Abstract
Bioimpedance spectroscopy (BIS) is an easily applicable tool to assess body composition. The three compartment model BIS (3C BIS) conventionally expresses body composition as lean tissue index (LTI) (lean tissue mass [LTM]/height in meters squared) and fat tissue index (FTI) (adipose tissue mass/height in meters squared), and a virtual compartment reflecting fluid overload (FO). It has been studied extensively in relation to diagnosis and treatment guidance of fluid status disorders in patients with advanced‐stage or end‐stage renal disease. It is the aim of this article to provide a narrative review on the relevance of 3C BIS in the nutritional assessment in this population. At a population level, LTI decreases after the start of hemodialysis, whereas FTI increases. LTI below the 10th percentile is a consistent predictor of outcome whereas a low FTI is predominantly associated with outcome when combined with a low LTI. Recent research also showed the connection between low LTI, inflammation, and FO, which are cumulatively associated with an increased mortality risk. However, studies toward nutritional interventions based on BIS data are still lacking in this population. In conclusion, 3C BIS, by disentangling the components of body mass index, has contributed to our understanding of the relevance of abnormalities in different body compartments in chronic kidney disease patients, and appears to be a valuable prognostic tool, at least at a population level. Studies assessing the effect of BIS guided nutritional intervention could further support its use in the daily clinical care for renal patients.
Keywords: Bioimpedance spectroscopy, body composition, chronic kidney disease, nutritional assessment
INTRODUCTION
Protein energy wasting (PEW) is highly prevalent in dialysis patients, but also in patients with earlier stages of chronic kidney disease (CKD).1 The cause of PEW, which is an important risk factor for mortality, is multifactorial.2
The diagnosis of PEW in renal failure is according to current convention based on four different criteria, that is, body mass (low body weight, weight loss, or decreased total fat mass [FM]), decreased muscle mass, serum chemistry, and an estimation of dietary intake.3 The estimation of body fat and muscle mass as proposed in expert panels or guidelines is generally left to the discretion of the clinician, and can include different methods such as anthropometry, dual X‐ray absorptiometry (DEXA), and bioimpedance analysis (BIA).3, 4 BIA is an easily applicable and operator independent method which has a long history of research in end‐stage renal disease (ESRD). Whereas various BIA applications are available, such as single‐frequency, including vector based methods,5 whole body (or more appropriately called “wrist‐to‐ankle”) bioimpedance spectroscopy (BIS) appears at present to be most frequently studied.6, 7 Approximately one decade ago, a three compartment (3C) BIS model was introduced, which differentiates between three relevant compartments, that is, lean tissue mass (LTM), adipose tissue mass (ATM), and a calculated virtual entity reflecting fluid overload (FO), which is in the literature commonly described as the “overhydration” (OH) compartment,8 and which will be a negative volume in case of fluid depletion.
Whereas most research on 3C BIS has been devoted to abnormalities in fluid state,6, 7, 9 recent papers also have shed more light on its potential use in nutritional assessment. The aim of this short review is to discuss the available evidence on the use of the 3C BIS model and to explore the potential role in monitoring and management of PEW and other abnormalities in body composition in adult patients with advanced or end‐stage kidney failure.
SHORT TECHNOLOGICAL BACKGROUND OF THE 3C BIS MODEL
It is not the aim of this article to discuss the basics of BIS into detail, for which there are recent reviews available.10, 11 Basically, its principle is based on the measurement of the impedance of tissue on a broad range of frequencies of an alternating current. At low frequencies, the current only passes through the extracellular fluids (ECF), whereas at high frequencies the current also passes through the cell membranes. Complex models have been developed to use the variation in impedance with frequency to derive estimates of the ECF and intracellular fluids (ICF) based on the original Hanai model.12, 13 Differences between the two compartment (2C) models and the 3C models can be explained by different arrangements of body composition compartments, which are based on differences in hydration status of the compartments as is shown in Figure 1. The 2C model divides body composition compartments into a fat free mass (FFM) compartment and a FM compartment, where FFM is estimated based on the assumption that fat free tissue contains 73% of water.15 As the 2C model cannot distinguish excess volume due to fluid retention, predictions of FFM are influenced by the presence of FO.14, 16
Figure 1.
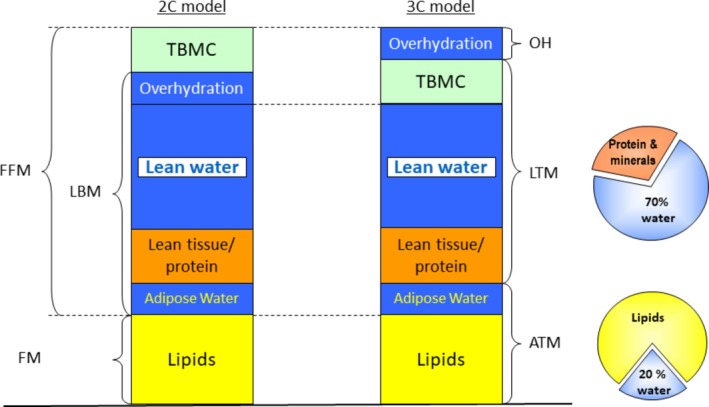
Distribution of body composition compartments 2C versus 3C model (by P. Wabel, Fresenius Medical Care D GmbH, Bad Homburg, Germany), as published by Broers et al.14 (reproduced with permission). 2C = two compartment; 3C = three compartment; ATM = adipose tissue mass; FFM = fat free mass; FM = fat mass; LBM = lean body mass; LTM = lean tissue mass; TBMC = total bone mineral content. [Color figure can be viewed at http://wileyonlinelibrary.com]
The 3C model was developed based on validation with tracer dilution techniques, DEXA, and air displacement plethysmography in healthy controls and dialysis patients.8, 12 The 3C model recognizes the presence of ECF and ICF in adipose tissue and calculates the so‐called OH compartment based on the assumption of normal hydration ratios for lean and adipose tissue, respectively.8 Thus, the 3C model expresses three body compartments: LTM, ATM, and the OH compartment as an indicator of FO8 (Figure 1), which can also be negative and in this case points to fluid depletion.7, 17 Lipid containing FM can be derived from ATM by taking into account the hydration state of adipose tissue.8 In addition, LTM and ATM are usually normalized by dividing by height in meters squared, and by convention usually expressed in the literature as lean tissue index (LTI) and fat tissue index (FTI).14 The algorithm embedded in one of most cited multifrequency bioimpedance devices (i.e., Body Composition Monitor, BCM®), which applies the 3C method, has been assessed in different populations, mostly Caucasian,12 and validated against reference methods both for fluid volume and nutritional findings.12, 18 It should be recognized that BIS does not directly measure body composition, but only electrical properties of tissue which are used for the calculation of body compartments based on empirically derived values for tissue coefficients.19
VALIDATION AGAINST REFERENCE METHODS
Comparison between body fluid compartments when assessed by tracer dilution techniques and BIS, in which a correction factor for body mass index (BMI) was included, have yielded acceptable limits of agreement (−0.4 ± 1.4 L [mean ± SD] for extracellular water [ECW] and 0.2 ± 2.0 L for intracellular water [ICW]).12 Volume estimation by BIS also seems to be reliable in patients with high BMI.20 However, it should be recognized that even different tracer dilution methods show disagreement between themselves in the assessment of fluid compartments which was comparable to the difference between tracer dilution methods and bioimpedance findings, and therefore the presence of real “gold standard” methods in this respect have been questioned.21 Using the 3C model, the mean difference in FM assessed between BIS and DEXA was 0.55 ± 3.3 kg in a Taiwanese population. The discrepancy between DEXA and BIS was not related to the degree of FO, but was more pronounced in patients with higher BMI, where BIS appeared to overestimate the fat compartment as compared to DEXA.22 In a study in 50 peritoneal dialysis (PD) patients, mean difference in estimated FM between 3C BIS and DEXA was 0.9 ± 5.7 kg (95% confidence interval [CI] = ‐10.5–12.3) and −0.3 ± 5.6 kg (95% CI = ‐11.8–10.8) for LTM.23 Relatively larger limits of agreement were also observed in the study of Zhou et al. in 120 patients with nondialysis dependent CKD.24 In order to facilitate the comparison, the authors calculated FFM by BIS by subtracting FM from body weight. FFM was higher in BIS as compared to DEXA by a mean difference of −2.8 kg (−12 to 6.5 kg), whereas mean FM was 3.1 kg lower when assessed by BIS as compared to DEXA (−6.8 to 13 kg).24 Importantly, in both studies, the discrepancy between DEXA and 3C BIS was related to the degree of FO, which has been shown to influence the estimation of body composition by DEXA.16 It is of importance to note that all of these studies have been performed in chronic disease population and none of them has been addressed to acutely ill patients.
In a study of 91 patients treated with online hemodiafiltration (OL‐HDF), 3C BIS was also compared between groups with or without PEW, as defined by the malnutrition‐inflammation score25 ≥ 5.26 In the PEW group, FTI but not LTI was lower as compared to the group without PEW. Notably, the prevalence of PEW was far lower (19.7%) as compared to that of LTI < 10th percentile, which was present in, respectively, 64.5% and 73.3% of patients with and without PEW.26
Data on the reproducibility of BIS in the nutritional assessment in renal patients are limited, but a study reported in abstract form showed a coefficient of variation of ECW and ICW in a time period of 30 days of 2.2% and 3.7%, respectively.27 However, although ECW and ICW are used for the calculation of body composition by 3C BIS, there are to the best of our knowledge no data yet on the reproducibility of FTI and LTI in the literature.
PREVALENCE OF ABNORMALITIES IN BODY COMPOSITION IN DIALYSIS PATIENTS
A low LTI is highly prevalent in dialysis patients as compared with the LTI of healthy aged matched controls for which reference ranges are available.28 In the literature, cutoff limits of <10th or >90th percentile of an age‐matched and sex‐matched healthy population are usually used. In a study of Marcelli et al.29 in 37,345 European hemodialysis (HD) patients, LTI < 10th percentile was present in 44% of patients. LTI and FTI < 10th percentile was less prevalent, and only observed in 4.2% of patients, which means that in general, a low LTI is accompanied by a normal or increased FTI. Still, the combination of LTI < 10th percentile and FTI > 90th percentile, which would coincide with sarcopenic obesity, was only present in 3.5% of patients, whereas the combination of LTI < 10th percentile and FTI between the 10th and 90th percentile was observed in 39% of patients.29 The shift to higher BMI in dialysis patients appears to be due to an altered distribution between LTI and FTI in this population. For example, the group of patients with low LTI had, respectively, a mean BMI of 19 ± 1.9 kg/m2 when accompanied with FTI < 10th percentile, or a BMI of 25.4 ± 3.9 kg/m2 when accompanied with FTI between the 10th and 90th percentile. A normal body composition (e.g., both LTI and FTI between the 10th and 90th percentile) coincided with a mean BMI of 27.6 ± 4.0 kg/m2.29
The high presence of a low LTI appears to be a global phenomenon. In a study in Argentina in 934 patients, 58.8% of patients had LTI < 10th percentile.30
It has also been suggested that BIS could also play a role in the diagnosis of sarcopenic obesity in dialysis patients by investigating the relationship between ATM and LTM.30, 31
Changes in LTI and FTI or differences between groups appear to be detectable by BIS, at least on a population level. In a recent study, Marcelli et al. observed an increase in FTI of 1.0 kg/m2, and a decline in LTI of 0.4 kg/m2 in the first 2 years after the start of HD.32 These changes coincided with a mean increase in BMI of 0.6 kg/m2. These data were later confirmed by a study from Keane et al., who observed a mean increase in FM of 0.7 kg and a decline in LTM of 0.9 kg over a 2‐year period following the start of dialysis.17 In a cohort of 824 PD patients, LTI declined by a mean of 1.1 kg/m2 whereas FTI increased by 1.9 kg/ m2,33 with a significant inverse relationship between changes in both body compartments. These observations are in agreement with earlier data obtained by DEXA in a smaller group of dialysis patients.34 Another study found a lower LTI, but a comparable FTI in HD as compared with a matched cohort of PD patients.35
Interestingly, also seasonal differences in body composition in HD patients were detected by 3C BIS, with higher FM and lower LTM in the winter period.36
RELATION BETWEEN ABNORMALITIES IN BODY COMPOSITION AND OUTCOME
Previous research showed that especially low BMI in dialysis patients is associated with adverse outcome, whereas higher BMI is actually protective.37 However, as BMI is a composite parameter without the possibility to differentiate between LTM and ATM, there is a clear rationale for investigating the relation between specific body compartments and outcome, because targeted interventions may differ. Various studies have explored the relation between body composition assessed by 3C BIS and outcome. Rosenberger et al. observed a relation between LTI < 10th percentile and increased mortality in a study of 960 HD patients after adjustment for case‐mix.38 In a study including 697 Portuguese HD patients, a low FTI was associated with reduced survival.39 In a smaller group of HD patients, those with LTI < 10th percentile had a significantly higher risk of mortality,40 whereas in a cohort of 6395 Spanish HD patients, LTI below the 10th percentile was associated with increased mortality.41 In a study in 824 PD patients, both LTI below the 10th percentile and FTI above the 90th percentile were associated with increased mortality, although the latter relation lost significance after adjustment for C‐reactive protein (CRP) and serum albumin.33 In an international cohort study in 37,345 HD patients, both LTI < 10th percentile (hazard ratio [HR] = 1.53), and FTI < 10th percentile (HR = 1.19) were associated with significantly increased mortality as compared to LTI and FTI between the 10th and 90th percentile. The highest mortality (HR = 2.51) was observed in the group with a combination of both FTI and LTI < 10th percentile.29 Furthermore, the interaction between FTI/LTI and outcome was analyzed by means of smoothing spline analysis of variance in this study (Figure 2).
Figure 2.
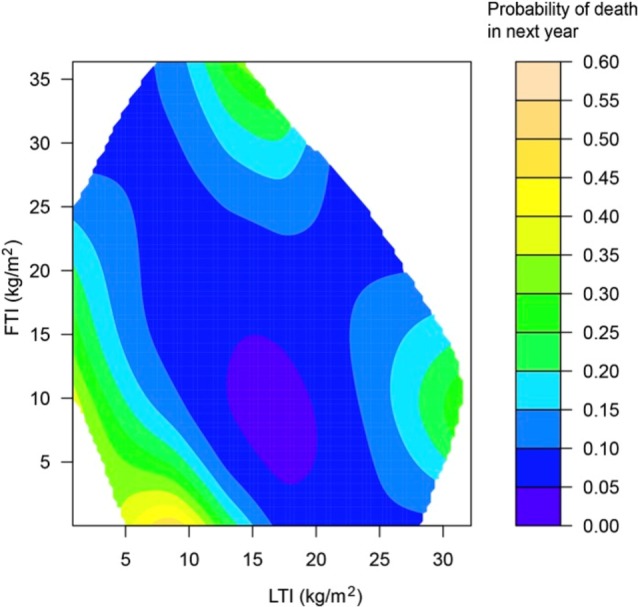
Interaction between LTI, FTI, and outcome in female HD patients by smoothing spline ANOVA (reproduced with permission from Marcelli et al.32). ANOVA = analysis of variance; FTI = fat tissue index; LTI = lean tissue index. [Color figure can be viewed at http://wileyonlinelibrary.com]
The relation between low LTI and outcome was confirmed in a meta‐analysis, in which LTI < 10th percentile was associated with mortality with a HR of 1.53.42
Also in patients with CKD stage 4–5, LTI appeared to be predictive for outcome. In a cohort of 356 patients, a lower LTI (defined as LTI < 14.1 kg/m2) was associated with an increased mortality during a mean follow‐up time of 22 months.43 These results were confirmed in a study in 326 patients with CKD stages 3–5, in whom LTI above the median value, but not high BMI or FTI, was associated with improved outcomes. Interestingly, the group with both LTI and FTI above the median showed the best outcomes.44
Whereas low FTI has been associated with adverse outcomes, as shown above, a very high FTI was associated with increased mortality as well. In an international study of our group,29 FTI > 90th percentile was associated with increased mortality without adjustment for LTI. However, in a combined model, those patients with LTI < 10th and FTI > 90th percentile had a lower mortality as compared to patients with a combination of LTI and FTI < 10th percentile, suggesting that high FTI in patients with significantly reduced LTI may actually be partially protective. Patients with normal LTI but FTI > 90th percentile also tended to have an increased mortality.29 Lee et al. observed a relation between the fat tissue/lean tissue (FM/LTM) ratio, as a proposed surrogate of sarcopenic obesity, and the risk of cardiac events and all‐cause mortality in a cohort of 130 HD patients.31 It should be noted that the quartile with the highest FM/LTM ratio was characterized both by the highest FM as well as the lowest LTM, which makes the interpretation of the individual contribution of the respective body compartments somewhat difficult to interpret.
Summarizing, a low LTI is associated with adverse outcomes, which also holds true for a low FTI in combination with a low LTI. This relation may be caused by the underlying disease state, leading to wasting, but also because skeletal muscle is an important reservoir of proteins.42, 44 Next to this, a low LTI contributes to muscle weakness and frailty with an increased risk of complications and may be associated with a reduced physical activity, which is at itself a risk factor for mortality.31, 44, 45 Moreover, also a low FTI may be a sign of serious underlying disorders, where severely depleted fat stores could interfere with the homeostatic response to a stressor such as infection or surgery, given the fact that fat is an important source of energy. In addition, the higher circulating lipoproteins associated with adipose tissue could provide prevention against endotoxins.46, 47, 48 On the other hand, visceral adiposity was related to an enhanced risk of cardiovascular complications in HD patients.49 Therefore, also in dialysis patients, extremes in body composition seem to be disadvantageous. A summary of articles reflecting body composition parameters in relation to outcome is presented in Table 1.
Table 1.
Articles reflecting body composition parameters in relation to outcome
| Group | Study population | Patient characteristics | Follow‐up period | Outcome |
|---|---|---|---|---|
| Rosenberger et al.38 |
HD (n = 748)*
*complete cases out of n = 960 |
Age: 63 (54–73) y Male (%): 54 |
Median (IQR): 17 (10–33) mo |
* fully adjusted model |
| Caetano et al.39 | HD (n = 697) |
Age: 67 (55.5–76) y Male (%): 56.5 |
12 mo |
Predictors of 1‐y all‐cause mortality*:
* fully adjusted model |
| Rymarz et al.40 | HD (n = 48) |
Age: 59.8 ± 15.5 y Male (%): 66.7 |
Mean ± SD: 29.93 ± 20.09 mo |
|
| Castellano et al.41 |
HD* (n = 6395)
*(Incident and prevalent patients) |
Age: 67.6 ± 14.7 y Male (%): 62.7 |
Not defined; Study period: January 2012–December 2014 |
* percentiles of LTI were calculated based on studied groups **multivariate regression. |
| Parthasarathy et al.33 | PD (n = 824) | Age: 55.9 (47–68) y Male (%): 64 | Up to 9 y |
|
| Marcelli et al.29 | HD (n = 37,345) |
Age: 62.7 ± 15.2 y Male (%): 57 |
Median (25th–75th percentile): 266 (132–379) d |
All‐cause mortality risks fully adjusted models:
HR only for LTI:
HRs only for FTI:
HRs for LTI + FTI combined:
|
| Hwang et al.42 | HD (3 studies) |
|
||
| Vega et al.43 | CKD4‐5 ND (n = 356) |
Age: 67 ± 13 y Male (%): 64 |
Median (range): 22 (3–49) mo |
*multivariate regression. |
| Lee et al.31 | HD (n = 131) |
Age: 60.7 ± 13.6 y Male (%): 55.7 |
Mean ± SD: 53.1 ± 10.9 mo |
* adjusted model |
Age is given in mean ± standard deviation (SD) or median with interquartile range. BMI = body mass index; CI = confidence intervals; FTI = fat tissue index; HD = hemodialysis; HR = hazard ratio; IQR = interquartile range; LTI = lean tissue index; OR = odds ratio.
ASSOCIATION OF ABNORMALITIES IN BODY COMPOSITION WITH OTHER RISK DOMAINS
Malnutrition often occurs in combination with abnormalities in other risk domains, most notably inflammation, and forms part of the so‐called Malnutrition Inflammation Atherosclerosis (MIA) syndrome.50 However, malnutrition also appears to be associated with abnormalities in fluid status. In a cohort of 338 patients with CKD stages 3–5, FO was inversely associated with LTI, and was incrementally associated with the MIA score,51 as well as with interleukin‐6.52 In a cohort of 478 patients with CKD stages 4 and 5, both LTI and FTI were lower in the fluid overloaded group, defined as an overhydration/extracellular volume (OH/ECV) ratio above 7%.53
Recently, we explored the association between LTM, inflammation, and FO. In a cohort of 8883 European prevalent dialysis patients, predialytic FO was more pronounced in patients with LTI < 10th percentile, and highest in the subgroup with the combined presence of LTI < 10th percentile and inflammation, defined as a high sensitive CRP (hsCRP) level above 6 mg/L.54 In 40% of this entire cohort, a low LTI was present in combination with either FO and/or inflammation, whereas in only 6.5% of patients, a low LTI was observed as an isolated phenomenon. Therefore, there are important arguments for a clustering of risk factors over different domains, which includes FO as an important novel risk factor. Furthermore, there also appears to be a clustering of abnormalities including those involved in the MIA syndrome with FO, although the relative contribution of abnormalities in the different risk domains might fluctuate between patients and over time.7, 54
Although in our study, the association between low LTI and mortality was highly apparent in combination with FO and/or inflammation, the association lost significance when corrected for other risk domains.54 However, in the study of Vega et al., a low LTI added independent prognostic information for risk of mortality,43 whereas in the study of Castellano et al., a low LTI remained predictive for mortality after adjustment for FO, serum albumin, and the Charlson comorbidity index.41 In a study in 529 PD patients, LTI was not significantly associated with outcome in a model adjusted for FO,55 whereas in another study in 824 patients, this relation remained significant after adjustment for FO.33
The mechanisms behind the relation between malnutrition, FO, and inflammation are likely complex (Figure 3) and may, in dialysis patients, include factors such as inflammation, hypoalbuminemia, and incorrect adjustment of target weight.56
Figure 3.
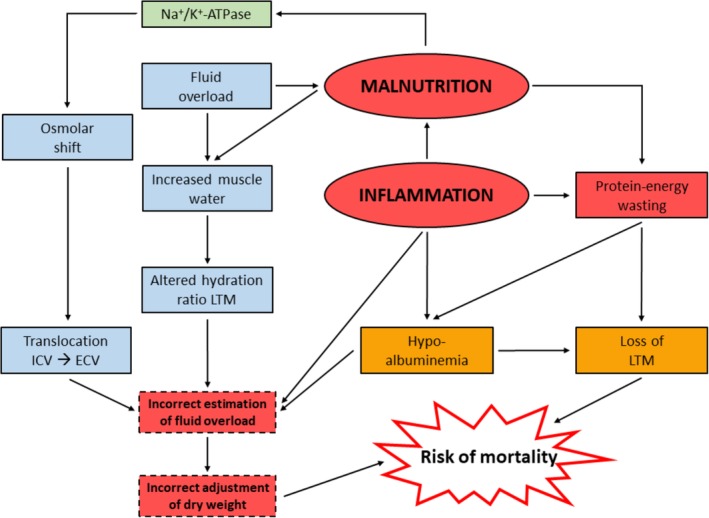
Hypothesized relation between malnutrition, fluid overload, and inflammation. ECV = extracellular volume; ICV = intracellular volume; LTM = lean tissue mass; Na+/K+‐ATPase = sodium‐potassium pump. [Color figure can be viewed at http://wileyonlinelibrary.com]
However, whereas in malnourished patients without renal failure, ECV remained stable in an absolute sense, but increased relatively to body weight,57, 58 also in the absence of hypoalbuminemia. Given the fact that adipose tissue, which was also lower in these patients, contains ECW, actually a reduction in ECV might have been expected. The mechanisms for this relative expansion of ECV in nonuremic malnutrition, other than explained by a loss of intracellular mass per se11 remain unclear,59 although a translocation of ICW to ECW based on an osmotic shift from the intracellular to the extracellular space, or abnormalities of the Na+‐K+‐ATPase pump have been postulated.60, 61 Inhibition of the Na+‐K+‐ATPase pump may lead to cell shrinkage by secondary accumulation of intracellular Ca2+ resulting in a loss of amino acids and ions.62
Interestingly, relative water content of muscles was also found to be increased in nonuremic malnourished subjects, for reasons that have not been completely elucidated. Although the increase in relative water content of muscle, the major component of LTM, was relatively small (±3%), it violates to some degree the assumption of a fixed hydration level of LTM of 3C‐BIS which is used to calculate the OH compartment,8 which should therefore, in our opinion, be interpreted with some caution in patients with severe PEW.26 Keane et al. also found an increased OH (mean 1.1 L) in nonuremic malnourished patients.63 However, in nonuremic subjects, no difference in the hydration of LTM was observed between patients with mild and severe malnutrition.64 Also, a study by Chazot et al. showed a relation between brain natriuretic peptide and malnutrition (defined according to serum [pre]albumin and normalized protein nitrogen appearance),65 whereas Arias‐Guillen et al. observed a higher prevalence of FO in malnourished patients according to the clinical PEW criteria mentioned in the introduction of this article.26 This supports the FO‐malnutrition relationship also by the use of other methodologies than BIS.
Whereas a low LTI was related to FO and inflammation, the study of Lee et al. showed that in male subjects, the FM/LTM ratio was also positively related to hsCRP and interleukin‐6.31 In PD patients, changes in FTI were also positively related to changes in inflammatory status.66 This has mainly been attributed to the relation between visceral adiposity and systemic inflammation.67 However, a recent study suggested that the production of inflammatory cytokines was actually higher in subcutaneous as compared to visceral adipose tissue.68
A summary of articles reflecting abnormalities in body composition in relation with other risk domains in patients with advanced or end stage kidney disease is presented in Table 2.
Table 2.
Abnormalities in body composition with other risk domains in patients with advanced or end stage kidney disease
| Group | Study population | Patient characteristics | Outcome |
|---|---|---|---|
| Hung et al.51 | CKD 3–5 (n = 338) |
Age: 65.7 ± 13.5 y Male (%): 68.9 |
|
| Wang et al.52 | CKD 3–5 (n = 326) |
Age: 65.8 ± 13.3 y Male (%): 68.7 |
|
| Tsai et al.53 | CKD 4 and 5 (n = 478) |
Age: 65.4 ± 12.7 y Male (%): 54.6 |
|
| Dekker et al.54 | Prevalent HD (n = 8883) |
Age: 63.5 ± 14.8 y Male (%): 57.2 |
|
| Vega et al.43 | CKD 4 and 5 (n = 356) |
Age: 67.0 ± 13.0 y Male (%): 64.0 |
|
| Castellano et al.41 | HD (n = 6395) |
Age: 67.6 ± 14.7 y Male (%): 62.7 |
|
| O'Lone et al.55 | PD (n = 529) |
Age: 57.0 (46.7–68.8) y Male (%): 62.0 |
|
| Parthasarathy et al.33 | PD (n = 824) |
Age: 55.9 (47.0–68.0) y Male (%): 64.0 |
|
| Chazot et al.65 | HD (n = 51) |
Age: 65.3 ± 14.2 y Male (%): 54.9 |
|
| Arias‐Guillén et al.26 | HD (n = 91) |
Age: 60.0 ± 14.0 y Male (%): 29.7 |
|
| Lee et al.31 | HD (n = 131) |
Age: 60.7 ± 13.6 y Male (%): 55.7 |
|
| Rincón Bello et al.66 | Prevalent PD (n = 31) |
Age: 57.4 ± 18.0 y Male (%): 45.2 |
|
| Delgado et al.67 | HD (n = 609) |
Age: 56.1 ± 14.3 y Male (%): 57.0 |
|
Age is given in mean ± standard deviation or median with interquartile range. CI = confidence intervals; CRP = C‐reactive protein; FO = fluid overload; FTI = fat tissue index; HD = hemodialysis; HR = hazard ratio; hs‐CRP = high sensitivity C‐reactive protein; LTI = lean tissue index; OR = odds ratio.
INTERVENTIONAL STUDIES USING 3C BIS
Important is whether changes in body composition, measured by BIS, respond to nutritional intervention. Preliminary evidence suggests that this is indeed the case. In a nonrandomized study in which patients with a serum albumin level below 38 g/L received a high protein meal during dialysis, their FTI increased in contrast to a decline in FTI in the control group. However, LTI decreased both in the interventional and in the control groups.69 In addition, the PESET study used 3C BIS to monitor changes in body composition assigned to HD or OL‐HDF and observed a decline in LTM and an increase in ATM in HD as compared to the OL‐HDF patients.70 No study has yet prescribed nutritional intervention according to abnormalities in body composition by BIS, although recently a proposal was made for nutritional monitoring and intervention based on FTI and LTI criteria.26
THE ROLE OF BIS IN THE INTEGRATED ASSESSMENT OF DIALYSIS PATIENTS
It is important to realize that assessment of body composition is only part of the nutritional and functional assessment of dialysis patients. Earlier recommendations have suggested to include body composition as one of the criteria for PEW.3, 71 In addition, a study of Chen et al. already showed the added value of measuring LTI with the BCM as a screening tool for nutritional status, given the fact that low LTI as a marker of protein wasting, is seen as the primary component of PEW.72 It is of importance to realize that abnormalities in body composition are part of a wider, but only partly overlapping spectrum, including physical inactivity, a reduction in muscle strength, and a reduction in health‐related quality of life14, 73, 74, 75 in which the latter may surpass a reduction of muscle mass in the prediction of outcome.76 Indeed, recent guidelines on sarcopenia recommend to include combined assessments of muscle mass, muscle strength, and physical performance.77 Next to this, it would be rational to include physical activity in this assessment as it plays an important role as a determinant of muscle strength and the frailty syndrome.78, 79 Therefore, to our opinion BIS should not be implemented as a single solution, but as part of a multimodal and recurrent assessment strategy in which the different dimensions of PEW are incorporated, next to a parameter for muscle strength, physical activity, physical performance, and health‐related quality of life. Easily applicable tools such as handgrip strength measurements, actometers, the 4‐meter gait speed test, and short form‐36 (SF‐36) questionnaires can assess all of these domains (Figure 4). However, further research is recommended with regard to the implementation of BIS measurements for determination of fluid status and nutritional guidance of ESRD patients to show the effect of using the BCM on clinical outcomes in this patient group.80
Figure 4.
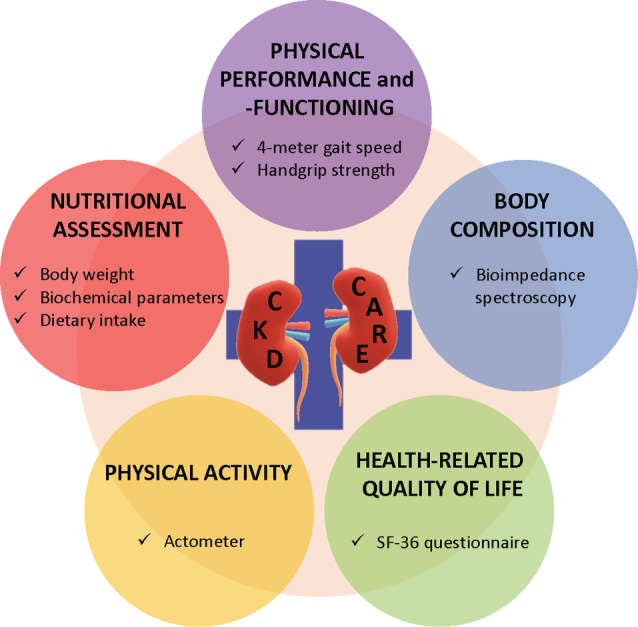
Proposed role of bioimpedance spectroscopy in the multidimensional assessment of nutritional and functional status in patients with advanced chronic kidney disease. SF‐36 = short form‐36. [Color figure can be viewed at http://wileyonlinelibrary.com]
CONCLUSION
In the last years, assessment of body composition using the 3C BIS model has provided a wealth of information, not only regarding the importance of FO, but also that of other abnormalities in body composition in patients with ESRD and earlier stages of CKD. Its ease of use has led to the availability of large cohorts in which the relation between body composition and outcome can be studied. The use of 3C BIS has also facilitated the interpretation of the relation between outcome and the interaction of abnormalities in different body compartments. Despite the relatively wide limits of agreement with reference techniques such as DEXA found in some studies, the available studies consistently show that a low LTI is related to increased mortality, and as such has predictive validity. It has also been shown that the concomitant presence of a low FTI can add to the risk associated with low LTI. On the other hand, excessively high FTI may also carry a risk for especially cardiovascular mortality. The available information thus suggests that assessment of body composition by the 3C BIS model provides highly relevant prognostic information, at least at a population level.
To the best of our knowledge, in contrast to studies using BIS for the assessment of fluid status,81 studies assessing the effect of nutritional interventions are still limited. Studies showing the validity of BIS‐guided nutritional intervention would provide a strong rationale for the implementation of BIS in the prevention of PEW. At present, the main argument for its use in clinical practice is its usefulness in risk stratification, in combination with assessment of FO, which provides prognostic information about different risk domains using a single measurement. This besides a role in a holistic nutritional and functional assessment using easily applicable tools in a dialysis population.
AUTHOR CONTRIBUTIONS
Manuscript draft: NJHB and JPK; Manuscript revision: BC, MJED, FMvdS, SS, PW, and JPK; Manuscript approval of final version: NJHB, BC, MJED, FMvdS, SS, PW, and JPK.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Conflict of Interest: Bernard Canaud, Stefano Stuard, and Peter Wabel are employees of Fresenius Medical Care Deutschland GmbH.
Disclosure of grants or other funding: BC has a consulting contract (part time) with FMC. SS is Vice President EMEA Clinical & Therapeutical Governance at FMC. PW is Vice President PSA Home Therapy & Fluid Management at FMC.
REFERENCES
- 1. Kovesdy CP, Kopple JD, Kalantar‐Zadeh K. Management of protein‐energy wasting in non‐dialysis‐dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obi Y, Qader H, Kovesdy CP, Kalantar‐Zadeh K. Latest consensus and update on protein‐energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fouque D, Kalantar‐Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein‐energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. [DOI] [PubMed] [Google Scholar]
- 4. Fouque D, Vennegoor M, ter Wee P, et al. EBPG guideline on nutrition. Nephrol Dial Transplant. 2007;22:ii45–ii87. [DOI] [PubMed] [Google Scholar]
- 5. Zhu F, Rosales L, Kotanko P. Techniques for assessing fluids status in patients with kidney disease. Curr Opin Nephrol Hypertens. 2016;25:473–479. [DOI] [PubMed] [Google Scholar]
- 6. Zoccali C, Moissl U, Chazot C, et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol. 2017;28:2491–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dekker MJ, Marcelli D, Canaud BJ, et al. Impact of fluid status and inflammation and their interaction on survival: A study in an international hemodialysis patient cohort. Kidney Int. 2017;91:1214–1223. [DOI] [PubMed] [Google Scholar]
- 8. Chamney PW, Wabel P, Moissl UM, et al. A whole‐body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85:80–89. [DOI] [PubMed] [Google Scholar]
- 9. Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Price KL, Earthman CP. Update on body composition tools in clinical settings: Computed tomography, ultrasound, and bioimpedance applications for assessment and monitoring. Eur J Clin Nutr. 2019;73:187–193. [DOI] [PubMed] [Google Scholar]
- 11. Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision‐making of volume assessments in dialysis patients. Kidney Int. 2014;86:489–496. [DOI] [PubMed] [Google Scholar]
- 12. Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–933. [DOI] [PubMed] [Google Scholar]
- 13. Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors. 2014;14:10895–10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Broers NJ, Martens RJ, Cornelis T, et al. Body composition in dialysis patients: A functional assessment of bioimpedance using different prediction models. J Ren Nutr. 2015;25:121–128. [DOI] [PubMed] [Google Scholar]
- 15. Molfino A, Don BR, Kaysen GA. Comparison of bioimpedance and dual‐energy x‐ray absorptiometry for measurement of fat mass in hemodialysis patients. Nephron Clin Pract. 2012;122:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konings CJ, Kooman JP, Schonck M, et al. Influence of fluid status on techniques used to assess body composition in peritoneal dialysis patients. Perit Dial Int. 2003;23:184–190. [PubMed] [Google Scholar]
- 17. Keane D, Gardiner C, Lindley E, Lines S, Woodrow G, Wright M. Changes in body composition in the two years after initiation of haemodialysis: A retrospective cohort study. Nutrients. 2016;8:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole‐body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ward LC. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr. 2019;73:194–199. [DOI] [PubMed] [Google Scholar]
- 20. Keane D, Chamney P, Heinke S, Lindley E. Use of the body composition monitor for fluid status measurements in subjects with high body mass index. Nephron. 2016;133:163–168. [DOI] [PubMed] [Google Scholar]
- 21. Raimann JG, Zhu F, Wang J, et al. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int. 2014;85:898–908. [DOI] [PubMed] [Google Scholar]
- 22. Lim PS, Chen CH, Zhu F, et al. Validating body fat assessment by bioelectric impedance spectroscopy in Taiwanese hemodialysis patients. J Ren Nutr. 2017;27:37–44. [DOI] [PubMed] [Google Scholar]
- 23. Popovic V, Zerahn B, Heaf JG. Comparison of dual energy X‐ray absorptiometry and bioimpedance in assessing body composition and nutrition in peritoneal dialysis patients. J Ren Nutr. 2017;27:355–363. [DOI] [PubMed] [Google Scholar]
- 24. Zhou Y, Hoglund P, Clyne N. Comparison of DEXA and bioimpedance for body composition measurements in nondialysis patients with CKD. J Ren Nutr. 2019;29:33–38. [DOI] [PubMed] [Google Scholar]
- 25. Kalantar‐Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition‐inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. [DOI] [PubMed] [Google Scholar]
- 26. Arias‐Guillen M, Perez E, Herrera P, et al. Bioimpedance spectroscopy as a practical tool for the early detection and prevention of protein‐energy wasting in hemodialysis patients. J Ren Nutr. 2018;28:324–332. [DOI] [PubMed] [Google Scholar]
- 27. Wabel PCP, Moissl U, Schultheiss B, Rode C, Wieskotten S, Wizemann V, Charra B. Reproducibility of bioimpedance spectroscopy (BIS) in health and disease. Neph Dia Trans. 2007;22(suppl 6):vi129–vi138. [Google Scholar]
- 28. Wieskotten S, Moissl U, Chamney P, Wabel P. Reference ranges for human body composition and fluid overload. Germany: Fres Medi Care. 2013. [Google Scholar]
- 29. Marcelli D, Usvyat LA, Kotanko P, et al. Body composition and survival in dialysis patients: Results from an international cohort study. Clin J Am Soc Nephrol. 2015;10:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valtuille R, Casos ME, Fernandez EA, Guinsburg A, Marelli C. Nutritional markers and body composition in hemodialysis patients. Int Sch Res Notices. 2015;2015:695263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee HS, Kim SG, Kim JK, et al. Fat‐to‐lean mass ratio can predict cardiac events and all‐cause mortality in patients undergoing hemodialysis. Ann Nutr Metab. 2018;73:241–249. [DOI] [PubMed] [Google Scholar]
- 32. Marcelli D, Brand K, Ponce P, et al. Longitudinal changes in body composition in patients after initiation of hemodialysis therapy: Results from an international cohort. J Ren Nutr. 2016;26:72–80. [DOI] [PubMed] [Google Scholar]
- 33. Parthasarathy R, Oei E, Fan SL. Clinical value of body composition monitor to evaluate lean and fat tissue mass in peritoneal dialysis. Eur J Clin Nutr. 2019;73:1520–1528. [DOI] [PubMed] [Google Scholar]
- 34. Pupim LB, Heimburger O, Qureshi AR, Ikizler TA, Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005;68:2368–2374. [DOI] [PubMed] [Google Scholar]
- 35. van Biesen W, Claes K, Covic A, et al. A multicentric, international matched pair analysis of body composition in peritoneal dialysis versus haemodialysis patients. Nephrol Dial Transplant. 2013;28:2620–2628. [DOI] [PubMed] [Google Scholar]
- 36. Broers NJ, Usvyat LA, Marcelli D, et al. Season affects body composition and estimation of fluid overload in haemodialysis patients: Variations in body composition; a survey from the European MONDO database. Nephrol Dial Transplant. 2015;30:676–681. [DOI] [PubMed] [Google Scholar]
- 37. Rahimlu M, Shab‐Bidar S, Djafarian K. Body mass index and all‐cause mortality in chronic kidney disease: A dose‐response meta‐analysis of observational studies. J Ren Nutr. 2017;27:225–232. [DOI] [PubMed] [Google Scholar]
- 38. Rosenberger J, Kissova V, Majernikova M, Straussova Z, Boldizsar J. Body composition monitor assessing malnutrition in the hemodialysis population independently predicts mortality. J Ren Nutr. 2014;24:172–176. [DOI] [PubMed] [Google Scholar]
- 39. Caetano C, Valente A, Oliveira T, Garagarza C. Body composition and mortality predictors in hemodialysis patients. J Ren Nutr. 2016;26:81–86. [DOI] [PubMed] [Google Scholar]
- 40. Rymarz A, Gibinska J, Zajbt M, Piechota W, Niemczyk S. Low lean tissue mass can be a predictor of one‐year survival in hemodialysis patients. Ren Fail. 2018;40:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Castellano S, Palomares I, Moissl U, et al. Risk identification in haemodialysis patients by appropriate body composition assessment. Nefrologia. 2016;36:268–274. [DOI] [PubMed] [Google Scholar]
- 42. Hwang SD, Lee JH, Lee SW, Kim JK, Kim MJ, Song JH. Risk of overhydration and low lean tissue index as measured using a body composition monitor in patients on hemodialysis: A systemic review and meta‐analysis. Ren Fail. 2018;40:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vega A, Abad S, Macias N, et al. Low lean tissue mass is an independent risk factor for mortality in patients with stages 4 and 5 non‐dialysis chronic kidney disease. Clin Kidney J. 2017;10:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin TY, Peng CH, Hung SC, Tarng DC. Body composition is associated with clinical outcomes in patients with non‐dialysis‐dependent chronic kidney disease. Kidney Int. 2018;93:733–740. [DOI] [PubMed] [Google Scholar]
- 45. Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. [DOI] [PubMed] [Google Scholar]
- 46. Kalantar‐Zadeh K, Rhee CM, Chou J, et al. The obesity paradox in kidney disease: How to reconcile it with obesity management. Kidney Int Rep. 2017;2:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalantar‐Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. [DOI] [PubMed] [Google Scholar]
- 48. Kaysen GA, Ye X, Raimann JG, et al. Lipid levels are inversely associated with infectious and all‐cause mortality: International MONDO study results. J Lipid Res. 2018;59:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Okamoto T, Morimoto S, Ikenoue T, Furumatsu Y, Ichihara A. Visceral fat level is an independent risk factor for cardiovascular mortality in hemodialysis patients. Am J Nephrol. 2014;39:122–129. [DOI] [PubMed] [Google Scholar]
- 50. Pecoits‐Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—the heart of the matter. Nephrol Dial Transplant. 2002;17:28–31. [DOI] [PubMed] [Google Scholar]
- 51. Hung SC, Kuo KL, Peng CH, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–709. [DOI] [PubMed] [Google Scholar]
- 52. Wang YW, Lin TY, Peng CH, Huang JL, Hung SC. Factors associated with decreased lean tissue index in patients with chronic kidney disease. Nutrients. 2017;9:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsai YC, Chiu YW, Tsai JC, et al. Association of fluid overload with cardiovascular morbidity and all‐cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol. 2015;10:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dekker MJE, Konings C, Canaud B, et al. Interactions between malnutrition, inflammation, and fluid overload and their associations with survival in prevalent hemodialysis patients. J Ren Nutr. 2018;28:435–444. [DOI] [PubMed] [Google Scholar]
- 55. O'Lone EL, Visser A, Finney H, Fan SL. Clinical significance of multi‐frequency bioimpedance spectroscopy in peritoneal dialysis patients: Independent predictor of patient survival. Nephrol Dial Transplant. 2014;29:1430–1437. [DOI] [PubMed] [Google Scholar]
- 56. Dekker MJE, van der Sande FM, van den Berghe F, Leunissen KML, Kooman JP. Fluid overload and inflammation axis. Blood Purif. 2018;45:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kerpel‐Fronius E. Volume and composition of the body fluid compartments in severe infantile malnutrition. J Pediatr. 1960;56:826–833. [DOI] [PubMed] [Google Scholar]
- 58. Kerpel‐Fronius E, Kovach S. The volume of extracellular body fluids in malnutrition. Pediatrics. 1948;2:21–23. [PubMed] [Google Scholar]
- 59. Nichols BL, Alleyne GA, Barnes DJ, Hazlewood CD. Relationship between muscle potassium and total body potassium in infants with malnutrition. J Pediatr. 1969;74:49–57. [DOI] [PubMed] [Google Scholar]
- 60. Dicker SE. Changes in the extracellular‐ and intracellular‐ fluid phases of tissues during water diuresis in normal and hypoproteinaemic rats. Biochem J. 1948;43:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patrick J, Reeds PJ, Jackson AA, Seakins A, Picou DI. Total body water in malnutrition: The possible role of energy intake. Br J Nutr. 1978;39:417–424. [DOI] [PubMed] [Google Scholar]
- 62. Smith TW, Rasmusson RL, Lobaugh LA, Lieberman M. Na+/K+ pump inhibition induces cell shrinkage in cultured chick cardiac myocytes. Basic Res Cardiol. 1993;88:411–420. [DOI] [PubMed] [Google Scholar]
- 63. Keane DF, Bowra K, Kearney K, Lindley E. Use of the body composition monitor for fluid status measurements in elderly malnourished subjects. ASAIO J. 2017;63:507–511. [DOI] [PubMed] [Google Scholar]
- 64. Barac‐Nieto M, Spurr GB, Lotero H, Maksud MG. Body composition in chronic undernutrition. Am J Clin Nutr. 1978;31:23–40. [DOI] [PubMed] [Google Scholar]
- 65. Chazot C, Jean G, Vo‐Van C, et al. The plasma level of brain natriuretic peptide is increased in malnourished hemodialysis patients. Blood Purif. 2009;28:187–192. [DOI] [PubMed] [Google Scholar]
- 66. Rincon Bello A, Bucalo L, Abad Estebanez S, et al. Fat tissue and inflammation in patients undergoing peritoneal dialysis. Clin Kidney J. 2016;9:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Delgado C, Chertow GM, Kaysen GA, et al. Associations of body mass index and body fat with markers of inflammation and nutrition among patients receiving hemodialysis. Am J Kidney Dis. 2017;70:817–825. [DOI] [PubMed] [Google Scholar]
- 68. Spoto B, Di Betta E, Mattace‐Raso F, et al. Pro‐ and anti‐inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr Metab Cardiovasc Dis. 2014;24:1137–1143. [DOI] [PubMed] [Google Scholar]
- 69. Caetano C, Valente A, Silva FJ, Antunes J, Garagarza C. Effect of an intradialytic protein‐rich meal intake in nutritional and body composition parameters on hemodialysis patients. Clin Nutr ESPEN. 2017;20:29–33. [DOI] [PubMed] [Google Scholar]
- 70. Molina P, Vizcaino B, Molina MD, et al. The effect of high‐volume online haemodiafiltration on nutritional status and body composition: The ProtEin Stores prEservaTion (PESET) study. Nephrol Dial Transplant. 2018;33:1223–1235. [DOI] [PubMed] [Google Scholar]
- 71. Marcelli D, Wabel P, Wieskotten S, et al. Physical methods for evaluating the nutrition status of hemodialysis patients. J Nephrol. 2015;28:523–530. [DOI] [PubMed] [Google Scholar]
- 72. Chen HS, Cheng CT, Hou CC, et al. A practical standardized composite nutrition score based on lean tissue index: Application in nutrition screening and prediction of outcome in hemodialysis population. J Ren Nutr. 2017;27:267–274. [DOI] [PubMed] [Google Scholar]
- 73. Kim JC, Kalantar‐Zadeh K, Kopple JD. Frailty and protein‐energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24:337–351. [DOI] [PubMed] [Google Scholar]
- 74. Broers NJH, Martens RJH, Cornelis T, et al. Physical activity in end‐stage renal disease patients: The effects of starting dialysis in the first 6 months after the transition period. Nephron. 2017;137:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Broers NJH, Martens RJH, Canaud B, et al. Health‐related quality of life in end‐stage renal disease patients: The effects of starting dialysis in the first year after the transition period. Int Urol Nephrol. 2018;50:1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9:1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marty E, Liu Y, Samuel A, Or O, Lane J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone. 2017;105:276–286. [DOI] [PubMed] [Google Scholar]
- 78. Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV‐infection. Curr HIV/AIDS Rep. 2014;11:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Souweine JS, Kuster N, Chenine L, et al. Physical inactivity and protein energy wasting play independent roles in muscle weakness in maintenance haemodialysis patients. PLoS One. 2018;13:e0200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. NICE ‐ National Institute for Health and Care Excellence . Multiple frequency bioimpedance devices to guide fluid management in people with chronic kidney disease having dialysis. 2017. https://www.nice.org.uk/guidance/dg29
- 81. Hur E, Usta M, Toz H, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: A randomized controlled trial. Am J Kidney Dis. 2013;61:957–965. [DOI] [PubMed] [Google Scholar]


