Abstract
Malfunction of the liver is a central factor in metabolic disease. Glucose production by liver is complex and controlled via indirect mechanisms; insulin regulates adipose tissue lipolysis, and free fatty acids in turn regulate liver glucose output. This latter concept is confirmed by studies in L-Akt-Foxo1 knockout mice. The adipocyte is a likely locus of hepatic insulin resistance. Also, kidneys play a role in regulating glucose production; denervated kidneys abrogate the effect of fat feeding to cause insulin resistance. Glucose itself is an important regulator of liver metabolism (“glucose effectiveness”); after entering liver, glucose is phosphorylated and can be exported as lactate. Using the dynamic glucose/lactate relationship, we have been able to estimate glucose effectiveness in intact animals and human subjects. Families have been identified with a glucokinase regulatory protein defect; modeling demonstrates elevated glucokinase activity. Insulin clearance by liver is highly variable among normal individuals, and is under environmental control: high fat diet reduces clearance by 30%. Liver insulin clearance is significantly lower in African American (AA) adults and children compared to European American participants, accounting for fasting hyperinsulinemia in AA. We hypothesize that reduced hepatic insulin clearance causes peripheral insulin resistance and increased Type 2 diabetes in AA.
Ancients saw the liver as the seat of the soul (1). Signals were proposed to go from liver to heart, and thence to the brain. While we no longer seem to view the liver as the seat of the soul, we can still appreciate the importance of the liver to regulating systems in the body, including blood glucose homeostasis. Production of glucose is one of the most important functions of the liver. This process is under complex control by substrates, hormones and nerves. The precise regulation of endogenous glucose production (liver and kidneys) mirrors the importance of an adequate supply of glucose to the brain, to guarantee the ability of the organism to respond to environmental threats. While a full accounting of the bevy of systems interacting with liver glucose handling is beyond the scope of this Commentary, we will take this opportunity to examine several recent observations which have altered our understanding of how liver function is regulated.
1. Control of Liver Glucose Output: Direct or Indirect?
It has long been accepted that the rate of endogenous glucose production (EGP) is related to the balance between insulin and glucagon levels in blood entering the liver (2). Glucagon acts via cyclic 3’5’ AMP to stimulate glycogenolysis and gluconeogenesis (3), and insulin is presumed to inhibit glucose production via phosphodiesterase, thus lowering the internal cAMP signal (4). However, there are reasons to believe that this “direct” effect of insulin to suppress glucagon’s effect might not be the “whole story.” The liver has enormous capacity to degrade insulin. In fact, about one-half of insulin entering the liver via the abdominal portal vein, or the hepatic artery, will not survive the passage, will bind to hepatic insulin receptors, be internalized into hepatocytes, and be therefore degraded, possibly by the protein CEACAM1 and insulin degrading enzyme (IDE) (5). If such a large mass of insulin is degraded each day (450,000 pM/day!!!), it may be difficult to imagine that portal vein insulin levels represent a “signal” to control liver glucose production. Based upon the latter consideration, some years ago we considered that the well documented effect of insulin to suppress liver glucose production may not be a direct effect of the hormone, but may be mediated by extrahepatic mechanisms.
1.1. Free Fatty Acids (FFA)
We performed experiments to examine whether the primary effect of insulin to suppress liver glucose output was a direct anti-glucagon effect as described above, or might be mediated indirectly. During euglycemic clamps in dogs, insulin was infused either directly into the abdominal portal vein, or systemically at half the portal infusion rate (6). This design resulted in matched peripheral insulin concentrations, but very different portal insulin concentrations, with intraportal infusion resulting portal hyperinsulinemia. Intraportally infused insulin was no more effective than peripherally infused insulin in suppression of liver glucose output, supporting the concept that insulin acts indirectly to regulate EGP. We examined a variety of possible signals which could mediate insulin’s peripheral action to suppress the liver. We reported that preventing the decline in FFA during insulin infusion also prevented the decline in EGP (7). These experimental studies supported the idea that to lower EGP, insulin first suppressed white adipose tissue lipolysis; lowering FFA in blood entering the liver, and thereby secondarily lowering liver glucose production.
On the basis of studies that investigated the intraportal versus systemic insulin infusion and transendothelial transport of insulin, we proposed the “single gateway hypothesis,” which proposes an indirect regulation of hepatic glucose production by insulin; the rate-limiting transport of insulin across the adipose tissue capillaries is responsible for the slow suppression of FFAs, which in turn is responsible for delayed suppression of hepatic EGP during insulin infusion (8). More recently, mice lacking hepatic Foxo1 in addition to Akt1 and Akt2 (L-AktFoxo1TKO), all required for insulin signaling, surprisingly showed normal glycemia (9). Inhibiting the fall of plasma FFAs in these mice prevented the suppression of EGP during a clamp, reaffirming that the site of insulin action to control EGP is extrahepatic (10).
Measuring whole-body turnover rates of glucose and FFAs in L-AktFoxo1TKO mice also confirmed that hepatic EGP was regulated by insulin-mediated control of FFAs (11). The knockout mouse model, in combination with sophisticated molecular techniques, confirmed our physiological findings and the single gateway hypothesis. The aforementioned studies suggest that in metabolic disease, with insulin resistance reflected as overproduction of glucose due to inadequate effect of insulin to lower FFA and hence EGP. Whereas therapies to normalize glucose levels have been directed at the liver, or at increased sensitization of skeletal muscle, the results discussed above suggest that the most effective way to control the fasting glucose level is via adipose tissue. Thus it would be important to identify molecules which suppress lipolysis, particularly in the morning, which in turn would reduce liver glucose output. Future studies are necessary to confirm the “single gateway hypothesis” and explore under what conditions (prediabetes, diabetes) suppression of lipolysis per se would mitigate overproduction by the liver. Such studies are progressing in our laboratory.
1.2. Kidneys
Recently there has been great interest in denervation of the kidneys as a treatment for primary hypertension resistant to pharmacologic treatment. In a relatively small double blind clinical trial (Symplicity HTN-2), evidence was presented for reduced blood pressure after radiofrequency ablation of renal nerves with an intra-renal arterial catheter (12). These studies encouraged the much larger international double blind Symplicity HTN3 trial (13). However, results were disappointing. The expected 5 mm Hg reduction in blood pressure with renal denervation was not achieved (14), leading to widespread disappointment with the denervation procedure to treat hypertension. A variety of reasons have been suggested for this lack of success; one possibility is that adequate ablation of the renal nerves was not accomplished by the ablation device employed in this large trial.
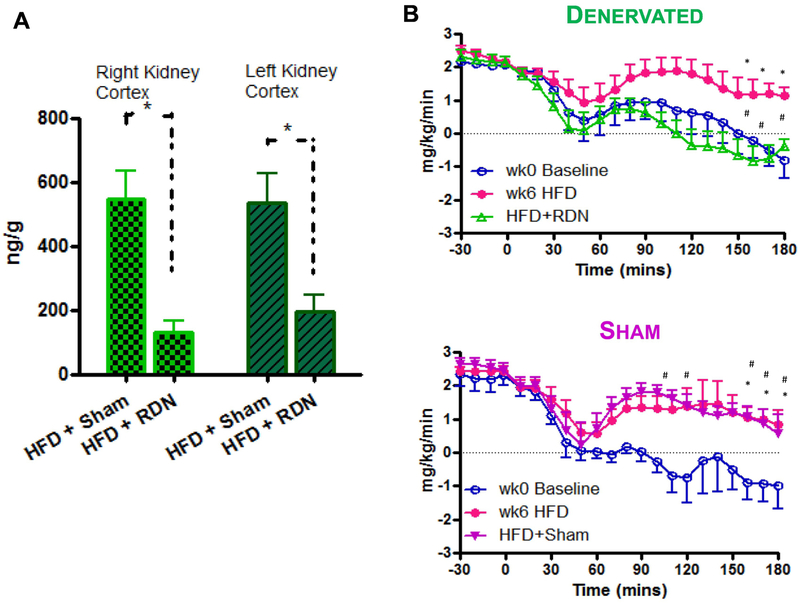
In our laboratory, we considered that in the face of possible inadequate ablation, we are able to perform total surgical ablation of the renal nerves in experimental animals. In collaboration with the late Ronald Victor, we designed a study to examine the effects of surgical ablation on carbohydrate homeostasis in the conscious dog model (15). Animals were made insulin resistant by feeding a high fat diet, and renal denervation (or sham procedure) was performed to determine whether resistance is ameliorated by denervation. Validation of the surgical ablation was confirmed by a marked reduction in norepinephrine content of the denervated kidneys (Fig. 1a). Renal nerve ablation totally reversed the diet-induced impairment of insulin’s effect to suppress endogenous glucose production during euglycemic clamps (Fig. 1b). This study, which was originally conceived based upon the idea that ablation of sympathetic renal nervous connection to liver would alter blood pressure, yielded a surprising and possibly more important effect, that renal innervation may play a role in control of endogenous glucose production.
Figure 1.
Renal denervation by surgical ablation in the canine model. (A) Validation of denervation; (B) Demonstration that hepatic resistance induced by fat feeding is reversed by renal denervation (bottom), but not sham procedure (top). Adapted from (15).
It is clear from these above results that the initial concept that insulin controls liver glucose production by suppression of the effect of glucagon may be not the only, or even the primary mechanism by which insulin controls glucose output from the liver. In fact, there are at least two extrahepatic signaling mechanisms; insulin can reduce endogenous glucose production by suppression of free fatty acids, which in turn act to lower glucose production independent of a direct effect of insulin per se. In addition, it is possible that the kidneys may play a previously unappreciated role on glucose production. Taken together, these studies change the original bi-hormonal model of glucose production regulation. It seems clear that there is more to be learned regarding the role(s) of cross talk from extra-hepatic tissues (adipose tissue and kidney) to regulation of endogenous glucose output.
It is known that the kidneys, via gluconeogenesis, contribute to overall glucose production. The studies discussed above suggest that the kidneys may also play a role in signaling the liver to produce glucose. If so, one may hypothesize that said signaling may be at least partially responsible for the overproduction of glucose in diabetes. It is also possible that the control of renal signaling to the liver may involve the sympathetic nervous system (SNS). It will be important in the future to further examine renal control of liver glucose production, and examine whether intervention via the SNS could provide yet another target which may result in improvement in insulin sensitivity of liver, and add to normalization of glucose. This putative renal mechanism further emphasizes the importance of indirect control of liver glucose production in the pathogenesis of hyperglycemia.
2. Direct control of hepatic glucose uptake by glucose per se.
Nutrient ingestion results in rapid disposal of ingested carbohydrate into skeletal muscle and the liver. Clearly, when said disposal is retarded, glucose intolerance and diabetes can ensue. Thus, it is important to understand the mechanisms involved in postprandial glucose disposal. Classically (and correctly), it is felt that carbohydrate ingestion stimulates a rapid insulin response; the insulin acts to mobilize glucose transporters (GLUT4) to the plasma membrane of insulin-sensitive tissues (primarily skeletal muscle), allowing the hexose to enter cells to be metabolized or stored (16). What then is the role of the liver? In fact, the liver is not acutely responsive to the hyperinsulinemia, yet it also plays an important role in glucose storage and utilization after nutrient meals. The significance of liver glucose uptake after meals has been underestimated. One reason is the difficulty of accurately assessing hepatic glucose uptake; turnover studies have utilized euglycemic clamp (EGC) methodologies; the latter method measures total glucose uptake and liver glucose production, yet it is challenging to assess specifically liver glucose uptake using the EGC method.
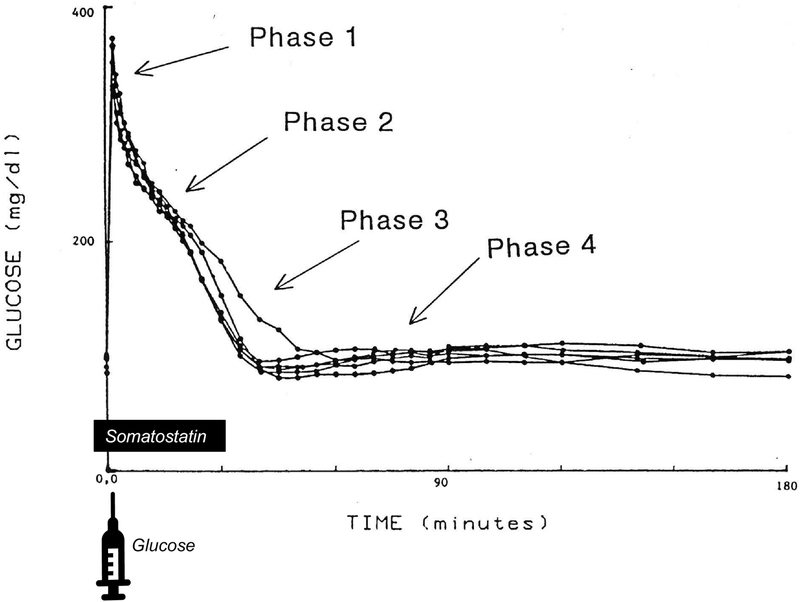
We have named the effect of plasma glucose to enhance net glucose uptake after carbohydrate ingestion “glucose effectiveness (GE) (17).” The latter is “the effect of an increment in plasma glucose to enhance glucose uptake independent of the acute change in insulin.” We have chosen to assess the importance of GE using the intravenous glucose bolus so that effects of glucose independent of gastrointestinal hormones can be examined. Fig. 2 reproduces an experiment done in our own laboratory observing the plasma glucose pattern after iv injection, in which the insulin response is delayed with somatostatin (18). Note that with a delayed insulin response, glucose is steadily normalized (“phase 2”) due to the effects of glucose per se. As the somatostatin effect dissipates and insulin levels increase, the rate of glucose normalization is accelerated (“phase 3”). The rate of glucose normalization in the absence of an insulin response reflects the effect of glucose on its own disappearance – glucose effectiveness.
Figure 2.
Phases of glucose normalization during the intravenous glucose tolerance test. Glucose was injected at t=0. Somatostatin was infused from −1 to 30 minutes to delay insulin response. Phase 1: glucose mixing; Phase 2: insulin-independent glucose disappearance (i.e. glucose effectiveness); Phase 3: insulin-dependent glucose disappearance; Phase 4: glucose renormalization. Adapted from (18).
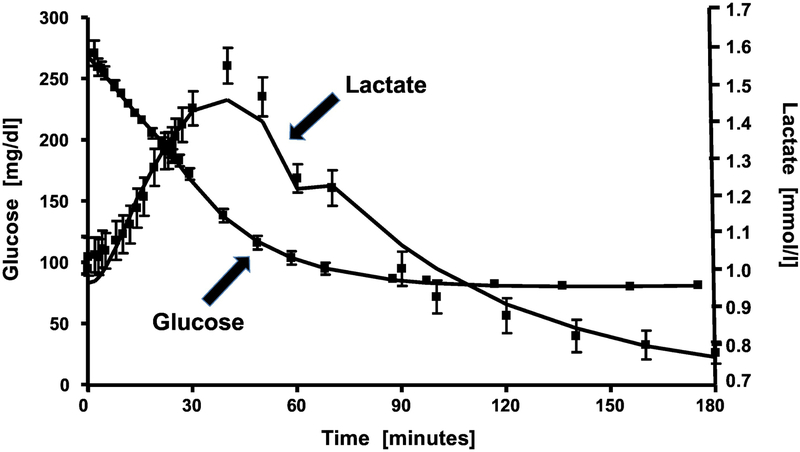
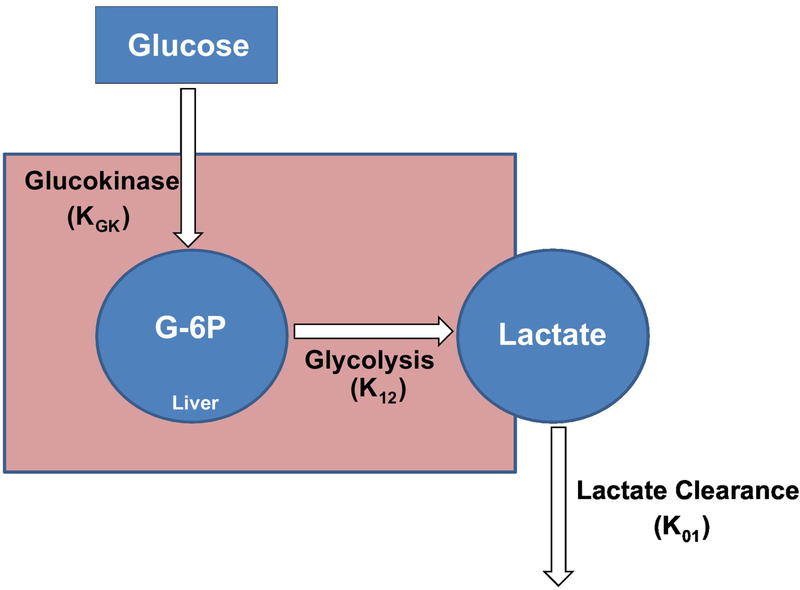
Recently we introduced a new approach to quantification of GE (19). Under resting conditions, injected glucose can enter the liver independent of the dynamic insulin response, and be phosphorylated by glucokinase. The intrahepatic fate of glucose-6-phosphate can include storage as liver glycogen, or glycolytic conversion to pyruvate which may enter the TCA cycle or be exported as lactate. Under resting (non-exercise) conditions, it may be expected that lactate production reflects liver phosphorylation by glucokinase. To assess glucose effectiveness, we observed the time-dependent relationship between plasma glucose during the frequently-sampled intravenous glucose tolerance test (FSIGT) and plasma lactate levels (Fig. 3). Note that following glucose injection, there is a retarded increase in plasma lactate which we assume primarily represents glucose entering the liver and being exported as lactate. The 3-carbon compound is renormalized following the return of glucose to basal. We introduced a simple mathematical model describing the glucose-lactate relationship (Fig. 4) which allows for the prediction of the rate of hepatic glucose phosphorylation (19). This model has been validated in a series of studies published by Michael Schwartz and his colleagues at the University of Washington (20). In their studies, the protein FGF 1 was injected into the central nervous system, resulting in a normalization of glucose levels in the ZDF rat model. The measured changes in glucokinase activity from biopsied liver tissue were almost exactly replicated by the estimate of glucokinase activity from the glucose-lactate model. Additionally, using the model, the increased glucokinase activity in members of a family with a genetic defect in glucokinase regulatory protein (GKRP) activity was detected using the glucose/lactate model (M. Boehnke, personal communication).
Figure 3.
Time course of plasma glucose and lactate during the intravenous glucose tolerance test.
Figure 4.
Two-compartment mathematical model of lactate kinetics. From the time course of plasma glucose and lactate from the intravenous glucose tolerance test, the model output includes parameter KGK represents glucokinase activity. Adapted from (19).
Glucose effectiveness is an important contributor to glucose disposal after intravenous or oral consumption of carbohydrate. Evidence exists that defects in GE may be an important factor in the pathogenesis of Type 2 diabetes, and hepatic glucokinase may represent may be an interesting and little appreciated target for diagnosis and treatment. It is important to reemphasize that despite its importance to overall glucose handling, glucose effectiveness has been little appreciated by the scientific community. The latter is because GE is difficult to measure in patients. Traditional turnover methods focus on insulin action per se, with little information regarding GE. Therefore it is important to continue to devise methods to assess GE (for example, the lactate model discussed above) to assess the importance of changes in GE to metabolic dysfunction in prediabetes or diabetes. GE is also a possible target for therapy.
3. Insulin Clearance
As discussed, much of the insulin released by the beta-cells of the pancreas (averaging about 50%) is cleared from the blood by the liver during the first pass through the organ (21). Years ago Robert Turner asked me – why did evolution “decide” that such a large fraction of secreted insulin be destroyed, even before it was able to increase glucose utilization and suppress glucose production (via FFA; see above)? In our laboratory we have recently been investigating this question.
3.1. Direct measure of insulin clearance in the large animal model
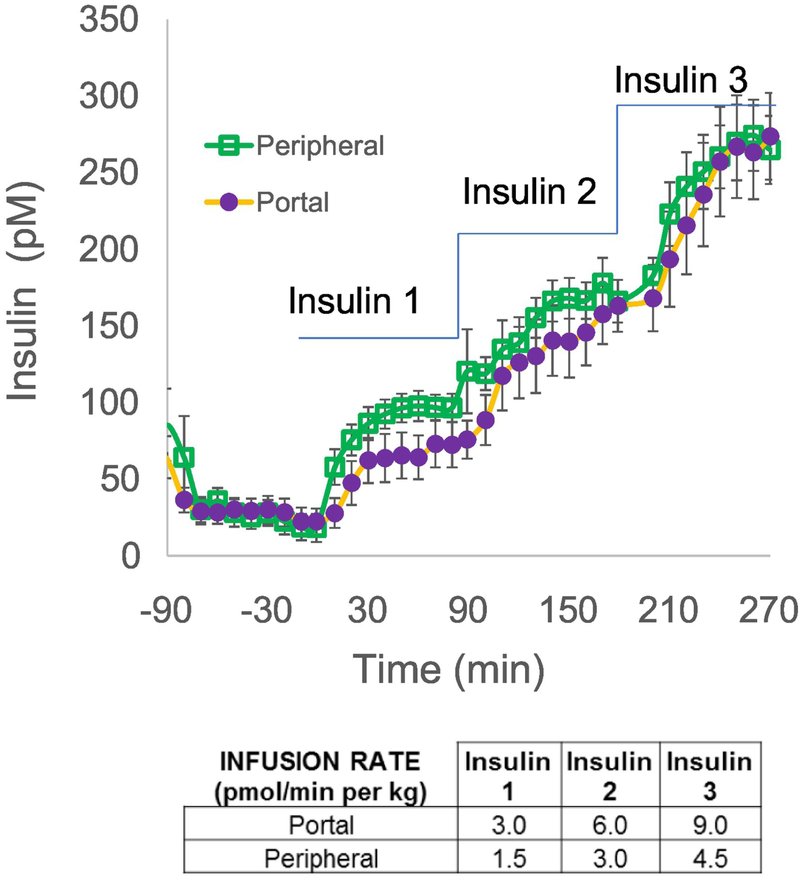
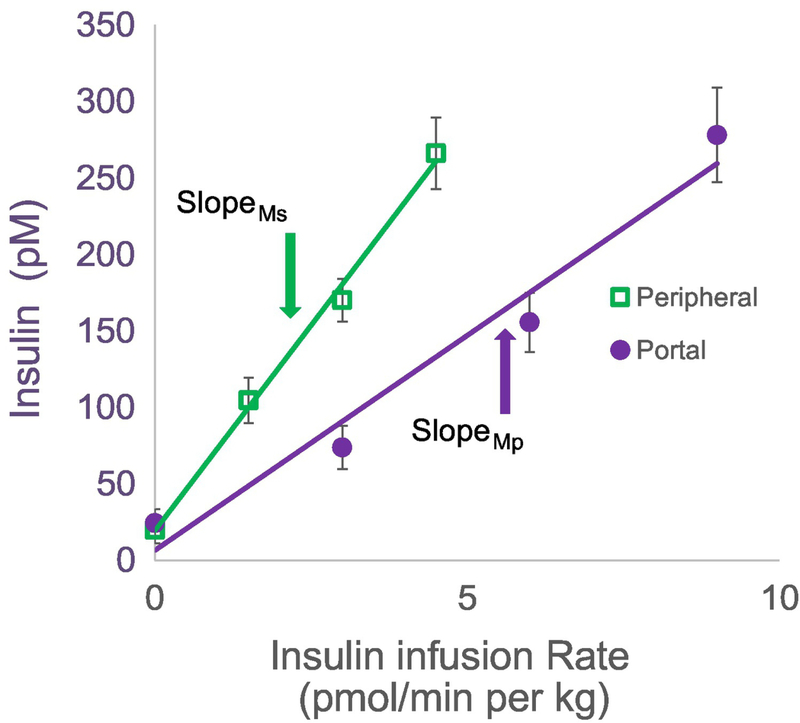
We have reintroduced the protocol by which insulin is infused into conscious animals (the dog) either into the portal vein, where it would immediately engage the liver, or into a peripheral vein, where it would be distributed into the general circulation (6). Because it is reasonable to expect that at least half the insulin entering the liver directly would be bound to hepatic insulin receptors, internalized and degraded, we infused insulin at twice the rate intraportally to attempt to achieve matched systemic concentrations with both intraportal versus systemic infusion. Fig. 5 demonstrates that this goal was achieved in dose-response EGC experiments (22). Relating insulin infusion rates to resulting insulinemia (Fig. 6), it is clear that the slope of such a relationship is considerably lower with intraportal infusion due to the first-pass degradation of the hormone via the intraportal route. It is easy to demonstrate that the ratio of the slopes from systemic intravenous versus intraportal infusion allows for a calculation of the first pass insulin extraction (FPE) (23):
| (Eq. 1) |
Figure 5.
Plasma insulin time courses during euglycemic clamps with either intraportal or peripheral insulin infusions. Insulin is infused at 3 sequential rates shown in the table. Note that intraportal infusion rates are twice the rates infused peripherally to account for ~50% first-pass hepatic extraction. As a result, insulin levels in the peripheral circulation were well-matched. Adapted from (22).
Figure 6.
Steady state plasma insulin versus insulin infusion rate from clamps in which insulin is infused at 3 sequential rates either intraportally or peripherally. First pass hepatic insulin extraction is calculated from the ratio of slopes from this dose response (see text). Adapted from (22).
where mS and mP represent the two slopes illustrated in Fig. 6. On average, first-pass extraction of insulin in the canine is 49%. Interestingly, among a small group of normal animals, the first pass extraction varied from 22 to 77 %, with a coefficient of variation of 20% (22).
The surprisingly large variance in measurements of insulin clearance in normal animals led us to question of whether clearance is not stable, but can be changed by environmental factors. Employing the double infusion technique described above, we examined whether first-pass insulin clearance can be changed by environmental factors, or whether FPE is unchangeable. We observed that placing animals on an elevated fat diet caused a time-dependent reduction in insulin clearance: FPE was reduced from 60% to 56% after 6 weeks, and to 44% after 12 weeks of an elevated fat diet (23).
Our experimental studies demonstrated that there is a wide range of insulin clearance in normal animals, and that environment (in this case, fat feeding) can act to reduce first-pass insulin clearance, and deliver a greater percentage of insulin secreted from the beta-cells to the extrahepatic, systemic circulation. Thus, the liver can be viewed as a “gateway” device, responsible for altering the fraction of insulin degraded, presumably to compensate for insulin resistance associated with fat feeding. Alternatively, changes in insulin clearance and insulinemia may precede insulin resistance, rather than compensate for existing resistance. It has been demonstrated that hyperinsulinemia can induce insulin resistance in peripheral tissues (24,25). Proposed mechanisms include desensitization of the insulin receptor on target tissues, reduction of cell surface receptors, or diminished action of post-receptor downstream molecules (26,27).
3.2. Insulin clearance in human participants
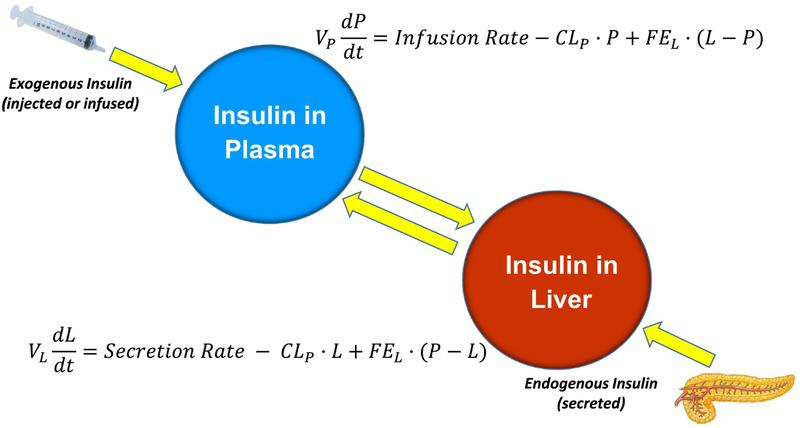
Obviously, it is impossible to apply the double infusion method to human subjects. Therefore, we turned to the mathematical modeling approach to estimate insulin clearance by the liver, and by extrahepatic tissues. The modelling was done in collaboration with David Polidori at Janssen Research Corporation and Anne Sumner at the NIH (28). We were able to obtain Dr. Sumner’s data which emerged from a population study she performed in African American women, in which insulin, glucose and C-peptide levels were measured during frequently-sampled intravenous glucose tolerance tests (FSIGTs). The model is presented in Fig. 7; calculated endogenous insulin secretion rates from the FSIGT in a single subject are entered in the model. The model yields the rates of hepatic fractional insulin extraction (FEL) and extra-hepatic insulin degradation.
Figure 7.
Mathematical model to estimate hepatic insulin extraction and extra-hepatic insulin clearance. Model input includes time course of insulin secretion, calculated by C-peptide deconvolution, and exogenous insulin administered during the IVGTT. Adapted from (28).
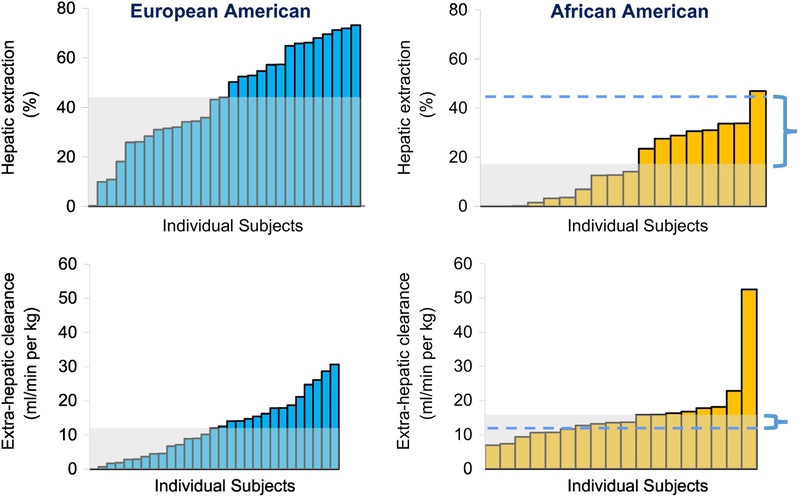
Additionally, we were extremely fortunate to obtain FSIGT data from Drs. Barbara Gower and Jose Fernandez from the University of Alabama. Dr. Gower had performed FSIGT protocols in 47 adult volunteers: 29 of European heritage (European American, EA) and 18 adults of African descent (African American, AA) (29). Similar to animal studies, there was large variance in FEL (Fig. 8). While extrahepatic insulin degradation was not different between ethnicities, there was a large and significant difference in hepatic insulin clearance: insulin clearance was 60% lower, on average, in AA compared to EA subjects. Reduced insulin clearance in AA (30,31) can explain the fasting hyperinsulinemia observed in African Americans in several recently published studies. It certainly is possible that the fasting hyperinsulinemia may result from both reduced clearance and increased secretion; our results reanalyzing Dr. Gower’s data encourage us to focus strongly on the reduced clearance component.
Figure 8.
Distribution of hepatic extraction and extra-hepatic clearance in subjects of European American or African American ethnicity. Gray shaded area represents median values. Horizontal dashed lines depict median value for European American cohort, and bracket demonstrates differences in median values between ethnic groups. Adapted from (29).
It was of interest to ask whether the reduced insulin clearance in AA was due to well-described differences in lifestyle between AA and EA adults (32), or whether there might be a genetic component. Analyzing data from Dr. Jose Fernandez, also from the University of Alabama, revealed that like adults, AA children have a relatively lower liver insulin clearance than EA youth of comparable age (31). These studies suggest, but certainly do not prove, that there may well be a genetic component to the lesser propensity of the liver to clear insulin in AA.
3.3. Significance of reduced insulin clearance
In the face of similar, or even greater insulin secretion from the beta cells, lower insulin clearance in some groups at higher risk for Type 2 diabetes may contribute to fasting hyperinsulinemia (c.f. Fig 8). Elevated insulin could in turn contribute to insulin resistance of peripheral tissues – i.e., skeletal muscle and adipose. Insulin resistance could stress the beta cells, which might oversecrete in an insulin resistant environment. Thus, we imagine this scenario in at-risk individuals:
Genetics → Reduced insulin clearance → Fasting and prandial hyperinsulinemia → insulin resistance → beta-cell stress → Type 2 diabetes (and other insulin resistance pathologies)
Additional experimental and population studies are required to examine the above hypothesis, to determine whether it applies for a few, some or even many individuals at risk for metabolic disease. In any event, it supports the previously underestimated importance of insulin clearance in the pathogenesis of metabolic disease.
4. Final comments
Is the liver the “seat of the soul?” Possibly not. However, it is clear from the above summary, and many other studies by outstanding investigators, that the liver plays a very central role in the regulation of metabolism. Production of glucose by the liver at normal rates is essential for normal blood glucose concentration, and the importance of the liver physiologically is supported by the many physiological signals which have evolved to regulate its function. These signals emanate not only from the pancreatic islets, but also the central nervous system, adipose tissue and the kidneys. To understand the overall function and interaction of these signals which guarantee an appropriate output of sugar into the bloodstream is a daunting task; however, such studies are critical to provide an overall view of how blood glucose is normally regulated, and how this regulation may be perturbed in an increasing number of individuals in the modern world.
Great effort has been expended to understand the pathogenesis of diabetes using so-called “modern methods,” including GWAS to identify disease variants. However, these approaches have yet to identify the specific loci which are responsible for the disease. The traditional “physiological” approach, as outlined in this document, continues to provide greater understanding of how carbohydrate and lipid is handled in vivo, and appears to provide new and previously unidentified targets to potentially treat disease in patients. Emphasized here are the “indirect” effect of insulin suggesting targets in adipose tissue and kidney (including SNS). Also emphasized is the glucose effectiveness mechanism wherein the role of hepatic glucokinase and GKRP must be emphasized. It is hoped that the results summarized herein provide a useful pathway forward for physiologically-based research to identify loci for treatment of metabolic disease. Clearly such treatment targets are sorely needed.
Highlights.
Control of glucose production
Hepatic and extrahepatic Insulin clearance
Glucose effectiveness and hepatic glucose uptake
Ethnic differences in insulin clearance
Pathogenesis of Type 2 diabetes
Acknowledgments
Funding. R.N.B. is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK27619, DK29867), Astra Zeneca and Janssen Research & Development.
Footnotes
DECLARATION OF INTERESTS
Duality of interest. R.N.B. is an Advisory Board Member of Ingredion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jastrow M: The liver in antiquity and the beginning of anatomy. Trans Col Phys Phila 1907;39:117–138 [Google Scholar]
- 2.Cherrington AD, Vranic M: Role of glucagon and insulin in control of glucose turnover. Metabolism 1971;20:625–628 [DOI] [PubMed] [Google Scholar]
- 3.Corvera S, Huerta-Bahena J, Pelton JT, Hruby VJ, Trivedi D, Garcia-Sainz JA: Metabolic effects and cyclic AMP levels produced by glucagon, (1-N alpha-Trinitrophenylhistidine,12-homoarginine)glucagon and forskolin in isolated rat hepatocytes. Biochim Biophys Acta 1984;804:434–441 [DOI] [PubMed] [Google Scholar]
- 4.Gabbay RA, Lardy HA: Site of insulin inhibition of cAMP-stimulated glycogenolysis. J Biol Chem 1984;259:6052–6055 [PubMed] [Google Scholar]
- 5.Poy MN, Yang Y, Rezaei K, Fernstrom MA, Lee AD, Kido Y, Erickson SK, Najjar SM: CEACAM1 regulates insulin clearance in liver. Nature Gen 2002;30:270–276 [DOI] [PubMed] [Google Scholar]
- 6.Ader M, Bergman RN: Peripheral effects of insulin dominate suppression of fasting hepatic glucose production. Am J Physiol 1990;258:E1020–E1032 [DOI] [PubMed] [Google Scholar]
- 7.Rebrin K, Steil GM, Mittelman S, Bergman RN: Causal linkage between insulin regulation of lipolysis and liver glucose output. J Clin Invest 1996;98:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman RN, Bradley DC, Ader M: On insulin action in vivo: the single gateway hypothesis. Adv Exp Med Biol 1993;334:181–198 [DOI] [PubMed] [Google Scholar]
- 9.Lu ML, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueda K, Kahn CR, Birnbaum MJ: Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 2012;18:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, Chen H, Monks BR, Chen J, Rabinowitz JD, Birnbaum MJ: Direct hepatocyte insulin signaling is required for lipogenesis but is dispensible for the suppression of glucose production. Cell Metab 2016;23:1154–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry RJ, Camporez JG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang XM, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI: Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 2015;160:745–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Investigators SH-, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M: Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial), a randomised control trial. Lancet 2010;376:1903–1909 [DOI] [PubMed] [Google Scholar]
- 13.Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G: Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012;35:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris G, Investigators SH-: A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–1401 [DOI] [PubMed] [Google Scholar]
- 15.Iyer MS, Bergman RN, Korman JE, Woolcott OO, Kabir M, Victor RG, Clegg DJ, Kolka C: Renal denervation reverses hepatic insulin resistance induced by high fat diet. Diabetes 2016;65:3453–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen PA, Gulve EA, Marshall BA, Gao J, Pessin JE, Holloszy JO, Mueckler M: Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J Biol Chem 1995;270:1679–1684 [DOI] [PubMed] [Google Scholar]
- 17.Bergman RN, Ider YZ, Bowden CR, Cobelli C: Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 18.Ader M: Physiologic principles underlying glucose effectiveness In The Minimal Model Approach and Determinants of Glucose Tolerance Bergman RN, Lovejoy JC, Eds. Rouge Baton, Louisiana State University Press, 1997 [Google Scholar]
- 19.Stefanovski D, Youn JH, Rees M, Watanabe RM, Ader M, Ionut V, Jackson AU, Boenke M, Collins FS, Bergman RN: Estimating hepatic glucokinase activity using a simple model of lactate kinetics. Diabetes Care 2012;35:1015–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarlett JM, Muta K, Brown JM, Rojas JM, Matsen ME, Acharya NK, Secher A, Ingvorsen C, Jorgensen R, Hoeg-Jensen T, Stefanovski D, Bergman RN, Piccinini F, Kaiyala KJ, Shiota M, Morton GJ, Schwartz MW: Peripheral mechanisms mediating the sustained anti-diabetic action of FGF1 in the brain. Diabetes 2018;Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrannini E, Cobelli C: The kinetics of insulin in man: II. Role of the liver. Diab Metab Rev 1987;3:365–397 [DOI] [PubMed] [Google Scholar]
- 22.Asare Bediako I, Paszkiewicz RL, Kim SP, Woolcott OO, Kolka CM, Burch MA, Kabir M, Bergman RN: Variability of directly measured first-pass hepatic insulin extraction and its association with insulin sensitivity and plasma insulin. Diabetes 2018;67:1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SP, Ellmerer M, Kirkman EL, Bergman RN: Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin resistant, fat-fed canine model. Am J Physiol 2007;292:1581–1589 [DOI] [PubMed] [Google Scholar]
- 24.Gray SL, Donald C, Jetha A, Covey SD, Kieffer TJ: Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta-cell leptin signaling. Endocrinology 2010;151:4178–4186 [DOI] [PubMed] [Google Scholar]
- 25.Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE: Production of insulin resistance by hyperinsulinemia in man. Diabetologia 1985;28:70–75 [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Desai KS, Steiner G: Receptor and postreceptor insulin resistance induced by in vivo hyperinsulinemia. Can J Physiol Pharmacol 1983;61:802–807 [DOI] [PubMed] [Google Scholar]
- 27.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J: Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diab Care 2008;31 (suppl 2):S262–S268 [DOI] [PubMed] [Google Scholar]
- 28.Polidori DC, Bergman RN, Chung ST, Sumner AE: Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes 2016;65:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccinini F, Polidori DC, Gower BA, Bergman RN: Hepatic but not extrahepatic insulin clearance is lower in African American than in European American women. Diabetes 2017;66:2564–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CC, Haffner SM, Wagenknecht LE, Lorenzo C, Norris JM, Bergman RN, Stefanovski D, Anderson AM, Rotter JI, Goodarzi MO, Hanley AJ: Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS family study. Diab Care 2013;36:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piccinini F, Polidori DC, Gower BA, Fernandez J, Bergman RN: Dissection of hepatic and extra-hepatic insulin clearance: ethnic differences in childhood. Diab Obes Metab 2018;12:2869–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haughton CF, Silfee VJ, Wang ML, Lopez-Cepero AC, Estabrook DP, Frisard C, Rosal MC, Pagoto SL, Lemon SC: Racial-ethnic representation in lifestyle weight loss intervention studies in the United States: a systematic review. Prev Med Rep 2018;9:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]