Abstract
Background
Malnutrition worsens health‐related quality of life (HRQoL) and the prognosis of patients with advanced cancer. This study aimed to assess the clinical benefits of parenteral nutrition (PN) over oral feeding (OF) for patients with advanced cancer cachexia and without intestinal impairment.
Material and Methods
In this prospective multicentric randomized controlled study, patients with advanced cancer and malnutrition were randomly assigned to optimized nutritional care with or without supplemental PN. Zelen's method was used for randomization to facilitate inclusions. Nutritional and performance status and HRQoL using the European Organization for Research and Treatment of Cancer QLQ‐C15‐PAL questionnaire were evaluated at baseline and monthly until death. Primary endpoint was HRQoL deterioration‐free survival (DFS) defined as a definitive deterioration of ≥10 points compared with baseline, or death.
Results
Among the 148 randomized patients, 48 patients were in the experimental arm with PN, 63 patients were in the control arm with OF only, and 37 patients were not included because of early withdrawal or refused consent. In an intent to treat analysis, there was no difference in HRQoL DFS between the PN arm or OF arm for the three targeted dimensions: global health (hazard ratio [HR], 1.31; 95% confidence interval [CI], 0.88–1.94; p = .18), physical functioning (HR, 1.58; 95% CI, 1.06–2.35; p = .024), and fatigue (HR, 1.19; 95% CI, 0.80–1.77; p = .40); there was a negative trend for overall survival among patients in the PN arm. In as treated analysis, serious adverse events (mainly infectious) were more frequent in the PN arm than in the OF arm (p = .01).
Conclusion
PN improved neither HRQoL nor survival and induced more serious adverse events than OF among patients with advanced cancer and malnutrition. Clinical trial identification number. NCT02151214
Implications for Practice
This clinical trial showed that parenteral nutrition improved neither quality of life nor survival and generated more serious adverse events than oral feeding only among patients with advanced cancer cachexia and no intestinal impairment. Parenteral nutrition should not be prescribed for patients with advanced cancer, cachexia, and no intestinal failure when life expectancy is shorter than 3 months. Further studies are needed to assess the useful period with a potential benefit of artificial nutrition for patients with advanced cancer.
Keywords: Palliative care, Malnutrition, Parenteral nutrition, Cancer, Zelen's method
Short abstract
Malnutrition impairs clinical outcome in patients with advanced cancer. This study compared parenteral nutrition with oral feeding for malnourished patients with advanced cancer and functional gastrointestinal tract.
Introduction
Malnutrition occurs in 50%–80% of patients with advanced cancer, according to the type of cancer and the stage. It can severely impair clinical outcomes among patients with cancer, increasing morbidity and mortality and reducing treatment efficacy 1, 2. Cachexia in advanced cancer is a multifactoriel syndrom that associates weight loss, sarcopenia and loss of fat tissue. Its pathophysiology is driven by various combinations of inadequate food intake and systemic inflammation response syndrome (SIRS), which in turn promotes metabolic disorders and catabolism, especially protein breakdown in skeletal muscle 3, 4. In a vicious circle, systemic inflammation‐induced fatigue contributes to decreased physical activity and thus reduces anabolic signals, promoting further muscle loss. Anticancer treatments can cause side effects that further compromise nutritional status, and muscle loss strongly predicts the development of chemotherapy toxicity 5, 6. Patients with cachexia and sarcopenia report worse quality of life (QoL) and more depression symptoms 7, 8. Furthermore, higher muscle strength at the start of palliative chemotherapy is associated with significantly better survival in older patients with advanced cancer 9, 10. Indeed, the management of malnutrition is a very important target of patient‐centered approach, as well as a necessity to increase anticancer treatment efficacy, which involves close collaboration between the oncologist and an integrated palliative care team 11, 12.
Nutritional guidelines for patients with advanced cancer recommend a multimodal management, including increasing food intake, promoting physical activity, and fighting against SIRS, alongside anticancer treatment 13, 14. Nutritional interventions should aim at improving clinical outcomes such as changes in physical function and QoL. In patients undergoing anticancer treatments, if oral food intake is inadequate despite counselling and oral nutritional support, supplemental enteral nutrition or parenteral nutrition (PN) may be implemented. Careful consideration of the prognosis is required to avoid overtreatment with artificial nutrition at the end of life 15, 16, 17, 18. Enteral nutrition should be first considered for patients with a normally functioning gastrointestinal tract, but adverse effects of enteral nutrition are frequent (e.g., early satiety, nausea and vomiting, pulmonary aspiration, and metabolic complications) 19, 20. It has been reported that most patients with advanced cancer do not wish to receive nasogastric tube feeding because of the psychological and social impact 21. PN may be more effective for more rapidly increasing calorie intake, with fewer adverse events except for infectious complications 18, 19, 20.
In this context, in order to increase the level of evidence, we performed the first multicentric randomized study to assess the clinical impact of PN among malnourished patients with advanced cancer without gastrointestinal dysfunction.
Materials and Methods
Study Design and Patients
The study is a prospective, national, multicenter, open‐label randomized, parallel‐group, controlled trial designed to compare PN with oral feeding (OF) for malnourished patients with advanced cancer and functional gastrointestinal tract. The detailed protocol has already been published 22, and we present here the outline.
Inclusion criteria were patients with malnutrition defined as a body mass index (BMI) <18.5 for patients aged less than 70 years and BMI <21 for those aged more than 70 years or as weight loss of 2% in 1 week, 5% in 1 month, or 10% in 6 months; life expectancy less than 12 months and more than 2 months; functional gastrointestinal tract without symptomatic peritoneal carcinomatosis or intestinal obstruction; and patients with a central venous catheter. Main exclusion criteria were patients with head and neck and esophageal‐gastric cancer and any contraindication for PN (such as poorly controlled diabetes, severe heart failure, or severe ascites and edema). To assess life expectancy clinicians could use the previously published “surprise” question (“Would I be surprised if this patient died in the next 12 months?”) for predicting death in seriously ill patients 23, 24.
All patients were systematically referred for a consultation with a dietician for assessment of symptoms limiting food intake; advice on hypercaloric, hyperproteic, and fractionated feeding; and prescription of oral nutritional complement if needed. Patients simultaneously received medical information and counselling about adapted physical activity. Patients were all already being followed by the palliative care team.
Patients were randomized following Zelen's single‐consent design, which allows physicians to randomize patients before consent and then obtain informed consent on the intervention only from those patients randomized to the experimental arm 25, 26. Patients in both arms gave their consent for the monthly follow‐up with quality of life questionnaires. This choice of randomization method was guided by the difficulty of randomly assigning patients between two treatments of unequal appearance. Implementing parenteral nutrition or continuing with oral feeding are such different treatments that relatives and patients themselves may have a strong preference for one or the other. It can be influenced by their willingness to take action or “give up” or their preconceived ideas of which type of nutrition may be more effective, more toxic, or both. The use of Zelen's method was approved by patient associations gathered within the “Collectif Interassociatif Sur la Santé” and the clinical ethics committee of the Besançon University Hospital. Letters of support have been produced by these committees, highlighting the sensitivity of informing and having a random assignment for the mode of feeding for this particularly fragile and vulnerable study population. Some experts initially considered the Zelen method to be a violation of the ethos; then the method was found to be attractive for research in situations of great precariousness and has been used in a variety of different contexts, including cancer treatment 26, 27, 28, 29, 30, 31. The Zelen procedure protects patients in the control arm who receive routine care from anxious questioning related to a randomization and offers a true informed choice and consent for parenteral nutrition for patients in the intervention arm. More formally, in accordance with the regulations applicable in France, the study protocol subsequently received a favorable opinion from the “Comité de Protection des Personnes” and the institutional review boards of the participating centers and an authorization from the national health authority (“Agence nationale de sécurité du médicament et des produits de santé”).
The study was performed in accordance with Good Clinical Practice guidelines and with the Declaration of Helsinki.
Intervention: Parenteral Nutrition
Parenteral nutrition was administered by central venous route using industrial ternary preparations and systematic daily addition of polyvitamins, trace elements, and electrolytes (sodium, potassium, vitamin K, magnesium, phosphorus), adapted as required. The dosage depended on the patient's food intake to achieve 30–35 kcal/kg/day with 1.2–1.5 g/kg/day of protein, without exceeding 1.25 times the resting state energy expenditure calculated according to the Harris‐Benedict equation. For patients who maintained an oral diet, a minimum intake of 1,000 kcal/day and 6 g of nitrogen was prescribed 5 days a week.
Objectives and Assessments
The primary objective was to assess the impact of PN on QoL for malnourished advancer cancer patients with no intestinal failure. Health‐related QoL (HRQoL) was assessed in each treatment arm at least once per month until death using the European Organization for Research and Treatment of Cancer (EORTC) QLQ‐C15‐PAL. This questionnaire is a validated tool in the French language to assess HRQoL in palliative cancer care patients 32.
The primary endpoint was HRQoL deterioration‐free survival defined as time from inclusion in the study to the first deterioration of ≥10 points in the HRQoL scale scores with no further improvement of at least 10 points as compared with the baseline score, or death 33. We targeted three dimensions of the EORTC QLQ‐C15‐PAL: overall quality of life, physical functioning, and fatigue.
Secondary endpoints were secondary HRQoL dimensions, nutritional parameter (food intake, digestive symptoms, weight, oral nutritional complement intake), adverse events, and survival.
Statistical Analysis
Sample size calculation was based on a median deterioration‐free survival of 1 month versus 2 months, with a hazard ratio (HR) of 0.50, a bilateral type I error of .0166 (three targeted dimensions), and a statistical power of 80%. This corresponded to a total enrolment of 96 patients followed and 89 events to be observed, that is, patients who had a significant deterioration in HRQoL or died, whichever occurred first. Considering a 10% rate of switching between treatment arms as a result of the use of Zelen's randomization, a total of 106 patients with available data were required.
The intent to treat (ITT) population was defined as all randomized patients score available, regardless of whether eligibility criteria were met and regardless of treatment received. The primary endpoint was analyzed in a modified intention‐to‐treat (mITT) population, that is, considering all ITT patients with at least a baseline HRQoL score available. A post hoc analysis for the primary endpoint was conducted for the first 6 months of follow‐up only in order to reduce the bias caused by long survivors. Deterioration‐free survival was estimated using the Kaplan‐Meier method. Adverse events were analyzed in the as treated population and were considered for patients receiving at least 1 day of treatment in the PN arm and only patients without artificial nutrition in the OF arm. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). All analyses were two sided, and the statistical significance value was fixed at .0166 to take account of the number of comparisons performed.
Results
Patient Characteristics
Between June 2012 and March 2017, 148 patients with cancer were randomized in the 13 participating centers. As some patients did not consent to the completion of the quality of life questionnaires (13 in PN arm and 10 in control arm) and early withdrawal was observed, a total of 111 patients were included: 48 (42.3%) in PN arm and 60 (54.1%) in the OF arm. Eight patients in the PN arm only consented to QoL questionnaire completion. Because of edema, five patients in each arm did not adhere to the inclusion criteria regarding weight loss and/or BMI. The flowchart for the study population is shown in Figure 1.
Figure 1.
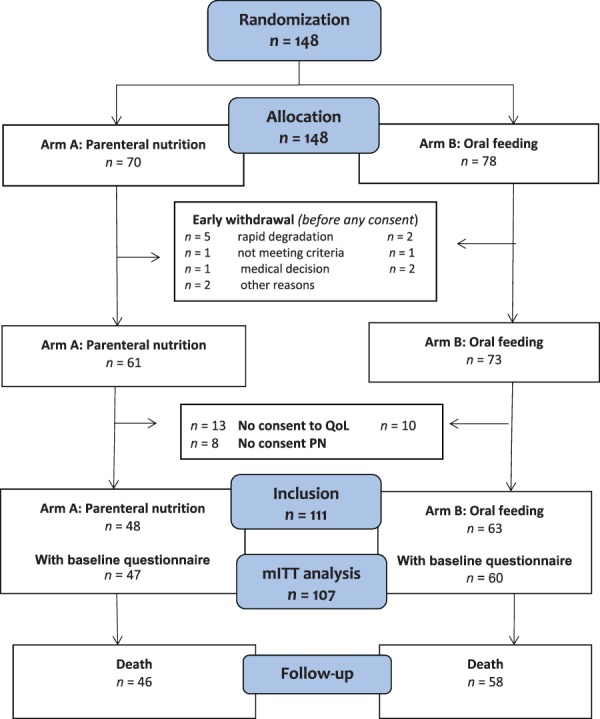
Flowchart of the study population.Abbreviations: mITT, modified intent to treat; PN, parenteral nutrition; QoL, quality of life questionnaire.
The baseline clinical and sociodemographic characteristics of the patients were well balanced between treatment arms (Table 1). The median age was 67 years (interquartile range, 60–72), 61 patients were women (55%), and the most common cancer localization was digestive cancer (28.8%). Almost all patients (98%) were metastatic, with a life expectancy of less than 1 year (for 14% it was less than 3 months, for 54% less than 6 months), and 49% were still on systemic anticancer treatment. The patients were malnourished with a median weight loss of 8.20 kg (range, 10–26.5) in the previous 6 months, 73% had low albumin, and the mean food intake was 40%.
Table 1.
Baseline characteristics of the patients included (n = 111)
| Characteristics | Parenteral nutrition arm (n = 48), n (%) | Oral feeding arm (n = 63), n (%) | All patients (n = 111), n (%) |
|---|---|---|---|
| Age | |||
| Mean ± SD | 66.6 ± 9.7 | 66.2 ± 9.2 | 66.3 ± 9.4 |
| Median (IQR) | 66.5 (61–75) | 67 (59–72) | 67 (60–72) |
| Gender | |||
| Male | 22 (45.8) | 28 (44.4) | 50 (45.05) |
| Female | 26 (54.2) | 35 (55.6) | 61 (54.95) |
| Cancer site | |||
| Digestive | 14 (29.17) | 18 (28.57) | 32 (28.83) |
| Pelvis | 8 (16.67) | 11 (17.46) | 19 (17.12) |
| Lung | 9 (18.75) | 12 (19.05) | 21 (18.92) |
| Prostate | 5 (10.42) | 7 (11.11) | 12 (10.81) |
| Sarcoma | 0 (0) | 4 (6.35) | 4 (3.60) |
| Breast | 11 (22.92) | 5 (7.94) | 16 (14.41) |
| Melanoma | 0 (0) | 1 (1.59) | 1 (0.90) |
| Other | 1 (2.08) | 5 (7.94) | 6 (5.41) |
| Number of metastases | |||
| Mean ± SD | 2.25 ± 1.03 | 2.24 ± 1.41 | 2.25 ± 1.26 |
| Median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| ECOG performance status | |||
| 1 | 4 (8.33) | 3 (4.92) | 7 (6.42) |
| 2 | 22 (45.83) | 26 (42.62) | 48 (44.04) |
| 3 | 18 (37.50) | 28 (45.90) | 46 (42.20) |
| 4 | 4 (8.33) | 4 (6.56) | 8 (7.34) |
| Chemotherapy | |||
| Ongoing | 21 (43.75) | 29 (46.03) | 50 (45.05) |
| Prior treatment | 25 (52.08) | 30 (47.62) | 55 (49.55) |
| Hormone therapy | |||
| Ongoing | 2 (4.17) | 1 (1.59) | 3 (2.7) |
| Prior treatment | 12 (25) | 9 (14.29) | 21 (18.92) |
| Targeted therapy | |||
| Ongoing | 0 (0) | 1 (1.59) | 1 (0.9) |
| Prior treatment | 5 (10.42) | 6 (9.52) | 11 (9.9) |
| Body mass index | |||
| Mean ± SD | 20.45 ± 4.39 | 20.68 ± 3.73 | 20.58 ± 4.01 |
| Median (IQR) | 19.03 (14.72–32.93) | 20.23 (12.29–31.88) | 19.87 (12.3–32.93) |
| Weight variation since last month | |||
| Weight gain | 5 (11.90) | 9 (15) | 14 (13.73) |
| 0%–5% loss | 20 (47.62) | 23 (38.33) | 43 (42.16) |
| 5%–10% loss | 8 (19.05) | 12 (20) | 20 (19.61) |
| >10% loss | 9 (21.3) | 16 (26.67) | 25 (24.51) |
| Albumin, g/L | |||
| Mean ± SD | 30 ± 7 | 29 ± 7 | 29 ± 7 |
| Median (IQR) | 30 (13–42) | 28 (17–43) | 29 (13–43) |
| CRP, mg/L | |||
| Mean ± SD | 72 ± 76 | 85 ± 72.51 | 79 ± 74 |
| Median (IQR) | 54 (1–363) | 71 (1–275) | 63 (1–363) |
| LDH, UI/L | |||
| Mean ± SD | 513 ± 579 | 508 ± 580 | 510 ± 577 |
| Median (IQR) | 306 (4–2,997) | 289 (107–3,809) | 289 (4–3,809) |
Abbreviations: CRP, C‐reactive protein; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range (quartile 1 to quartile 3); LDH, lactate dehydrogenase.
HRQoL Deterioration‐Free Survival
In the mITT analysis, there was no difference on HRQoL deterioration‐free survival in the oral nutrition group versus the PN arm for the three dimensions targeted: global QoL (HR, 1.31; 95% confidence interval [CI], 0.88–1.94; p = .18), physical functioning (HR, 1.58; 95% CI, 1.06–2.35; p = .024), and fatigue (HR, 1.19; 95% CI, 0.80–1.77; p = .393; Fig. 2). The post hoc analysis excluding data beyond 6 months of follow‐up showed a statistically significant increase in deterioration‐free survival for physical functioning, with a median of 2.23 months for the OF arm versus 1.05 months for the PN arm (HR, 2.03; 95% CI, 1.33–3.12; p = .0008).
Figure 2.
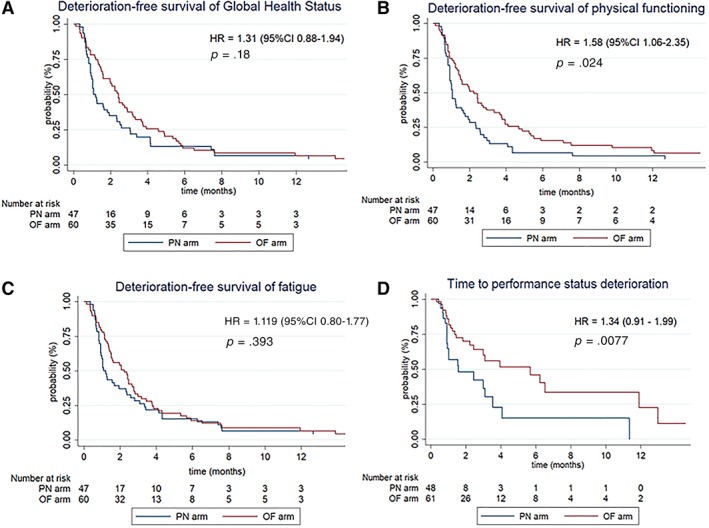
Kaplan‐Meier curves of health‐related quality of life deterioration‐free survival for the three target dimensions and time to performance status deterioration.Abbreviations: CI, confidence interval; HR, hazard ratio; OF, oral feeding; PN, parenteral nutrition.
For secondary HRQoL dimensions there was a statistically significant increase in deterioration‐free survival for the pain dimension (HR, 1.79; 95% CI, 1.20–2.66; p = .004) in the OF arm versus the PN arm and a trend in favor of OF with an HR >1 for the other dimensions (Table 2).
Table 2.
Quality of life deterioration‐free survival for each dimension in the modified intention‐to‐treat population
| Dimensions | n (events) | Patients event free at 1 month, % (95% CI) | Median (95% CI) | HR (95% CI) | p value |
|---|---|---|---|---|---|
| Targeted dimensions | |||||
| Global health status | |||||
| Oral feeding arm | 60 (57) | 78 (65.1–86.6) | 2.43 (1.61–3.22) | 1 | .18 |
| Parenteral nutrition arm | 47 (46) | 60.9 (45.3–73.3) | 1.15 (0.99–2.33) | 1.31 (0.88–1.94) | |
| Physical functioning | |||||
| Oral feeding arm | 60 (57) | 74.6 (61.4–83.8) | 2.23 (1.48–3.65) | 1 | .024 |
| Parenteral nutrition arm | 47 (45) | 56.5 (41.1–69.4) | 1.05 (0.92–1.77) | 1.58 (1.06–2.35) | |
| Fatigue | |||||
| Oral feeding arm | 60 (56) | 78 (65.1–86.6) | 2.23 (1.51–2.76) | 1 | .393 |
| Parenteral nutrition arm | 47 (45) | 58.7 (43.2–71.3) | 1.15 (0.95–2.37) | 1.19 (0.80–1.77) | |
| Secondary dimensions | |||||
| Emotional functioning | |||||
| Oral feeding arm | 60 (58) | 72.9 (59.6–82.4) | 2.07 (1.48–2.89) | 1 | .753 |
| Parenteral nutrition arm | 47 (45) | 54.3 (39–67.4) | 1.05 (0.92–2.37) | 1.07 (0.72–1.58) | |
| Nausea | |||||
| Oral feeding arm | 60 (57) | 79.7 (67–87.9) | 2.66 (1.58–3.78) | 1 | .0283 |
| Parenteral nutrition arm | 47 (46) | 60.9 (45.3–73.3) | 1.23 (0.99–2.37) | 1.56 (1.05–2.31) | |
| Pain | |||||
| Oral feeding arm | 60 (57) | 78 (65.1–86.6) | 2.23 (1.58–2.99) | 1 | .004a |
| Parenteral nutrition arm | 47 (46) | 50 (34.9–63.3) | 1.00 (0.92–1.25) | 1.79 (1.20–2.66) | |
| Dyspnea | |||||
| Oral feeding arm | 59 (56) | 67.2 (53.6–77.7) | 1.69 (1.22–2.66) | 1 | .389 |
| Parenteral nutrition arm | 47 (46) | 58.7 (43.2–71.3) | 1.05 (0.95–2.53) | 1.19 (0.80–1.76) | |
| Insomnia | |||||
| Oral feeding arm | 60 (56) | 78 (65.1–86.6) | 2.43 (1.61–2.96) | 1 | .0442 |
| Parenteral nutrition arm | 47 (46) | 60.9 (45.3–73.3) | 1.10 (0.99–2.33) | 1.50 (1.01–2.23) | |
| Appetite loss | |||||
| Oral feeding arm | 60 (56) | 79.7 (67–87.9) | 2.46 (1.91–3.65) | 1 | .233 |
| Parenteral nutrition arm | 47 (46) | 69.6 (54.1–80.7) | 1.45 (1.05–2.79) | 1.27 (0.86–1.88) | |
| Constipation | |||||
| Oral feeding arm | 57 (53) | 80.4 (67.3–88.6) | 2.07 (1.51–2.99) | 1 | .166 |
| Parenteral nutrition arm | 47 (46) | 63 (47.5–75.2) | 1.23 (1.05–2.53) | 1.33 (0.89–1.98) |
Statistically significant at the level of .016.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Survival
The median follow‐up was 33.8 months (95% CI, 14.6–not available). In total 104 patients died: 46 in the PN arm and 58 in the OF arm. For the whole population, the Kaplan‐Meier median survival was 2.66 months (95% CI, 1.97–3.09), with 59.6% of patients alive at 2 months and 20.8% alive at 6 months (Fig. 3). There was no statistically significant difference in overall survival, with a median of 3 months (95% CI, 2.1–3.9) for the OF arm versus 2 months (95% CI, 1.2–3.0) for the PN arm (HR, 1.34; 95% CI, 0.91–1.99; p = .14). At each time point, overall survival for PN arm was below that of the OF arm.
Figure 3.
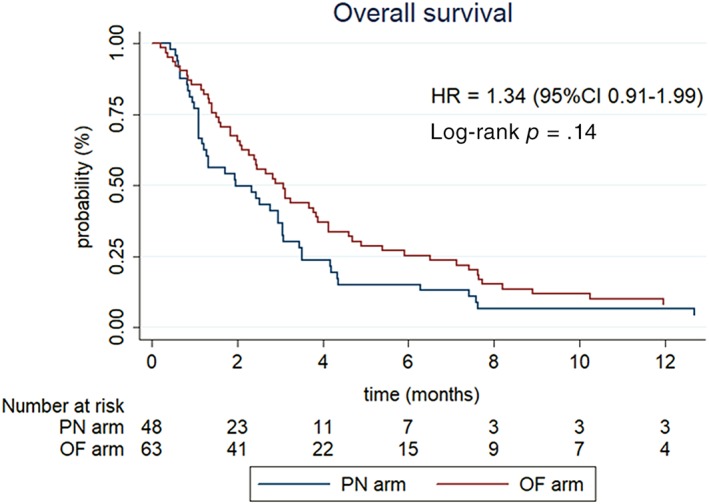
Overall survival curve according to the Kaplan‐Meier estimate per treatment arm (intention‐to‐treat population).Abbreviations: CI, confidence interval; HR, hazard ratio; OF, oral feeding; PN, parenteral nutrition.
Nutritional Parameters
For all patients in both arms, the mean ± SD change between baseline and last available measure in the first 2 months was a gain of 0.44 ± 2.13 for visual analog scale of ingesta and 0.33 kg ± 3.09 for weight, with no statistically significant difference observed between treatment arms. The time to performance status deterioration was significantly longer in the OF group, with a median of 1.6 months (95% CI, 0.92–3.5) in the PN arm versus 5.7 months (95% CI, 2.5–11.9) in the OF arm (HR, 2.24; 95% CI, 1.21–4.15; p = .008).
Toxicities
In as treated analyses, severe adverse effects were higher in the PN arm than in the OF arm, with seven patients within the PN arm versus only one patient in the OF arm (p = .0105). The main severe adverse events were catheter infection (n = 5), infection (n = 1), and acute pulmonary edema (n = 1).
Discussion
In this study, PN failed to improve QoL for patients with cancer‐related cachexia as well as survival. Moreover, PN caused more serious adverse events. This is the first study to assess PN for patients with advanced cancer with estimated life expectancy under 1 year, so comparison with data in the literature is difficult. In the only previous study (in Sweden), 339 patients were randomized if they had cancer‐related cachexia and functional gastrointestinal tract to receive nutritional support (including possibility of home PN) or not 34. Unfortunately, HRQoL was not assessed, and the median overall survival did not differ in the ITT analysis. The authors mentioned the complexity of their current model, as additional interventions (i.e., cyclooxygenase inhibitors and erythropoietin) were offered to the best possible extent for patients in both the study and control arms. The authors concluded by not excluding the possibility that an interaction could have been overlooked by their relatively straightforward two‐group analysis of a single intervention (i.e., nutritional support). On the other hand, numerous studies have shown clinical benefit of PN among malnourished patients with cancer and gastrointestinal dysfunction, improving HRQoL, performance and nutritional status, and sometimes survival 35, 36, 37, 38, 39, 40, 41.
Several reasons could explain this lack of efficacy of PN for patients with advanced cancer and cancer‐related cachexia in our study. Trends in reduced survival and tumor response, as well as increased incidence of infectious complications in patients receiving PN, were reported in a meta‐analysis performed years ago 42. The short survival time of study population is certainly the major cause of the failure of PN, as a crucial issue is the timing of nutritional interventions. A window of anabolic potential seems to exist when survival is greater than 90 days, creating a chance for nutritional intervention to stop or reverse cachexia 43. Artificial nutrition can maintain or improve nutritional status in patients with cancer, but only if depletion of muscular mass is not extreme, and can be more successful if started earlier. A recent study has assessed PN for 47 patients with incurable gastrointestinal cancer who were not malnourished but nutritionally at risk 44. The results of this study show that HRQoL was better at 12 weeks, fat‐free mass increased significantly, and the median overall survival was around 5 months. Indeed, implementing earlier PN in the course of the disease has some big promise in the management of malnutrition.
Several limitations in this study should be kept in mind. First, anthropometric criteria (weight loss and body mass index reduction) are insufficient to define malnutrition for patients with advanced cancer, who frequently suffer complications such as edema, ascites, or pleural effusion. Better selection of malnourished patients would have possible using bioelectrical impedance or skeletal muscle measures on computed tomography scans, which would have permitted more precise measures for malnutrition screening and assessment 45, 46. Second, the slow accrual of patients in this study confirms the difficulty of performing clinical trials in this setting. Many patients did not complete the planned follow‐up, mainly because of deterioration of their condition or death, a common difficulty in the field of palliative care clinical research. Finally, we observed a shorter median overall survival in the study population than expected, given that one of the inclusion criteria was a life expectancy of less than 1 year according to the “surprise” question. Prediction of the life expectancy is one of the most difficult tasks in oncology, relying on either clinical estimation or prognostics factors that can be added to build different scores. Today the optimal prognostic factors in patients with advanced cancer are not known. Prognostic models such as the Glasgow Prognostic Score, Palliative Performance Scale, Palliative Prognostic Score, Palliative Prognostic Index, or Prognosis in Palliative Care Study predictor model may augment the clinician prediction of survival 47. However, care must be taken to select the appropriate tool because prognostic accuracy varies by patient population, setting, and time frame of prediction. Results of a recent study on 478 patients with a median survival of 4.2 months showed that the modified Glasgow Prognostic Score was one of the most effective tools 48. But even if prognostication is still a challenge, this might not be the only reason why clinicians have mainly included patients with life expectancy under 3 months. Bad representation of PN could have prevented physicians from including patients with good prognosis 49. It seems like a bad general condition was unconsciously a necessary condition to consider prescription of PN.
Conclusion
PN improved neither HrQoL nor survival for patients with advanced cancer and cancer‐related cachexia and caused more serious side effects. This study increases the level of evidence and supports the recommendation not to prescribe PN for patients with advanced cancer with life expectancy under 3 months and functional intestinal tract. Zelen's method can be useful in an ethical point of view allowing randomized study in an advanced care setting. On the other hand, a more accurate prognostic assessment is a key point to obtain a homogeneous study population. Further studies are needed to assess the best ways to use artificial nutrition for patients with advanced cancer with life expectancy of more than 3 months and how to overcome reluctance from clinicians to prescribe artificial nutrition in this situation.
Author Contributions
Conception/design: Carole Bouleuc, Ghislain Grodard, Lionel Pazart, Régis Aubry
Provision of study material or patients: Carole Bouleuc, Antoine Thiery‐Vuillemin, Olivier Dubroeucq, Nathalie Crétineau, Véronique Frasie, Vincent Gamblin, Gisèle Chvetzoff, Laure Favier, Christophe Tournigand, Marie‐Christine Grach, Bruno Raynard, Sébastien Salas, Géraldine Capodano
Collection and/or assembly of data: Amélie Anota, Cécile Cornet, Lionel Pazart
Data analysis and interpretation: Carole Bouleuc, Amélie Anota, Cécile Cornet, Ghislain Grodard, Lionel Pazart, Régis Aubry
Manuscript writing: Carole Bouleuc, Amélie Anota, Lionel Pazart, Régis Aubry
Final approval of manuscript: Carole Bouleuc, Amélie Anota, Cécile Cornet, Ghislain Grodard, Antoine Thiery‐Vuillemin, Olivier Dubroeucq, Nathalie Crétineau, Véronique Frasie, Vincent Gamblin, Gisèle Chvetzoff, Laure Favier, Christophe Tournigand, Marie‐Christine Grach, Bruno Raynard, Sébastien Salas, Géraldine Capodano, Lionel Pazart, Régis Aubry
Disclosures
Amélie Anota: AstraZeneca, Roche (C/A), Bristol‐Myers Squibb, Roche (H); Antoine Thiery‐Vuillemin: Pfizer, AstraZeneca, Sanofi, Janssen, Novartis, Ipsen, Roche/Genentech, Bristol‐Myers Squibb, Merck Sharp Dohme, Astellas Pharma (C/A, H), Pfizer (RF—institution). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank all the investigators and teams for their contributions to reaching the number of patients needed for the study. This study received only public grants from the French Ministry of Health and National Cancer Institute (PHRC 20110728). C.B. is currently affiliated with the Department of Supportive Care, Institut Curie, Paris, France.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Hébuterne X, Lemarié E, Michallet M et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014;38:196–204. [DOI] [PubMed] [Google Scholar]
- 2. Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 3. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 4. Arends J. Struggling with nutrition in patients with advanced cancer: Nutrition and nourishment‐focusing on metabolism and supportive care. Ann Oncol 2018;29(suppl 2):ii27–ii34. [DOI] [PubMed] [Google Scholar]
- 5. Prado CM, Baracos VE, McCargar LJ et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 6. Prado CM, Antoun S, Sawyer MB et al. Two faces of drug therapy in cancer: Drug‐related lean tissue loss and its adverse consequences to survival and toxicity. Curr Opin Clin Nutr Metab Care 2011;14:250–254. [DOI] [PubMed] [Google Scholar]
- 7. Lis CG, Gupta D, Lammersfeld CA et al. Role of nutritional status in predicting quality of life outcomes in cancer–A systematic review of the epidemiological literature. Nutr J 2012;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nipp RD, Fuchs G, El‐Jawahri A et al. Sarcopenia is associated with quality of life and depression in patients with advanced cancer. The Oncologist 2018;23:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryan AM, Prado CM, Sullivan ES et al. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 2019;67–68:110539. [DOI] [PubMed] [Google Scholar]
- 10. Versteeg KS, Blauwhoff‐Buskermolen S, Buffart LM et al. Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. The Oncologist 2018;23:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hui D, Kim YJ, Park JC et al. Integration of oncology and palliative care: A systematic review. The Oncologist 2015;20:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannon B, Swami N, Pope A et al. Early palliative care and its role in oncology: A qualitative study. The Oncologist 2016;21:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arends J, Baracos V, Bertz H et al. ESPEN expert group recommendations for action against cancer‐related malnutrition. Clin Nutr 2017;36:1187–1196. [DOI] [PubMed] [Google Scholar]
- 14. Arends J, Bachmann P, Baracos V et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [DOI] [PubMed] [Google Scholar]
- 15. Baldwin C, Spiro A, Ahern R et al. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta‐analysis. J Natl Cancer Inst 2012;104:371–385. [DOI] [PubMed] [Google Scholar]
- 16. Bozzetti F. Nutritional intervention is indicated in malnourished cancer patients. Clin Nutr 2019;38:477. [DOI] [PubMed] [Google Scholar]
- 17. Marín Caro MM, Laviano A, Pichard C. Nutritional intervention and quality of life in adult oncology patients. Clin Nutr 2007;26:289–301. [DOI] [PubMed] [Google Scholar]
- 18. Muscaritoli M, Molfino A, Laviano A et al. Parenteral nutrition in advanced cancer patients. Crit Rev Oncol Hematol 2012;84:26–36. [DOI] [PubMed] [Google Scholar]
- 19. Cotogni P. Enteral versus parenteral nutrition in cancer patients: Evidences and controversies. Ann Palliat Med 2016;5:42–49. [DOI] [PubMed] [Google Scholar]
- 20. Chow R, Bruera E, Chiu L et al. Enteral and parenteral nutrition in cancer patients: A systematic review and meta‐analysis. Ann Palliat Med 2016;5:30–41. [DOI] [PubMed] [Google Scholar]
- 21. Amano K, Morita T, Miyamoto J et al. Perception of need for nutritional support in advanced cancer patients with cachexia: A survey in palliative care settings. Support Care Cancer 2018;26:2793–2799. [DOI] [PubMed] [Google Scholar]
- 22. Pazart L, Cretin E, Grodard G et al. Parenteral nutrition at the palliative phase of advanced cancer: The ALIM‐K study protocol for a randomized controlled trial. Trials 2014;15:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moss AH, Lunney JR, Culp S et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med 2010;13:837–840. [DOI] [PubMed] [Google Scholar]
- 24. Downar J, Goldman R, Pinto R et al. The “surprise question” for predicting death in seriously ill patients: A systematic review and meta‐analysis. CMAJ 2017;189:E484–E493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zelen M. A new design for randomized clinical trials. N Engl J Med 1979;300:1242–1245. [DOI] [PubMed] [Google Scholar]
- 26. Gore SM. Zelen randomisation. Lancet 1984;2:226–227. [DOI] [PubMed] [Google Scholar]
- 27. Ellenberg SS. Informed consent: Protection or obstacle? Some emerging issues. Control Clin Trials 1997;18:628–636; discussion 661–666. [DOI] [PubMed] [Google Scholar]
- 28. Adamson J, Cockayne S, Puffer S et al. Review of randomised trials using the post‐randomised consent (Zelen's) design. Contemp Clin Trials 2006;27:305–319. [DOI] [PubMed] [Google Scholar]
- 29. Fisher B, Bauer M, Margolese R et al. Five‐year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665–673. [DOI] [PubMed] [Google Scholar]
- 30. Siva S, Ball D. Curing operable stage I non‐small cell lung cancer with stereotactic ablative body radiotherapy: The force awakens. The Oncologist 2016;21:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sauer R, Becker H, Hohenberger W et al.; German Rectal Cancer Study Group . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 32. Groenvold M, Petersen MA, Aaronson NK et al.; EORTC Quality of Life Group . The development of the EORTC QLQ‐C15‐PAL: A shortened questionnaire for cancer patients in palliative care. Eur J Cancer 2006;42:55–64. [DOI] [PubMed] [Google Scholar]
- 33. Anota A, Hamidou Z, Paget‐Bailly S et al. Time to health‐related quality of life score deterioration as a modality of longitudinal analysis for health‐related quality of life studies in oncology: Do we need RECIST for quality of life to achieve standardization? Qual Life Res 2015;24:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lundholm K, Daneryd P, Bosaeus I et al. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: Effects on survival, metabolism, and function. Cancer 2004;100:1967–1977. [DOI] [PubMed] [Google Scholar]
- 35. Naghibi M, Smith TR, Elia M. A systematic review with meta‐analysis of survival, quality of life and cost‐effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr 2015;34:825–837. [DOI] [PubMed] [Google Scholar]
- 36. Bozzetti F, Santarpia L, Pironi L et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: A multi‐centre observational study with prospective follow‐up of 414 patients. Ann Oncol 2014;25:487–493. [DOI] [PubMed] [Google Scholar]
- 37. Cotogni P, De Carli L, Passera R et al. Longitudinal study of quality of life in advanced cancer patients on home parenteral nutrition. Cancer Med 2017;6:1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vashi PG, Dahlk S, Popiel B et al. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 2014;14:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brard L, Weitzen S, Strubel‐Lagan SL et al. The effect of total parenteral nutrition on the survival of terminally ill ovarian cancer patients. Gynecol Oncol 2006;103:176–180. [DOI] [PubMed] [Google Scholar]
- 40. Fan BG. Parenteral nutrition prolongs the survival of patients associated with malignant gastrointestinal obstruction. JPEN J Parenter Enteral Nutr 2007;31:508–510. [DOI] [PubMed] [Google Scholar]
- 41. Tobberup R, Thoresen L, Falkmer UG et al. Effects of current parenteral nutrition treatment on health‐related quality of life, physical function, nutritional status, survival and adverse events exclusively in patients with advanced cancer: A systematic literature review. Crit Rev Oncol Hematol 2019;139:96–107. [DOI] [PubMed] [Google Scholar]
- 42. McGeer AJ, Detsky AS, O'Rourke K. Parenteral nutrition in cancer patients undergoing chemotherapy: A meta‐analysis. Nutrition 1990;6:233–240. [PubMed] [Google Scholar]
- 43. Prado CM, Sawyer MB, Ghosh S et al. Central tenet of cancer cachexia therapy: Do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012–1019. [DOI] [PubMed] [Google Scholar]
- 44. Obling SR, Wilson BV, Pfeiffer P et al. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin Nutr 2019;38:182–190. [DOI] [PubMed] [Google Scholar]
- 45. Cotogni P, Monge T, Fadda M et al. Bioelectrical impedance analysis for monitoring cancer patients receiving chemotherapy and home parenteral nutrition. BMC Cancer 2018;18:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teigen LM, Kuchnia AJ, Nagel E et al. Impact of software selection and ImageJ tutorial corrigendum on skeletal muscle measures at the third lumbar vertebra on computed tomography scans in clinical populations. JPEN J Parenter Enteral Nutr 2018;42:933–941. [DOI] [PubMed] [Google Scholar]
- 47. Hui D, Paiva CE, Del Fabbro EG et al. Prognostication in advanced cancer: Update and directions for future research. Support Care Cancer 2019;27:1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simmons C, McMillan DC, Tuck S et al; IPAC Study Group . “How long have I got?” ‐ a prospective cohort study comparing validated prognostic factors for use in patients with advanced cancer. The Oncologist 2019;24:e960–e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Durán‐Poveda M, Jimenez‐Fonseca P, Sirvent‐Ochando M et al. Integral nutritional approach to the care of cancer patients: Results from a Delphi panel. Clin Transl Oncol 2018;20:1202–1211. [DOI] [PubMed] [Google Scholar]


