Abstract
Objectives
To investigate the clinical and chest CT characteristics of COVID-19 pneumonia and explore the radiological differences between COVID-19 and influenza.
Materials and methods
A total of 122 patients (61 men and 61 women, 48 ± 15 years) confirmed with COVID-19 and 48 patients (23 men and 25 women, 47 ± 19 years) confirmed with influenza were enrolled in the study. Thin-section CT was performed. The clinical data and the chest CT findings were recorded.
Results
The most common symptoms of COVID-19 were fever (74%) and cough (63%), and 102 patients (83%) had Wuhan contact. Pneumonia in 50 patients with COVID-19 (45%) distributed in the peripheral regions of the lung, while it showed mixed distribution in 26 patients (74%) with influenza (p = 0.022). The most common CT features of the COVID-19 group were pure ground-glass opacities (GGO, 36%), GGO with consolidation (51%), rounded opacities (35%), linear opacities (64%), bronchiolar wall thickening (49%), and interlobular septal thickening (66%). Compared with the influenza group, the COVID-19 group was more likely to have rounded opacities (35% vs. 17%, p = 0.048) and interlobular septal thickening (66% vs. 43%, p = 0.014), but less likely to have nodules (28% vs. 71%, p < 0.001), tree-in-bud sign (9% vs. 40%, p < 0.001), and pleural effusion (6% vs. 31%, p < 0.001).
Conclusions
There are significant differences in the CT manifestations of patients with COVID-19 and influenza. Presence of rounded opacities and interlobular septal thickening, with the absence of nodules and tree-in-bud sign, and with the typical peripheral distribution, may help us differentiate COVID-19 from influenza.
Key Points
• Typical CT features of COVID-19 include pure ground-glass opacities (GGO), GGO with consolidation, rounded opacities, bronchiolar wall thickening, interlobular septal thickening, and a peripheral distribution.
• Presence of rounded opacities and interlobular septal thickening, with the absence of nodules and tree-in-bud sign, and with the typical peripheral distribution, may help us differentiate COVID-19 from influenza.
Keywords: Coronavirus infections; Tomography, x-ray computed; Pneumonia, viral; Influenza; Human
Introduction
In December 2019, an outbreak of COVID-19 (formerly known as 2019-nCoV) pneumonia began in Wuhan (Hubei Province, China). The COVID-19 spread rapidly across China and many other countries. As of January 30, 2020, the World Health Organization (WHO) has designated this outbreak as a global health emergency. By April 1, 2020, 823,626 confirmed cases and 40,598 death cases have been officially reported by the WHO [1]. Respiratory droplets and direct contact are likely to be the most important routes of transmission [2, 3]. Therefore, early diagnosis and isolation becomes essential against novel coronavirus. Reverse transcriptase polymerase chain reaction (RT-PCR) is the reference standard to confirm the diagnosis of COVID-19 infection. However, more and more false negative RT-PCR results have been reported recently [4, 5], making the diagnosis rather challenging. Currently, chest CT is one of the best tools for screening, primary diagnosis, estimation of disease severity, and prediction of the prognosis.
Chest CT manifestations of COVID-19 pneumonia have not been well known. Recent studies showed that typical CT findings included bilateral pulmonary parenchymal ground-glass and consolidative pulmonary opacities, with a peripheral lung distribution [6, 7]. However, the CT features of COVID-19 are very diverse, and it is difficult to differentiate it from other kinds of viral pneumonia. Recently, Bai et al compared the chest CT features of COVID-19 pneumonia and non-COVID-19 viral pneumonia, and found that COVID-19 pneumonia was more likely to have a peripheral distribution and ground-glass opacity [8]. However, there are several kinds of respiratory tract viruses that may lead to viral pneumonia, and the CT features of the different kinds of viral pneumonia may be different [9]. Influenza pneumonia is among the most common viral pneumonia, and now it is just during the “flu season”; therefore, it is essential to differentiate COVID-19 pneumonia from influenza. Herein, we described and summarized the chest CT findings of 122 patients with COVID-19 pneumonia and 48 patients with influenza, and tried to explore the radiological differentiation between the two groups.
Materials and methods
Patients
This study was approved by the institutional review board. Written informed consent was waived for this retrospective study. Laboratory-confirmed COVID-19 patients in the inpatient databases of The First Affiliated Hospital of Chongqing Medical University and Chongqing Three Gorges Center Hospital from January 2020 to February 2020 were consecutively collected. In addition, patients with influenza in the inpatient database of The First Affiliated Hospital of Chongqing Medical University from January 2015 to February 2020 were consecutively enrolled. Diagnosis of COVID-19 and influenza was based on the positive results by real-time RT-PCR. Our exclusion criteria were (1) patients without CT scan; (2) patients comorbid with other infections; and (3) patients with a past history of interstitial lung disease. Clinical data and CT images of the patients were reviewed.
Image acquisition
All of the chest CT scans followed usual clinical acquisition parameters according to local protocols. A total of 119 patients were imaged with 1.5-mm-thick slices with a Siemens SOMATOM Sensation scanner (Siemens Healthineers), and 51 patients were imaged with 1-mm-thick slices with a Siemens SOMATOM Perspective scanner (Siemens Healthineers). The scanning parameters were as follows: 120 kVp, 150 mA, 1.5 mm collimition, reconstruction matrix of 512 × 512, slice thickness of 1.0 mm, and high spatial resolution algorithm. Radiation dose of chest CT was as follows: volume CT dose index, 6.24 mGy; dose-length product, 208.45 mGy; effective dose, 2.98 mSv. The scanning range included the whole chest from the first ribs to the diaphragm. All the patients were examined without injection of contrast media. Images were obtained with mediastinal (width, 350–450 HU; level, 20–40 HU) and parenchymal (width, 1200–1600 HU; level, − 500 to − 700 HU) window settings.
Image interpretation
Two thoracic radiologists (with 8 years and 25 years of experience, respectively) blinded to the clinical data reviewed the CT images independently, and the discrepancies were resolved by consensus. CT images of each patients were assessed for the presence and distribution of parenchymal abnormalities, including (1) pure ground-glass opacities (GGO), which was defined as a hazy increase in lung attenuation with no obscuration of the underlying vessels; (2) GGO with consolidation, which was defined as an area of opacification obscuring the underlying vessels in GGO; (3) consolidation without GGO; (4) rounded opacities, which was defined as opacities with a rounded morphology [7]; (5)nodules; (6) tree-in-bud sign; (7) air bronchogram; (8) interlobular septal thickening; (9) bronchiolar wall thickening; (10) crazy paving pattern, which was defined as the appearance of GGO with superimposed interlobular septal thickening and intralobular septal thickening; (11) halo sign, which was defined as the GGO surrounding a pulmonary nodule or mass; (12) cavitation; (13) pleural effusion; (14) pericardial effusion; and (15) lymphadenopathy, which was defined as a lymph node > 1 cm in short-axis diameter. In addition, the outer one-third of the lung was defined as peripheral, and the rest was defined as central.
Statistical analysis
Statistical analyses were performed with the SPSS for Windows software package (version 17.0, SPSS Inc.). Data were expressed as mean ± standard deviation (SD), and each discrete variables were compared using the independent t test or λ2 test depending on parametric or non-parametric data. A p value < 0.05 was considered statistically significant. The p values for multiple comparisons of the lesion distribution and chest CT findings between the patients with COVID-19 and those with influenza were not adjusted in our study.
Results
Clinical characteristics of patients
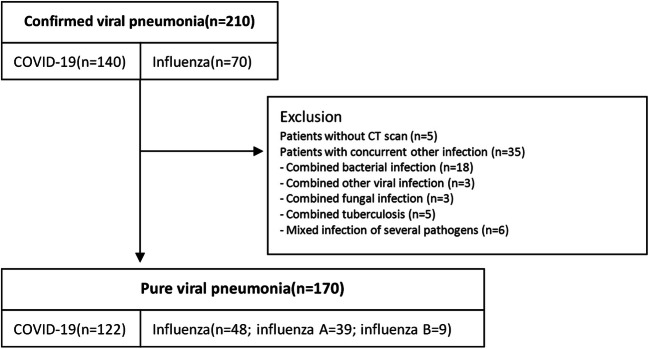
A total of 210 patients were confirmed with COVID-19 and influenza. Of these patients, 5 patients did not undergo CT scans, and 35 patients who had concurrent infection with other pathogens were also excluded (see Fig. 1). Finally, 122 patients with COVID-19 and 48 patients with influenza (39 with influenza A and 9 with influenza B) were collected in the present study. The COVID-19 group included 61 (50%) men and 61 (50%) women, with ages ranging from 15 to 80 years (48 ± 15 years). The influenza group included 23 (48%) men and 25 (23%) women, with ages ranging from 15 to 86 years (47 ± 19 years). There was no statistically significant difference in age or sex (p = 0.886, 0.807, respectively). Among the COVID-19 group, a hundred and two patients (83%) had contact with individuals from Wuhan (traveled to/or lived in Wuhan, or were in contact with confirmed COVID-19 patients). The most common symptoms of COVID-19 were fever (74%) and cough (63%). The less common symptoms were headache (14%), sputum (12%), dyspnea (10%), sore throat (8%), and stuffy and runny nose (7%). Seven patients (6%) of COVID-19 were asymptomatic. The most common symptoms of patients with influenza were fever (83%) and cough (77%). The less common symptoms were stuffy and runny nose (23%), sputum (21%), dyspnea (15%), sore throat (15%), and headache (8%). Three patients of the COVID-19 group and 2 patients of the influenza group died of respiratory failure or pulmonary embolism, and the rest discharged after marked improvement. The clinical characteristics of these patients are shown in Table 1.
Fig. 1.
Flow diagram of the study
Table 1.
Demographic and clinical characteristics of the patients included in the present study
| Characteristics | COVID-19 group (N = 122) | Influenza group (N = 48) | p value |
|---|---|---|---|
| Sex | |||
| Male | 61 (50%) | 23 (48%) | 0.807 |
| Female | 61 (50%) | 25 (52%) | |
| Age (years) | 48 ± 15 (range 15–80) | 47 ± 19 (range 15–86) | 0.886 |
| Wuhan contact | |||
| Yes | 102 (83%) | – | |
| No | 20 (17%) | – | |
| Comorbidity | |||
| Diabetes | 13 (11%) | 2 (4%) | 0.179 |
| Hypertension | 10 (8%) | 3 (6%) | 0.667 |
| Chronic obstructive pulmonary disease | 4 (3%) | 6 (13%) | 0.021 |
| Cardiovascular disease | 3 (2%) | 3 (6%) | 0.228 |
| Hepatic disease | 3 (2%) | 0 | 0.273 |
| Malignancy | 2 (1%) | 1 (2%) | 0.843 |
| Symptoms | |||
| No obvious symptoms | 7 (6%) | 0 | 0.090 |
| Fever | 90 (74%) | 40 (83%) | 0.186 |
| Cough | 77 (63%) | 37 (77%) | 0.081 |
| Sputum | 15 (12%) | 10 (21%) | 0.157 |
| Stuffy and runny nose | 8 (7%) | 11 (23%) | 0.002 |
| Dyspnea | 12 (10%) | 7 (15%) | 0.153 |
| Headache | 17 (14%) | 4 (8%) | 0.318 |
| Nausea and vomit | 3 (2%) | 2 (4%) | 0.553 |
| Chest pain | 3 (2%) | 2 (4%) | 0.553 |
| Sore throat | 10 (8%) | 7 (15%) | 0.211 |
| Diarrhea | 4 (3%) | 2 (4%) | 0.778 |
| Clinical outcome | |||
| Died | 3 (2%) | 2 (4%) | 0.553 |
| Discharged | 119(98%) | 46 (96%) | |
Italicized entries when P < 0.05
Chest CT evaluation
The median intervals between disease onset and chest CT scan were 6.2 ± 4.8 days for the COVID-19 group and 6.0 ± 3.9 days for the influenza group, respectively, which did not differ statistically (p = 0.713). The distribution of the CT lesions is listed in Table 2. Briefly, ten patients (8%) with COVID-19 were negative for CT, while up to 13 patients (27%) with influenza were CT-negative. Among the CT-positive patients, all the five lobes were affected in most of the patients in both groups. The frequency of lobe affected did not differ across the two groups. There were 50 COVID-19 patients (45%) with lesions distributed in the peripheral/subpleural regions of the lung, and the majority of the influenza patients (63%) showed mixed distribution, which differed significantly (p = 0.022).
Table 2.
Distribution of the lesions in the patients with COVID-19 and influenza
| Characteristics | COVID-19 group (N = 122) | Influenza group (N = 48) | p value |
|---|---|---|---|
| Unilateral lung affected | 8 (7%) | 8 (17%) | 0.067 |
| Bilateral lung affected | 104 (85%) | 27 (56%) | |
| Numbers of lobes affected | |||
| 0 | 10 (8%) | 13 (27%) | 0.669 |
| 1 | 10 (8%) | 5 (10%) | |
| 2 | 15 (12%) | 5 (10%) | |
| 3 | 7 (6%) | 3 (6%) | |
| 4 | 28 (23%) | 5 (10%) | |
| 5 | 52 (43%) | 17 (35%) | |
| Frequency of lobe affected | |||
| Right upper lobe | 95 (78%) | 28 (58%) | 0.749 |
| Right middle lobe | 85 (69%) | 18 (38%) | 0.058 |
| Right lower lobe | 104 (85%) | 27 (56%) | 0.085 |
| Left upper lobe | 99 (81%) | 27 (56%) | 0.275 |
| Left lower lobe | 104 (85%) | 30 (63%) | 0.493 |
| Predominant distribution | |||
| Central | 2 (2%) | 2 (6%) | 0.022 |
| Peripheral | 50 (45%) | 7 (20%) | |
| Mixed | 60 (53%) | 26 (74%) | |
Italicized entries when P < 0.05
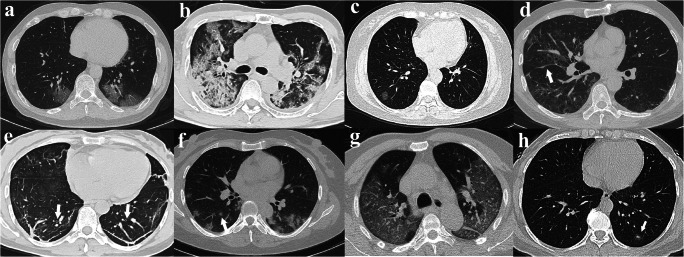
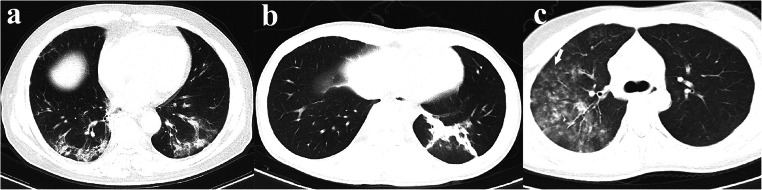
The radiological signs of the lesions were reviewed subsequently and are listed in Table 3. The most common CT features of the COVID-19 group were pure GGO (36%, Fig. 2a), GGO with consolidation (51%, Fig. 2b), rounded opacities (35%, Fig. 2c), linear opacities (64%), interlobular septal thickening (66%, Fig. 2d), and bronchiolar wall thickening (49%, Fig. 2e). The less common CT features included nodules (28%, Fig. 2f), crazy paving pattern (21%, Fig. 2g), air bronchogram (20%), and pure consolidation (13%). The rare CT features were tree-in-bud sign (9%), pleural effusion (6%), halo sign (3%, Fig. 2h), pericardial effusion (3%), and lymphadenopathy (3%). On the contrary, main CT features in the influenza group were GGO with consolidation (63%, Fig. 3a), nodules (71%, Fig. 3c), linear opacities (71%), interlobular septal thickening (43%), and tree-in-bud sign (40%, Fig. 3c). Pure GGO (20%), consolidation without GGO (17%, Fig. 3b), rounded GGO (17%), crazy paving pattern (17%), air bronchogram (37%), bronchiolar wall thickening (34%), and pleural effusion (31%) were less common CT findings of the patients with influenza. Compared with influenza pneumonia, COVID-19 pneumonia was more likely to have rounded opacities (35% vs. 17%, p = 0.048) and interlobular septal thickening (66% vs. 43%, p = 0.014), but less likely to have nodules (28% vs. 71%, p < 0.001), tree-in-bud sign (9% vs. 40%, p < 0.001), and pleural effusion (6% vs. 31%, p < 0.001). In addition, we found the following combinations were helpful to differentiate COVID-19 from influenza: (1) presence of pure GGO and absence of nodules (29% vs. 11%, p < 0.001); (2) presence of pure GGO and interlobular septal thickening (21% vs. 6%, p = 0.042); (3) presence of rounded opacities and absence of nodules (22% vs. 0%, p = 0.002); (4) presence of interlobular septal thickening and absence of nodules (45% vs. 6%, p < 0.001); (5) presence of rounded opacities and interlobular septal thickening and absence of pleural effusion (19% vs. 3%, p = 0.021).
Table 3.
Chest CT findings in the patients with COVID-19 and influenza
| Characteristics | COVID-19 group | Influenza group | p value |
|---|---|---|---|
| Interval between onset and CT scan (days) | 6.2 ± 4.8 | 6.0 ± 3.9 | 0.713 |
| Ground-glass opacities and consolidation | |||
| Pure GGO | 40 (36%) | 7 (20%) | 0.082 |
| GGO with consolidation | 57 (51%) | 22 (63%) | 0.215 |
| Pure consolidation | 15 (13%) | 6 (17%) | 0.580 |
| Opacity characteristics | |||
| Linear opacities | 72 (64%) | 25 (71%) | 0.436 |
| Rounded opacities | 39 (35%) | 6 (17%) | 0.048 |
| Crazy paving pattern | 24 (21%) | 6 (17%) | 0.583 |
| Halo sign | 3 (3%) | 3 (8%) | 0.124 |
| Nodules | 31 (28%) | 25 (71%) | < 0.001 |
| Tree-in-bud sign | 10 (9%) | 14 (40%) | < 0.001 |
| Air bronchogram | 22 (20%) | 13 (37%) | 0.264 |
| Interlobular septal thickening | 74 (66%) | 15 (43%) | 0.014 |
| Bronchiolar wall thickening | 55 (49%) | 12 (34%) | 0.124 |
| Cavitation | 0 (0%) | 0 (0%) | – |
| Pleural effusion | 7 (6%) | 11 (31%) | < 0.001 |
| Pericardial effusion | 2 (3%) | 0 (0%) | 0.426 |
| Lymphadenopathy | 2 (3%) | 0 (0%) | 0.426 |
| Combinations | |||
| Pure GGO (+) + nodules (−) | 32 (29%) | 4 (11%) | < 0.001 |
| Pure GGO (+) + interlobular septal thickening(+) | 23 (21%) | 2 (6%) | 0.042 |
| Rounded opacities (+) + interlobular septal thickening(+) | 21 (19%) | 2 (6%) | 0.064 |
| Rounded opacities (+) + nodules (−) | 25 (22%) | 0 (0%) | 0.002 |
| Interlobular septal thickening(+) + nodules (−) | 50 (45%) | 2 (6%) | < 0.001 |
| Rounded opacities (+) + interlobular septal thickening (+) + pleural effusion(−) | 21 (19%) | 1 (3%) | 0.021 |
Italicized entries signify p < 0.05
Fig. 2.
CT features of COVID-19-related pneumonia. a Ground-glass opacities with a peripheral distribution. b Ground-glass opacities with consolidation. c Rounded GGO. d Interlobular septal thickening. e Bronchiolar wall thickening (arrow). f Nodule (arrow). g Crazy paving pattern. h Halo sign (arrow)
Fig. 3.
Most common CT features of influenza pneumonia. a Ground-glass opacities with consolidation with a peripheral distribution. b Consolidation without ground-glass opacities. c Multiple nodules and tree-in-bud sign (arrow)
Discussion
Eighty-three percent of the patients with COVID-19 in the present study had a history of Wuhan contact, which was very important for diagnosis. However, a considerable portion of patients with Wuhan contact were finally diagnosed with influenza or bacterial pneumonia in our hospital. Therefore, the etiological tests of other pathogens of the respiratory tract are needed for the patients with negative PCR for COVID-19. The total positive rate of initial RT-PCR for throat swab samples was reported to be about 30 to 60% [10]. Xie et al found positive CT findings on patients with COVID-19 who had negative initial RT-PCR [4]. In addition, the sensitivity of chest CT was reported to be greater than that of RT-PCR (98% vs. 71%) [11]. Therefore, chest CT differentiation was of high importance. However, patients with PCR-confirmed COVID-19 may have normal CT findings at admission [12], and thus, a normal CT scan cannot rule out the diagnosis. Therefore, the combination of chest CT and RT-PCR is necessary for the diagnosis of COVID-19.
We have confirmed in the present study that the lesion distribution of the two diseases has much in common. Both COVID-19 and influenza groups involved bilateral lungs with multiple lung lobes, and a majority of patients in both groups involved all five lobes, which was consistent with previous studies [9, 13–16]. However, the COVID-19 group mainly involved peripheral part of the lung, which was in line with other coronavirus infection such as SARS [17], while influenza pneumonia was more diffuse and involved both central and peripheral parts. Our results showed that the most common imaging signs of the COVID-19 group were pure GGO, GGO with consolidation, rounded opacities, bronchiolar wall thickening, and interlobular septal thickening, which were in line with previous studies [8, 18–20]. Only few patients with COVID-19 appeared as pure consolidation. According to a recent study, more consolidation would be found in patients with COVID-19 as the disease course increased, and more consolidation lesions were found in elder patients (> 50 years) than in the younger patients [14, 21]. Since a substantial amount of patients with COVID-19 in the present study were at early stage, and the median age of the patients was just 48 years, this might be a possible explanation why there were less complete consolidation lesions in the COVID-19 group. Follow-up CT scan should be done to validate this speculation. Interlobular septal thickening is a common sign on thin-slice chest CT; it may be seen in the presence of interstitial fluid, cellular infiltration, or fibrosis. This sign can be found in various viral pneumonias such as parainfluenza, Hantavirus, and SARS [9, 22]. Edema and smooth muscle hyperplasia could result in bronchiolar wall thickening, which has been reported in influenza, parainfluenza, and adenovirus [17, 22]. Nodule size is helpful in the differential diagnosis of infectious causes of nodules. It is reported that patients whose nodules were smaller than 10 mm in diameter were most likely to have a viral infection [23]. Nodules were present in 71% of the patients with influenza but in only 28% patients with COVID-19, which was consistent with a previous study [13]. Although each of the above sign is not specific, some combinations of the CT features may help better differentiate COVID-19 from influenza pneumonia: presence of pure GGO/rounded opacities/interlobular septal thickening and the absence of nodules; presence of pure GGO and interlobular septal thickening; presence of rounded opacities and interlobular septal thickening; and the absence of pleural effusion. Combining the above CT signs with predominant distribution in peripheral part of the lungs may help us differentiate COVID-19 from influenza.
Our study had several limitations. Firstly, the present study was a retrospective study, and the radiological differences between the COVID-19 group and influenza group in our study could be data-driven, so external validation was needed before our findings can be widely used in the clinical practice.
Secondly, the sample size was relatively small, especially the influenza group. Finally, we did not review the follow-up CT. Pulmonary GGO and consolidation lesions may change as the disease progresses [24, 25], so analyzing the following up CT may help us better understand the dynamics of the disease. In the future, we will investigate the chest CT features of differential stages of patients with COVID-19 by using larger and more diverse samples.
Conclusions
In conclusion, the most common imaging patterns of COVID-19 pneumonia are pure GGO, GGO with consolidation, rounded opacities, linear opacities, interlobular septal thickening, and bronchiolar wall thickening, with predominant distribution in bilateral lower lobes and in peripheral part of the lungs. Presence of rounded opacities and interlobular septal thickening, with the absence of nodules and tree-in-bud sign, and with the typical peripheral distribution, may help us differentiate COVID-19 from influenza.
Acknowledgments
We thank Dr. Huaming Mou for assistance with data collection.
Abbreviations
- GGO
Ground-glass opacities
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SARS
Severe acute respiratory syndrome
Funding information
The authors state that this work has not received any funding.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Kaihu Xiao.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained (Certification No: ChiCTR2000029952).
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported before.
Methodology
• retrospective
• cross sectional study
•multicenter study
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fajin Lv, Email: fajinlv@163.com.
Kaihu Xiao, Email: xiaokh1@163.com.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 72. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2. Accessed 1 Apr 2020
- 2.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 10.1148/radiol.2020200343 [DOI] [PMC free article] [PubMed]
- 5.Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed]
- 6.Pan Y, Guan H (2020) Imaging changes in patients with 2019-nCov. Eur Radiol. 10.1007/s00330-020-06713-z [DOI] [PMC free article] [PubMed]
- 7.Chung M, Bernheim A, Mei X et al (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed]
- 8.Bai HX, Hsieh B, Xiong Z et al (2020) Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 10.1148/radiol.2020200823 [DOI] [PMC free article] [PubMed]
- 9.Kim MC, Kim MY, Lee HJ, et al. CT findings in viral lower respiratory tract infections caused by parainfluenza virus, influenza virus and respiratory syncytial virus. Medicine (Baltimore) 2016;95:e4003. doi: 10.1097/MD.0000000000004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Yang M, Shen C et al (2020) Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. https://www.medrxiv.org/content/10.1101/2020.02.11.20021493v2. Accessed 17 Feb 2020
- 11.Fang Y, Zhang H, Xie J et al (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed]
- 12.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;3:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 13.Pan Y, Guan H, Zhou S et al (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed]
- 14.Song F, Shi N, Shan F et al (2020) Emerging coronavirus 2019-nCoV pneumonia. Radiology. 10.1148/radiol.2020200274
- 15.Lei J, Li J, Li X, Qi X (2020) CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 10.1148/radiol.2020200236 [DOI] [PMC free article] [PubMed]
- 16.Shi H, Han X, Zheng C (2020) Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology. 10.1148/radiol.2020200269 [DOI] [PMC free article] [PubMed]
- 17.Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38:719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 18.Li K, Wu J, Wu F et al (2020) The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed]
- 19.Wu J, Wu X, Zeng W et al (2020) Chest CT findings in patients with Corona virus disease 2019 and its relationship with clinical features. Invest Radiol. 10.1097/RLI.0000000000000670 [DOI] [PMC free article] [PubMed]
- 20.Zu ZY, Jiang MD, Xu PP et al (2020) Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 10.1148/radiol.2020200490 [DOI] [PMC free article] [PubMed]
- 21.Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 10.1148/radiol.2020200370
- 22.Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260:18–39. doi: 10.1148/radiol.11092149. [DOI] [PubMed] [Google Scholar]
- 23.Franquet T, Muller NL, Gimenez A, Martinez S, Madrid M, Domingo P. Infectious pulmonary nodules in immunocompromised patients: usefulness of computed tomography in predicting their etiology. J Comput Assist Tomogr. 2003;27:461–468. doi: 10.1097/00004728-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Shi H, Han X, Jiang N et al (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed]
- 25.Bernheim A, Mei X, Huang M et al (2020) Chest CT findings in coronavirus Disease-19 (COVID-19): relationship to duration of infection. Radiology. 10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed]