Abstract
Gonadotropin releasing hormone (GnRH) is a highly conserved neuroendocrine decapeptide that is essential for the onset of puberty and the maintenance of the reproductive state. First identified in mammals, the GnRH signaling pathway is found in all classes of vertebrates; homologues of GnRH have also been identified in invertebrates. In addition to its role as a hypothalamic releasing hormone, GnRH has multiple functions including modulating neural activity within specific regions of the brain. These various functions are mediated by multiple isoforms, which are expressed at diverse locations within the central nervous system. Here we discuss the GnRH signaling pathways in light of new reports that reveal that some vertebrate genomes lack GnRH1. Not only do other isoforms of GnRH not compensate for this gene loss, but elements upstream of GnRH1, including kisspeptins, appear to also be dispensable. We discuss routes that may compensate for the loss of the GnRH1 pathway.
Keywords: Zebrafish, genome, evolution, GnIH, domestication, kisspeptin, synteny, gene loss
1. Introduction
Gonadotropin-releasing hormone (GnRH) is the pivotal neuropeptide regulating fertility and reproduction in vertebrates [1]. In vertebrates, at least fifteen GnRH isoforms have been identified [2], where any given species has two or three forms of GnRH, each encoded by different genes. The different forms of GnRH are expressed in both neuronal and non-neuronal tissues and serve a number of distinct functions. Historically, GnRH has been known primarily as a hypothalamic hormone controlling the onset of puberty and the maintenance of fertility through the release of the gonadotropes, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), from the pituitary. For the purposes of this review the name “hypophysiotropic” will be used to refer to the hypothalamic reproductive form of GnRH.
GnRH has been extensively studied in mammals and fishes and as different isoforms of GnRH were discovered they were named according to the animal in which they were found, thus leading to a confusing body of literature. However, when the corresponding genes were identified, the different GnRH peptides could be classified based on the sequence of the corresponding genes and their location in the genome, clarifying the naming of GnRH isoforms.
In considering the function of the hypothalamic GnRH neurons, it is important to note that there are significant differences between the anatomy of fish and mammalian brains, both important model systems used to study the neuroendocrinology of GnRH. In mammals, GnRH is released from nerve endings into the hypophyseal portal system in a pulsatile manner to stimulate the synthesis of the gonadotropes, FSH and LH, from the anterior pituitary. Thus, the communication between the hypothalamic GnRH cells and the pituitary occurs via a localized circulatory system called the median eminence in which hypothalamic-releasing and -inhibiting hormones converge onto the portal capillary system of the ventral hypothalamus (Figure 1A) [3], [4]. However, this elaborate pituitary vasculature connecting the hypothalamus to the anterior pituitary gland typical of tetrapods is notably lacking in teleost fish [5], in which axons of the GnRH neurons project directly to post-synaptic sites in the pituitary (Figure 1B). In addition, the neurohypophysis is greatly reduced in size. While structurally different from mammals, the teleost fish brain contains all the hypothalamic cell types identified in mammals [6], including the GnRH containing cells localized to the parvocellular nucleus [7]. The lack of a median eminence in teleost fish is believed to be a derived trait because primitive fishes such as coelacanth, sturgeons, and lungfish [8; 9], as well as cartilaginous fishes [10], contain a median eminence. Although hypothalamic structures differ between teleosts and mammals, it is generally thought that GnRH has remained the principal peptide controlling fertility, reproduction, and behaviors associated with reproduction, in these groups of vertebrates.
Figure 1. Structure of the vertebrate hypothalamus and description of gnrh/GnRH containing cells in the adult zebrafish brain.
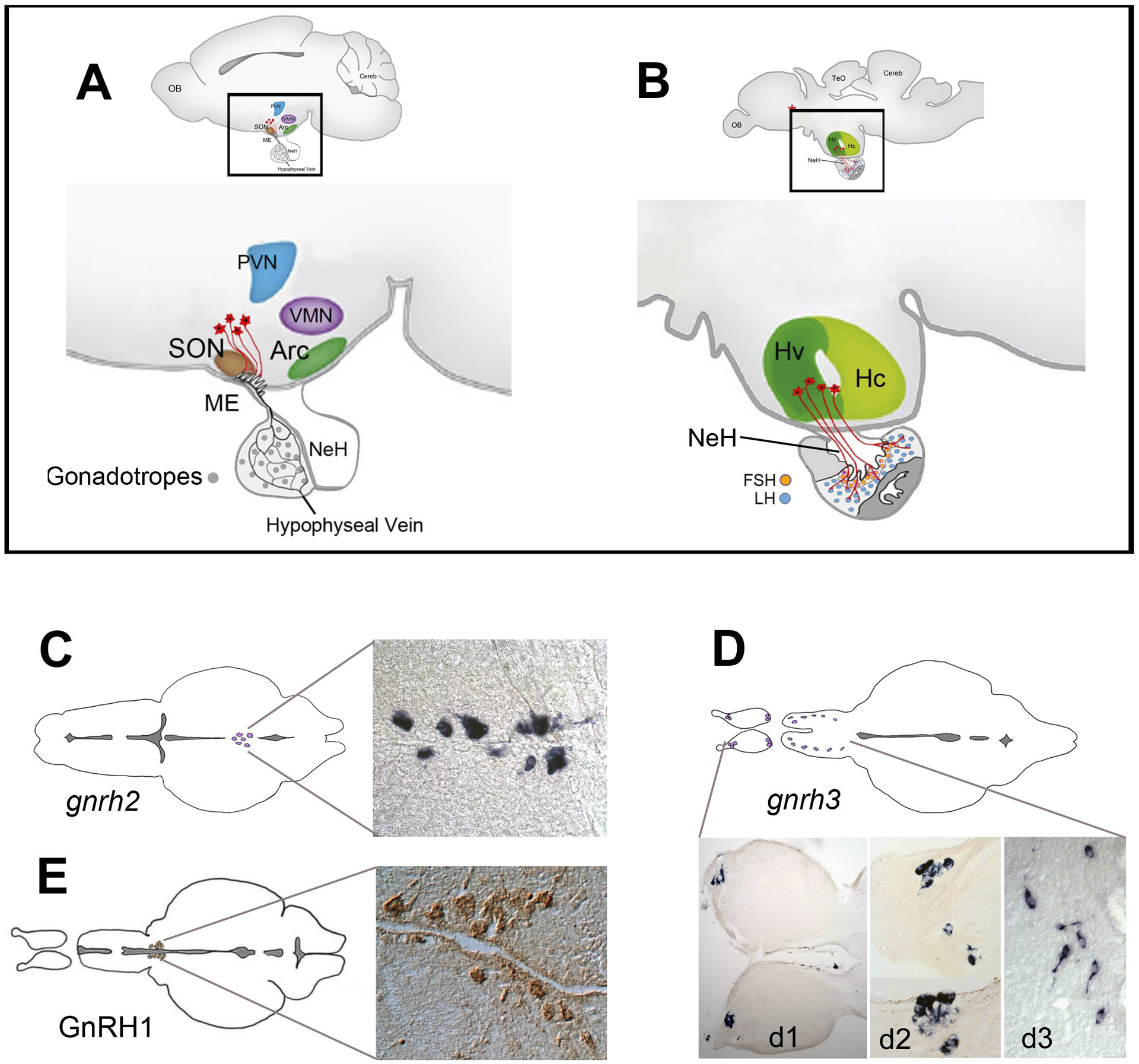
(A, B) Schematic diagram of a lateral view of the mouse (A) and the zebrafish (B) brain. In mammals (A), the GnRH cells (red) project to the median eminence, a highly vascularized connection to the pituitary. In teleosts (B), the neurohypophysis (NeH) is reduced and the GnRH cells make neural connections with the pituitary. (C-E) gnrh2 and gnrh3 expression detected by in situ hybridization in the midbrain (C), and terminal nerve (D, d1, d2) and ventral telencephalon (D, d3) [133] In contrast, GnRH cells in the parvocellular nucleus of the hypothalamus can be detected only by immunohistochemistry (E from [62]). There are no convincing data supporting cellular expression of any gnrh gene in the parvocellular nucleus of the adult zebrafish. Abbreviations: Arc, arcuate nucleus; Hv, ventral zone of periventricular hypothalamus; Hc, caudal zone of periventricular hypothalamus; (red asterisk), neurosecretory preoptic area; ME, median eminence; NeH, neurohypophysis; OB, olfactory bulb; PVN, paraventricular nucleus; SON, supraoptic nucleus; TeO, tectum opticum; VMN, ventromedial nucleus. (A, B modified from [3], [4]).
In addition to playing a crucial role in the regulation of reproduction, GnRH also has a variety of functions outside of the hypothalamic-pituitary-gonadal axis as reflected by the variety of isoforms and receptors that are expressed throughout the brain. GnRH-containing neurons are located in the terminal nerve (cranial nerve zero) [11], the ventral telencephalon, the hypothalamus, and the midbrain. Although the general function of GnRH is highly conserved across vertebrates, there are notable differences in the locations and functions of specific GnRH isoforms in the brain. In most vertebrates, the hypophysiotropic form (the form essential for puberty that controls the release gonadotropes from the pituitary) is called GnRH1, and its primary site of synthesis and release is from neurons in the hypothalamus. A second more highly conserved isoform, GnRH2, is found in neurons of the midbrain tegmentum. This form of GnRH is generally thought to be involved in the regulation of behaviors associated with reproduction [12]; in some animals it has been coopted as the hypothalamic releasing hormone acting on the pituitary [13] [14]. A third isoform, GnRH3, is specific to fish and its cell bodies are located in the terminal nerve and the ventral telencephalon. GnRH3 is usually neuromodulatory and does not control puberty and reproduction through the liberation of pituitary gonadotropes. However it may be indirectly linked to the reproductive neuroendocrine axis by modulating functions of the nervous system that relate to social behaviors important for reproduction [15].
Several groups have reported that the gene encoding GnRH1 does not exist in the zebrafish genome and, based on a limited number of analyses, has been suggested to also be absent from the genome of other cyprinid fish [16], [17], [18], [19]. Here we discuss the implications of this finding by considering which peptide might replace GnRH1 in the hypothalamus. We also consider the possibility that the loss of GnRH1 in laboratory strains of zebrafish is a consequence of domestication. Indeed, domesticated (i.e., laboratory) zebrafish have acquired a mechanism of sex determination that is not found in wild zebrafish [20], and this change could affect the hypothalamic-pituitary axis, including the functions normally subserved by GnRH1. Finally, we consider the mechanisms by which puberty and reproduction might be controlled in zebrafish and possibly in other cyprinid fishes.
2.1. GnRH isoforms and their functions
Mammalian brains typically express two different forms of GnRH (GnRH1 and GNRH2), whereas fish typically express three isoforms (GnRH1–3) [21]. GnRH was first discovered in the mammalian brain [22] [23] [24] and its sequence shown to be: pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2. This isoform is universally called GnRH1 with the understanding that this is the hypothalamic version of the hormone. Following the initial discovery of GnRH1, other forms of GnRH were isolated and characterized in a variety of vertebrates. Thus, a second form of GnRH, which is universally present from jawed fish to humans and is also highly conserved between these groups, is the form originally named chicken GnRH II (cGnRH: pGlu-His-Trp-Ser-His-Gly-Trp-Tyr-Pro-Gly-NH2) [25], [14; 26] [27; 28; 29], which is now called GnRH2. Finally GnRH3 (pGlu-His-Trp-Ser-Try-Gly-Trp-Leu-Pro-Gly-NH2) [25] is an isoform specific to fishes, which has been proposed to be a form extant in early vertebrates and secondarily lost in tetrapods [30].
All GnRHs are encoded as a preprohormone whose cleavage product generates the GnRH decapeptide and the GnRH Associated Protein (GAP). The genes encoding the GnRH preprohormone share the same basic structure that was originally described for mammalian GnRH [31]: They all contain four exons where the first exon encodes only the 5 prime–UTR; exon 2 encodes the signal peptide, the GnRH decapeptide, the proteolytic cleavage site, and the N-terminus of the GAP; exons 3 encodes the central portion and the C terminus of the GAP; and exon 4 encodes the 3 prime-UTR. Five of the ten amino acids present in the mature GnRH hormone are invariant whereas two other positions show conservative changes. An unusual aspect of the GnRH preprohormone is that in spite of extremely high conservation of the GnRH gene structure and peptide sequences, the GAP coding sequences are highly divergent even within closely related groups of animals [32], [33] [34]. The high level of conservation of GnRH peptide sequence and gene structure suggests that all GnRH genes may have arisen through gene duplication of a single ancestral GnRH whose origin predates the diversification of vertebrates.
GnRH as a neuromodulator
The reproductive, releasing, form of GnRH is expressed in the hypothalamus but the various GnRH isoforms and their receptors are found throughout the brain where they may act to control multiple higher functions such as feeding and reproductive behaviors as well as learning and memory [35; 36]. The majority of the literature concentrates on the role of GnRH as the pivotal peptide controlling reproduction in vertebrates. Often overlooked is the role of GnRH as a neuromodulatory peptide in the brain. In all vertebrates, GnRH-containing cells can be found not only in the hypothalamus but also in the terminal nerve, the ventral forebrain, and the midbrain (Figure 1C–E) to mention the most common regions when comparing across species.
GnRH containing neurons of the Terminal Nerve (Cranial Nerve 0)
The GnRH isoform expressed within the hypothalamus versus the terminal nerve/ventral telencephalic networks can vary depending upon the animal, but is generally either GnRH1 or GnRH3; GnRH2, the form with the most conserved amino acid sequence, is consistently expressed in neurons of the midbrain in most vertebrate groups. The terminal nerve/ventral telencephalic network is part of the centrifugal visual system, a visually driven retinal feedback projection ([37] for review) that is present in tetrapods and teleosts, reflecting the importance of GnRH not only as a hypophysiotropic releasing hormone but also as a neuromodulatory peptide within the brain. In fishes, the GnRH3 positive neurons of the terminal nerve (Figure 1D, d1, d2) send their axons to a variety of regions within the central nervous system [11], most notably the retina, where they synapse in the inner plexiform layer (IPL) [38]. In the white perch, for example, they have been shown to stimulate the release of dopamine from interplexiform cells [39]. Interestingly, FMRFamide, which is also present in the terminal nerve neurons terminating in the retina, has no physiological effects on its own yet has been reported to block the effects of GnRH on the retina [39]. In zebrafish, the terminal nerve contains both GnRH3 and a homologue of the PQRF subfamily of RFamides, which is expressed in the same neurons that express GnRH3 [40]. In zebrafish and medaka, GnRH3 is present not only in the terminal nerve, but also in the trigeminal nerves (cranial nerve V) of developing embryos [41], [42]. In zebrafish a Tg(gnrh3:emd) reporter line was used to show that, starting at two days post-fertilization, the neuromodulatory GnRH3:EMD cells of the terminal nerve initiate spontaneous firing, followed by a bursting pattern of activity that matures into a final tonic firing pattern by three days post-fertilization [43].
The GnRH population expressed in neurons of the terminal nerve has also been identified in a variety of mammals including rats [44], [45] and dolphins [46]. In the big brown bat (Eptesicus fuscus), GnRH and FMRFamide have been detected in the terminal nerve, and their projections extend throughout the basal forebrain with additional intense labeling in cell bodies within the arcuate nucleus [47]. Thus, the GnRH positive cells in the brain form a complex consisting of two principal GnRH neuronal networks: one associated with the POA/hypothalamus, which projects to the pituitary (hypophysiotropic function), and a second terminal nerve/ventral telencephalic population, which send axons to peripheral targets and multiple regions of the brain, but never do so to the pituitary [48].
The different physiological roles of GnRH are correlated with the developmental origin of the GnRH cells [49], [50]. Indeed, the GnRH3 cells of the hypothalamus do not originate from the region of the terminal nerve/olfactory sensory system, but from within the hypothalamus [51]. This suggests a separate developmental origins for the neuromodulatory vs. the reproductive GnRH cells, as has been suggested for other animals including chick [52], [53], medaka [54], and monkey (rhesus macaque), where two distinct GnRH cell populations have been reported, one associated with the olfactory system and a second arising independently from within the central nervous system and which populates the hypothalamus [55].
The role of GnRH in longevity
More recently, an additional role for GnRH has been added to the already extensive list of GnRH functions, and that is as a peptide important for longevity. Linked to the control of reproduction, hypothalamic GnRH appears to prevent the onset of ageing. Recent studies in mouse have examined the effects of GnRH on ageing and uncovered an intriguing potential pathway. Normally proteins involved in hypothalamic immunity, IκB kinase-β (IKK-β) and nuclear factor κB (NF-κB), inhibit GnRH during the ageing process, and recently it has been shown that blocking this pathway results in the restoration of GnRH in the hypothalamus in both male and female mice [56]. Additionally, treatment with GnRH to compensate for the aging-related decline in GnRH resulted in neurogenesis in brain regions including the hypothalamus and interestingly, the hippocampus [57].
2.2. Evolutionary genomics of GnRH
The number of gnrh genes varies across genomes of modern-day vertebrates. The gnrh1 gene exists broadly across species but has been lost in certain teleostean clades within the Cyprinidae and Salmonidae families (see below). The gnrh2 gene is consistently found in most vertebrate groups ranging from fish to humans, but has been lost in rodents [12]. Finally, gnrh3 appears to be a teleost-specific gene. In species with three GnRH isoforms, gnrh1 is expressed in the hypothalamus and is the hypothalamic releasing hormone. Overall at least thirteen different GnRH isoforms and up to four GnRH receptors have been identified in vertebrates. Genome sequencing has revealed the genomic location of these genes in both model and non-model systems, providing an opportunity to independently confirm the sequence of various reported GnRH peptides and that of their receptors. In mammals no gene encoding for GnRH3 has been identified thus far, but both gnrh1 and gnrh2 genes have been identified in most mammalian genomes. A notable exception to the highly conserved presence of gnrh2 is the lack of a gnrh2 gene in the mouse genome [12]. The majority of fishes have three genes encoding GnRH: gnrh1 (the hypophysiotropic form), gnrh2, and gnrh3 (the neuromodulatory form). In some species, such as the goldfish (Carassius), which has no GnRH1, either GnRH2 or GnRH3 is the hypothalamic releasing form [58], [13]. Yet, in other species of fishes, such as the zebrafish, GnRH1 appears to be absent and, furthermore, no hypophysiotropic form can be detected in the hypothalamus.
2.3. GnRH and the zebrafish genome
The three extant gnrh genes are proposed to have arisen from a common ancestor where, at the origin of vertebrates, two rounds of genome duplication produced four paralogous chromosomal regions, each with a GNRH gene, followed by loss of GNRH3 and GNRH4 in the human lineage [30]. The gene encoding GnRH1 (the hypothalamic isoform) is found in the majority of fishes and is encoded by a single gene, in spite of the genome duplication that occurred at the radiation of the teleosts [59]. Based on the identification of gnrh2 [60], gnrh3 [61], [60], and the presence of GnRH-immunolabeling in the hypothalamus [50], [62], it was proposed that zebrafish would have three forms of GnRH (Figure 1C–E). In zebrafish, the gnrh genes were initially localized to LG21 (gnrh2) and LG17 (gnrh3), whereas the ortholog to human GNRH1 appeared to be lacking [63]. Subsequent synteny analyses confirmed the presence of gnrh2 on (now) chromosome 21 and gnrh3 on chromosome 17, as well as the lack of gnrh1, in spite of retaining neighboring syntenic genes [63] [64] (Figure 2A).
Figure 2. Genomic rearrangements in the syntenic region of GnRH1 shows a loss of gnrh1 in zebrafish.
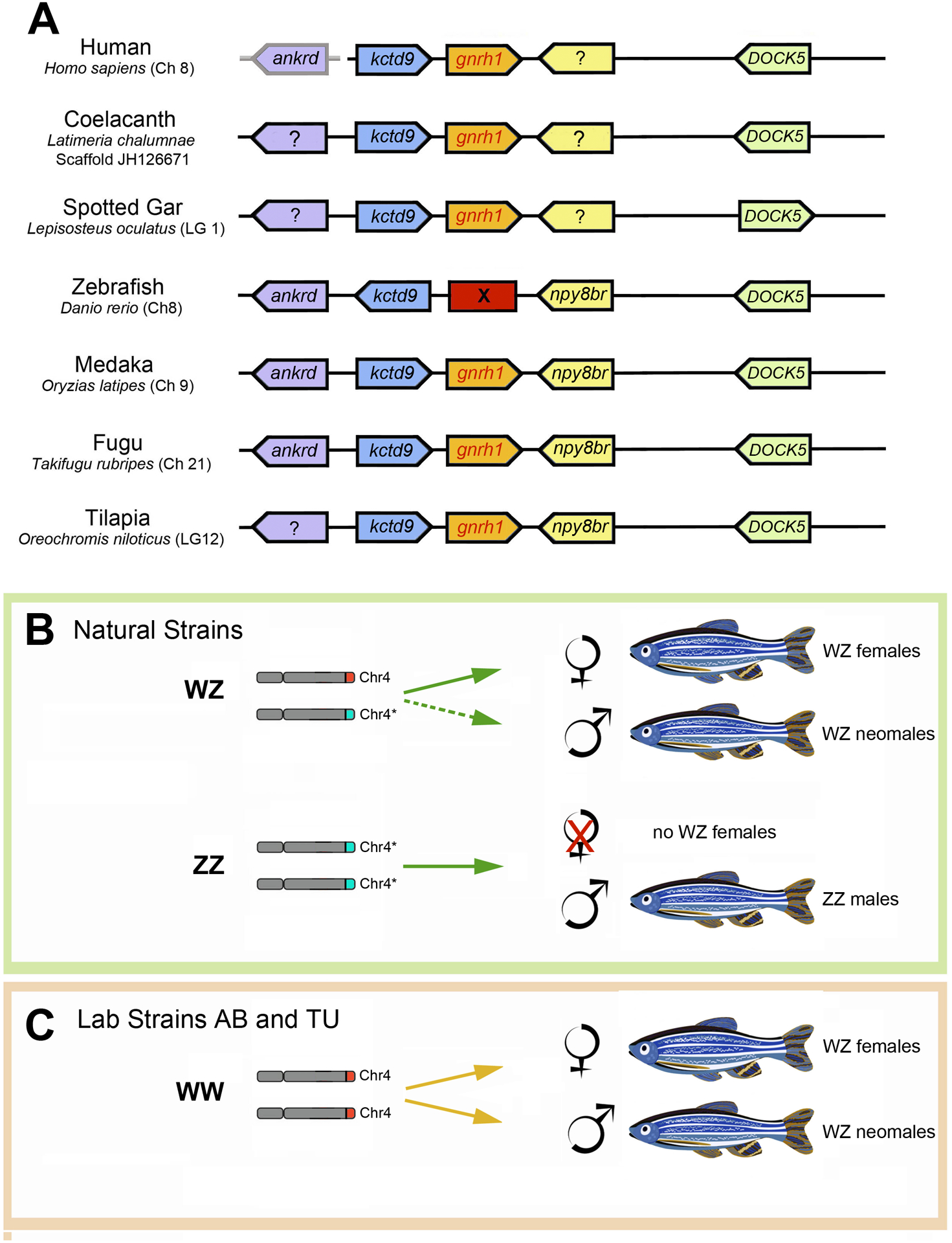
(A) Analysis of conserved syntenies in the region of the gnrh1 gene reveals an inversion of the kctd9 gene in zebrafish relative to the other species, suggesting a re-arrangement in this region (see text for details). Human ankrd is boxed in grey because it is located on a separate chromosome. (B, C) Genes controlling sex determination in zebrafish have been altered by domestication. In wild-caught zebrafish (B), the majority of fish heterozygous at the Z locus (Chr4*/Chr4) become females (solid arrow). Animals with two copies of the Z locus (light blue band) on chromosome 4 (Chr4*/Chr4*) become males. Under specific conditions Chr4*/Chr4 animals can become neomales (dashed arrow, see text). In contrast, laboratory strains AB and TU (C) have lost this locus (Chr4*) on chromosome 4 and the population exists as Chr4/Chr4 where both females and neomales are generated (solid yellow arrows) albeit often in skewed sex-ratios.
Because of the gaps in the zebrafish genome sequence at the time of previous publications (the zebrafish genome was only officially released in 2013 [65]), there existed the possibility that the small gnrh1 gene does exist in the zebrafish genome but was missing from the zebrafish genome assembly due to incomplete coverage. For this reason, we reanalyzed the gnrh1 region of the zebrafish genome using the most recent release (Z11) of the zebrafish genome. Figure 2A shows the genomic region surrounding GNRH1 gene orthologs in humans, among lobe fin vertebrates (e.g., the coelacanth Latimeria chalumnae), and in fugu (Takifugu rubripes), tilapia (Oreochromis nitoticus), spotted Gar (Lepisosteus oculatus), medaka (Oryzias latipes), and zebrafish (Danio rerio) among ray fin vertebrates. In all species of the latter group except zebrafish, gnrh1 is flanked by the kctd9 gene on the left (Figure 2A, blue), and (where annotated) by the npy8br gene on the right (Figure 2A, yellow). In contrast, no gnrh1 is present between kctd9 and npy8br in the zebrafish genome. Furthermore, in zebrafish the kctd9 gene is in the opposite orientation in comparison to the other genomes, which is the signature of a small inversion in the gnrh1 syntenic region. In the zebrafish genome database this region was notated as having less than optimum coverage. To confirm the genomic situation in zebrafish, we re-sequenced the BAC containing the Ankrd9-npy8br region (CH211–178D20). Our data showed that the reference sequence contains the same sequence as the BAC, confirming that the zebrafish genome lacks the ortholog of gnrh1 found in tetrapods and other fishes. We conclude that gnrh1 disappeared from its ancestral location due to a genomic rearrangement, including at least one inversion, at the position of the ancestral gnrh1 gene. The loss of gnrh1 in zebrafish was further confirmed by analyses of RNA-seq data from our lab [66] as well as from data deposited at NCBI (https://www.ncbi.nlm.nih.gov/) for zebrafish brains. These analyses readily detected transcripts for gnrh2 and gnrh3 but failed to pick up transcripts for gnrh1.
Despite the lack of a gnrh1 gene, zebrafish do show GnRH immunoreactivity in the hypothalamus (Figure 1E) in the region known to contain GnRH-positive cells in other animals. In order to investigate the origin of this immunoreactivity we performed a proteome/peptidome analysis of tissues extracted form the adult hypothalamus of zebrafish. Consistent with the genomic analysis, GnRH1 was not detected in MALDITOF or Orbitrap analyses. Furthermore, this same analysis failed to reveal the presence of GnRH2 or GnRH3 peptides in these tissues (Christian Wegener, Neurobiology and Genetics, University of Würzburg, Germany personal communication). Thus, the origin of GnRH immunoreactivity in the zebrafish hypothalamus remains unresolved (Figure 1E). Indeed, this immunoreactivity is detected using an antibody that recognizes GnRH1 and GnRH3, yet, to date, there are no reports of cell bodies expressing detectable levels of gnrh1 or gnrh3 in the parvocellular nucleus of the hypothalamus of adult zebrafish. Previously, GnRH3:GFP positive fibers were reported in the pre-optic area (POA) of developing zebrafish [51] and GnRH3-immunoreactive fibers were described in the POA of adult zebrafish using an antibody produced against the GnRH-associated peptide (GAP) fragment of the preproGnRH3 of sea bass (which shows 80% identity with the GAP fragment of zebrafish GnRH3, [67]). Taken together, these results suggest the presence of low levels of GnRH3 peptide in the hypothalamus. Yet, elimination of GnRH3 does not affect zebrafish fertility (see section 2.5, below), leaving unanswered the question of which would be this fish’s hypophysiotropic releasing hormone.
2.4. The effects of domestication on the genome
The modification of the genome by directed mutagenesis or through persistent selection for a given trait creates invisible ripples through the genome that can manifest themselves in unintended ways. The consequences of human intervention on animal genomes are most notable in the intentional domestication of plants and animals for horticulture and agriculture. As observed by Darwin [68], the domestication of animals leads to a striking increase in variability, yet many features associated with domestication are similar in different animals, such as the white star/blaze and floppy ears observed in domesticated mammals [69]. The process of domestication also often de-couples reproduction from the environmental photoperiod [70]. This change enables animals such as chickens, for instance, to mature their gametes throughout the year, and at the same time tends to attenuate the role of the hypothalamic-pituitary-adrenal (HPA) axis [71]. A particularly striking example of the effects of selective domestication is the case of the silver fox of Russia. These initially aggressive animals were domesticated for the fur industry. Selection for submissive behaviors led to dramatic changes in other features of these animals, including their physical appearance, physiology, and genome. Thus, the domesticated foxes have white markings, curled tails, droopy ears, and behave much like a dog [69]. Importantly, this selection also resulted in dramatic changes in endocrine profiles of male and female silver foxes where levels of proopiomenanocorticotropin (POMC)(anterior pituitary) and corticotropin releasing hormone (hypothalamus) gene expression were reduced [72], [69], as were the levels of pituitary and plasma adrenocorticotropic hormone (ACTH) [73]. Finally, the selection for tame foxes was associated with increased level of neurogenesis in the hippocampus of adult foxes [74].
Domestication-induced genomic alterations have also been observed in aquaculture, where the collection of salmon from the wild for hatchery stocks has led to changes in their genome within the first three generations [75]. In research animals, inbred lab strains generally show genomic variations that correlate with the number of generations maintained in captivity.
A dramatic example of genomic effect of domestication in a model system is evident in the sex determination system of the zebrafish. First introduced to the lab setting by Dr. George Streisinger [76], the lines of zebrafish most researchers use (called AB and TU, [77]) have been maintained in closed breeding populations with no introduction from wild populations. It is known that stress can affect the sex ratio in zebrafish, such that reduced food, high temperatures, and low oxygen, can drive the sex ratio towards an increased production of males [78], [79]. Furthermore, zebrafish can change sex under conditions in which germ cell signaling is disrupted [80]. Juvenile animals that lack oocytes will develop as phenotypic males [81], [82]. And strikingly, the depletion of oocytes, but retention of germline stem cells, causes adult females to sex-revert to sperm-producing males [83], [84]. These studies were performed using lab strains, yet cytogenetic studies on zebrafish captured in India (this fish is native of India and Southeast Asia [85] [86]); have revealed females to be the heterogametic sex with a defined female-specific chromosome [87].
Research into the mechanisms that control sex determination in zebrafish have reconciled these conflicting results and led to the surprising finding that domestication of zebrafish has led to the loss of sex-determining genetic loci (Figure 2B,C). Studies comparing wild-caught zebrafish versus zebrafish maintained for generations in captivity has revealed that the natural strains (named EKW, Nadia, WIK, and Cooch Behar), have retained a region of chromosome 4 that is genetically different between phenotypic females and most phenotypic males, consistent with a ZZ(male)/ZW(female) sex determination system [20] (Figure 2B). All phenotypic females have a W allele, no ZZ individuals ever become females, but some ZW fish sex-reverse to become males (neo-males). In contrast, lab strains AB and TU lack the alleles strongly associated with sex determination [20], although there is evidence that weak or polymorphic sex biasing genes are present in other parts of the genome in some lab strains [88], [89], [90], [91], [92], [93], [94] (Figure 2C). The AB lab strain originated from pet store animals (1970s, Oregon, USA) that were selected to produce many offspring and were subjected to several rounds of gynogenesis to make them free of lethal mutations, thereby creating a genetic bottleneck [95]. Likewise, the TU lab strain originated from a fish pet store in Germany, and was also heavily selected to produce large numbers of offspring free of lethal mutations [77]. By contrast, the EKW strain has been maintained since 1962 in large populations in outdoor ponds without selection for specific characteristics (EkkWill Waterlife Resources).
These observations on the altered genome of “domesticated” zebrafish raise the possibility that domestication itself may have led to the loss of the gnrh1 gene in this species. Indeed, some of the aforementioned changes seen in domesticated mammals, such as the de-coupling of reproduction from the natural cycles, increased fecundity, and the effects on the HPA axis, suggest that the loss of the gnrh1 gene in zebrafish (and potentially also in other domesticated Cyprinids) may have resulted from the selective pressures imposed by human desires. This hypothesis predicts that gnrh1 would be found in the genome of the unselected EKW line and absent from the reference zebrafish genome (currently GRCz11), since this latter sequence was obtained from lab-selected fish. Nevertheless, we found that the genome of the unselected EKW strain of zebrafish also lacks a gnrh1 gene (Figure 3), indicating that the absence of a gnrh1 gene in the reference zebrafish genome sequence is not a consequence of domestication (Figure 3A, B). In contrast, Medaka, (Oryzias latipes) another fish used in biomedical research [96] retains the gnrh1 gene ([41], Figure 3C). Yet, the analysis of other fish genomes revealed that the loss of the gnrh1 gene (Figure 3D) is not exclusive to zebrafish but also occurred in other fishes, such as the Mexican cavefish (Astyanax mexicanus). A finer analysis of this species’ Ankrd9-npy8br genomic region is not yet possible given the current quality of the cavefish genome sequence, but it will be interesting to determine if the loss of the gnrh1 gene is associated with the same inversion observed in zebrafish, as this would suggest that gene loss occurred before the divergence of the Astyanax (Order Characiformes, Family Characidae) and zebrafish (Order Cypriniformes, Family Cyprinidae) lineages (Figure 3E).
Figure 3. Loss of gnrh1 pre-dates the domestication of zebrafish.
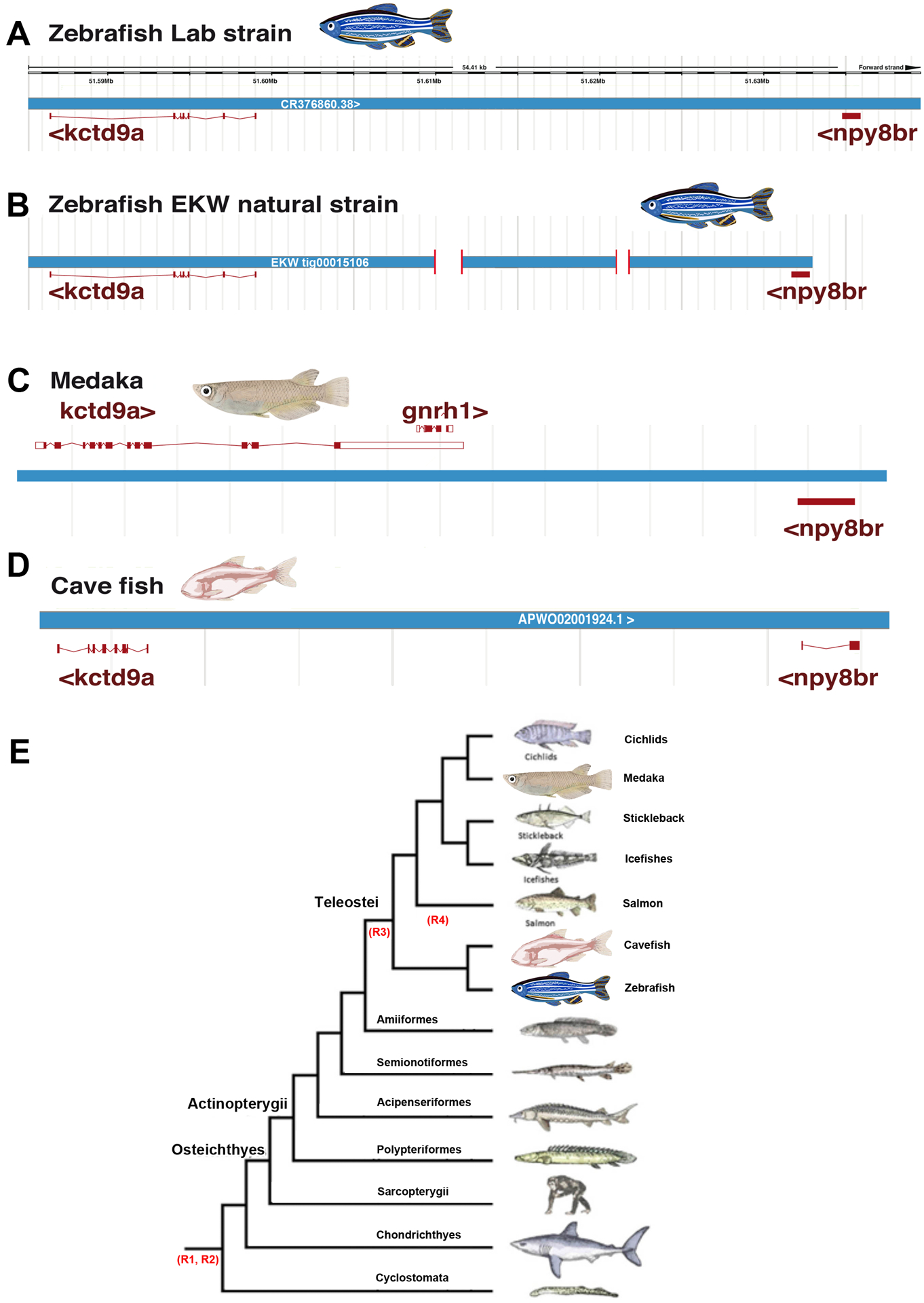
(A-D) neither lab strains (A) nor wild-caught (B) zebrafish contain the gnrh1 gene. In wild-caught zebrafish this interval contains small deletions not present in the lab stains (indicated as gaps in the schematic). In contrast to zebrafish, medaka contains gnrh1 (C). Similar to zebrafish, the cavefish Astyanax mexicanus lacks the gnrh1 gene (D). (E) Simplified lineage tree of fishes. Similarly to zebrafish (Danio rerio, Order Cypriniformes, Family Cyprinidae; panels C and D), the genome of the cavefish (Astyanax mexicanus, Order Characiformes, Family Characidae; panel B) lacks gnrh1 (E adapted from [134])
2.5. Potential alternative pathways to GnRH in Zebrafish
Loss of gnrh1 and/or gnrh3 does not result in infertility
The absence of the gnrh1 gene in the zebrafish (and likely in cypriniformes in general) raises the possibility that GnRH3 may be the hypophysiotropic form in these fish. In a study to determine the potential role of GnRH3 in zebrafish reproduction, cells containing GnRH3, as identified in vivo using a Tg(gnrh3:egfp) reporter line [42], were laser ablated during early development [97]. The resulting adult fish lacked GnRH3:GFP positive neurons and showed arrested oocyte development and reduced average oocyte diameter, suggesting that GnRH3 might play a role in reproduction. However, because these ablations eliminated neurons that may contain not only GnRH3 and its GAP but potentially also other peptides such those in the FMRFamide family (which are known to be co expressed with GnRH [40]); the possibility existed that it was the loss of other peptides (alone or in combination with GnRH3) that may have caused an unintended trophic effect on the development of the HPA axis [51]. Consistent with this possibility, two different groups recently reported that the deletion of the gnrh3 gene using TALEN technologies did not affect the fertility of adult zebrafish. One group [98] reported limited effects on mRNA expression of follicle-stimulating hormone beta (fshb), luteinizing hormone beta (lhb), and chorionic gonadotropin subunit alpha (cga) during early development. Yet, these changes in expression were not present in the adult animals and there were no adverse effects on reproduction in either male or female gnrh3−/− adult fish [98]. These data are consistent with those reported by a second study showing that gnrh3−/− mutants retained full reproductive capacity, although in this second study [99], unlike Spicer et al. (2016) [98] no changes in expression of the pituitary gonadotropins fshb, lhb, and cga were found in female or male gnrh3 mutant fish but it is unclear whether they were sampling the same stages of development. Thus, knocking-out gnrh3 in zebrafish, which already lack the gnrh1 gene, resulted animals that were fertile, with normal gametogenesis and reproductive performance in both males and females.
Loss of GnRH2 does not affect fertility
If zebrafish lacking GNRH3 are fertile, the only GnRH hormone left that could play a role in reproduction is GnRH2. The role of GnRH2 in reproduction and behavior is relatively unknown, including its potential relationship with the GnRH3 network within the HPA axis. Recently, transgenic reporter lines, Tg(GnRH2:eGFP) and Tg(GnRH3:tdTomato; GnRH2:eGFP), were used to visualize the GnRH2 and GnRH3 networks in intact developing zebrafish. These analyses showed that GnRH2 neurons have extensive projections in the brain and spinal cord with potential projections to the pituitary [100]. Zebrafish have four GnRH receptors (zfGnRHR1, zfGnRHR2, zfGnRHR3, zfGnRHR4; [101], [102]) where zfGnRHR1 and zfGnRHR3 have a greater affinity for GnRH2 than for GnRH3, and zfGnRHR2 and zfGnRHR4 have the same affinity for GnR2 and GnRH3 [102]. Since the various GnRH receptors are expressed in the brain, eye, and ovaries and testes (although zfGnRHR3 shows very reduced expression in the brain and the ovaries [102]) the possibility exists that GnRH2 could replace GnRH3 as the hypophysiotropic hormone controlling reproduction in zebrafish. Because of the promiscuity of ligand/receptor interactions and the reported GnRH2 projections to the pituitary, the gnrh2 gene was knocked out in a gnrh3−/− background to determine whether this peptide could compensate for the loss of the gnrh3 gene function. Surprisingly, whereas age-matched adult male and female gnrh2−/−; gnrh3−/− double knockout fish exhibited up-regulation of several genes in the brain, including the gonadotropin inhibitory hormone (gnih; also known as Lpxrfa), they showed no reproductive defects [103]. Since these mutant animals lack all forms of GnRH, these results show that in zebrafish, neither GnRH2 nor GnRH3 replaces the function of GnRH1, raising the possibility that other, non-GnRH, peptides replace GnRH1 as the hypophysiotropic releasing hormone.
Loss of gnrh3 and kisspeptin does not affect reproduction
An essential hypothalamic peptide that regulates GnRH is kisspeptin (Kiss), an obligate upstream regulator of gonadotropin-releasing hormone secretion in mammals. Kiss is so important in this process that it has been proposed to be the “master molecule” in reproductive events, not only during puberty but also in adulthood [104], [105]. In teleosts there are two kiss genes, called kiss1 and kiss2 [106], and two Kiss receptors encoded by kiss1ra and kiss1rb [107]. Subsequent analysis has shown that in zebrafish, Kiss2 is important for reproductive events, whereas the function of Kiss1 remains to be established [67]. The Kiss peptides are part of the GnRH pathway in animals in which GnRH acts as the hypophysiotropic hormone. In zebrafish, animals homozygous for TALEN-generated knockouts of both kiss1 and kiss2 genes showed normal puberty and gonadal development [67]. Furthermore, the inactivation of the kiss genes in a gnrh3−/− mutant background did not result in impaired puberty or reproductive defects. Interestingly, the expression of neuropeptide Y (npy), tachykinin 3 (tac3), and secretogranin-II (sgII), all neuropeptides reported to stimulate gonadotropin release, were significantly increased in the triple knockout mutant [99]. These results suggest that zebrafish must use a different non-Kiss- and non-GnRH-based mechanism to regulate reproduction. If GnRH1 does not exist in zebrafish, and its function has not been replaced by GnRH2, GnRH3, Kiss1, or Kiss2, which peptide has taken its place?
2.6. Potential alternative pathways to GnRH in Zebrafish
Approximately half of the extant vertebrates are fish and, of this group, greater than 95% are teleosts (numbering over 30,000 species), thus making teleosts the dominant class of not only fish but of vertebrates on the Earth [108]. Teleosts have secondarily lost the median eminence characteristic of vertebrate ancestors and of other extant vertebrates, which allows for direct neuroendocrine regulation of the brain-pituitary axes. The list of potential regulators of reproduction is long, including neuropeptides such as gonadotropin inhibitory hormone (GnIH), aminergic neurotransmitters such as dopamine and serotonin, and amino acid neurotransmitters such as glutamate and GABA (for review see [109]). Thus, the perplexing situation of the reproductive control in zebrafish could possibly be explained by a variety of pathway modifications, such as a) Cooption of a known peptide, such as neuropeptides or neurotransmitter, in the reproductive cascade, or b) Use of an unrelated and as yet unidentified peptide.
Duplication of one of the other forms of GnRH
With the diversity of the teleost fishes also comes a diversity in the mechanisms of sexual plasticity and their mechanics of gametogenesis and reproduction [3] and underlying this diversity is the evolution of a fantastically diverse genome. Goldfish, one of the earliest domesticated fish, [110], also lacks the gnrh1 gene, and has undergone an additional round of whole genome duplication (WGD) relative to zebrafish, although both are cyprinid fishes [111]. Interestingly, this WGD appears to have arisen through allopolyploidzation (a process in which chromosomes are derived from one of two or more species) of hybrid offspring of ancestors of common carp (Cyprinus carpio) and goldfish (Carassius sp.) [112]. In goldfish (Carassius auratus), there are two isoforms of GnRH3 (originally named sGnRH-I and II) and two gnrh2 genes (originally named cGnRH-I and II) [16], [13], and both GnRH2 and GnRH3 can trigger spawning in female goldfish [113]. Like goldfish, salmonids (genera of the subfamily Salmoninae), rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar), and Arctic charr (Salvelinus alpinus), lack gnrh1 and their genome has also undergone an additional WGD compared to that of the zebrafish [114]. The salmonid genome duplication resulted in a duplication of the gnrh3 gene (each copy encoding GNRH3A and GNRH3B) (formerly known as sGnRH1 and sGnRH2, [114]) and in this group of fishes, GnRH3A appears to be the hypophysiotropic form used to control spawning [115]. Thus, to date the only fishes where a specific GnRH has been duplicated (cf, GNRH3A and GNRH3B) are those that have undergone an additional round of WGD (R4: Figure 3E). Yet, in spite of a duplication at the base of the teleost lineage (R3: Figure 3E), zebrafish do not have duplicates of GnRH genes, unlike other genes in their genome.
Coopting a peptide known to be part of the reproductive cascade
One neurohormone proposed to potentially modulate the reproductive axis in the zebrafish is GnRH-Inhibitory hormone (Figure 4A, B; GnIH, also called LPXFR), a hypothalamic peptide first discovered in the Japanese quail, where it inhibits gonadal development and gonadotropin release [116], [117]. GnIH belongs to the RFamide family and inhibits the brain-pituitary reproductive axis in mammals and birds [118], [119]). In addition, GnIH can have a stimulatory effect on gonadotropin secretion in some fish, including tilapia [120] and goldfish [121], as well as in a variety of other fishes (for review see [122]). The GnIH orthologs in fish are often referred to as “LPXRFamide (Lpxrfa) peptides”, of which zebrafish has three: Lpxrfa-1, Lpxrfa-2, and Lpxrfa-3. Lpxfra peptides are cleaved from a 198-amino-acid precursor peptide, and act on three GPCRs (Lpxrf-R1, Lpxrf-R2, and Lpxrf-R3) [123]. Most recently, the Lpxfra-3 peptide was shown to reduce the expression of lsh and cga in pituitary explants of zebrafish, thereby inhibiting the reproductive axis, and also reduced the expression of gnrh3 in neurons in the brain [124]. Therefore, reproductive inhibitory neuropeptides acting through Lpxrfa may interact with GnRH3 neurons in the brain and with pituitary gonadotropes, while also potentially utilizing the Kiss2/Kiss1ra pathway [124]. To date, these data do not support a potential role for GnIH/Lpxfra in the stimulation of the reproductive axis in the zebrafish, although the functions of Lpxfra-1 and Lpxfra-2 need to be better defined because they could be bypassing the kisspeptin pathway (Figure 4B, red).
Figure 4. Potential alternative pathways replacing GnRH1 signaling in zebrafish.
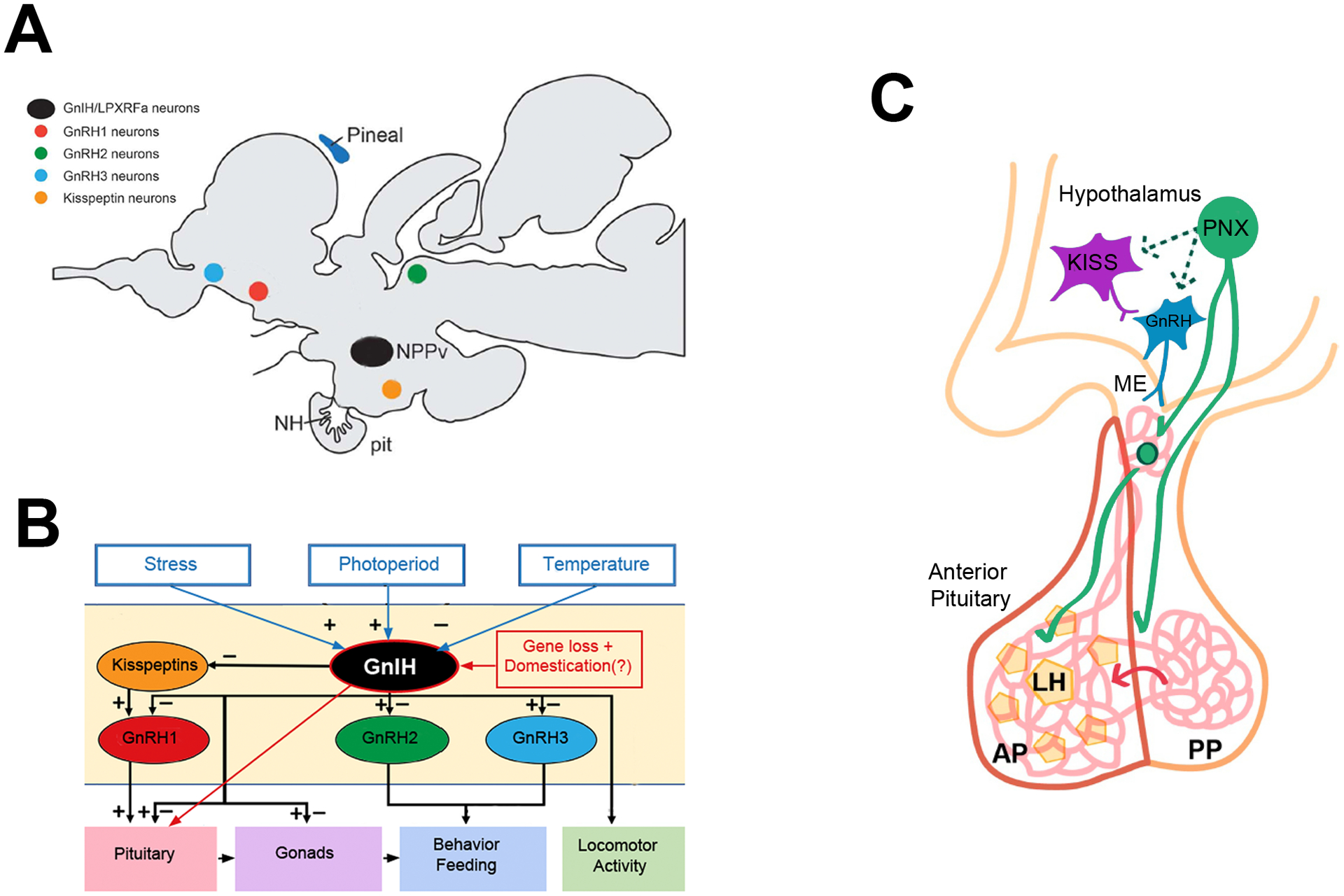
(A) Cells containing GnRH-Inhibitory hormone (GnIH) (black) are shown relative to the populations of cells with which GnIH might interact: GnRH1 (red), GnRH2 (green), and GnRH3 (blue), and Kisspeptins (orange). (B) GnIH signaling pathways in the brain pituitary axis. In zebrafish, the loss of gnrh1 and loss-of-function mutations in gnrh2, gnrh3, kiss1, and kiss2, do not result in infertility, thus suggesting a potential direct interaction of GnIH on the pituitary gonadotropes (red arrow). This unusual loss of signaling may be due to intense selective pressures (red box: gene loss + domestication) (A modified from [134]; B modified from [122]). (C) The recently discovered reproductive peptide, phoenixin (PNX), can stimulate kisspeptin (purple) and GnRH (blue) release in hypothalamic cell lines. In intact animals PNX can stimulate LH release from the hypothalamus (green cell with solid arrows) although the exact details have yet to be uncovered. PNX is conserved in fish and is a candidate peptide for hypothalamic-pituitary interactions in zebrafish (C from [135]).
Use of an unrelated peptide
It is often overlooked that there are still many “orphan” receptors, which suggests the existence of uncharacterized ligands with potentially new functions. One such recently discovered protein is phoenixin (Figure 4C), originally identified using a bioinformatic approach to screen the human genome for sequences predicted to encode previously unrecognized secreted peptides [125]. Phoenixin was shown to be highly conserved from humans to zebrafish [125], is cleaved from the C-terminus of the so-called small integral membrane protein 20 (SMIM20). The 14 and 20 amino acid cleavage products, PNX-14 and PNX-20, have been shown in rats to act on gonadotropes through the orphan GPCR, Gpr173 [126], and thus appear to affect gonadotropin release from the pituitary via modulation of GnRHR expression [125], [126]. Furthermore, the presence of PNX peptides has been confirmed in different regions of the mammalian brain including the hypothalamus, sensory ganglia, and spinal cord [127]. Because PNX can regulate the expression of kisspeptin, GnRH, GnRH receptor, and LH (see for review [128]), PNX peptides have been proposed to be hypothalamic factors that could potentiate the action of pituitary gonadotropes [129], [130], thereby acting as reproductive peptides. Because of the potential reproductive functions of PNX, we have confirmed the expression of PNX in developing and adult zebrafish (Ceriani, Calfun and Whitlock, in preparation), which opens the door for the analysis of this peptide as a potential regulator of the brain-pituitary axis in the zebrafish.
A final part of the GnRH mystery, yet to be solved, is a strikingly specific GnRH immunoreactivity pattern in the parvocellular nucleus of the adult zebrafish (Figure 1E, [62]). Previously, we used antibodies recognizing GnRH1 [131] to localize cells in the hypothalamus [62]. Because of their location and the fact that to date there is no convincing evidence showing expression of gnrh3 in the parvocellular region of hypothalamus in the adult zebrafish, we classified the immunopositive cells as GnRH1 cells. In light of the overwhelming evidence that a gnrh1 gene does not exist in the genome of the zebrafish and that neither MALDITOF nor Orbitrap analyses of hypothalamic tissues detected any GnRH-like peptide in this tissue, the anti-GnRH antibodies must be recognizing a protein immunologically similar to, but unrelated to, GnRH1. We hope that future analysis will uncover the basis for this perplexing, highly specific expression pattern.
3. Conclusions
Here we have presented an overview of the fascinating situation of the brain-pituitary axis in zebrafish. In spite of an extra round of duplication relative to mammals, the genome of zebrafish lacks the gene encoding GnRH1 and this loss is not compensated for by either gnrh2 or gnrh3. Furthermore, knocking out genes encoding several key peptides involved in reproduction, most importantly kisspeptin, does not cause defects in fertility in the adult fish. Interestingly, we now know that the zebrafish genome has been highly modified by domestication to the extent of altering the genetic mechanisms of sex determination relative to the wild populations. Although domestication does not explain the loss of the gnrh1 gene, it must be kept in mind when working with highly selected lines developed for medical research, aquaculture, or the pet trade industry, especially when investigating the endocrine control of the reproductive axis.
Here we have shown that the “usual suspects” are not involved in regulating the hypothalamic-pituitary-gonadal axis in zebrafish, and there are hints that a similar situation may occur in other fishes that also lack GnRH1. It is interesting to note that in humans the availability of genome-wide analyses has revealed that only ~30% of the cases of idiopathic hypogonadic hypogonadism, which was once considered a monogenic disorder, are explained by mutations in one of eleven known genes [132]. This suggests that there may be more flexibility than expected in this pathway even beyond fishes; it could also be that physiological and environmental factors may also be playing a role.
Acknowledgments:
We thank Ricardo Ceriani for performing dissections of the adult hypothalami necessary for the peptide analysis, Christian Wegener for performing MALDITOF or Orbitrap and Catherine Wilson for assistance in genomic analysis.
Funding: This work was supported by grants FONDECYT 1160076 (KEW); FONDECYT 1180403 JE); Centro Interdisciplinario de Neurociencia de Valparaíso (CINV) Millennium Institute grant P09-022-F, supported by the Millennium Scientific Initiative of the Ministerio de Economía, Fomento y Turismo (K.E.W; J.E.); and grants R01 OD011116 and R01 GM085318 (J.H.P.).
References:
- [1].Gore AC, GnRH: Tha Master Molecule of Reproduction, Kluwer Academic Publishers, Massachussetts, 2002. [Google Scholar]
- [2].Lethimonier C, Madigou T, Munoz-Cueto JA, Lareyre JJ, and Kah O, Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish. Gen Comp Endocrinol. 135 (2004) 1–16. [DOI] [PubMed] [Google Scholar]
- [3].Kitahashi T, Shahjahan MD, and Parhar I, Hypothalamic Regulation of Pituitary Gonadotropins, Nova Science Publishers, Inc., 2013. [Google Scholar]
- [4].Biran J, Tahor M, Wircer E, and Levkowitz G, Role of developmental factors in hypothalamic function. Front Neuroanat. 9:47 (2015) 10.3389/fnana.2015.00047. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peter RE, Yu K-L, Marchant TA, and Rosenblum PM, Direct Regulation of the Teleost Adenohypophysis. Journal of Experimental Zoology Supplement (1990) 84–89. [Google Scholar]
- [6].Machluf Y, Gutnick A, and Levkowitz G, Development of the zebrafish hypothalamus. Ann N Y Acad Sci. 1220:93–105. (2011) 10.1111/j.1749-6632.2010.05945.x. [DOI] [PubMed] [Google Scholar]
- [7].Gomes CC, Costa FG, and Borella MI, Distribution of GnRH in the brain of the freshwater teleost Astyanax altiparanae. Micron. 52–53:33–8. (2013) 10.1016/j.micron.2013.07.008. Epub 2013 Aug 7. [DOI] [PubMed] [Google Scholar]
- [8].Ball JN, Hypothalamic control of the pars distalis in fishes, amphibians, and reptiles. Gen Comp Endocrinol. 44 (1981) 135–70. [DOI] [PubMed] [Google Scholar]
- [9].Gorbman A, Olfactory origins and evolution of the brain-pituitary endocrine system: facts and speculation. Gen Comp Endocrinol. 97 (1995) 171–8. doi: 10.1006/gcen.1995.1016. [DOI] [PubMed] [Google Scholar]
- [10].Gaillard AL, Tay BH, Perez Sirkin DI, Lafont AG, De Flori C, Vissio PG, Mazan S, Dufour S, Venkatesh B, and Tostivint H, Characterization of Gonadotropin-Releasing Hormone (GnRH) Genes From Cartilaginous Fish: Evolutionary Perspectives. Front Neurosci. 12:607 (2018) 10.3389/fnins.2018.00607. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Whitlock KE, Development of the nervus terminalis: origin and migration. Microsc Res Tech 65 (2004) 2–12. [DOI] [PubMed] [Google Scholar]
- [12].Desaulniers AT, Cederberg RA, Lents CA, and White BR, Expression and Role of Gonadotropin-Releasing Hormone 2 and Its Receptor in Mammals. Front Endocrinol (Lausanne). 8:269 (2017) 10.3389/fendo.2017.00269. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yu KL, He ML, Chik CC, Lin XW, Chang JP, and Peter RE, mRNA expression of gonadotropin-releasing hormones (GnRHs) and GnRH receptor in goldfish. Gen Comp Endocrinol. 112 (1998) 303–11. doi: 10.1006/gcen.1998.7137. [DOI] [PubMed] [Google Scholar]
- [14].King JA, and Millar RP, Structure of chicken hypothalamic luteinizing hormone-releasing hormone. II. Isolation and characterization. J Biol Chem. 257 (1982) 10729–32. [PubMed] [Google Scholar]
- [15].Oka Y, Three types of gonadotrophin-releasing hormone neurones and steroid-sensitive sexually dimorphic kisspeptin neurones in teleosts. J Neuroendocrinol. 21 (2009) 334–8. doi: 10.1111/j.1365-2826.2009.01850.x. [DOI] [PubMed] [Google Scholar]
- [16].Lin XW, and Peter RE, Expression of salmon gonadotropin-releasing hormone (GnRH) and chicken GnRH-II precursor messenger ribonucleic acids in the brain and ovary of goldfish. Gen Comp Endocrinol. 101 (1996) 282–96. doi: 10.1006/gcen.1996.0031. [DOI] [PubMed] [Google Scholar]
- [17].Penlington MC, Williams MA, Sumpter JP, Rand-Weaver M, Hoole D, and Arme C, Isolation and characterisation of mRNA encoding the salmon- and chicken-II type gonadotrophin-releasing hormones in the teleost fish Rutilus rutilus (Cyprinidae). J Mol Endocrinol. 19 (1997) 337–46. [DOI] [PubMed] [Google Scholar]
- [18].Steven C, Lehnen N, Kight K, Ijiri S, Klenke U, Harris WA, and Zohar Y, Molecular characterization of the GnRH system in zebrafish (Danio rerio): cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. Gen Comp Endocrinol. 133 (2003) 27–37. [DOI] [PubMed] [Google Scholar]
- [19].Filby AL, van Aerle R, Duitman J, and Tyler CR, The kisspeptin/gonadotropin-releasing hormone pathway and molecular signaling of puberty in fish. Biol Reprod. 78 (2008) 278–89. doi: 10.1095/biolreprod.107.063420. Epub 2007 Oct 31. [DOI] [PubMed] [Google Scholar]
- [20].Wilson CA, High SK, McCluskey BM, Amores A, Yan YL, Titus TA, Anderson JL, Batzel P, Carvan MJ 3rd, Schartl M, and Postlethwait JH, Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics. 198 (2014) 1291–308. doi: 10.1534/genetics.114.169284. Epub 2014 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Choi D, Evolutionary Viewpoint on GnRH (gonadotropin-releasing hormone) in Chordata - Amino Acid and Nucleic Acid Sequences. Dev Reprod. 22 (2018) 119–132. doi: 10.12717/DR.2018.22.2.119. Epub 2018 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsuo H, Baba Y, Nair RM, Arimura A, and Schally AV, Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 43 (1971) 1334–9. [DOI] [PubMed] [Google Scholar]
- [23].Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, and Guillemin R, Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) (LH-hypothalamus-LRF-gas chromatography-mass spectrometry-decapeptide-Edman degradation). Proc Natl Acad Sci U S A. 69 (1972) 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tan L, and Rousseau P, The chemical identity of the immunoreactive LHRH-like peptide biosynthesized in the human placenta. Biochem Biophys Res Commun. 109 (1982) 1061–71. [DOI] [PubMed] [Google Scholar]
- [25].Sherwood NM, Evolution of a Neuropeptide Family: Gonadotropin-Releasing Hormone. American Society of Zoologists 26 (1986) 1041–1054. [Google Scholar]
- [26].King JA, and Millar RP, Structure of chicken hypothalamic luteinizing hormone-releasing hormone. I. Structural determination on partially purified material. J Biol Chem. 257 (1982) 10722–8. [PubMed] [Google Scholar]
- [27].Miyamoto K, Hasegawa Y, Igarashi M, Chino N, Sakakibara S, Kangawa K, and Matsuo H, Evidence that chicken hypothalamic luteinizing hormone-releasing hormone is [Gln8]-LH-RH. Life Sci. 32 (1983) 1341–7. [DOI] [PubMed] [Google Scholar]
- [28].Miyamoto K, Hasegawa Y, Minegishi T, Nomura M, Takahashi Y, Igarashi M, Kangawa K, and Matsuo H, Isolation and characterization of chicken hypothalamic luteinizing hormone-releasing hormone. Biochem Biophys Res Commun. 107 (1982) 820–7. [DOI] [PubMed] [Google Scholar]
- [29].Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, and Matsuo H, Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci U S A. 81 (1984) 3874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tostivint H, Evolution of the gonadotropin-releasing hormone (GnRH) gene family in relation to vertebrate tetraploidizations. Gen Comp Endocrinol. 170 (2011) 575–81. doi: 10.1016/j.ygcen.2010.11.017. Epub 2010 Nov 29. [DOI] [PubMed] [Google Scholar]
- [31].Seeburg PH, and Adelman JP, Characterization of cDNA for precursor of human luteinizing hormone releasing hormone. Nature. 311 (1984) 666–8. [DOI] [PubMed] [Google Scholar]
- [32].White SA, Kasten TL, Bond CT, Adelman JP, and Fernald RD, Three gonadotropin-releasing hormone genes in one organism suggest novel roles for an ancient peptide. Proc Natl Acad Sci U S A. 92 (1995) 8363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kasten TL, White SA, Norton TT, Bond CT, Adelman JP, and Fernald RD, Characterization of two new preproGnRH mRNAs in the tree shrew: first direct evidence for mesencephalic GnRH gene expression in a placental mammal. Gen Comp Endocrinol. 104 (1996) 7–19. doi: 10.1006/gcen.1996.0135. [DOI] [PubMed] [Google Scholar]
- [34].White RB, and Fernald RD, Genomic structure and expression sites of three gonadotropin-releasing hormone genes in one species. Gen Comp Endocrinol. 112 (1998) 17–25. doi: 10.1006/gcen.1998.7125. [DOI] [PubMed] [Google Scholar]
- [35].Okuyama T, Yokoi S, and Takeuchi H, Molecular basis of social competence in medaka fish. Dev Growth Differ. 59 (2017) 211–218. doi: 10.1111/dgd.12359. Epub 2017 May 26. [DOI] [PubMed] [Google Scholar]
- [36].Hough D, Bellingham M, Haraldsen IR, McLaughlin M, Robinson JE, Solbakk AK, and Evans NP, A reduction in long-term spatial memory persists after discontinuation of peripubertal GnRH agonist treatment in sheep. Psychoneuroendocrinology. 77:1–8. (2017) 10.1016/j.psyneuen.2016.11.029. Epub 2016 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Reperant J, Ward R, Miceli D, Rio JP, Medina M, Kenigfest NB, and Vesselkin NP, The centrifugal visual system of vertebrates: a comparative analysis of its functional anatomical organization. Brain Res Rev. 52 (2006) 1–57. doi: 10.1016/j.brainresrev.2005.11.008. Epub 2006 Feb 15. [DOI] [PubMed] [Google Scholar]
- [38].Zucker CL, and Dowling JE, Centrifugal fibres synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 330 (1987) 166–8. doi: 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]
- [39].Umino O, and Dowling JE, Dopamine release from interplexiform cells in the retina: effects of GnRH, FMRFamide, bicuculline, and enkephalin on horizontal cell activity. J Neurosci. 11 (1991) 3034–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oehlmann VD, Korte H, Sterner C, and Korsching SI, A neuropeptide FF-related gene is expressed selectively in neurons of the terminal nerve in Danio rerio. Mech Dev. 117 (2002) 357–61. [DOI] [PubMed] [Google Scholar]
- [41].Okubo K, Sakai F, Lau EL, Yoshizaki G, Takeuchi Y, Naruse K, Aida K, and Nagahama Y, Forebrain gonadotropin-releasing hormone neuronal development: insights from transgenic medaka and the relevance to X-linked Kallmann syndrome. Endocrinology. 147 (2006) 1076–84. doi: 10.1210/en.2005-0468. Epub 2005 Nov 17. [DOI] [PubMed] [Google Scholar]
- [42].Abraham E, Palevitch O, Ijiri S, Du SJ, Gothilf Y, and Zohar Y, Early development of forebrain gonadotrophin-releasing hormone (GnRH) neurones and the role of GnRH as an autocrine migration factor. Journal of Neuroendocrinology 20 (2008) 394–405. [DOI] [PubMed] [Google Scholar]
- [43].Ramakrishnan S, Lee W, Navarre S, Kozlowski DJ, and Wayne NL, Acquisition of spontaneous electrical activity during embryonic development of gonadotropin-releasing hormone-3 neurons located in the terminal nerve of transgenic zebrafish (Danio rerio). Gen Comp Endocrinol 168 (2010) 401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zheng LM, Caldani M, and Jourdan F, Immunocytochemical identification of luteinizing hormone-releasing hormone-positive fibres and terminals in the olfactory system of the rat. Neuroscience. 24 (1988) 567–78. [DOI] [PubMed] [Google Scholar]
- [45].Merchenthaler I, Culler MD, Petrusz P, Flerko B, and Negro-Vilar A, Immunocytochemical localization of the gonadotropin-releasing hormone-associated peptide portion of the LHRH precursor in the hypothalamus and extrahypothalamic regions of the rat central nervous system. Cell Tissue Res. 255 (1989) 5–14. [DOI] [PubMed] [Google Scholar]
- [46].Demski LS, Ridgway SH, and Schwanzel-Fukuda M, The terminal nerve of dolphins: gross structure, histology and luteinizing-hormone-releasing hormone immunocytochemistry. Brain Behav Evol 36 (1990) 249–61. doi: 10.1159/000115311. [DOI] [PubMed] [Google Scholar]
- [47].Oelschlager HA, Helpert C, and Northcutt RG, Coexistence of FMRFAMIDE-like and LHRH-like immunoreactivity in the terminal nerve and forebrain of the big brown bat, Eptesicus fuscus. Brain Behav Evol 52 (1998) 139–47. doi: 10.1159/000006558. [DOI] [PubMed] [Google Scholar]
- [48].Wirsig-Wiechmann CR, Wiechmann AF, and Eisthen HL, What defines the nervus terminalis? Neurochemical, developmental, and anatomical criteria. Prog Brain Res 141:45–58. (2002) 10.1016/S0079-6123(02)41083-7. [DOI] [PubMed] [Google Scholar]
- [49].Whitlock KE, Wolf CD, and Boyce ML, Gonadotropin-releasing hormone (GnRH) cells arise from cranial neural crest and adenohypophyseal regions of the neural plate in the zebrafish, Danio rerio. Dev Biol 257 (2003) 140–52. [DOI] [PubMed] [Google Scholar]
- [50].Whitlock KE, Illing N, Brideau NJ, Smith KM, and Twomey S, Development of GnRH cells: Setting the stage for puberty. Mol Cell Endocrinol 254–255 (2006) 39–50. [DOI] [PubMed] [Google Scholar]
- [51].Zhao Y, Lin MC, Farajzadeh M, and Wayne NL, Early development of the gonadotropin-releasing hormone neuronal network in transgenic zebrafish. Front Endocrinol (Lausanne). 4:107 (2013) 10.3389/fendo.2013.00107. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].el Amraoui A, and Dubois PM, Experimental evidence for an early commitment of gonadotropin-releasing hormone neurons, with special regard to their origin from the ectoderm of nasal cavity presumptive territory. Neuroendocrinology. 57 (1993) 991–1002. doi: 10.1159/000126490. [DOI] [PubMed] [Google Scholar]
- [53].Witkin JW, Dao D, Livne I, Dunn IC, Zhou XL, Pula K, and Silverman AJ, Early expression of chicken gonadotropin-releasing hormone-1 in the developing chick. J Neuroendocrinol. 15 (2003) 865–70. [DOI] [PubMed] [Google Scholar]
- [54].Parhar IS, Soga T, Ishikawa Y, Nagahama Y, and Sakuma Y, Neurons synthesizing gonadotropin-releasing hormone mRNA subtypes have multiple developmental origins in the medaka. J Comp Neurol. 401 (1998) 217–26. [PubMed] [Google Scholar]
- [55].Quanbeck C, Sherwood NM, Millar RP, and Terasawa E, Two populations of luteinizing hormone-releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol. 380 (1997) 293–309. [PubMed] [Google Scholar]
- [56].Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, and Cai D, Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 497 (2013) 211–6. doi: 10.1038/nature12143. Epub 2013 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tang Y, and Cai D, Hypothalamic inflammation and GnRH in aging development. Cell Cycle. 12 (2013) 2711–2. doi: 10.4161/cc.26054. Epub 2013 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kobayashi M, Furukawa K, Kim MH, and Aida K, Induction of male-type gonadotropin secretion by implantation of 11-ketotestosterone in female goldfish. Gen Comp Endocrinol. 108 (1997) 434–45. doi: 10.1006/gcen.1997.6993. [DOI] [PubMed] [Google Scholar]
- [59].Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, and Postlethwait JH, Zebrafish hox clusters and vertebrate genome evolution. Science. 282 (1998) 1711–4. [DOI] [PubMed] [Google Scholar]
- [60].Gopinath A, Andrew Tseng L, and Whitlock KE, Temporal and spatial expression of gonadotropin releasing hormone (GnRH) in the brain of developing zebrafish (Danio rerio). Gene Expr Patterns 4 (2004) 65–70. [DOI] [PubMed] [Google Scholar]
- [61].Torgersen J, Nourizadeh-Lillabadi R, Husebye H, and Aleström P, In silico and in situ characterization of the zebrafish (Danio rerio) gnrh3 (sGnRH) gene. BMC Genomics 3 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cortes-Campos C, Letelier J, Ceriani R, and Whitlock KE, Zebrafish adult-derived hypothalamic neurospheres generate gonadotropin-releasing hormone (GnRH) neurons. Biol Open 24 (2015) 010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kuo MW, Lou SW, Postlethwait J, and Chung BC, Chromosomal organization, evolutionary relationship, and expression of zebrafish GnRH family members. J Biomed Sci 12 (2005) 629–39. doi: 10.1007/s11373-005-7457-z. Epub 2005 Nov 10. [DOI] [PubMed] [Google Scholar]
- [64].Kim DK, Cho EB, Moon MJ, Park S, Hwang JI, Kah O, Sower SA, Vaudry H, and Seong JY, Revisiting the evolution of gonadotropin-releasing hormones and their receptors in vertebrates: secrets hidden in genomes. Gen Comp Endocrinol. 170 (2011) 68–78. doi: 10.1016/j.ygcen.2010.10.018. Epub 2010 Oct 29. [DOI] [PubMed] [Google Scholar]
- [65].Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, et al. , The zebrafish reference genome sequence and its relationship to the human genome. Nature. 496 (2013) 498–503. doi: 10.1038/nature12111. Epub 2013 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Calfun C, Dominguez C, Perez-Acle T, and Whitlock KE, Changes in Olfactory Receptor Expression Are Correlated With Odor Exposure During Early Development in the zebrafish (Danio rerio). Chem Senses. 41 (2016) 301–12. doi: 10.1093/chemse/bjw002. Epub 2016 Feb 17. [DOI] [PubMed] [Google Scholar]
- [67].Servili A, Le Page Y, Leprince J, Caraty A, Escobar S, Parhar IS, Seong JY, Vaudry H, and Kah O, Organization of two independent kisspeptin systems derived from evolutionary-ancient kiss genes in the brain of zebrafish. Endocrinology. 152 (2011) 1527–40. doi: 10.1210/en.2010-0948. Epub 2011 Feb 15. [DOI] [PubMed] [Google Scholar]
- [68].CR D, The variation of animals and plants under domestication, John Murray, London, 1875. [Google Scholar]
- [69].Trut L, Oskina I, and Kharlamova A, Animal evolution during domestication: the domesticated fox as a model. Bioessays. 31 (2009) 349–60. doi: 10.1002/bies.200800070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Asher GW, Monfort SL, and Wemmer C, Comparative reproductive function in cervids: implications for management of farm and zoo populations. J Reprod Fertil Suppl 54 (1999) 143–56. [PubMed] [Google Scholar]
- [71].Rauw WM, Johnson AK, Gomez-Raya L, and Dekkers JCM, A Hypothesis and Review of the Relationship between Selection for Improved Production Efficiency, Coping Behavior, and Domestication. Front Genet. 8:134 (2017) 10.3389/fgene.2017.00134. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gulevich RG, Oskina IN, Shikhevich SG, Fedorova EV, and Trut LN, Effect of selection for behavior on pituitary-adrenal axis and proopiomelanocortin gene expression in silver foxes (Vulpes vulpes). Physiol Behav. 82 (2004) 513–8. doi: 10.1016/j.physbeh.2004.04.062. [DOI] [PubMed] [Google Scholar]
- [73].Hekman JP, Johnson JL, Edwards W, Vladimirova AV, Gulevich RG, Ford AL, Kharlamova AV, Herbeck Y, Acland GM, Raetzman LT, Trut LN, and Kukekova AV, Anterior Pituitary Transcriptome Suggests Differences in ACTH Release in Tame and Aggressive Foxes. G3 (Bethesda). 8 (2018) 859–873. doi: 10.1534/g3.117.300508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Huang S, Slomianka L, Farmer AJ, Kharlamova AV, Gulevich RG, Herbeck YE, Trut LN, Wolfer DP, and Amrein I, Selection for tameness, a key behavioral trait of domestication, increases adult hippocampal neurogenesis in foxes. Hippocampus. 25 (2015) 963–75. doi: 10.1002/hipo.22420. Epub 2015 Mar 26. [DOI] [PubMed] [Google Scholar]
- [75].Bicskei B, Bron JE, Glover KA, and Taggart JB, A comparison of gene transcription profiles of domesticated and wild Atlantic salmon (Salmo salar L.) at early life stages, reared under controlled conditions. BMC Genomics. 15:884 (2014) 10.1186/1471-2164-15-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Varga M, The Doctor of Delayed Publications: The Remarkable Life of George Streisinger (1927–1984). Zebrafish. 15 (2018) 314–319. doi: 10.1089/zeb.2017.1531. Epub 2018 Jan 5. [DOI] [PubMed] [Google Scholar]
- [77].Mullins MC, Hammerschmidt M, Haffter P, and Nusslein-Volhard C, Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol. 4 (1994) 189–202. [DOI] [PubMed] [Google Scholar]
- [78].Shang EH, Yu RM, and Wu RS, Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio). Environ Sci Technol. 40 (2006) 3118–22. [DOI] [PubMed] [Google Scholar]
- [79].Villamizar N, Ribas L, Piferrer F, Vera LM, and Sanchez-Vazquez FJ, Impact of daily thermocycles on hatching rhythms, larval performance and sex differentiation of zebrafish. PLoS One 7 (2012) e52153. doi: 10.1371/journal.pone.0052153. Epub 2012 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dranow DB, Hu K, Bird AM, Lawry ST, Adams MT, Sanchez A, Amatruda JF, and Draper BW, Bmp15 Is an Oocyte-Produced Signal Required for Maintenance of the Adult Female Sexual Phenotype in Zebrafish. PLoS Genet. 12 (2016) e1006323. doi: 10.1371/journal.pgen.1006323. eCollection 2016 Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Slanchev K, Stebler J, de la Cueva-Mendez G, and Raz E, Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci U S A. 102 (2005) 4074–9. doi: 10.1073/pnas.0407475102. Epub 2005 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Siegfried KR, and Nusslein-Volhard C, Germ line control of female sex determination in zebrafish. Dev Biol. 324 (2008) 277–87. doi: 10.1016/j.ydbio.2008.09.025. Epub 2008 Oct 7. [DOI] [PubMed] [Google Scholar]
- [83].Rodriguez-Mari A, Canestro C, Bremiller RA, Nguyen-Johnson A, Asakawa K, Kawakami K, and Postlethwait JH, Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 6 (2010) e1001034. doi: 10.1371/journal.pgen.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dranow DB, Tucker RP, and Draper BW, Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev Biol. 376 (2013) 43–50. doi: 10.1016/j.ydbio.2013.01.016. Epub 2013 Jan 21. [DOI] [PubMed] [Google Scholar]
- [85].Engeszer RE, Patterson LB, Rao AA, and Parichy DM, Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 4 (2007) 21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- [86].Parichy DM, Advancing biology through a deeper understanding of zebrafish ecology and evolution. Elife. 4 (2015) 10.7554/eLife.05635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sharma KK, Sharma OP, and Tripathi NK, Female heterogamety in Danio rerio (Cypriniformes: Cyprinidae). Proc. Natl. Acad. Sci. INDIA 68(B) (1998) 123–126. [Google Scholar]
- [88].Orban L, Sreenivasan R, and Olsson PE, Long and winding roads: testis differentiation in zebrafish. Mol Cell Endocrinol. 312 (2009) 35–41. doi: 10.1016/j.mce.2009.04.014. Epub 2009 May 5. [DOI] [PubMed] [Google Scholar]
- [89].Siegfried KR, In search of determinants: gene expression during gonadal sex differentiation. J Fish Biol. 76 (2010) 1879–902. doi: 10.1111/j.1095-8649.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- [90].Tong SK, Hsu HJ, and Chung BC, Zebrafish monosex population reveals female dominance in sex determination and earliest events of gonad differentiation. Dev Biol. 344 (2010) 849–56. doi: 10.1016/j.ydbio.2010.05.515. Epub 2010 Jun 8. [DOI] [PubMed] [Google Scholar]
- [91].Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, and Smith JR, An SNP-Based Linkage Map for Zebrafish Reveals Sex Determination Loci. G3 (Bethesda). 1 (2011) 3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Anderson JL, Rodriguez Mari A, Braasch I, Amores A, Hohenlohe P, Batzel P, and Postlethwait JH, Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One 7 (2012) e40701. doi: 10.1371/journal.pone.0040701. Epub 2012 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liew WC, and Orban L, Zebrafish sex: a complicated affair. Brief Funct Genomics. 13 (2014) 172–87. doi: 10.1093/bfgp/elt041. Epub 2013 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, and Orban L, Polygenic sex determination system in zebrafish. PLoS One 7 (2012) e34397. doi: 10.1371/journal.pone.0034397. Epub 2012 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Streisinger G, Walker C, Dower N, Knauber D, and Singer F, Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature. 291 (1981) 293–6. [DOI] [PubMed] [Google Scholar]
- [96].Kirchmaier S, Naruse K, Wittbrodt J, and Loosli F, The genomic and genetic toolbox of the teleost medaka (Oryzias latipes). Genetics. 199 (2015) 905–18. doi: 10.1534/genetics.114.173849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Abraham E, Palevitch O, Gothilf Y, and Zohar Y, Targeted gonadotropin-releasing hormone-3 neuron ablation in zebrafish: effects on neurogenesis, neuronal migration, and reproduction. Endocrinology. 151 (2010) 332–40. doi: 10.1210/en.2009-0548. Epub 2009 Oct 27. [DOI] [PubMed] [Google Scholar]
- [98].Spicer OS, Wong TT, Zmora N, and Zohar Y, Targeted Mutagenesis of the Hypophysiotropic Gnrh3 in Zebrafish (Danio rerio) Reveals No Effects on Reproductive Performance. PLoS One. 11 (2016) e0158141. doi: 10.1371/journal.pone.0158141. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liu Y, Tang H, Xie R, Li S, Liu X, Lin H, Zhang Y, and Cheng CH, Genetic Evidence for Multifactorial Control of the Reproductive Axis in Zebrafish. Endocrinology. 158 (2017) 604–611. doi: 10.1210/en.2016-1540. [DOI] [PubMed] [Google Scholar]
- [100].Xia W, Smith O, Zmora N, Xu S, and Zohar Y, Comprehensive analysis of GnRH2 neuronal projections in zebrafish. Sci Rep. 4:3676 (2014) 10.1038/srep03676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Roch GJ, Busby ER, and Sherwood NM, GnRH receptors and peptides: skating backward. Gen Comp Endocrinol. 209:118–34. (2014) 10.1016/j.ygcen.2014.07.025. Epub 2014 Aug 5. [DOI] [PubMed] [Google Scholar]
- [102].Tello JA, Wu S, Rivier JE, and Sherwood NM, Four functional GnRH receptors in zebrafish: analysis of structure, signaling, synteny and phylogeny. Integrative and Comparative Biology 48 (2008) 570–87. [DOI] [PubMed] [Google Scholar]
- [103].Marvel M, Spicer OS, Wong TT, Zmora N, and Zohar Y, Knockout of the Gnrh genes in zebrafish: effects on reproduction and potential compensation by reproductive and feeding-related neuropeptides. Biol Reprod. 99 (2018) 565–577. doi: 10.1093/biolre/ioy078. [DOI] [PubMed] [Google Scholar]
- [104].Dungan HM, Clifton DK, and Steiner RA, Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 147 (2006) 1154–8. doi: 10.1210/en.2005-1282. Epub 2005 Dec 22. [DOI] [PubMed] [Google Scholar]
- [105].Seminara SB, and Crowley WF Jr., Kisspeptin and GPR54: discovery of a novel pathway in reproduction. J Neuroendocrinol. 20 (2008) 727–31. doi: 10.1111/j.1365-2826.2008.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kanda S, Akazome Y, Matsunaga T, Yamamoto N, Yamada S, Tsukamura H, Maeda K, and Oka Y, Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually dimorphic kisspeptin neurons in medaka (Oryzias latipes). Endocrinology. 149 (2008) 2467–76. doi: 10.1210/en.2007-1503. Epub 2008 Jan 17. [DOI] [PubMed] [Google Scholar]
- [107].van Aerle R, Kille P, Lange A, and Tyler CR, Evidence for the existence of a functional Kiss1/Kiss1 receptor pathway in fish. Peptides. 29 (2008) 57–64. doi: 10.1016/j.peptides.2007.10.018. Epub 2007 Oct 25. [DOI] [PubMed] [Google Scholar]
- [108].P.F. H., J.C. M., and H.J. B., Vertebrate Life, Pearson Prentice Hall, Upper Saddle River New Jersey, 2005. [Google Scholar]
- [109].Trudeau VL, Facing the Challenges of Neuropeptide Gene Knockouts: Why Do They Not Inhibit Reproduction in Adult Teleost Fish? Front Neurosci. 12:302 (2018) 10.3389/fnins.2018.00302. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Komiyama T, Kobayashi H, Tateno Y, Inoko H, Gojobori T, and Ikeo K, An evolutionary origin and selection process of goldfish. Gene. 430 (2009) 5–11. doi: 10.1016/j.gene.2008.10.019. Epub 2008 Nov 6. [DOI] [PubMed] [Google Scholar]
- [111].Kuang YY, Zheng XH, Li CY, Li XM, Cao DC, Tong GX, Lv WH, Xu W, Zhou Y, Zhang XF, Sun ZP, Mahboob S, Al-Ghanim KA, Li JT, and Sun XW, The genetic map of goldfish (Carassius auratus) provided insights to the divergent genome evolutions in the Cyprinidae family. Sci Rep. 6:34849 (2016) 10.1038/srep34849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ma W, Zhu ZH, Bi XY, Murphy RW, Wang SY, Gao Y, Xiao H, Zhang YP, and Luo J, Allopolyploidization is not so simple: evidence from the origin of the tribe Cyprinini (Teleostei: Cypriniformes). Curr Mol Med 14 (2014) 1331–8. [DOI] [PubMed] [Google Scholar]
- [113].Volkoff H, and Peter RE, Actions of two forms of gonadotropin releasing hormone and a GnRH antagonist on spawning behavior of the goldfish Carassius auratus. Gen Comp Endocrinol. 116 (1999) 347–55. doi: 10.1006/gcen.1999.7377. [DOI] [PubMed] [Google Scholar]
- [114].Leder EH, Danzmann RG, and Ferguson MM, Comparison of GNRH3 genes across salmonid genera. Anim Genet. 35 (2004) 126–9. doi: 10.1111/j.1365-2052.2004.01104.x. [DOI] [PubMed] [Google Scholar]
- [115].Ando H, and Urano A, Molecular regulation of gonadotropin secretion by gonadotropin-releasing hormone in salmonid fishes. Zoolog Sci. 22 (2005) 379–89. doi: 10.2108/zsj.22.379. [DOI] [PubMed] [Google Scholar]
- [116].Tsutsui K, Osugi T, Son YL, and Ubuka T, Review: Structure, function and evolution of GnIH. Gen Comp Endocrinol. 264:48–57. (2018) 10.1016/j.ygcen.2017.07.024. Epub 2017 Jul 25. [DOI] [PubMed] [Google Scholar]
- [117].Ubuka T, Ueno M, Ukena K, and Tsutsui K, Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system. J Endocrinol. 178 (2003) 311–8. [DOI] [PubMed] [Google Scholar]
- [118].Osugi T, Ubuka T, and Tsutsui K, Review: evolution of GnIH and related peptides structure and function in the chordates. Front Neurosci. 8:255 (2014) 10.3389/fnins.2014.00255. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tsutsui K, and Ubuka T, GnIH Control of Feeding and Reproductive Behaviors. Front Endocrinol (Lausanne). 7:170 (2016) 10.3389/fendo.2016.00170. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ogawa S, Sivalingam M, Biran J, Golan M, Anthonysamy RS, Levavi-Sivan B, and Parhar IS, Distribution of LPXRFa, a gonadotropin-inhibitory hormone ortholog peptide, and LPXRFa receptor in the brain and pituitary of the tilapia. J Comp Neurol. 524 (2016) 2753–75. doi: 10.1002/cne.23990. Epub 2016 Mar 7. [DOI] [PubMed] [Google Scholar]
- [121].Moussavi M, Wlasichuk M, Chang JP, and Habibi HR, Seasonal effects of GnIH on basal and GnRH-induced goldfish somatotrope functions. J Endocrinol. 223 (2014) 191–202. doi: 10.1530/JOE-14-0441. [DOI] [PubMed] [Google Scholar]
- [122].Munoz-Cueto JA, Paullada-Salmeron JA, Aliaga-Guerrero M, Cowan ME, Parhar IS, and Ubuka T, A Journey through the Gonadotropin-Inhibitory Hormone System of Fish. Front Endocrinol (Lausanne) 8 (2017) 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhang Y, Li S, Liu Y, Lu D, Chen H, Huang X, Liu X, Meng Z, Lin H, and Cheng CH, Structural diversity of the GnIH/GnIH receptor system in teleost: its involvement in early development and the negative control of LH release. Peptides. 31 (2010) 1034–43. doi: 10.1016/j.peptides.2010.03.003. Epub 2010 Mar 11. [DOI] [PubMed] [Google Scholar]
- [124].Spicer OS, Zmora N, Wong TT, Golan M, Levavi-Sivan B, Gothilf Y, and Zohar Y, The gonadotropin-inhibitory hormone (Lpxrfa) system’s regulation of reproduction in the brain-pituitary axis of the zebrafish (Danio rerio). Biol Reprod. 96 (2017) 1031–1042. doi: 10.1093/biolre/iox032. [DOI] [PubMed] [Google Scholar]
- [125].Yosten GL, Lyu RM, Hsueh AJ, Avsian-Kretchmer O, Chang JK, Tullock CW, Dun SL, Dun N, and Samson WK, A novel reproductive peptide, phoenixin. J Neuroendocrinol. 25 (2013) 206–15. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, and Yosten GL, Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol. 311 (2016) R489–96. doi: 10.1152/ajpregu.00191.2016. Epub 2016 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lyu RM, Huang XF, Zhang Y, Dun SL, Luo JJ, Chang JK, and Dun NJ, Phoenixin: a novel peptide in rodent sensory ganglia. Neuroscience. 250:622–31. (2013) 10.1016/j.neuroscience.2013.07.057. Epub 2013 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Yuan T, Sun Z, Zhao W, Wang T, Zhang J, and Niu D, Phoenixin: A Newly Discovered Peptide with Multi-Functions. Protein Pept Lett 24 (2017) 472–475. doi: 10.2174/0929866524666170207154417. [DOI] [PubMed] [Google Scholar]
- [129].Treen AK, Luo V, and Belsham DD, Phoenixin Activates Immortalized GnRH and Kisspeptin Neurons Through the Novel Receptor GPR173. Mol Endocrinol. 30 (2016) 872–88. doi: 10.1210/me.2016-1039. Epub 2016 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Palasz A, Janas-Kozik M, Borrow A, Arias-Carrion O, and Worthington JJ, The potential role of the novel hypothalamic neuropeptides nesfatin-1, phoenixin, spexin and kisspeptin in the pathogenesis of anxiety and anorexia nervosa. Neurochem Int. 113:120–136. (2018) 10.1016/j.neuint.2017.12.006. Epub 2017 Dec 15. [DOI] [PubMed] [Google Scholar]
- [131].Park MK, and Wakabayashi K, Preparation of a monoclonal antibody to common amino acid sequence of LHRH and its application. Endocrinol Japan 33 (1986) 257–72. [DOI] [PubMed] [Google Scholar]
- [132].Pitteloud N, Durrani S, Raivio T, and Sykiotis GP, Complex genetics in idiopathic hypogonadotropic hypogonadism. Front Horm Res 39:142–53. (2010) 10.1159/000312700. Epub 2010 Apr 8. [DOI] [PubMed] [Google Scholar]
- [133].Whitlock KE, Origin and development of GnRH neurons. Trends Endocrinol Metab 16 (2005) 145–51. [DOI] [PubMed] [Google Scholar]
- [134].Casane D, and Retaux S, Evolutionary Genetics of the Cavefish Astyanax mexicanus. Adv Genet 95:117–59. (2016) 10.1016/bs.adgen.2016.03.001. Epub 2016 Jun 13. [DOI] [PubMed] [Google Scholar]
- [135].Stein LM, Haddock CJ, Samson WK, Kolar GR, and Yosten GLC, The phoenixins: From discovery of the hormone to identification of the receptor and potential physiologic actions. Peptides. 106:45–48. (2018) 10.1016/j.peptides.2018.06.005. Epub 2018 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]


