Abstract
Immune checkpoint blockade (ICB) is highly effective for the treatment of metastatic cancers, but its side effects are incompletely understood. The objective of this article is to highlight hypertrophic lichen planus (HLP) with histological features diagnosed as squamous cell carcinoma (SCC), which is a potential cutaneous reaction to ICB. Two patients (75 and 69 years) presented with lesions diagnosed as SCC on biopsy, which developed after 3–9 months on ICB therapy. Biopsies demonstrated endophytic, atypical, or cystic squamous proliferations consistent with cutaneous SCC. However, the clinical presentation including monomorphic nature of the lesions and lichenoid inflammation in the background were consistent with HLP. Patients initially received topical 5‐fluorouracil (5‐FU) to reduce the hyperkeratotic lesions followed by topical steroids. The eruptions readily responded to this treatment regimen. Dermatologic immune‐related adverse events (irAEs) are the most common irAEs associated with ICB therapy. Our findings indicate that HLP resembling SCC on biopsy is a potential side effect of ICB that can be correctly diagnosed on careful clinical exam and is responsive to ICB cessation and topical steroid with or without 5‐FU treatment.
Key Points
Immune checkpoint blockade is associated with cutaneous immune‐related adverse events including lichen planus.
Hypertrophic lichen planus can appear as squamous cell carcinoma histologically and clinical context is key for the proper diagnosis.
Hypertrophic lichen planus can be safely treated with topical steroids with or without topical 5‐fluorouracil in cases with severe hyperkeratotic lesions.
Immune checkpoint blockade may be safely continued if clinical presentation is consistent with hypertrophic lichen planus.
Short abstract
Immune checkpoint blockade immunotherapy has revolutionized cancer treatment but is associated with dermatologic adverse events. This brief report highlights hypertrophic lichen planus with histological features diagnosed as squamous cell carcinoma on biopsy, featuring two successfully treated patients.
Introduction
Squamous cell carcinoma (SCC) is a cutaneous malignancy associated with chronic UV exposure and immunosuppression that presents as scaly, well‐demarcated, erythematous papules or plaques. Simultaneous development of multiple SCCs is unusual but has been described secondary to arsenic‐containing traditional medicine administration, immunosuppression, and allergic contact dermatitis secondary to tattoo ink 1, 2. Immune checkpoint blockade (ICB) therapy has been associated with hypertrophic lichen planus (HLP), a pink, papular, inflammatory dermatosis that can mimic SCC and coincide with the development of multiple keratoacanthomas (KAs) 3, 4.
ICB therapies targeting the programmed cell death pathway play a critical role in the treatment of metastatic cancers including cutaneous SCC, metastatic melanoma, non‐small cell lung cancer, and Merkel cell carcinoma. ICB is associated with several dermatologic immune‐related adverse events (irAEs), including pruritus, morbilliform drug eruption, lichen planus (LP), atopic dermatitis, bullous disorders, and vitiligo 5, 6, 7. We report a case duet of HLP associated with ICB therapy that were diagnosed as SCC on biopsy.
Report of Cases
Case 1
A 75‐year‐old woman was diagnosed with an unresectable SCC of the oral cavity, treated previously with brachytherapy followed by carboplatin and paclitaxel (Table 1). Her disease progressed, prompting pembrolizumab therapy. Physical examination demonstrated scaly, pink papules and plaques, consistent with HLP due to the extensive distribution, monomorphic nature, and the rapid onset of the lesions (Fig. 1A). Biopsy of a left upper arm lesion revealed a crateriform, cystic squamous proliferation most consistent with well‐differentiated SCC, KA type (Fig. 1B, 1C). Biopsy of a separate lesion on her right thigh also showed well‐differentiated SCC. 5‐Fluorouracil (5‐FU) was initially applied to hyperkeratotic lesions on the legs, which further helped the diagnosis of HLP as primary dermatosis become apparent. Subsequently, she responded well to topical betamethasone dipropionate 0.05% ointment and triamcinolone‐acetonide 0.1% ointment twice daily (Fig. 1D). Pembrolizumab was discontinued because of cancer progression.
Table 1.
Patients’ clinical data
| Patient/sex/age | Primary malignancy | Prior therapy | ICB agent, dose, and frequency | ICB duration, mo | Rash onset relative to start of ICB, mo | Anatomic distribution | Treatment |
|---|---|---|---|---|---|---|---|
| Pt1/F/75 | mSCC | Brachytherapy, carboplatin, taxol | Pembrolizumab 200 mg IV every 3 wk | 11 | 3 | Back, bilateral lower extremities | Betamethasone, triamcinolone, 5‐FU, ICB cessation |
| Pt2/M/69 | mNSCLC | None | Pembrolizumab 200 mg IV every 3 wk | 10 | 9 | Bilateral lower extremities | Triamcinolone, 5‐FU |
Cutaneous lesions developed three to nine months after therapy initiation, and were responsive to steroid therapy, 5‐FU, and ICB cessation.
Abbreviations: F, female; 5‐FU, 5‐fluorouracil; ICB, immune checkpoint blockade; IV, intravenous; M, male; mNSCLC, metastatic non‐small cell lung cancer; mSCC, mucosal squamous cell carcinoma; Pt, patient.
Figure 1.
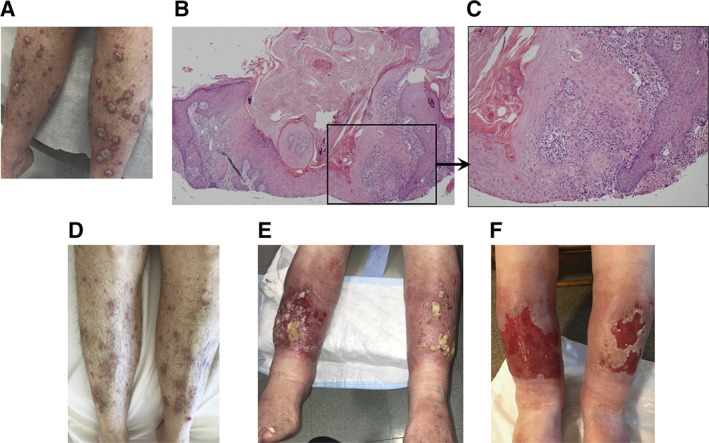
Clinical and pathological examination of the patients' skin lesions. Physical examination of patient (Pt)1 (A) and Pt2 (E) revealed scattered, annular, flat‐topped, frequently scaly, pink papules and plaques, clinically consistent with HLP. Low‐ (B) and high‐ (C) power H&E stains of a skin biopsy from Pt1 demonstrated a crateriform, cystic squamous proliferation most consistent with a well‐differentiated squamous cell carcinoma, keratoacanthoma type, extending to the peripheral and deep tissue edges. Physical exam findings of Pt1 (D) and Pt2 (F) demonstrated improvement with topical 5‐fluorouracil followed by topical steroids.
Case 2
A 69‐year‐old man was diagnosed with stage IV lung adenocarcinoma and was started on palliative pembrolizumab because a lung biopsy demonstrated high programmed death‐ligand 1 (PD‐L1) expression (Table 1). Physical examination demonstrated flat‐topped, pink papules and plaques with ulcerations consistent with clinical diagnosis of HLP (Fig. 1E). Biopsy of a lesion in the right proximal pretibial region demonstrated an atypical endophytic squamous proliferation, consistent with SCC. The eruption was initially treated with topical 5‐FU twice daily for 4 weeks to treat his large hyperkeratotic lesions on the lower legs that were concerning for multifocal eruptive squamous atypia (Fig. 1E). After 4 weeks, triamcinolone ointment was applied under occlusion for an additional 4 weeks, with excellent results (Fig. 1F). The remaining skin erosions healed over the following 3 months. His malignant disease remains stable on ICB therapy.
Discussion
We present two cases of hyperkeratotic lichenoid reaction diagnosed as SCC on biopsy following ICB therapy. The patients developed skin eruptions a median of 6 months after ICB therapy initiation. Biopsies were consistent with SCC in both patients. Widespread and monomorphic presentation of the papulosquamous lesions is a highly unusual presentation for SCC. The consistency of the clinical findings with HLP in the setting of ICB therapy led to the use of topical steroids as appropriate treatment for this condition, with complete resolution of the cutaneous lesions in both patients (Table 1).
ICB therapy is associated with irAEs, such as dermatologic toxicities 5, 6, 7, caused by possible antigen “unmasking” in which PD‐1 pathway blockade allows for the body to mount an immune response to the uncovered antigens 8. Lichenoid reactions observed in the context of anti‐PD‐1 therapy support the possible role of the PD‐1 pathway in lichen planus 6, 7, 9. One study of approximately 80 patients receiving either pembrolizumab or nivolumab noted a lichenoid eruption in nearly one‐fifth of patients 10. In a case series of 20 patients receiving anti‐PD‐1 therapy who had at least one cutaneous adverse event, nine of ten patients on nivolumab monotherapy developed lichenoid dermatitis 8. One of the nine patients developed hypertrophic plaques of the lower extremities, requiring oral prednisone. Another study showed that administration of pembrolizumab therapy for metastatic melanoma led to a papular eruption approximately two months after the start of therapy 11. These rashes were not severe, resolving with topical steroids or with no treatment.
Although several reports have evaluated LP reactions to ICB therapy, only a few previous reports have described a hypertrophic lichen planus mimicking SCC in this setting 3, 6, 7. Characteristics in favor of HLP over SCC are hyperkeratotic raised lesions on the distal extremities and absence of actinic damage or other risk factors for development of simultaneous SCCs. Distinguishing traits on biopsy that could favor HLP over SCC are eosinophilic dermatitis with lichenoid characteristics, hyperkeratosis, presence of Civatte bodies, absence of dysplasia, and lack of pronounced elastosis or lesional involvement in the deep dermis 3. In the cases presented here, the simultaneous appearance of multiple papules and plaques was more suggestive of HLP.
Conclusion
We present two cases of hyperkeratotic lichenoid reaction resembling SCC on biopsy following ICB therapy responsive to ICB discontinuation, topical 5‐FU, and topical steroids treatment. Although discontinuing ICB therapy may have contributed to clinical improvement in one patient, it is likely the eruption would have been safely managed with concurrent ICB therapy if continued ICB therapy were clinically indicated. Further investigation will be critical to elucidate the molecular basis for ICB‐induced HLP.
Author Contributions
Conception/design: Amir H. Ameri, Ruth K. Foreman, Shadmehr Demehri
Provision of study material or patients: Shadmehr Demehri
Collection and/or assembly of data: Amir H. Ameri, Ruth K. Foreman, Shadmehr Demehri
Data analysis and interpretation: Amir H. Ameri, Ruth K. Foreman, Shadmehr Demehri
Manuscript writing: Amir H. Ameri, Shadmehr Demehri
Final approval of manuscript: Amir H. Ameri, Ruth K. Foreman, Priyanka Vedak, Steven Chen, David M. Miller, Shadmehr Demehri
Disclosures
Ruth K. Foreman: Fate Therapeutics, Inc. (IP), Stemcell Biology (IP‐patent royalties); David M. Miller: Merck, Regeneron, Pfizer, Sanofi Genzyme (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Institutional review board (IRB) approval status was reviewed and approved by Massachusetts General Hospital IRB.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Siefring ML, Lu D, States JC et al. Rapid onset of multiple concurrent squamous cell carcinomas associated with the use of an arsenic‐containing traditional medicine for chronic plaque psoriasis. BMJ Case Rep 2018;30:2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maxim E, Higgins H, D'Souza A. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Women Dermatol 2017;3:228–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fontecilla NM, Kittler NW, Lopez A et al. Programmed cell death protein‐1 inhibitor‐induced granuloma annulare and hypertrophic lichen planus masquerading as squamous cell carcinoma. JAAD Case Rep 2018;4:636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freites‐Martinez A, Kwong BY, Rieger KE et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol 2017;153:694–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tetzlaff MT, Nagarajan P, Chon S et al. Lichenoid dermatologic toxicity from immune checkpoint blockade therapy: A detailed examination of the clinicopathologic features. Am J Dermatopathol 2017;39:121–129. [DOI] [PubMed] [Google Scholar]
- 6. Massey PR, Jones KM, Fox MC. New yellow plaques in a patient taking pembrolizumab. JAMA Oncol 2017;3:119–120. [DOI] [PubMed] [Google Scholar]
- 7. Coscarat A Martel J, Lee MP et al. Pembrolizumab‐induced pseudoepitheliomatous eruption consistent with hypertrophic lichen planus. J Cutan Pathol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Shi VJ, Rodic N, Gettinger S et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti‐programmed cell death 1 and anti‐programmed cell death ligand 1 immunotherapy. JAMA Dermatol 2016;152:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou G, Zhang J, Ren XW et al. Increased B7‐H1 expression on peripheral blood T cells in oral lichen planus correlated with disease severity. J Clin Immunol 2012;32:794–801. [DOI] [PubMed] [Google Scholar]
- 10. Hwang SJ, Carlos G, Wakade D et al. Cutaneous adverse events (AEs) of anti‐programmed cell death (PD)‐1 therapy in patients with metastatic melanoma: A single‐institution cohort. J Am Acad Dermatol 2016;74:455–461.e1. [DOI] [PubMed] [Google Scholar]
- 11. Joseph RW, Cappel M, Goedjen B et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti‐PD‐1 therapy. Cancer Immunol Res 2015;3:18–22. [DOI] [PubMed] [Google Scholar]


