Abstract
Background
Pulmonary embolism (PE) is a common and life-threatening medical condition with non-specific clinical presentation. Computed tomography pulmonary angiography (CT-PA) has been the diagnostic modality of choice, but its use is not without risks. Clinical decision rules have been established for the use of diagnostic modalities for patients with suspected PE. This study aims to assess the adherence of physicians to the diagnostic algorithms and rules.
Methods
A retrospective observational study examining the utilization of CT-PA in the Emergency Department of King Fahd Hospital of Imam Abdulrahman Bin Faisal University for patients with suspected PE from May 2016 to December 2019. The electronic health records were used to collect the data, including background demographic data, clinical presentation, triage vital signs, D-dimer level (if ordered), risk factors for PE, and the CT-PA findings. The Wells score and pulmonary embolism rule-out (PERC) criteria were calculated retrospectively without knowledge of the results of D-dimer and the CT-PA.
Results
The study involved a total of 353 patients (125 men and 228 women) with a mean age of 46.7 ± 18.4 years. Overall, 200 patients (56.7%) were classified into the “PE unlikely” group and 153 patients (43.3%) in the “PE likely” group as per Wells criteria. Out of all the CT-PA, 119 CT-PA (33.7%) were requested without D-dimer assay (n = 114) or with normal D-dimer level (n = 5) despite being in the “PE unlikely” group. Only 49 patients had negative PERC criteria, of which three patients had PE.
Conclusions
The study revealed that approximately one-third of all CT-PA requests were not adhering to the clinical decision rules with a significant underutilization of D-dimer assay in such patients. To reduce overutilization of imaging, planned interventions to promote the adherence to the current guidelines seem imperative.
Keywords: Computed Tomography, Pulmonary embolism, Wells criteria, Guidelines
Background
Pulmonary embolism (PE) is a common and potentially life-threatening medical condition. Every year, there are approximately 100,000 deaths from PE in the USA [1]. PE has a wide range of presenting symptoms ranging from no symptoms to sudden death. Due to its non-specific signs and symptoms, the diagnosis of PE is often missed or delayed [2]. Early diagnosis and management are crucial as the mortality rate reaches nearly 30% for untreated PE [3]. Computed tomography pulmonary angiography (CT-PA) has become the modality of choice in the evaluation of patients with suspected PE because of its high sensitivity and accuracy [4]. However, an increase in the use of computed tomography scans has become a subject of concern as it may subject patients to ionizing radiations and contrast-related complications and increase the hospital stay and expenditure [5–7]. Of note, it has been reported that the USA spends approximately twice as much as other high-income countries on the healthcare and overutilization of diagnostic imaging was the main reason [8].
Diagnostic algorithms and clinical decision rules have been established to guide in the management of patients with suspected PE. Such criteria include the Wells criteria and the pulmonary embolism rule-out criteria (PERC) which were found to have high predictive accuracy [9, 10]. In the modified Wells criteria, the patient who has a score ≤ 4 is considered unlikely to have PE and should have D-dimer testing. If the D-dimer level is normal, no further workup is warranted. The CT-PA is recommended in case of elevated D-dimer level or for Wells score > 4 [11]. Similarly, the patient who does not meet any of the PERC criteria should not have any further workup for PE [10]. The implementation of the diagnostic algorithms is essential and advocated by national guidelines [12, 13].
In Saudi Arabia, however, there has been no previous study investigating the adherence of physicians to the clinical decision rules for patients with suspected PE. Therefore, this study was designed to assess the adherence to these rules in the emergency department (ED).
Methods
Study design
We conducted a retrospective study examining the adherence of physicians to the clinical decision rules for PE among patients with suspected PE in the ED.
Study setting
The study was conducted in King Fahd Hospital of the Imam Abdulrahman Bin Faisal University, which is a 440-bed capacity academic center and is located in the Eastern Province of Saudi Arabia. Approximately 250,000 patients visit the ED and 20,000 patients are admitted every year. In our institution, CT-PA can only be requested by board-certified consultant physicians.
Study population
Eligible patients included all adults (≥ 18 years) with suspected PE who underwent CT-PA. The radiology information system was utilized to identify all CT-PA requests from the ED from May 2016 to December 2019.
Data collection
A web-based structured data collection form was used to collect the data from the electronic health record. The collected data included background demographic data, clinical presentation, D-dimer level (if ordered), risk factors for PE (immobilization or surgery within past 4 weeks, previous deep venous thrombosis or PE, and exogenous estrogen use), and the CT-PA findings.
The imaging results were collected from the electronic reports for assessing the presence of pulmonary artery filling defects. In case the patient had more than one CT-PA scan, only the most recent was included in our study. The Wells and PERC scores were calculated retrospectively by investigators without the knowledge of D-dimer and CT-PA results. The patients were categorized into the “PE likely” group if the Wells score was > 4 and the “PE unlikely” group if the Wells score was ≤ 4.
Imaging technique
The scans were performed from the diaphragm to the lung apices, using one of the two multidetector scanners:
The dual-source 128-slice scanner (Somatom Definition Flash, Siemens; Erlangen, Germany) with the administration of 25-ml non-ionic contrast material with timed intravenous pump infusion followed by a 25-ml saline flush, after the test bolus.
The 64-slice scanner (Somatom Definition AS, Siemens; Erlangen, Germany) with the administration of 60-ml non-ionic contrast material with timed intravenous pump infusion followed by a 40-ml saline flush, with bolus tracking.
D-dimer assay
A rapid quantitative immunoturbidimetric assay (Advanced D-dimer; Dade Behring, Marburg, Germany) was used for the D-dimer assay. The results were expressed as mg/ml and the normal range was defined as < 500 mg/ml. The result was usually reported within 1 h.
Statistical analysis
The obtained data were compiled using the QuestionPro platform and analyzed using IBM SPSS for Windows, version 25 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation. Descriptive statistics, such as percentages and frequency distribution of different characteristics, were used as appropriate. Categorical variables were compared using chi-square test. The statements of statistical significance were based on a significance level of α = 0.05.
Results
Patients characteristics
The study involved a total of 353 patients, including 125 men and 228 women, representing 35.4% and 64.6% of the study population, respectively. The mean age of patients was 46.7 ± 18.4 years (range, 18–96 years).
Decision rules
Wells criteria
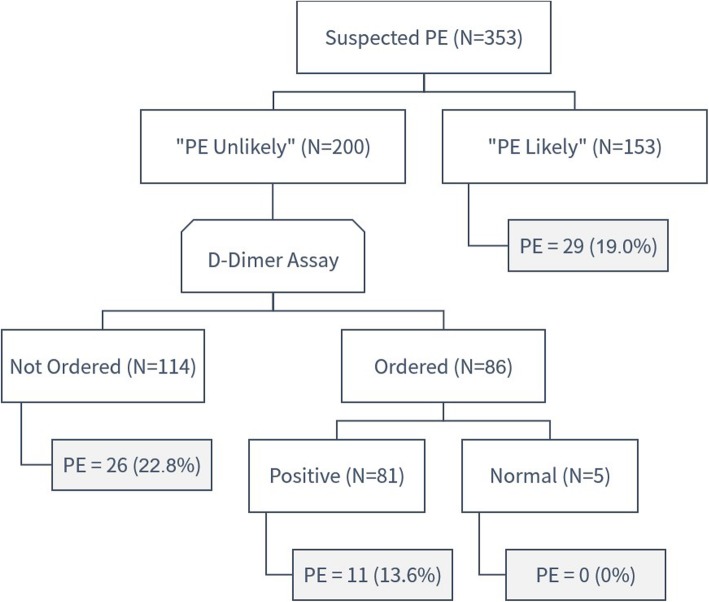
Based on Wells criteria, 200 patients (56.7%) were classified into the “PE unlikely” group and 153 patients (43.3%) in the “PE likely” group (Fig. 1). Interestingly, there were no significant differences between the two groups in terms of age, gender, D-dimer order rate and result, and even the positive CT-PA rate (Table 1).
Fig. 1.
Positive CT-PA rate based on Wells criteria. N number of patients, PE pulmonary embolism
Table 1.
Characteristics of the patients in the “PE likely” and “PE unlikely” group
| Characteristics | “PE unlikely” (total n = 200) | “PE likely” (total n = 153) | χ2 (P value) | ||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | ||||
| Age group (years) | 18–30 | 43 | (21.5) | 34 | (22.2) | 3.417 | (0.332) |
| 31–50 | 83 | (41.5) | 71 | (46.4) | |||
| 51–70 | 47 | (23.5) | 24 | (15.7) | |||
| > 70 | 27 | (13.5) | 24 | (15.7) | |||
| Gender | Male | 75 | (37.5) | 50 | (32.7) | 0.881 | (0.348) |
| Female | 125 | (62.5) | 103 | (67.3) | |||
| D-dimer assay order | Ordered | 86 | (43.0) | 63 | (41.2) | 0.118 | (0.731) |
| Not ordered | 114 | (57.0) | 90 | (58.8) | |||
| D-dimer level (mg/dL) | ≤ 0.5 | 5 | (5.8) | 1 | (1.6) | 1.681 | (0.195) |
| > 0.5 | 81 | (94.2) | 62 | (98.4) | |||
| CT-PA result | Negative | 163 | (81.5) | 124 | (81.0) | 0.012 | (0.914) |
| Positive | 37 | (18.5) | 29 | (19.0) | |||
n number of patients, CT-PA computed tomography pulmonary angiography
“PE likely” group
Out of the 153 patients in the “PE likely” group, 29 patients (19%) had PE including 15 men and 14 women. In 63 patients (41.2%), the CT-PA was performed following D-dimer assay. The D-dimer assay was elevated in all these patients, except for one patient who had normal level and subsequently had a normal CT-PA result.
“PE unlikely” group
Among the “PE unlikely” group, the D-dimer was ordered in 86 patients (43%) only. Thus, there were 114 patients (57%) in the “PE unlikely” group who had CT-PA as the first investigation for PE. Of the 114 patients, 88 patients (77.2%) had a negative CT-PA. The CT-PA was performed despite a negative D-dimer for 5 patients from the “PE unlikely” group and none of them had a CT-PA positive for PE. Out of all the CT-PA, 119 CT-PA (33.7%) were requested without D-dimer assay (n = 114) or with normal D-dimer level (n = 5) despite being in the “PE unlikely” group.
PERC criteria
There were 49 patients (13.9%) having negative PERC criteria (Table 2). Only three of these 49 patients had a CT-PA positive for PE, giving a negative predictive value of 93.9%.
Table 2.
PERC criteria for the patients with suspected PE
| 1.Item | No | ||
|---|---|---|---|
| n | (%) | ||
| 1 | Age ≥ 50 years | 230 | (65.2) |
| 2 | Heart rate ≥ 100 | 182 | (51.6) |
| 3 | Oxygen saturation on room air < 95% | 275 | (77.9) |
| 4 | Unilateral leg swelling | 335 | (94.9) |
| 5 | Hemoptysis | 338 | (95.8) |
| 6 | Recent surgery or trauma | 318 | (90.1) |
| 7 | Prior PE or DVT | 300 | (85.0) |
| 8 | Hormone use | 346 | (98.0) |
| Overall | 49 | (13.9) | |
PE pulmonary embolism, DVT deep vein thrombosis
Discussion
Our study revealed that one-third of all CT-PA performed in our institution were not in line with the clinical decision rules. This is because the CT-PA had been requested without having a positive D-dimer level despite the patient being in the “PE unlikely” group. Because the Wells score includes a subjective item which is “an alternative diagnosis is less likely than PE” that has a large contribution of three points to the score, it was initially proposed that the high proportion of unjustified CT-PA scans may be attributed to the retrospective nature of the study. However, we calculated the Wells score again with the assignment of these three points to all patients. Interestingly, the analysis showed that the D-dimer level was not ordered in 18.7% of patients despite being advised by the decision rules, thus, confirming our initial finding.
The reason behind the underutilization of the D-dimer assay is unclear, but the increased patient load and time constraints in the ED might play a role. Furthermore, the use of CT-PA is often justified due to the finding of alternative diagnoses explaining the patient’s clinical presentation, though some evidence did not support this argument [14, 15]. An important contributing factor is the widespread availability of CT with a rapid turnaround time that is available free of charge for all citizens.
Additionally, we had five cases (1.4%) in the “PE unlikely” group who underwent CT-PA despite a normal D-dimer level. Although this seems a very low rate, these were unjustified given the high sensitivity of D-dimer assay which reaches 99% [16]. Notably, none of these cases had PE, thereby confirming the safety of not performing CT-PA scans in these patients.
The finding of inadequate adherence to clinical decision rules is not unique. Several previous studies from different institutions showed comparable results [17–19]. For instance, the National Quality Forum showed that the imaging was avoidable in 32% of patients with suspected PE [20]. A recent study by Kline et al. involving 23 hospitals revealed that out of the 25,870 patients who underwent CT-PA, 59% have not had a D-dimer before the imaging. The same study found a significant positive correlation between the increase in D-dimer assay ordering and the positive CT-PA rate [21].
We identified that 6.1% of patients had PE despite having negative PERC criteria. The PERC criteria were designed to identify patients in whom the risk of unnecessary workup outweighs the risk of PE. A prospective study involving 8138 patients with negative PERC criteria showed that less than 1% had PE [10]. Our finding revealed a relatively high proportion of PE among patients with negative PERC criteria. However, it should be noted that we only had 49 patients with negative PERC criteria. Additionally, Hugli et al. have shown that the prevalence of PE was 5.4% among patients with negative PERC criteria suggesting that the criteria could not safely identify the patients in whom the PE can be excluded without any further workup [22].
The CT-PA remains the first-choice diagnostic modality for the diagnosis of PE because of its high accuracy, especially when incorporated into the clinical decision rules [23]. However, the bedside echocardiography can be more feasible when the rapid or presumptive diagnosis is needed in hemodynamically unstable patients to justify the use of thrombolytic therapy [24]. While the echocardiography may demonstrate the presence of clot or right ventricle strain, it is generally considered to have limited diagnostic value in most cases and it is most beneficial for its prognostic value in patients with confirmed PE [24, 25].
Different strategies have been proposed to increase adherence to the clinical decision rules in the diagnosis of PE. These include the clinical decision support interventions which are based on a computerized physician order entry that prompts the physician to use the Wells criteria when ordering a CT-PA scan. Clinical performance and feedback reports to physicians about their practice and outcome are another intervention to improve the utilization of CT-PA. Formal educational interventions with training sessions have been advised. It has been suggested that the combination of these interventions may have a more significant impact [26].
This study is the first to investigate the utilization of CT-PA in Saudi Arabia. However, the present study has some limitations. The retrospective observational nature of the study is an important limitation. The radiological images were not reviewed for the confirmation of the reported findings. Also, the study did not involve the patients with suspected PE who had not undergone CT-PA. Lastly, the complete reliance on the radiology report for the diagnosis of PE is another limitation given that some studies had shown some concern about the overdiagnosis of clinically insignificant PE [27].
Conclusion
The study revealed that one-third of all CT-PA was not adhering to the clinical decision rules with significant underutilization of D-dimer assay when indicated. Further studies are needed to explore the barriers leading to inadequate adherence to these rules. To reduce overutilization of imaging, planned interventions to promote the adherence to the current guidelines seem imperative.
Acknowledgements
Not applicable
Abbreviations
- CI
Confidence interval
- CT-PA
Computed tomography pulmonary angiography
- ED
Emergency department
- OR
Odd ratio
- PERC criteria
Pulmonary embolism rule-out criteria
Authors’ contributions
Conceptualization: OA, AH, and SS; data analysis: AH; methodology: AH and AN; writing-original draft: AH and MQ; writing-review and editing: HA, AZ, and MA; and supervision: OA. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board at the Imam Abdulrahman Bin Faisal University (IAU IRB No. 2020-01-138). All collected data were de-identified. The data was stored electronically and only the principal investigator has access to view the stored data.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Omran Al Dandan and Ali Hassan contributed equally to this work.
Contributor Information
Omran Al Dandan, Email: odandan@iau.edu.sa.
Ali Hassan, Email: alihy8@gmail.com.
Afnan Alnasr, Email: afnannasr@iau.edu.sa.
Mohammed Al Gadeeb, Email: m.baqiri.j@hotmail.com.
Hossain AbuAlola, Email: hossainabuaola@iau.edu.sa.
Sarah Alshahwan, Email: sshahwan@iau.edu.sa.
Malak Al Shammari, Email: moalshammari@iau.edu.sa.
Alaa Alzaki, Email: azaki@iau.edu.sa.
References
- 1.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711–1717. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Martinez JL, Sanchez FJ, Echezarreta MA. Delay and misdiagnosis in sub-massive and non-massive acute pulmonary embolism. Eur J Intern Med. 2010;21(4):278–282. doi: 10.1016/j.ejim.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/S0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 4.Perrier A, Roy P-M, Sanchez O, Le Gal G, Meyer G, Gourdier A-L, Furber A, Revel M-P, Howarth N, Davido A. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352(17):1760–1768. doi: 10.1056/NEJMoa042905. [DOI] [PubMed] [Google Scholar]
- 5.De González AB, Mahesh M, Kim K-P, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morabito S, Pistolesi V, Benedetti G, Di Roma A, Colantonio R, Mancone M, Sardella G, Cibelli L, Ambrosino M, Polistena F, et al. Incidence of contrast-induced acute kidney injury associated with diagnostic or interventional coronary angiography. J Nephrol. 2012;25(6):1098–1107. doi: 10.5301/jn.5000101. [DOI] [PubMed] [Google Scholar]
- 7.Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039. doi: 10.1001/jama.2018.1150. [DOI] [PubMed] [Google Scholar]
- 9.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, Forgie M, Kovacs G, Ward J, Kovacs MJ. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135(2):98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 10.Kline JA, Courtney DM, Kabrhel C, Moore C, Smithline H, Plewa M, Richman P, O'NEIL B, Nordenholz K. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772–780. doi: 10.1111/j.1538-7836.2008.02944.x. [DOI] [PubMed] [Google Scholar]
- 11.Douma RA, Gibson NS, Gerdes VE, Büller HR, Wells PS, Perrier A, Le Gal G. Validity and clinical utility of the simplified Wells rule for assessing clinical probability for the exclusion of pulmonary embolism. Thromb Haemost. 2009;101(01):197–200. doi: 10.1160/TH08-07-0444. [DOI] [PubMed] [Google Scholar]
- 12.Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701–711. doi: 10.7326/M14-1772. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, Huisman MV, Humbert M, Jennings CS, Jiménez D et al: 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2019;41(4):543–603. [DOI] [PubMed]
- 14.Chandra S, Sarkar PK, Chandra D, Ginsberg NE, Cohen RI. Finding an alternative diagnosis does not justify increased use of CT-pulmonary angiography. BMC Pulm Med. 2013;13:9. doi: 10.1186/1471-2466-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Es J, Douma RA, Schreuder SM, Middeldorp S, Kamphuisen PW, Gerdes VEA, Beenen LFM. Clinical impact of findings supporting an alternative diagnosis on CT pulmonary angiography in patients with suspected pulmonary embolism. Chest. 2013;144(6):1893–1899. doi: 10.1378/chest.13-0157. [DOI] [PubMed] [Google Scholar]
- 16.den Exter PL, van Es J, Erkens PM, van Roosmalen MJ, van den Hoven P, Hovens MM, Kamphuisen PW, Klok FA, Huisman MV. Impact of delay in clinical presentation on the diagnostic management and prognosis of patients with suspected pulmonary embolism. Am J Respir Crit Care Med. 2013;187(12):1369–1373. doi: 10.1164/rccm.201212-2219OC. [DOI] [PubMed] [Google Scholar]
- 17.Costantino MM, Randall G, Gosselin M, Brandt M, Spinning K, Vegas CD. CT angiography in the evaluation of acute pulmonary embolus. American Journal of Roentgenology. 2008;191(2):471–474. doi: 10.2214/AJR.07.2552. [DOI] [PubMed] [Google Scholar]
- 18.Perelas A, Dimou A, Saenz A, Rhee JH, Teerapuncharoen K, Rowden A, Eiger G. CT pulmonary angiography utilization in the emergency department: diagnostic yield and adherence to current guidelines. Am J Med Qual. 2015;30(6):571–577. doi: 10.1177/1062860614543302. [DOI] [PubMed] [Google Scholar]
- 19.Shujaat A, Shapiro JM, Eden E. Utilization of CT pulmonary angiography in suspected pulmonary embolism in a major urban emergency department. Pulm Med. 2013;2013:915213. doi: 10.1155/2013/915213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesh AK, Kline JA, Courtney DM, Camargo CA, Plewa MC, Nordenholz KE, Moore CL, Richman PB, Smithline HA, Beam DM, et al. Evaluation of pulmonary embolism in the emergency department and consistency with a national quality measure: quantifying the opportunity for improvement. Arch Intern Med. 2012;172(13):1028–1032. doi: 10.1001/archinternmed.2012.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline JA, Garrett JS, Sarmiento EJ, Strachan CC, Courtney DM. Over-testing for suspected pulmonary embolism in American emergency departments: the continuing epidemic. Circ Cardiovasc Qual Outcomes. 2020;13(1):e005753. doi: 10.1161/CIRCOUTCOMES.119.005753. [DOI] [PubMed] [Google Scholar]
- 22.Hugli O, Righini M, Le Gal G, Roy PM, Sanchez O, Verschuren F, Meyer G, Bounameaux H, Aujesky D. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J Thromb Haemost. 2011;9(2):300–304. doi: 10.1111/j.1538-7836.2010.04147.x. [DOI] [PubMed] [Google Scholar]
- 23.Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Leeper KV, Jr, Popovich J, Jr, Quinn DA, Sos TA, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 24.Fields JM, Davis J, Girson L, Au A, Potts J, Morgan CJ, Vetter I, Riesenberg LA, et al. J Am Soc Echocardiogr. 2017;30(7):714–723.e714. doi: 10.1016/j.echo.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Dabbouseh NM, Patel JJ, Bergl PA. Role of echocardiography in managing acute pulmonary embolism. Heart. 2019;105(23):1785–1792. doi: 10.1136/heartjnl-2019-314776. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Lepanto L: Interventions to reduce the overuse of imaging for pulmonary embolism: a systematic review. www journalofhospitalmedicine com 2018, 13(1):52. [DOI] [PubMed]
- 27.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831–837. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.