treatment: A Japanese multi‐institutional study. Eur Urol Focus 2016;2:303–309. [DOI] [PubMed] [Google Scholar]
- 10. Stukalin I, Wells C, Fraccon AP et al. Fourth‐line therapy in metastatic renal cell carcinoma (mRCC): Results from the International mRCC Database Consortium (IMDC). J Clin Oncol 2017;35(suppl 6):498A.27918720 [Google Scholar]
- 11. Wells JC, Stukalin I, Norton C et al. Third‐line targeted therapy in metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2017;71:204–209. [DOI] [PubMed] [Google Scholar]
- 12. Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 13. Tannir NM, Frontera OA, Hammers HJ et al. Thirty‐month follow‐up of the phase III CheckMate 214 trial of first‐line nivolumab + ipilimumab (N+I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol 2019;37(suppl 7):547A. [Google Scholar]
- 14. Calvo E, Porta C, Grünwald V et al. The current and evolving landscape of first‐line treatments for advanced renal cell carcinoma. The Oncologist 2019;24:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reinhorn D, Sarfaty M, Leshno M et al. A cost‐effectiveness analysis of nivolumab and ipilimumab versus sunitinib in first‐line intermediate‐ to poor‐risk advanced renal cell carcinoma. The Oncologist 2019;24:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao X, McDermott DF. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert Opin Biol Ther 2018;18:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. FDA approves pembrolizumab plus axitinib for advanced renal cell carcinoma [news release]. Silver Spring, MD: U.S. Food and Drug Administration, April 22, 2019. Available at https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma. Accessed May 23, 2019. [Google Scholar]
- 18. FDA approves avelumab plus axitinib for renal cell carcinoma [news release]. Silver Spring, MD: U.S. Food and Drug Administration, May 15, 2019. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma. Accessed May 23, 2019. [Google Scholar]
- 19. Motzer RJ, Penkov K, Haanen J et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Powles T, Plimack ER, Stus V et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first‐line therapy for locally advanced or metastatic renal cell carcinoma (mRCC): Phase III KEYNOTE‐426 study. J Clin Oncol 2019;37(suppl 7):543A. [Google Scholar]
- 21. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Kidney Cancer. Version 2.2019. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2019.
- 22. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rini BI, Plimack ER, Stus V et al.; KEYNOTE‐426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables


