In comparing the disease course of newly diagnosed subjects with VEO-IBD with older pediatric IBD, we found that subjects with VEO-IBD had more severe disease than older children. There were more medication failures and colectomies and persistent growth failure.
Keywords: very early onset-inflammatory bowel disease, growth failure, disease course
Abstract
Background
Insight into the pathogenesis of very early onset-inflammatory bowel disease (VEO-IBD) has expanded through the identification of causative monogenic defects detected in a subset of patients. However, the clinical course of this population remains uncertain. The study objective is to determine whether VEO-IBD is associated with more severe disease, defined as increased surgical intervention and growth failure, than older pediatric IBD. Secondary outcomes included therapeutic response and hospitalizations.
Methods
Subjects with IBD diagnosed younger than 6 years old (VEO-IBD) were compared with children diagnosed 6 to 10 (intermediate-onset) and older than 10 years of age (older-onset IBD). Metadata obtained from the medical record included age of onset, disease phenotype and location, surgeries, medical therapy, and comorbid conditions. Length of follow-up was at least 1 year from diagnosis.
Results
There were 229, 221, and 521 subjects with VEO, intermediate-onset, and older-onset IBD, respectively. Very early onset-inflammatory bowel disease subjects underwent more diverting ileostomies (P < 0.001) and colectomies (P < 0.001) than the older children. There was less improvement in weight- and height-for-age Z scores during the follow-up period in subjects with VEO-IBD. Additionally, subjects with VEO-IBD had higher rates of medication failure at 1 year and were more frequently readmitted to the hospital. Targeted therapy was successfully used almost exclusively in VEO-IBD.
Conclusion
Patients with VEO-IBD can have a more severe disease course with increased surgical interventions and poor growth as compared with older-onset IBD patients. Further, VEO-IBD patients are more likely to be refractory to conventional therapies. Strategies using targeted therapy in these children can improve outcome and, in some cases, be curative.
Introduction
Inflammatory bowel disease (IBD) is a complex disease resulting from an aberrant immune response to environmental exposures, most notably the gut microbiome,1, 2 in a genetically susceptible host. The role that these factors play in disease development differs among patient populations. In older pediatric and adult patients, the disease is most often polygenic, involving over 230 risk loci identified mainly through genome-wide association studies.3, 4 This is in contrast with patients with very early onset-IBD (VEO-IBD), which comprises children diagnosed with IBD at 6 years of age or younger. A subset of patients with VEO-IBD have a stronger genetic contribution to their disease, including those in whom monogenic or digenic causative defects have been identified, largely through whole exome sequencing (WES) and targeted sequencing studies.5–9 Many of these identified variants are in primary immunodeficiency genes or are associated with epithelial defects.
Currently, there are conflicting data describing the disease course of VEO-IBD, largely due to the phenotypic and genetic heterogeneity of the population.10–12 Furthermore, the prognosis of this disease is relatively underexplored. Determining the optimal therapy for children with VEO-IBD, therefore, can be challenging, particularly when there is aggressive epithelial disease or marked immune dysregulation in the absence of an identified genetic defect. Subsequently, patients are often exposed to broad immunosuppressive agents typically used to treat older-onset pediatric IBD, which can be inappropriate in the setting of immune dysfunction.7, 11, 13 Better insight into the mechanisms of disease and disease progression is critically needed to develop targeted therapeutic approaches for these children and improve their disease outcome.
Our prior work in this cohort using WES demonstrated an enrichment of rare variants in genes associated with primary immunodeficiencies in patients with VEO-IBD, as compared with older-onset pediatric and adult IBD.14 Our clinical observations have suggested that VEO-IBD is frequently a different disease process and more likely to follow a severe and refractory course than older-onset pediatric IBD.15, 16 Therefore, to better characterize the disease course in children with VEO-IBD, we have established a VEO-IBD registry with detailed genomic and phenotypic data. The aim of this study is to objectively evaluate whether VEO-IBD is indeed associated with a more severe disease course, defined as increased rate of surgery and persistent growth failure, as compared with pediatric IBD diagnosed older than 6 years of age. Our secondary aim is to compare the medication use and hospitalizations between VEO-IBD and pediatric IBD.
MATERIALS AND METHODS
Study Cohort
This was a retrospective single-center review of patients with IBD followed at the Children’s Hospital of Philadelphia (CHOP) from 2008 to 2016. Patients were recruited from the Center for Pediatric IBD at CHOP, and 3 cohorts were included in this study: 1) patients with VEO-IBD or IBD diagnosed at 6 years or younger, 2) intermediate-onset IBD, defined as patients diagnosed with IBD between ages 6 and 10 years, and 3) older-onset pediatric IBD, defined as children diagnosed with IBD at ≥10 years or older (but less than 18) years of age. Inclusion criteria were as follows: patients with newly confirmed diagnosis of IBD at CHOP between 2008 and 2016 by standard methods of endoscopic, radiologic, laboratory and clinical evaluation, and whose primary gastrointestinal (GI) care was at CHOP for at least 1 year from diagnosis. Exclusion criteria included patients with a prior diagnosis of other intestinal disease (including chronic allergic or inflammatory diseases) or patients with IBD with less than 1 year of follow-up from diagnosis or incomplete data.
Patients were followed from the date of diagnosis, defined as the date that the first definitive diagnostic test was performed. Follow-up continued until either the patient transferred their care to another institution or the end of the available data at the time the present article was submitted. As decribed later on, specific follow-up points were used for some of the individual analyses.
Clinical Data
Clinical information was obtained from the electronic medical records of the study population. Subjects were categorized into 3 groups based on age at diagnosis, as described previously. The following variables were obtained: date of birth, sex, date of IBD diagnosis, age at time of diagnosis, weight, height, body mass index (BMI) at baseline and follow-up time point, Z scores, IBD diagnosis (Crohn’s disease [CD], ulcerative colitis (UC), IBD-unclassified [IBD-U]), disease phenotype by Paris classification,17 macroscopic location of disease, diagnostic gastrointestinal pathology results, IBD-related medication history, IBD-related surgical history, and number and length of hospitalizations. Crohn’s disease was diagnosed when any of the following features were present in the diagnostic evaluation: endoscopic skip lesions or ileal inflammation in the presence of a normal cecum, histologic evidence of epithelioid granulomas or chronic ileitis, radiologic evidence of thickened small bowel loops, or evidence of intestinal fistulizing disease or perianal disease. Ulcerative colitis was diagnosed in cases of diffuse continuous mucosal ulceration of varying severity extending through the colon proximally from the rectum. Inflammatory bowel disease–unclassified was diagnosed in cases consistent with ulcerative colitis but with at least 1 of the following additional features: rectal sparing, macroscopic duodenal or esophageal ulcers without comorbidities, or numerous gastric aphthous lesions without additional etiology. In addition, IBD-U was diagnosed in children with aphthous lesions in the colon without additional histologic findings.17, 18
For weight and height, the World Health Organization (WHO) growth curves were used for children 0 to 24 months, and the Centers for Disease Control (CDC) growth curves were used for children older than 2 years of age. In an effort to further delineate disease severity in VEO-IBD, we compared their therapeutic response to anti-TNF therapy and immunomodulators to older-onset pediatric IBD (older than 10 years of age) at 52 weeks. We limited the scope of this analysis to these 2 cohorts due to the need for extensive manual review of the medication data performed by 2 clinicians. The review included date of initiation and discontinuation of therapy, response to therapy, and reasons for the discontinuation.
Outcome Definition
The primary outcome measures were progression to surgery and growth failure. These outcome measures were chosen because of their clinical importance and the fact that they are typically well documented in the medical record. Surgery in particular was selected as an indicator of disease severity as it is most frequently a last resort intervention, signifying that all other therapies have failed to induce remission. We defined relevant surgeries as ileocecal resection, diverting ileostomy, colostomy, colectomy, and hemicolectomy. Age at time of surgery and indication for surgery (categorized as medically refractory inflammatory disease, refractory perianal disease, perforation, abscess, bowel obstruction/stricturing disease, penetrating disease, and growth failure) were obtained. Our second primary outcome was growth failure as measured by the change in weight, height, and BMI Z scores, calculated through the WHO (for patients 2 years or younger) and CDC (for patients older than 2 years) from diagnosis to follow-up. For this outcome measure, we included patients who had data obtained at a follow-up visit between 3 to 5 years after diagnosis. Subjects with only shorter or longer follow-up time data were excluded from this analysis to provide adequate time for growth assessment and to ensure the analyses were comparable.
As secondary outcomes, we included rate and length of hospitalizations and medication use. Medication failure was defined as steroid dependence, failure to achieve clinical remission, discontinuation of therapy or escalation to another medication at 1 year, or surgery. Steroid dependence was defined as glucocorticoid therapy that was unable to be tapered to less than prednisone 10 mg/day (or therapeutic equivalent) within 3 months of starting steroids without recurrent disease or if symptoms recurred within 3 months of stopping steroids.
Statistical Analysis
Standard descriptive statistics were used to describe subject characteristics stratified by age and IBD classification. Summary statistics such as medians, means, quartiles, and standard deviations were compiled for all measured variables. Binary data were analyzed using Fisher exact test. The Wilcoxon signed rank test was used to compare Z scores within groups and the Wilcoxon rank sum test was used to compare Z scores and number of hospitalizations across the different groups. Statistical significance was determined at the 2-sided α = 0.05 level for all tests. All statistical analyses were done in R (v3.2.2).19
Ethical Considerations
This study was approved by the Institutional Review Board of The Children’s Hospital of Philadelphia (Protocols 14-010826 and 13-010430).
RESULTS
There were 3091 patients diagnosed between the ages of 6 and 18 with older-onset IBD and 503 patients diagnosed with VEO-IBD before age 6 who were followed at our center from 2008 to 2015. A total of 420 patients diagnosed with VEO-IBD and 815 older children with IBD were identified for this study based on inclusion and exclusion criteria. From this identified cohort, patients were excluded if initial diagnostic data was missing, if there was insufficient follow-up time as a primary patient at this institution (<1 year), or if there was incomplete data. Thus, 229 patients with VEO-IBD, 221 patients with intermediate-onset IBD (age 6 to 10) and 521 with older-onset IBD (age 10 to 18) with complete data were ultimately included. This was an unselected cohort, with heterogeneous disease presentation and severity.
The median age of diagnosis in the VEO-IBD cohort was 3.9 years (interquartile range [IQR] 2.5, 4.8) as compared with 8.0 years (IQR 6.9, 9.2) in the intermediate-onset group and 13.3 (IQR 11.7, 15.3) in older-onset IBD. Males represented 58.5% of patients in the VEO-IBD group, 53.4% in the intermediate-onset IBD group, and 56.4% in older-onset IBD group (Table 1).
Table 1.
Cohort Demographics
| VEO | Intermediate | Older Onset | |||
|---|---|---|---|---|---|
| (n = 229) | (n = 221) | (n = 521) | P VEO vs Intermediate | P VEO vs Older Onset | |
| Female | 95 (41.5) | 103 (46.6) | 227 (43.6) | ns | ns |
| Age at Diagnosis (Years) a | 3.9 (2.5–4.8) | 8.0 (6.9–9.2) | 13.3 (11.7–15.3) | n/a | n/a |
| Ethnicity | P < 0.001 | P < 0.0001 | |||
| Not Hispanic or Latino | 200 (87.3) | 204 (92.3) | 494 94.8) | ||
| Hispanic or Latino | 28 (12.2) | 7 (3.2) | 24 (4.6) | ||
| Unknown | 1 (0.4) | 10 (4.5) | 3 (0.6) | ||
| Race | ns | P < 0.01 | |||
| White | 188 (82.1) | 177 (80.1) | 413 (79.3) | ||
| Black | 16 (7.0) | 27 (12.2) | 60 (11.5) | ||
| Asian | 15 (6.6) | 8 (3.6) | 8 (1.5) | ||
| American Indian or Alaska Native | 0 (0) | 1 (0.5) | 2 (0.4) | ||
| Multiple Races | 4 (1.7) | 1 (0.5) | 7 (1.3) | ||
| Unknown | 6 (2.6) | 7 (3.2) | 31 (6.0) | ||
| Continent of Origin | ns | ns | |||
| Europe | 73 (31.9) | 14 (6.3) | 11 (2.1) | ||
| North America | 14 (6.1) | 9 (4.1) | 5 (1.7) | ||
| Asia | 14 (6.1) | 4 (1.8) | 0 (0) | ||
| Middle East Region | 4 (1.7) | 0 (0) | 0 (0) | ||
| South America | 3 (1.3) | 0 (0) | 0 (0) | ||
| Africa | 2 (0.9) | 0 (0) | 0 (0) | ||
| Multiple | 48 (21.0) | 8 (3.6) | 1 (0.2) | ||
| Unknown | 71 (31.0) | 186 (84.2) | 504 (96.7) | ||
| Disease Type | ns | ns | |||
| Crohn’s Disease | 129 (56.3) | 130 (58.8) | 328 (63.0) | ||
| Ulcerative Colitis | 30 (13.1) | 26 (11.8) | 64 (12.3) | ||
| IBD-U | 70 (30.6) | 65 (29.4) | 129 (24.8) | ||
| Crohn’s Disease Phenotype | P < 0.05 | P < 0.0001 | |||
| B1 Inflammatory, Nonpenetrating and Nonstricturing | 112 (86.8) | 98 (75.4) | 217 (66.2) | ||
| B2 Stricturing | 12 (9.3) | 22 (16.9) | 62 (18.9) | ||
| B3 Penetrating | 5 (3.9) | 4 (3.1) | 20 (6.1) | ||
| B2B3 Stricturing and Penetrating | 0 (0) | 6 (4.6) | 29 (8.8) | ||
| Crohn’s Disease Location | P < 0.0001 | P < 0.0001 | |||
| L2 Colonic Only | 59 (45.7) | 29 (22.3) | 50 (15.2) | ||
| L3 Ileocolonic | 54 (41.9) | 89 (68.5) | 233 (71.0) | ||
| L4a/L4b Small Bowel | 3 (2.3) | 12 (9.2) | 45 (13.7) | ||
| Panenteric | 13 (10.1) | 0 (0) | 0 (0) | ||
| Perianal | 29 (24) | 22 (17) | 59 (18) | ns | ns |
| Extent of Ulcerative Colitis | ns | ns | |||
| E1 Ulcerative Proctitis (rectum only) | 1 (3) | 1 (4) | 5 (8) | ||
| E2 Left sided UC (distal to the splenic flexure only) | 7 (23.3) | 5 (19.2) | 11 (17.2) | ||
| E3 Extensive UC (extends proximal to the splenic flexure) | 2 (6.7) | 2 (7.7) | 10 (15.6) | ||
| E4 Pancolitis | 20 (66.7) | 18 (69.2) | 38 (59.4) |
Total counts (percentages) are shown unless otherwise noted.
aReported as median (IQR).
Paris Classification of Pediatric IBD
Crohn’s disease was diagnosed in 56.3% of VEO-IBD patients as compared with 58.8% in intermediate-onset IBD and 63.0% of patients with older-onset IBD. Similarly, there were comparable rates of IBD-U and UC among all 3 age cohorts (P > 0.05). Among the VEO-IBD subjects with CD, the disease behavior was overwhelmingly inflammatory, nonstricturing, nonpenetrating (B1), and there were no cases of penetrating and stricturing disease (B3). Conversely, 66.2% of older-onset IBD had an inflammatory (B1) phenotype, and the rest had complicated disease including 18.9% stricturing (B2), 6.1% penetrating (B3), and 8.8% penetrating and stricturing disease (B2B3). The extent of disease in UC was similar in all 3 cohorts (Table 1).
Crohn’s Disease Location
Overall, there were significant differences in location of disease between VEO-IBD and intermediate-onset IBD and older-onset IBD (P < 0.0001). Despite the considerable number of subjects diagnosed with CD, there was more isolated endoscopic colonic disease in subjects with VEO-IBD (45.7%) as compared with intermediate-onset (22.3%; P < 0.001) and older-onset IBD (15.2%; P < 0.0001). There was no difference in the frequency of perianal disease in subjects with VEO-IBD compared with intermediate-onset or older-onset IBD (P > 0.05) (Table 1)
Change in Diagnosis
During the study window, subjects underwent repeat endoscopy and colonoscopy when clinically indicated. There were similar rates of change of diagnosis in the 3 cohorts. Ten subjects with VEO-IBD (4.3%) had the diagnosis changed from IBD-U to CD based on the subsequent identification of granulomas, small bowel disease, or perianal disease during the follow-up period. There were 2 subjects with VEO-IBD whose diagnosis was changed from IBD-U to UC after colectomy (0.9%). In intermediate-onset IBD, there were 5 subjects (2.3%) whose diagnosis changed to CD from IBD-U based on the development of perianal disease or small bowel disease. One patient in the intermediate-onset IBD cohort had the diagnosis changed to UC following colectomy (0.5%). In the older-onset IBD group, there were 22 (4.2%) changes in diagnoses from IBD-U to CD based on development of perianal or small bowel disease.
Surgical Outcomes
The proportion of patients who underwent IBD-related surgeries were similar in all cohorts: 13.1% for VEO-IBD, 10.9% for intermediate-onset and 14.6% for older-onset IBD (P > 0.05). The average number of surgeries per patient was significantly higher in children with VEO-IBD (1.5 surgeries/patient) than both intermediate (1.2 surgeries/patient, P < 0.01) and older-onset IBD (1 surgery/patient, P < 0.0001). There were 16 subjects (53.3%) with VEO-IBD who had more than 2 surgeries as compared with 4 subjects (16.7%; P < 0.01) with intermediate-onset IBD and 3 older-onset IBD subjects (3.9%; P < 0.0001).
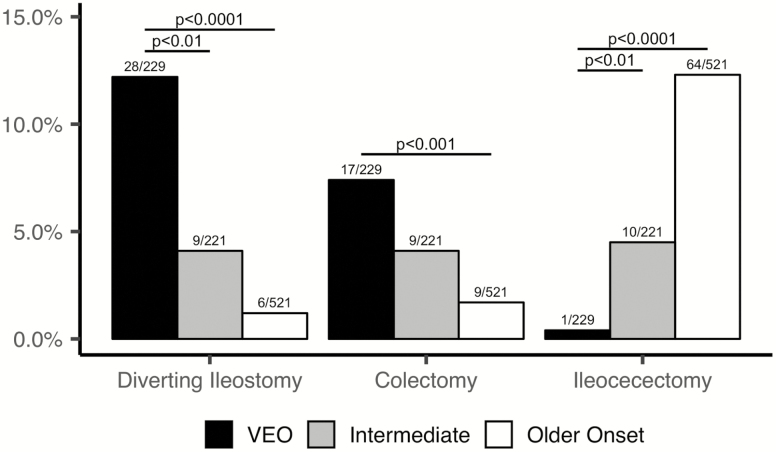
The location and phenotype of disease dictated the specific surgeries performed. Subjects with VEO-IBD underwent more diverting ileostomies compared with intermediate-onset IBD and older-onset pediatric IBD (12.2% vs 4.1%, P < 0.01; and 1.2%, P < 0.0001). There were still significantly more diverting ileostomies in VEO-IBD when excluding those with monogenic defects (10.5%) in comparison with the intermediate (P < 0.05) or older-onset IBD groups (P < 0.0001). In addition, VEO-IBD subjects had significantly more colectomies (7.4%) as compared with older-onset pediatric IBD (1.7%) (P < 0.001). Similarly, although not statistically significant, the VEO-IBD cohort had more colectomies than intermediate-onset IBD (4.1%) (Fig. 1). These findings were unchanged when removing the VEO-IBD subjects with monogenic defects, as well. However, consistent with the differences in the phenotype, the rate of ileocecal resections was significantly greater in older-onset IBD (P < 0.0001) and intermediate-onset IBD (P < 0.01) compared with subjects with VEO-IBD. Indication for surgery in the VEO-IBD subjects included medication failure primarily with subsequent persistent severe colonic disease but also growth failure, colonic stricture, colonic perforation, and perianal disease (Table 2B). The older-onset IBD subjects more commonly had surgical intervention for penetrating and stricturing disease (Table 2A).
Figure 1.
Frequency of inflammatory bowel disease–related surgeries among the 3 study groups.
Table 2A.
Types of Surgery by Age Group and Disease Type. (Data Is Shown as Counts.) Patients Can Present With More Than 1 Surgery.
| VEO | Intermediate | Older Onset | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Surgery | CD | IBD-U | UC | CD | IBD-U | UC | CD | IBD-U | UC |
| Diverting ileostomy | 14 | 11 | 3 | 6 | 2 | 1 | 3 | 2 | 1 |
| Colectomy | 6 | 7 | 4 | 1 | 5 | 3 | 4 | 1 | 4 |
| Ileocecectomy | 1 | 0 | 0 | 8 | 2 | 0 | 55 | 9 | 0 |
Table 2B.
Indications for Surgery. Data Is Shown as Counts (percentage). Patients Can Present With More Than 1 Indication.
| VEO | Intermediate | Older Onset | |
|---|---|---|---|
| Diverting Ileostomy | n = 28 | n = 9 | n = 6 |
| Growth Failure | 13 (46.4) | 1 (11.1) | 0 (0) |
| Severe Colitis | 12 (42.9) | 5 (55.6) | 4 (66.7) |
| Perianal Disease | 6 (21.4) | 2 (22.2) | 0 (0) |
| Colonic Stricture | 1 (3.6) | 1 (11.1) | 0 (0) |
| Intestinal Perforation | 1 (3.6) | 0 (0) | 2 (33.3) |
| Colectomy | n = 17 | n = 9 | n = 9 |
| Severe Colitis | 8 (47.1) | 7 (77.8) | 4 (44.4) |
| Growth Failure | 6 (35.3) | 1 (11.1) | 0 (0) |
| Perianal Disease | 3 (17.6) | 0 (0) | 0 (0) |
| Colonic Stricture | 1 (5.9) | 0 (0) | 3 (33.3) |
| Intestinal Perforation | 1 (5.9) | 1 (11.1) | 1 (11.1) |
| Penetrating | 0 (0) | 0 (0) | 1 (11.1) |
| Ileocecectomy | n = 1 | n = 10 | n = 64 |
| Abscess | 1 (100) | 0 (0) | 9 (14.1) |
| Stricture | 0 (0) | 6 (60.0) | 56 (87.5) |
| Phlegmon/Fistula | 0 (0) | 3 (30.0) | 20 (31.3) |
| Perforation | 0 (0) | 1 (10.0) | 2 (3.1) |
Growth
At baseline, the median weight for age and height-for-age z scores were similar among all 3 age groups (Fig. 2A, C, E). When comparing the change from diagnosis to the follow-up point, there was no improvement in median weight-for-age z scores in VEO-IBD compared with a significant improvement in intermediate-onset IBD (P < 0.05) and in older-onset IBD cohorts (P < 0.0001). In addition, there was no improvement in the BMI-for-age z score in the VEO-IBD population as compared with significant improvement in the older-onset IBD cohort (P < 0.0001).
Figure 2.
Growth parameters for 3 study groups. A, Height Z score by study group at diagnosis (visit 1) and follow-up time point (follow-up). B, Percentage of patients with stunting defined as height Z score less than negative two by study group at baseline (visit 1) and follow-up time point. C, Weight Z score by study group at diagnosis (visit 1) and follow-up time point (follow-up). D, Percentage of patients underweight defined as height Z score less than negative two by study group at baseline (visit 1) and follow-up time point. E, BMI Z score by study group at diagnosis (visit 1) and follow-up time point (follow-up). F, Percentage of patients with malnutrition defined as height Z score less than negative two by study group at baseline (visit 1) and follow-up time point.
Analysis of growth parameters was performed to characterize the frequency of poor nutrition (defined by stunting or low weight) among the cohorts as a marker of disease severity. At diagnosis, there were similar rates of stunted growth (height-for-age z score less than negative two) and low weight (weight-for-age z score less than negative two) among all 3 age groups (Fig. 2B, D, E). However, there were significantly more VEO-IBD subjects who remained stunted relative to older-onset IBD at the end of the follow-up period (P < 0.05). There was also a higher percentage of VEO-IBD subjects with stunted growth compared with the intermediate-onset cohort, although it did not reach statistical significance. There was less improvement in the weight, height, or BMI less than negative two z scores among the subjects with VEO-IBD or intermediate-onset IBD compared with older-onset IBD (P < 0.05).
Medication Use
Subjects with VEO-IBD were treated with immunomodulators (6-MP, azathioprine, and methotrexate) as monotherapy more frequently than older-onset pediatric IBD (35.4% vs 7.9%; P < 0.0001). Within immunomodulators, all cohorts were exposed more frequently to AZA/6MP than methotrexate as monotherapy. Both cohorts had similar exposures to antitumor necrosis factor-alpha (TNFα) agents, such as infliximab (IFX) and adalimumab (ADA) (58.1% vs 69.5%).
As described previously, treatment failure was defined as steroid dependence, discontinuation of therapy, and/or failure to achieve clinical remission at 1 year. Very early onset-IBD subjects failed IFX more frequently at 1 year from initiation of therapy as compared with subjects with older-onset IBD (62.4% vs 14.6%; P < 0.0001). Similar findings were seen when comparing ADA use, with VEO-IBD subjects failing this therapy more frequently compared with older-onset IBD (53.2% vs 7.2%; P < 0.0001). In addition, subjects with VEO-IBD failed immunomodulatory therapy more frequently than older pediatric IBD (56 of 81, 69.1% vs 8 of 41, 19.5%; P < 0.0001) as shown in Figure 3.
Figure 3.
Medication failure rate of infliximab, all anti-TNF alpha therapies, and immunomodulators in VEO-IBD vs older pediatric onset IBD subjects.
Due to the 9-year study window that spans a paradigm shift in pediatric IBD therapy, we performed a subanalysis of therapies and outcomes before December 31, 2011, to those diagnosed on January 1, 2012, or after. There was no significant difference in the rate of infliximab use (88 of 147, 59.9% vs 42 of 82, 51.2% P > 0.05) or infliximab failure (52 of 76, 68.4% vs 16 of 33, 48.5%, P > 0.05) between these 2 time periods. Similarly, there were no differences in rate of adalimumab use or failure. Of note, more VEO-IBD subjects were treated with immunomodulators if diagnosed before December 31, 2011, than those diagnosed after January 1, 2012 (71 of 147, 48.3% vs. 10 of 82, 12.2%, P < 0.0001), but there were no significant differences in the failure rate (pre: 49 of 71, 69%; post: 7 of 10, 70%; P > 0.05).
We found higher use of immunomodulator monotherapy in the earlier period, before 2012 in all cohorts, compared with 2012 and after.
Subjects with VEO-IBD were more often treated with therapies traditionally used for immunodeficiency or other indications and targeted therapy. These therapies included anti-IL-18 monoclonal antibody, sirolimus, tacrolimus, IL-1 blockade (anakinra or canakinumab), and intravenous immunoglobulin therapy (IVIg). In addition, 4 subjects with VEO-IBD in this cohort underwent hematopoietic stem-cell transplantation (HSCT). These therapies were utilized after genetic evaluation and/or immunophenotyping. There were no older-onset IBD subjects who received these therapies. Furthermore, taking into consideration that anti-TNF agents were used as first-line therapy in the second half of the study period (after 2012), we compared surgical rates in subjects diagnosed through 2011 with those diagnosed in 2012 and after and found no difference in the rate of surgery among study groups.
Hospitalization Days
Subjects with older-onset IBD were hospitalized at a significantly higher rate than subjects with VEO-IBD (59.5% vs 47.6%; P < 0.01). Subjects with intermediate-onset IBD were hospitalized more often (54.8%) than VEO-IBD, as well, but this did not reach statistical significance. However, among those who required hospitalization, length of stay was significantly longer in children with VEO-IBD, with an average of 31.6 days per patient, as compared with older-onset children with IBD, with an average of 16.0 days (P < 0.05). Additionally, subjects with VEO-IBD had higher readmission rates than older subjects (average of 4.0 vs 2.5 admissions per patient; P < 0.01).
Discussion
The incidence of pediatric IBD is increasing, and recent epidemiologic evidence demonstrates this increase is even more pronounced in children diagnosed at less than 6 years of age, with a rapid rise in some geographic regions of about 7.2% per year.20 This is a sobering finding because a subset of these children can be particularly challenging to treat, in part due to the unique pathogenesis of the disease, which can involve monogenic or digenic defects in immune-mediated pathways. Here, we set out to characterize the differences in the phenotype and disease course between VEO-IBD and older-onset pediatric IBD. These results may provide insight into the underlying disease process that ultimately impacts the distinct clinical course of each patient.
To accomplish these goals, we looked at several variables to characterize the severity of disease in subjects with VEO-IBD as compared with older-onset pediatric IBD and found that, overall, subjects with VEO-IBD had a more severe disease course. This was demonstrated by higher rates of failure of medical therapy, including immunomodulators and anti-TNFα therapy, than in older-onset pediatric IBD when assessed 1 year after initiation of therapy. Despite the severe disease at presentation, we found that the approach toward therapy was different in some cases of VEO-IBD than older patients, with more frequent use of immunomodulatory monotherapy. This may reflect the difficulty in obtaining insurance approval for biologics in the young children and the historical hesitation of using biologic therapy in children with VEO-IBD.
Our data also showed that the medical failure rate translated into subsequent increased diverting ileostomies and colectomies in children with VEO-IBD as compared with older children with IBD who underwent more ileocecectomies. While stricturing disease was the most common indication for surgery in the older children, severe refractory colonic inflammatory disease was the primary indication for surgical intervention in the younger children. This is partially reflective of the differences in location of disease between the cohorts, with a more significant endoscopic colonic distribution in subjects with VEO-IBD at diagnosis, which is similar to what has been shown in prior studies.5, 21 Interestingly, despite the colonic disease location, our data showed a majority of our subjects with VEO-IBD had definitive features of CD on the diagnostic endoscopy or upon repeat endoscopy, which is similar to other pediatric age groups. Thus, in addition to the refractory disease, these findings are potentially indicative of the unique pathogenesis and the need for an innovative therapeutic approach in young children.
To demonstrate that the severity in VEO-IBD exists even with optimized medication regimens that have evolved over time, we compared medication rates, failures, and surgical rates in the VEO-IBD cohort in those diagnosed through 2011 to those diagnosed during and after 2012. There was a significant difference in the frequency of immunomodulators use before 2012 compared with 2012 and after. This is consistent with our paradigm shift away from immunomodulatory monotherapy at our center. Overall failure rates of immunomodulator and biologic therapies remained consistent over the years even with the implementation of therapeutic drug monitoring. Other measures of disease severity examined in this study included growth parameters. Poor growth and both BMI and weight as measured by Z scores persisted at follow-up in the VEO-IBD cohort as compared with older children who showed significant improvement in growth over time. In addition, stunted growth measured by height-for-age Z scores was more prevalent over time in subjects with VEO-IBD compared with the older-onset IBD. This may reflect the persistent active and refractory disease in some younger children despite medical therapy. This finding, similar to the location of disease described previously, may also potentially shed light on an underlying cause of disease. Malnutrition, including poor weight gain and linear growth, have historically been a component of the key presenting features in patients with primary immunodeficiencies.22 Moreover, several studies have found that malnutrition is one the main clinical predictors of primary immunodeficiencies in children.22–24 A recent study looking at the US Immunodeficiency Network (USIDNET) data from 514 adults and 653 children showed a significantly higher prevalence of patients with an underweight status as compared with the healthy cohorts.25 Therefore, persistent poor growth in children with VEO-IBD may, in some cases, be indicative of an immune dysregulation that is driving the disease process.
We also looked at the frequency of hospitalizations as a measure of disease severity. Although older children with IBD were hospitalized at a higher rate, subjects with VEO-IBD had more readmissions and longer lengths of stay. This data highlights the significant burden of disease that children with VEO-IBD experience and the high cost of medical care in these young children that places a strain on the individual family’s finances and on the health care system. The overall data presented in this study supports the aggressive nature of VEO-IBD and indicates the need to use more effective and—when possible—targeted therapy early in the disease course.
This study is the largest single-center study to date focusing on disease phenotype of patients with VEO-IBD. There are some limitations to this work, however, including the retrospective nature of this study. In all cohorts, subjects without complete available data were excluded, and this may have impacted our results. Despite these limitations, this large cohort analysis showed that children with VEO-IBD have a more severe disease course, with failure of conventional therapies more frequently than older children. It is also important to recognize that there is great heterogeneity within the VEO-IBD population and that although a subset has an aggressive disease course, there are some children with VEO-IBD who have mild disease and respond well to medical therapy. It is, therefore, imperative that each patient be evaluated and treated based on their individual presentation and disease course.
Although this study did not focus on the genetics of VEO-IBD, included in this cohort were 10 subjects with VEO-IBD who had monogenic defects identified during the study period. Due to the small number, we were unable to perform statistical analyses to determine the disease course of patients with monogenic disease. However, it should be noted that 7 of the 8 subjects with monogenic defects failed medical treatment, and half of the subjects with monogenic defects required surgical intervention. Furthermore, these children all presented with stunted growth, required hospitalization, and experienced conventional medication failure. Although a larger genetic study is underway to understand the disease course and phenotypic findings in monogenic disease, the observations made on this small subset may prompt a search for an underlying genetic defect in patients who present with a similar signature of disease severity. Our own work and the work of others have shown that there is an enrichment of variants in primary immunodeficiency genes in patients with VEO-IBD.5, 6 Almost all pathways of immune function have been associated with VEO-IBD, including T cell, regulatory pathways, and pathways involving B cell differentiation. Some of these genes involved in immunodeficiency have resulted in severe intestinal disease, poor growth, and systemic autoimmmunity or comorbid conditions.7, 9, 26–34
This phenotype of monogenic disease may be used to generate mechanistic insight into the disease process of children who have similar disease characteristics but do not have a known causative defect. A precision medicine approach has been used in some cases of monogenic VEO-IBD and, in our cohort, has frequently led to improvement, if not full remission, of the disease. However, there remain many patients with VEO-IBD in whom we have not identified an underlying cause or conclusive effective therapy. This is also true for some cases of severe older-onset IBD, which may benefit from the discoveries made in children with monogenic disease. Further larger prospective studies are needed in VEO-IBD and pediatric IBD to develop novel therapeutic strategies by identifying genetic defects in therapeutically tractable pathways in both immune and epithelial cells. In the interim, the data from this current study may help identify patients that are likely to have a refractory course and will require aggressive therapy. It may be possible to apply the therapeutic strategies successfully employed in cases of identified monogenic defects to children with phenotypic similarities but in whom genetic testing is pending or is unrevealing.
CONCLUSION
These results demonstrate that children with VEO-IBD overall have a more severe disease course than older pediatric IBD, manifested by inadequate response to conventional therapy, increased surgical intervention, and persistent poor growth. These distinct features may have value as a prognostic tool for the clinician, as predictors of a severe disease course and indicative of the need for early use of effective therapy. Ultimately, as we are able to combine the genomic, immunologic, and microbial evaluation, we will improve our understanding of VEO-IBD and apply a precision medicine approach to more patients with this disease.
Acknowledgments
The authors would like to thank the entire VEO-IBD Program and collaborators, including the Children’s Hospital of Philadelphia Division of Genomic Diagnostics, the CHOP IBD Center, and the Department of Biomedical and Health Informatics. They would also like to thank the CURE for IBD for their generous support of the CHOP IBD Center. Finally, they would like to thank Dr. Petar Mamula for his careful review of this manuscript.
Glossary
Abbreviations
- VEO-IBD
very early onset-IBD
- WES
whole exome sequencing
- BMI
body mass index
- WHO
World Health Organization
- CDC
Center for Disease Control
Author Contributions: All authors contributed to this study. JRK, MAC, TP, ND, KES, and MD contributed to the study concept and design. JRK, RS, AM, and KM contributed to the acquisition of data. JRK, ND, TP, MAC, KES, and MC contributed to the analysis and interpretation of data. JRK, ND, MAC, TP, KES, and MD contributed to the drafting of the manuscript. JRK, ND, TP, MAC, KES, and MD contributed to the critical revision of analysis of the manuscript for important intellectual content. JRK, ND, and MD contributed to the statistical analysis. ND and KM contributed to the technical or material support. JRK supervised the study.
Supported by: This study was supported in part by R01DK111843 (MD). JRK is supported by the National Institutes of Health (K23DK100461-01A1), Broad Research 479578, and the Keystone Team Science Award.
References
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torres J, Colombel JF. Genetics and phenotypes in inflammatory bowel disease. Lancet. 2016;387:98–100. [DOI] [PubMed] [Google Scholar]
- 3. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium (IIBDGC) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu JZ, van Sommeren S, Huang H, et al. ; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uhlig HH, Schwerd T, Koletzko S, et al. ; COLORS in IBD Study Group and NEOPICS The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelsen JR, Dawany N, Moran CJ, et al. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology. 2015;149:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Worthey EA, Mayer AN, Syverson GD, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13:255–262. [DOI] [PubMed] [Google Scholar]
- 9. Avitzur Y, Guo C, Mastropaolo LA, et al. Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease. Gastroenterology. 2014;146:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benchimol EI, Mack DR, Nguyen GC, et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. 2014;147:803–813.e7; quiz e14. [DOI] [PubMed] [Google Scholar]
- 11. Glocker E, Grimbacher B. Inflammatory bowel disease: is it a primary immunodeficiency? Cell Mol Life Sci. 2012;69:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruemmele FM, El Khoury MG, Talbotec C, et al. Characteristics of inflammatory bowel disease with onset during the first year of life. J Pediatr Gastroenterol Nutr. 2006;43:603–609. [DOI] [PubMed] [Google Scholar]
- 13. Cannioto Z, Berti I, Martelossi S, et al. IBD and IBD mimicking enterocolitis in children younger than 2 years of age. Eur J Pediatr. 2009;168:149–155. [DOI] [PubMed] [Google Scholar]
- 14. Kelsen JR, Dawany N, Moran CJ, et al. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology. 2015;149:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelsen JR, Grossman AB, Pauly-Hubbard H, et al. Infliximab therapy in pediatric patients 7 years of age and younger. J Pediatr Gastroenterol Nutr. 2014;59:758–762. [DOI] [PubMed] [Google Scholar]
- 16. Kelsen JR, Dawany N, Martinez A, et al. A de novo whole gene deletion of XIAP detected by exome sequencing analysis in very early onset inflammatory bowel disease: a case report. BMC Gastroenterol. 2015;15:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. [DOI] [PubMed] [Google Scholar]
- 18. Levine A, Koletzko S, Turner D, et al. ; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. [DOI] [PubMed] [Google Scholar]
- 19. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 20. Benchimol EI, Bernstein CN, Bitton A, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017;112:1120–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kammermeier J, Dziubak R, Pescarin M, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J Crohns Colitis. 2017;11:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lankisch P, Schiffner J, Ghosh S, et al. The Duesseldorf warning signs for primary immunodeficiency: is it time to change the rules? J Clin Immunol. 2015;35:273–279. [DOI] [PubMed] [Google Scholar]
- 23. Subbarayan A, Colarusso G, Hughes SM, et al. Clinical features that identify children with primary immunodeficiency diseases. Pediatrics. 2011;127:810–816. [DOI] [PubMed] [Google Scholar]
- 24. Reda SM, El-Ghoneimy DH, Afifi HM. Clinical predictors of primary immunodeficiency diseases in children. Allergy Asthma Immunol Res. 2013;5:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruffner MA, Sullivan KE; USIDNET Body Weight Group Complications associated with underweight primary immunodeficiency patients: prevalence and associations within the USIDNET registry. J Clin Immunol. 2018;38:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glocker EO, Frede N, Perro M, et al. Infant colitis–it’s in the genes. Lancet. 2010;376:1272. [DOI] [PubMed] [Google Scholar]
- 27. Shim JO, Hwang S, Yang HR, et al. Interleukin-10 receptor mutations in children with neonatal-onset Crohn’s disease and intractable ulcerating enterocolitis. Eur J Gastroenterol Hepatol. 2013;25:1235–1240. [DOI] [PubMed] [Google Scholar]
- 28. Neven B, Mamessier E, Bruneau J, et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood. 2013;122:3713–3722. [DOI] [PubMed] [Google Scholar]
- 29. Alangari A, Alsultan A, Adly N, et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130:481–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shearer WT, Dunn E, Notarangelo LD, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133:1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2012. J Allergy Clin Immunol. 2013;131:675–682. [DOI] [PubMed] [Google Scholar]
- 32. Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Canna SW, Girard C, Malle L, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]