The outbreak of the COVID-19 pandemic in early 2020 poses dramatic problems to the health systems as no vaccine or truly effective drugs are yet available. The international scientific community is struggling to find new substances capable of contrasting the SARS-CoV-2 virus. A straightforward strategy to disclose compounds readily available to clinicians is drug repurposing, i.e. the use of drugs that were previously approved by the FDA for a different therapeutic indication. A few promising compounds against SARS-CoV-2 were identified through drug repurposing, e.g. remdesivir, chloroquine and hydroxychloroquine, tocilizumab, etc., but their therapeutic efficacy in COVID-19 patients is still debated. On the other hand, extensive screenings are conducted on thousands of novel molecules using combinatorial libraries or in silico docking experiments to discover new effective antiviral agents. Despite the intense research efforts, we were surprised to learn that no metal compound is currently being tested against the SARS-CoV-2 virus.1 Metal based agents form a variegate and attractive class of drugs with a number of therapeutic applications: we strongly encourage the international scientific community to fill this gap quickly and explore the potential of metallodrugs against COVID-19 disease.
A simple approach might be represented by the repurposing of clinically approved metal-based drugs. The ideal candidate should associate a good antiviral activity and a tolerable toxicity. To this end we recommend a rapid evaluation of auranofin (Ridaura) (Figure 1), AF hereafter.
Figure 1.
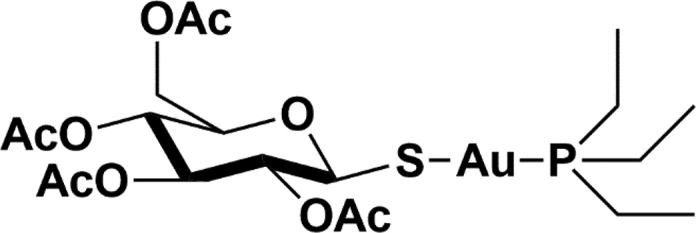
Chemical structure of auranofin.
AF is a drug approved by the FDA in 1985 for the treatment of rheumatoid arthritis mainly acting through a modulation of the immune response. AF shows an acceptable toxicity profile and is safe for human use. The exact mechanism of action of AF, most likely a multitarget one, is still debated. Yet, there is a growing consent in considering thioredoxin reductase 1 as the primary target, leading to perturbation of the main oxidoreductase pathways, dysregulation of intracellular redox homeostasis and reactive oxygen species (ROS) induction; the proteasome is a secondary but still important target.2−4
AF prompted a lot of interest during the last years for its versatility and for the chance to be repurposed for different therapeutic indications such as an antibacterial, anticancer, or antiparasitic agent.2 Significant activity against HIV was reported as well; AF entered accordingly clinical trials as an antiretroviral agent. It is worth reminding that AF, in the case of HIV infection, was found to be more effective than hydroxychloroquine and chloroquine in interfering with several processes involved in viral production, latency, and viral reactivation as well as in the reduction of the viral reservoir.5 Similarly to tocilizumab, AF was reported to interfere with the interleukin 6 signaling by inhibiting phosphorylation of JAK1 and STAT3, to inhibit few selected proteases and to bind preferentially to free cysteine residues in proteins, e.g. in cysteine proteases.6Based on these arguments, we support the off label quick evaluation of AF in COVID-19 patients.
Remarkably, during the review process of this manuscript, an article concerning AF and COVID-19 appeared in the public domain.7 In this paper the authors report that AF, at a low micromolar concentration, strongly inhibits SARS-COV-2 replication in human cells with a spectacular 95% reduction in the viral RNA. In addition, AF was found to dramatically reduce the expression of SARS-COV-2-induced cytokines in human cells, in line with the previous observations. These results offer an excellent support to our proposal and suggest that AF, owing to its favorable and multitarget mechanism, might be a useful drug to limit SARS-CoV-2 infection and treat the associated pneumonia.
Beyond the specific suggestion of auranofin and related gold compounds, we believe that extensive in vitro testing of a larger panel of representative metal-based agents containing a variety of metal centers, e.g. ruthenium and bismuth, should be pursued. We may reasonably expect that such unusual and unique metal centers, in a few cases, will produce important and favorable effects on this new pathogen that are difficult to predict a priori.
Acknowledgments
The authors thank the CIRCMSB (Consorzio Interuniversitario di Ricerca in Chimica dei Metalli nei Sistemi Biologici), Italy. Beneficentia Stiftung (Vaduz, Liechtenstein) and Fondazione CR Firenze (Firenze, Italy) are also acknowledged.
Views expressed in this editorial are those of the authors and not necessarily the views of the ACS.
References
- Gordon D. E.; Jang G. M.; Bouhaddou M.; Xu J.; Obernier K.; White K. M.; O’Meara M. J.; Rezelj V. V.; Guo J. Z.; Swaney D. L.; Tummino T. A.; Huettenhain R.; Kaake R. M.; Richards A. L.; Tutuncuoglu B.; Foussard H.; Batra J.; Haas K.; Modak M.; Kim M.; Haas P.; Polacco B. J.; Braberg H.; Fabius J. M.; Eckhardt M.; Soucheray M.; Bennett M. J.; Cakir M.; McGregor M. J.; Li Q.; Meyer B.; Roesch F.; Vallet T.; Mac Kain A.; Miorin L.; Moreno E.; Chi Naing Z. Z.; Zhou Y.; Peng S.; Shi Y.; Zhang Z.; Shen W.; Kirby I. T.; Melnyk J. E.; Chorba J. S.; Lou K.; Dai S. A.; Barrio-Hernandez I.; Memon D.; Hernandez-Armenta C.; Lyu J.; Mathy C. J. P.; Perica T.; Pilla K. B.; Ganesan S. J.; Saltzberg D. J.; Rakesh R.; Liu X.; Rosenthal S. B.; Calviello L.; Venkataramanan S.; Liboy-Lugo J.; Lin Y.; Huang X.-P.; Liu Y. F.; Wankowicz S. A.; Bohn M.; Safari M.; Ugur F. S.; Koh C.; Savar N. S.; Tran Q. D.; Shengjuler D.; Fletcher S. J.; O’Neal M. C.; Cai Y.; Chang J. C. J.; Broadhurst D. J.; Klippsten S.; Sharp P. P.; Wenzell N. A.; Kuzuoglu D.; Wang H.-Y.; Trenker R.; Young J. M.; Cavero D. A.; Hiatt J.; Roth T. L.; Rathore U.; Subramanian A.; Noack J.; Hubert M.; Stroud R. M.; Frankel A. D.; Rosenberg O. S.; Verba K. A.; Agard D. A.; Ott M.; Emerman M.; Jura N.; von Zastrow M.; Verdin E.; Ashworth A.; Schwartz O.; d’Enfert C.; Mukherjee S.; Jacobson M.; Malik H. S.; Fujimori D. G.; Ideker T.; Craik C. S.; Floor S. N.; Fraser J. S.; Gross J. D.; Sali A.; Roth B. L.; Ruggero D.; Taunton J.; Kortemme T.; Beltrao P.; Vignuzzi M.; García-Sastre A.; Shokat K. M.; Shoichet B. K.; Krogan N. J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder C.; Thomson M. J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R&D 2015, 15, 13–20. 10.1007/s40268-015-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Selvaraju K.; Saei A. A.; D’Arcy P.; Zubarev R. A.; Arnér E. S.; Linder S. Repurposing of auranofin: Thioredoxin reductase remains a primary target of the drug. Biochimie 2019, 162, 46–54. 10.1016/j.biochi.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Liu N.; Li X.; Huang H.; Zhao C.; Liao S.; Yang C.; Liu S.; Song W.; Lu X.; Lan X.; Chen X.; Yi S.; Xu L.; Jiang L.; Zhao C.; Dong X.; Zhou P.; Li S.; Wang S.; Shi X.; Dou P. Q.; Wang X.; Liu J. Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget 2014, 5, 5453–5471. 10.18632/oncotarget.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A.; Shytaj I. L. Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology 2015, 12, 51. 10.1186/s12977-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. H.; Lee M. Y.; Park S. J.; Choi J. S.; Oh M. K.; Kim I. S. Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology 2007, 122, 607–614. 10.1111/j.1365-2567.2007.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H. A.; Stone S.; Natekar J.; Kumari P.; Arora K.; Kumar M. The FDA-approved gold drug Auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Biorxiv.org 2020, 10.1101/2020.04.14.041228. [DOI] [PMC free article] [PubMed] [Google Scholar]


