Abstract
Background
On stopping bisphosphonate treatment, bone resorption may increase before evidence of a decrease in bone density. Offset of bisphosphonate effect may therefore be monitored by measuring C-terminal telopeptide (CTX) following long-term bisphosphonate treatment to inform clinical decisions on drug holiday.
Methods
Retrospective analysis of 158 patients (83% female, mean age 71 years) starting a drug holiday had plasma CTX measured at discontinuation (baseline), n=138 and 4 months and n=136, and 12 months (n=100). Premenopausal mean CTX levels and the least significant change (LSC) detectable were used to define target thresholds for bone turnover.
Results
Following long-term bisphosphonate treatment (69% alendronic acid, 33% risedronate, mean duration 8 years SD 2.7), 32% patients had CTX above target (0.19 μg/L). In those with baseline CTX below threshold, 28% increased CTX to >0.19 μg/L and > LSC (0.06 μg/L) by 4 months (mean CTX increase 0.05 μg/L [95% confidence interval (CI): 0.04–0.06; p<0.0001]) and 53% by 12 months (mean CTX increase 0.09 μg/L [95% CI: 0.07–0.10; p<0.0001]), whilst 47% had no detectable changes in CTX over 12 months.
Conclusion
A third of patients showed inadequate suppression of CTX at baseline, despite long-term bisphosphonate treatment. Drug holiday may not be appropriate for this group, showing a poor therapeutic response or poor adherence. For more than a quarter of patients, bisphosphonate effects were wearing off at 4 months and around half by 12 months. We suggest CTX monitoring could identify those not experiencing a sustained bisphosphonate effect, including poorly adherence to therapy, and may be used during a drug holiday to prompt recommencement of therapy.
Keywords: diphosphonates, bone resorption, osteoporosis, bone remodelling, collagen type 1, risedronic acid, alendronate
Introduction
Evidence for using 5 years of bisphosphonate (BP) treatment in the management of osteoporosis is well established,1,2 though the value of continuing treatment beyond this time is less clear. The emergence of rare adverse effects with longer-term therapy, such as atypical fracture and osteonecrosis of the jaw, has led to concerns that bone quality may be not be sustained indefinitely with ongoing treatment.3 In addition, several studies have demonstrated the continued effect of BPs following treatment cessation as a result of their long residency time in the bone.1,2,4 This has led to the increasing use of so-called drug holidays in clinical practice, whereby treatment is interrupted for up to 3 years to potentially minimise longer-term adverse effects whilst remaining at a relatively reduced risk of fracture.5,6 Zoledronic acid appears to have the longest duration of action after discontinuation, followed by alendronic acid, whilst risedronate appears to wear off more quickly, within a year of therapy cessation.1,2,4 Whilst these clinical trials have demonstrated persistent effects of BPs following treatment cessation, less is known about the real-world consequence of stopping BPs. Individual effects of BP treatment may vary, as may adherence in this setting, so discontinuation of prescription may not be associated with persistence of benefit in routine clinical practice. It is likely that adherence is higher in randomised controlled trial settings, where patients are contacted regularly to encourage adherence and are assessed with pill counts and questioning. Furthermore, analysis of bone turnover markers in the Fracture Intervention Trial Long-Term Extension (FLEX) trial was based on a subgroup of women who had high adherence.1 Application of trial findings may not be relevant if adherence has been suboptimal.
Bone turnover markers, including C-terminal crosslinking telopeptides of type 1 collagen (CTX), have been used as a means of monitoring osteoporosis therapies as a measure of metabolic activity of the bone,7,8 with suppression of CTX seen on initiation of BP therapy.8,9 Conversely, with treatment cessation, CTX levels increase, followed by slower changes in bone mineral density (BMD).1 These biomarkers could have a role in monitoring patients whilst on a drug holiday to ensure persistence of antiresorptive effect. They may also aid identification of patients who have poor adherence at baseline and would therefore not benefit from a drug holiday. We recognise that there are some circumstances in which this would be less appropriate, such as in poor renal function (CTX is renally cleared) or where a recent fracture has occurred (increased bone remodelling).
Our aim was to analyse changes in CTX on stopping long-term BPs in a real-world population to see whether persistence of BP effect was maintained. Key objectives were to assess whether long-term use of BPs results in an appropriate CTX level below the premenopausal mean target; to identify whether CTX increases are evident at 4 and 12 months to indicate return to normal bone turnover; and to identify any variance in persistence of effect between different BPs.
Methods
Patients attending an outpatient specialist bone clinic, who had been prescribed BP therapy for at least 5 years, from June 2012 until October 2014, were identified from monitoring records (n=158). This coincided with a change in routine clinical practice, as patients were offered a supervised drug holiday from therapy. This comprised a baseline measurement of serum CTX with subsequent reassessment at 4 and 12 months. Retrospective data were collected on age, gender, drug, duration of BP therapy and serum CTX level on initiation of drug holiday (‘baseline’ n=138) and at 4 months (n=136) and 12 months (n=100) following treatment cessation.
Least significant change (LSC) from baseline values were calculated for CTX at 4 and 12 months, using a one-sided probability of 0.05 applied to the formula √2 × 1.65 × CV, where CV is the combined coefficient of variation (√[CVanalytic2 + CVintra-individual2]).8 Analytic coefficient of variation was calculated from local laboratory data (5.8%, Roche immunoassay; intra-individual coefficient of variation was identified from the literature [13.4%]).10 The LSC was 33%; absolute LSC based on the premenopausal mean value from earlier studies (0.19 μg/L) was 0.06 μg/L.8,11,12
Comparisons of change in CTX at baseline, 4 and 12 months were made using t-tests, and 95% confidence intervals were constructed from these data (all STATA 15.0, Texas). CTX levels were compared to a target healthy premenopausal mean level (0.19 μg/L) at baseline, 4 and 12 months; normal bone turnover was said to have resumed if CTX was above target (0.19 μg/L) and above absolute LSC (0.06 μg/L). This was incorporated into a clinical algorithm for considering drug holidays.13
This paper presents an analysis of routine patient care, which was evaluated as a clinical service development. Data were collected in accordance with the Caldicott principles, which govern the handling of patient information in the United Kingdom, and the results have been used to inform clinical practice locally and service development (Caldicott approval 3082, Newcastle upon Tyne Hospitals NHS Foundation Trust, date 12 March 2014).
Results
Data were available for 131 women and 27 men, with a mean age of 71 years who had received BP therapy for a mean duration of 8 years (SD 2.74, range 3–15 years). The majority of participants had been taking alendronic acid (59%, n=93) or risedronate (33%, n=52) with other BPs accounting for a small number of cases (ibandronic acid 6%, n=9; zoledronic acid 2%, n=3; cyclical etidronate 1%, n=1). There were two patients with a CTX level ≥ 0.51 μg/L who were excluded from further analysis.11 CTX levels were available at both baseline and 4 months for 117 patients, at both 4 and 12 months for 89 patients and at all three time points for 83 patients.
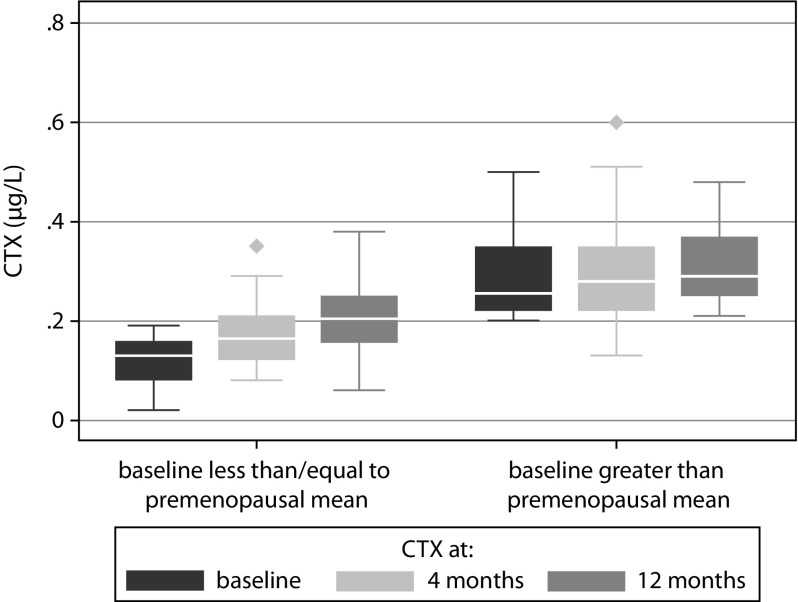
At baseline, 68% of patients had a CTX level below the premenopausal mean target. In this subset of patients, there was a mean increase in CTX by 0.05 μg/L (95% confidence interval [CI]: 0.04–0.06; p<0.0001) at 4 months and an increase by 0.09 μg/L (95% CI: 0.07–0.10; p<0.0001) at 12 months. This represented an increase in CTX by the LSC that was also above the premenopausal mean in 28% patients at 4 months and 53% patients at 12 months. In contrast, for those who had a baseline CTX above the premenopausal mean, there was no significant difference in CTX at 4 months (+0.01 μg/L; 95% CI: 0.01–0.04; p=0.31), though a significant increase was seen by 12 months (+0.05 μg/L; 95% CI: 0.01–0.09; p=0.01) (see Figure 1).
Figure 1.
CTX at 0, 4 and 12 months for patients below or above premenopausal mean CTX level at baseline.
CTX, C-terminal telopeptide.
Overall, following BP cessation, at 4 months, mean (median, interquartile range [IQR], %) serum CTX levels increased by 0.04 μg/L (0.04, 0.01–0.08, 42%) with a rise by at least the LSC (of 33%) in 47% patients. At 12 months, there was a rise by 0.08 μg/L (0.09, 0.04–0.12, 59%) with an increase by at least the LSC in 69%.
There was a greater increase in CTX on stopping risedronate at 4 months (+0.05 μg/L [0.06, 0.03–0.07]) and 12 months (0.09 μg/L [0.06, 0.07–0.12]) than alendronic acid (+0.04 μg/L [0.06, 0.02–0.05]) at 4 months and (0.07 μg/L [0.07, 0.05–0.09]) at 12 months, although this difference was not statistically significant (p=0.31 and p=0.12, respectively).
Discussion
We found that the majority of patients (68%) taking long-term BPs did appear to have an appropriate level of CTX suppression at the time of treatment cessation, meeting the target CTX threshold at or below the target premenopausal mean level (0.19 μg/L). These patients, who had appropriate CTX suppression at baseline, saw increases in CTX by the LSC and to above the premenopausal mean at 4 and 12 months in 28% and 53% of patients, respectively, demonstrating potential return to normal bone turnover within these timeframes. The remaining 32% of patients who did not meet the premenopausal target mean at baseline, despite taking long-term BP treatment, saw a significant rise in CTX by 12 months, but not as soon as 4 months.
We used the definition of an appropriate level of CTX suppression as being at or below a healthy premenopausal mean, as the lower half of the premenopausal reference intervals have been used as a target for those on BP treatment and gives an indication of appropriate suppression of bone turnover by BP.11 This revealed a significant number of patients who did not demonstrate biochemical evidence of suppression of bone resorption despite being on BP treatment. There are several possible explanations for this, including lack of response to treatment as a result of poor adherence, malabsorption, drug interactions, improper administration, limitations in interpreting CTX levels or for unknown reasons. Some patients may have had a partial response to treatment with suppression of CTX to a level above the premenopausal mean. One of the most likely explanations for this finding is the impact of adherence, as previous studies have found adherence to be poor in patients on oral BPs14 and bone turnover markers have been used to identify patients who are not adherent to BP therapy.12 This is supported by the lack of significant change in CTX at 4 months in those who did not reach the CTX target at baseline; consequently, no real change was seen on ‘cessation’ of treatment. Whilst a significant difference was noted in this group at 12 months, the confidence interval was undoubtedly wider and therefore less reliable [Figure 1]. The proportion of patients who did not appear to reach the target CTX response despite being on long-term treatment is concerning. The level of adherence should always be fully considered prior to initiation of a drug holiday and, as patients may not always admit to or realise the extent of their own level of adherence, estimation of bone turnover markers may add confidence and support the decision-making process from this point of view.
The rise in CTX following BP treatment is in line with published literature,1,2,4 with some patients experiencing a sustained response to BPs for up to 12 months post treatment cessation. The early increases in CTX at 4 months were not explained by the use of different BPs in our study, as although we saw increased percentage change in CTX in risedronate compared to alendronic acid, this was not statistically significant. It is possible that this may have become significant with an increased sample size as the number of patients taking risedronate was comparatively smaller.
We considered that the two-thirds of patients who were established on BP and maintained a target CTX after at least 5 years of treatment could be deemed suitable for a drug holiday to reduce the risk of rare longer-term adverse effects such as atypical fracture. By 12 months, over half of patients had a significant rise in CTX, which was also above the premenopausal mean, and we concluded that recommencement of treatment may be appropriate. It is clear that in a real-world clinical setting, longer-term BP persistence is not sustained for all patients, and therefore it is important that treatment is reviewed. Those who have elevated CTX levels are likely to be at higher risk of osteoporosis rather than atypical fracture and therefore may be less suitable for drug holiday. These findings have been used to guide our clinical practice in the management of long-term BP treatment.13
Assessment of BMD plays a particularly important role in assessing fracture risk at the time of deliberating a drug holiday, contributing to estimated 10-year fracture risk (FRAX/NOGG). We believe that bone turnover markers, which change much more rapidly than changes in bone density, could help to support early decisions on drug holiday monitoring to ensure that non-persistent responders who are likely to be at higher risk of low trauma fracture and may be less suitable for a drug holiday are identified. The markers could also potentially help to determine timing of recommencement of therapy. Current guidance,15 which was not available at the time of conducting this study, recommends that where a drug holiday is initiated, patients should be reviewed after 18 months to 3 years, or sooner if a fracture occurs. An increase in bone turnover could occur in a significant number of patients before changes in BMD can be seen and could potentially help to support decisions at an earlier stage. It would therefore be useful to apply NOGG thresholds to our CTX/LSC data with fracture, BMD and steroid history in order to assess application of these parameters to a clinical practice setting.
We recognise that whilst this analysis of our clinical data gives insight into the continued impact of BPs on bone resorption following cessation, the size of the study group means that impact on risk of fracture is not available. We did not perform a sample size calculation, as we studied all patients who had been initiated on a drug holiday in our clinic at the point of data collection. Serum CTX levels were assessed as per clinical practice; therefore, samples were always taken in the morning, though fasting states were not guaranteed; the healthy premenopausal reference intervals used also represented non-fasting levels. Other factors that may affect the interpretation of CTX reading, such as impaired renal function, liver function or recent fracture, were not separately analysed, though they should also be properly considered when making clinical decisions regarding drug holidays. We also note that the change in bone turnover markers after discontinuing therapy varies between antiresorptive agents and, particularly for denosumab, persistence of effect is much shorter.16,17 We would therefore not recommend this approach to monitoring off-set of therapy for denosumab, although we have adopted this practice for oral BPs in our clinic.
It was beyond the scope of this study to consider other turnover markers, as CTX is currently the only marker that we have available in our clinical setting. It is possible that markers of bone formation such as procollagen type 1 N propeptide (P1NP) could be used to assess bone turnover following BP cessation to indirectly measure BP effect, though changes in levels would not be as rapid. P1NP does have other advantages in terms of having less variability with respect to circadian rhythm and being less affected by food intake.
In summary, this paper demonstrates that in a real-world setting, persistent BP effect may not be evident in some patients, despite at least 5 years of BP treatment. Significant increases in the bone turnover marker CTX were seen as soon at 4 months following treatment cessation in some patients. The use of bone turnover markers may potentially aid clinical decision-making to highlight patients who may be less suitable for a drug holiday due to perceived lack of response despite long-term treatment or who should potentially restart treatment sooner. We suggest a practical threshold/LSC approach for aiding decisions related to drug holidays in a clinical practice setting.
Acknowledgements
Study results were presented as an oral presentation and poster at ECTS conference. May 14, 2016.
Footnotes
Contributions: LS, TJA, and SA designed the study. LS and SA collected data. LS wrote the research article with TJA by a process of iterative drafting. All authors reviewed the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: All authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/04/dic.2020-1-3-COI.pdf
Funding declaration: There was no funding received for this research or associated with the preparation of this article.
Correct attribution: Copyright © 2020 Statham L, Abdy S, Aspray TJ. https://doi.org/10.7573/dic.2020-1-3. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 1 April 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Black DM, Schwartz AV, Ensrud KE, et al. FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal. Fracture Trial (PFT) J Bone Miner Res. 2012;7:243–254. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shane E, Burr C, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 4.Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporosis Int. 2008;19(3):365–372. doi: 10.1007/s00198-007-0460-7. [DOI] [PubMed] [Google Scholar]
- 5.Compston JE, Bilezikian JP. Bisphosphonate therapy for osteoporosis: the long and short of it. J Bone Miner Res. 2012;27(2):240–242. doi: 10.1002/jbmr.1542. [DOI] [PubMed] [Google Scholar]
- 6.Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelsen J, Wallaschofski H, Friedrich N, et al. Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone. 2013;57(2):399–404. doi: 10.1016/j.bone.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporosis Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 9.Eekman DA, Bultink IEM, Heijboer AC, et al. Bone turnover is adequately suppressed in osteoporotic patients treated with bisphosphonates in daily practice. BMC Musculoskelet Disord. 2011;12(167):1471–2474. doi: 10.1186/1471-2474-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christgau S, Bitsch-Jensen O, Bjarnason NH, et al. Serum crosslaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone. 2000;26(5):505–511. doi: 10.1016/S8756-3282(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 11.Gossiel F, Finigan J, Jacques R, et al. Establishing reference intervals for bone turnover markers in healthy postmenopausal women in a nonfasting state. Bonekey Rep. 2014;3:573. doi: 10.1038/bonekey.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA. Bone turnover markers: are they clinically useful? Eur J Endocrinol. 2018;178:R19–R31. doi: 10.1530/EJE-17-0585. [DOI] [PubMed] [Google Scholar]
- 13.Statham L, Aspray T, Abdy S. Can bone turnover markers help to define the duration of bisphosphonate drug holidays? Bone Abstracts. 2016;5:P410. doi: 10.1530/boneabs.5.P410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imaz I, Zegarra P, Gonzalez-Enriquez J, Rubio B, Alcazar R, Amate JM. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporosis Int. 2010;21(11):1943–1951. doi: 10.1007/s00198-009-1134-4. [DOI] [PubMed] [Google Scholar]
- 15.Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–980. doi: 10.1210/jc.2010-1502. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2017;33(2):190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]