Abstract
Intranasally administered influenza vaccines could be more effective than injected vaccines, because intranasal vaccination can induce virus‐specific immunoglobulin A (IgA) antibodies in the upper respiratory tract, which is the initial site of infection. In this study, immune responses elicited by an intranasal inactivated vaccine of influenza A(H5N1) virus were evaluated in healthy individuals naive for influenza A(H5N1) virus. Three doses of intranasal inactivated whole‐virion H5 influenza vaccine induced strong neutralizing nasal IgA and serum IgG antibodies. In addition, a mucoadhesive excipient, carboxy vinyl polymer, had a notable impact on the induction of nasal IgA antibody responses but not on serum IgG antibody responses. The nasal hemagglutinin (HA)‐specific IgA antibody responses clearly correlated with mucosal neutralizing antibody responses, indicating that measurement of nasal HA‐specific IgA titers could be used as a surrogate for the mucosal antibody response. Furthermore, increased numbers of plasma cells and vaccine antigen‐specific Th cells in the peripheral blood were observed after vaccination, suggesting that peripheral blood biomarkers may also be used to evaluate the intranasal vaccine‐induced immune response. However, peripheral blood immune cell responses correlated with neutralizing antibody titers in serum samples but not in nasal wash samples. Thus, analysis of the peripheral blood immune response could be a surrogate for the systemic immune response to intranasal vaccination but not for the mucosal immune response. The current study suggests the clinical potential of intranasal inactivated vaccines against influenza A(H5N1) viruses and highlights the need to develop novel means to evaluate intranasal vaccine‐induced mucosal immune responses.
Keywords: HA‐specific nasal IgA, immunological surrogates, inactivated whole‐virion influenza vaccine, influenza A(H5N1) virus, intranasal inactivated influenza vaccine
Abbreviations
- BCA
bicinchoninic acid assay
- CVP
carboxy vinyl polymer
- HA
hemagglutinin
- NT
neutralizing test
- PBMCs
peripheral blood mononuclear cells
- S‐IgA
secretory IgA
- TCID50
50% infectious dose in tissue culture
- UMIN
University Medical Information Network
1. INTRODUCTION
Both secretory immunoglobulin A (S‐IgA) and IgG antibodies contribute to protection against influenza virus in the respiratory tract. 1 , 2 Current intramuscular or subcutaneous influenza vaccines, which include detergent‐disrupted split‐virus vaccines and subunit vaccines, predominantly induce systemic IgG antibodies. This strategy results in a significant reduction in mortality and morbidity because these antibodies have an important role in suppressing virus growth, especially in the lower respiratory tract. 3 , 4 , 5 , 6 However, the relatively small amount of serum IgG antibodies binding to the surface of the mucosal epithelia is insufficient to prevent virus infection in the upper respiratory tract, including the nasal and tracheal mucosa. 3 , 4 These IgG antibodies are highly protective against viruses antigenically homologous to the administered vaccine virus but not against heterologous viruses with different antigenicity because of antigenic drift. 7 These observations suggest that conventional influenza vaccines are effective at reducing the disease severity of influenza but not at providing protection from influenza virus infection. By contrast, intranasal vaccination, a vaccination mode that mimics natural infection, can induce virus‐specific S‐IgA antibodies in the upper respiratory mucosa as well as IgG antibodies in serum. 1 , 2 S‐IgA antibodies in the nasal mucosa are cross‐protective against not only antigenically homologous viruses but also antigenically heterologous viruses, and exist in the form of multimers such as trimers and tetramers. These multimeric S‐IgA antibodies display superior neutralizing potency against influenza A viruses compared with dimeric S‐IgA antibodies. 8 , 9 We previously showed that in the case of seasonal influenza vaccination, two doses of an intranasal inactivated whole‐virion vaccine could successfully induce S‐IgA and IgG responses in the nasal mucosa and serum, respectively, in healthy adults. 9 , 10 Measurement of the serum virus‐neutralizing antibody titers of the study participants prior to vaccination revealed that they already possessed baseline immunity against seasonal influenza virus. This indicated that the immune responses observed in those studies were due to the ability of the intranasal inactivated vaccine to boost this baseline immunity, leading to increased virus‐specific antibody responses. Therefore, the intranasal vaccination protocol implemented for seasonal influenza virus vaccination may not be suitable to induce sufficient virus‐specific mucosal S‐IgA and serum IgG responses against virus strains to which most humans are immunologically naive, such as avian influenza virus strains of the A(H5N1) subtype. Furthermore, in previous studies, the virus‐neutralizing antibody titers in serum and nasal wash specimens have been shown to be immunological surrogates of intranasal vaccine‐induced immune responses in adults. 9 , 10 , 11 To date, other immunological surrogates that could be measured in specimens besides mucosal wash and serum have not been reported. Although measurement of neutralizing antibodies enables quantitative evaluation of vaccine‐induced immune responses by a relatively convenient method, a nasal washing procedure, which is usually done by the study participants themselves, is required to collect samples for antibody titer measurement. 9 , 10 , 11 Therefore, it is challenging to measure the nasal antibody response in the elderly and the young, who are unable to perform the nasal washing procedure without assistance. Intranasal inactivated influenza vaccines are expected to work by an immune mechanism different from that of injected vaccines. Therefore, the immune responses induced by intranasal vaccination should be characterized further, and exploration of novel immunological surrogates for intranasal vaccine evaluation is important.
In this, peripheral blood mononuclear cells (PBMCs) and serum and nasal wash samples were collected from human participants who had been administered an intranasal inactivated vaccine of influenza A(H5N1) virus. By analyzing the immune response induced by intranasal vaccination, the feasibility of developing an intranasal inactivated influenza vaccine against nonseasonal influenza viruses, to which humans do not possess basic immunity, was evaluated. A novel method for evaluating intranasal vaccine‐induced immune responses using peripheral blood was also examined.
2. MATERIALS AND METHODS
2.1. Ethics
The study protocol and other relevant documentation for human studies were reviewed and approved by the Medical Research Ethics Committee of the National Institute of Infectious Diseases (University Medical Information Network Clinical Trials Registry ID: UMIN000008279). All participants consented in writing to participate in the study before enrollment and after being informed of the nature of the study, its risks, and its potential benefits. All participants have been fully anonymized.
2.2. Human participants and vaccination protocol
The study participants were 63 healthy volunteers between 21 and 64 years of age (mean age, 34.6 years; 31 male participants). The participants were vaccinated intranasally with an inactivated whole‐virion H5 influenza vaccine (45 µg hemagglutinin [HA]/dose) three times, on days 0, 21, and 280. The participants were divided into two groups, one with 32 participants (average age, 34.9 years; 16 males) and the other with 31 participants (average age, 34.3 years; 15 males), who received intranasal vaccination with or without carboxy vinyl polymer (CVP) as a mucoadhesive excipient, respectively. The vaccine was prepared from purified virus of the vaccine candidate IBCDC‐RG2, which was generated by reverse genetic engineering from A/Indonesia/5/05 (H5N1). The vaccine used in this study (lot number FPBMW1005‐d) was an experimental vaccine, and was manufactured according to good manufacturing practice guidelines and appropriately released by the Research Foundation for Microbial Disease of Osaka University (BIKEN, Kanonji, Kagawa, Japan). The virus was purified by sucrose gradient ultracentrifugation and treated with formalin according to the method of Davenport et al. 12 and then mixed with CVP (lot number INF‐632) from Toko Pharmaceutical Industrial Co. (Tateyama, Toyama, Japan). Intranasal vaccination was performed by spraying 0.25 ml of the vaccine into each nostril (0.5 ml total) using an atomizer (Keytron, Ichikawa, Chiba, Japan).
2.3. Serum, peripheral blood, and nasal wash sample collection
Serum and nasal wash samples were collected from each participant on days 0, 21, 42, 280, and 301. Approximately 100 ml nasal wash was collected by washing the nasal cavity several times with a nasal irrigation device (Hananoa; Kobayashi Pharmaceutical Co., Osaka, Osaka, Japan). 10 , 11 The nasal wash samples were filtered and concentrated as described previously. 10 The total protein concentration in these nasal wash samples was measured using the bicinchoninic acid assay (BCA) Protein Assay Kit (Thermo Fisher Scientific, Grand Island, NY, USA). The concentrated nasal wash samples were stored at −80°C until use. 10 , 11 For analysis of immune cell responses, peripheral blood samples were collected from 17 vaccine recipients into sterile blood collection tubes containing buffered sodium heparin anticoagulant on days 0, 7, 28, and 42.
2.4. Neutralization test assays
Neutralization titers of serum and nasal wash samples collected on days 0, 21, 42, 280, and 301 were determined using a previously described microneutralization assay 10 , 11 with the wild‐type influenza A(H5N1) virus strains A/Indonesia/5/05, A/Vietnam/1194/04, and A/Laos/JP127/07. Because there were several specimens that could not be analyzed in each assay, generally due to insufficient sample volume, the exact numbers of specimens evaluated in each assay are summarized in Table 1. The viruses were propagated in the allantoic cavities of 10 days old embryonated chicken eggs and purified from the allantoic fluid. The TCID50 of the virus was estimated using previously described methods. 10 In brief, 10‐fold serial dilutions of allantoic fluid containing the virus were used to inoculate MDCK cells (ATCC no. CCL‐34) in a 96 well culture plate and were incubated for 3 days at 37°C in a humidified 5% CO2 atmosphere. The cytopathic effect in the virus‐containing wells was evaluated under a microscope, and the TCID50 was calculated. Each standardized nasal wash was obtained by adjusting the concentrated nasal wash for 1 mg of total protein per milliliter based on BCA measurements and used in neutralization test assays. Standardized nasal wash contains one‐tenth of nasal IgA antibodies in nasal mucus. 10 , 11 Twofold serial dilutions of serum or nasal wash were mixed with an equal volume of diluent containing an influenza virus equivalent of 100 TCID50 and were added to the wells of 96 well plates containing a monolayer of MDCK cells. Four control wells containing virus or diluent alone were included on each plate. The plates were incubated for 3 or 4 days at 37°C in a humidified 5% CO2 atmosphere. All wells were observed for the presence or absence of cytopathicity, and then fixed with 10% formalin in PBS for 5 min at room temperature and stained with Naphthol Blue Black. The titers were recorded as the reciprocal of the highest dilution without a cytopathic effect. Microneutralization analysis was performed in a Biosafety Level 3 facility.
Table 1.
The number of participants and serum and nasal wash samples analyzed in NT assay and ELISA
| Day 0 | Day 21 | Day 42 | Day 280 | Day 301 | |||
|---|---|---|---|---|---|---|---|
| CVP+ | Number of participants vaccinated | 31 | 31 | ND | 25 | ND | |
| Number of serum samples collected | 31 | 31 | 31 | 25 | 25 | ||
| Analyzed for NT titer | A/Indonesia/5/05 | 31 | 31 | 31 | 25 | 25 | |
| A/Vietnam/1194/04 | 31 | nd | 31 | 25 | 25 | ||
| A/Laos/JP127/07 | 31 | nd | 31 | 25 | 25 | ||
| Analyzed for antibody titer | A/Indonesia/5/05 | 31 | nd | 31 | 25 | 25 | |
| Number of nasal wash samples collected | 31 | 31 | 31 | 25 | 25 | ||
| Analyzed for NT titer | A/Indonesia/5/05 | 31 | 27 | 31 | 25 | 25 | |
| A/Vietnam/1194/04 | 31 | nd | 31 | 25 | 25 | ||
| A/Laos/JP127/07 | 31 | nd | 31 | 25 | 25 | ||
| Analyzed for antibody titer | A/Indonesia/5/05 | 31 | nd | 31 | 25 | 25 | |
| CVP– | Number of participants vaccinated | 32 | 32 | ND | 24 | ND | |
| Number of serum samples collected | 32 | 32 | 32 | 24 | 24 | ||
| Analyzed for NT titer | A/Indonesia/5/05 | 32 | 32 | 32 | 24 | 24 | |
| A/Vietnam/1194/04 | 32 | nd | 32 | 24 | 24 | ||
| A/Laos/JP127/07 | 32 | nd | 32 | 24 | 24 | ||
| Analyzed for antibody titer | A/Indonesia/5/05 | 32 | nd | 32 | 24 | 24 | |
| Number of nasal wash samples collected | 32 | 32 | 32 | 24 | 24 | ||
| Analyzed for NT titer | A/Indonesia/5/05 | 32 | 24 | 31 | 24 | 24 | |
| A/Vietnam/1194/04 | 31 | nd | 31 | 24 | 24 | ||
| A/Laos/JP127/07 | 30 | nd | 30 | 23 | 23 | ||
| Analyzed for antibody titer | A/Indonesia/5/05 | 31 | nd | 31 | 24 | 24 | |
Abbreviations: ND, not done; nd, not determined.
2.5. Enzyme‐linked immunosorbent assay
Titers of IgA and IgG antibodies specific for the HA antigen of influenza A(H5N1) virus in the serum and standardized nasal wash samples collected on day 0 and 301 were determined by standard ELISA. 10 , 11 Here as well not all the specimens could be analyzed due to insufficient specimen volume, and the exact numbers of specimens evaluated are summarized in Table 1. Purified HA antigen, which was used as the coating antigen, was prepared from IBCDC‐RG2 virus according to the procedure of Phelan et al. 13 Half‐area flat‐bottomed microtiter plates (Costar, Corning, NY, USA) were coated (50 ng/well) overnight at 4°C with the HA antigen. Plates were blocked for 1 hr at 37°C with 1% BSA in PBS (pH 7.4), and serially diluted serum and standardized nasal wash samples were added to each well. Following incubation for 2 hr at 37°C, wells were washed three times with PBS containing 0.05% Tween 20. After addition of a diluted HRP‐conjugated goat antihuman IgA antibody (Bethyl Laboratories, Montgomery, TX, USA) or an HRP‐conjugated goat antihuman IgG‐Fc fragment antibody (Bethyl Laboratories), plates were incubated for 1 hr at 37°C, washed three times, and incubated with One‐Step Ultra Tetramethylbenzidine ELISA HRP substrate solution (Thermo Fisher Scientific). The reaction was stopped with H2SO4. The absorbance at 450 nm (reference, 655 nm) was measured in an iMark Microplate Reader (Bio‐Rad, Hercules, CA, USA). The HA‐specific antibody titer was calculated as the reciprocal of the highest dilution of the test sample that gave an absorbance greater than a cut‐off value equal to the mean absorbance of the dilution buffer plus two SDs.
2.6. Measurement of the plasma cell number in the peripheral blood
PBMCs were isolated from peripheral blood samples collected on days 0 (n = 9), 7 (n = 17), and 28 (n = 17) using Lymphoprep (Abbott Diagnostics, Lake Forest, IL, USA), and were stained with CD2 (clone RPA‐2.10; BioLegend, San Diego, CA, USA), CD3 (clones HIT3a and UCHT1; BioLegend), CD4 (clone RPA‐T4; BioLegend), CD10 (clone eBioCB‐CALLA; Thermo Fisher Scientific), CD19 (clone HIB19; BioLegend), CD20 (clone 2H7; BioLegend), CD27 (clone O323; Thermo Fisher Scientific), CD38 (clone HIT2; Thermo Fisher Scientific), and IgD (clone IA6‐2; BD Biosciences, San Jose, CA, USA) in the presence of human Fc receptor (FcR) block (Miltenyi Biotec, Bergisch Gladbach, Germany). CD19+ CD38++ CD27++ cells that were negative for CD2, CD3, CD4, CD10, IgD, and CD20 were counted as plasma cells. 14 Data were acquired on an FACS Canto II (BD Biosciences) and analyzed using FlowJo software (FlowJo, Ashland, OR, USA).
2.7. Estimating vaccine antigen‐specific Th cell numbers in the peripheral blood
Heparinized whole blood collected on days 0 (n = 17) and 42 (n = 17) was left unstimulated or stimulated in the final 1 µg HA/ml of inactivated whole‐virion influenza A(H5N1) vaccine at 37°C and 5% CO2 for 6 hr, in the presence of costimulatory antibodies against CD28 (clone CD28.2) and CD49d (clone 9F10) (final concentration, 1 µg/ml). Inactivated whole virions of X‐187 (a vaccine strain of A/Victoria/210/09 [H3N2]), which is an antigenically distinct strain from the vaccine strain virus used in this study, were used as a control stimulus (provided by BIKEN). After 6 hr of incubation, 100 µl of 20 mm EDTA in PBS was added to the samples to stop the activation process. Two hours before activation was stopped, brefeldin A was added to each whole blood sample at a final concentration of 10 µg/ml. Hemolysis and sample fixation were performed with FACS Lysing Solution (BD Biosciences). After permeabilization of fixed samples with FACS Permeabilizing Solution II (BD Biosciences), samples were stained with fluorescent antibodies against CD3 (clone UCHT1), CD4 (clone RPA‐T4), CD154 (clone TRAP1), TNF‐α (clone MAb11), IFN‐γ (clone 4S.B1), and IL‐2 (clone MQ1‐17H12) in the presence of human FcR block (Miltenyi Biotec). All antibodies were purchased from BD Biosciences. Data were acquired on an FACS Canto II (BD Biosciences) and analyzed using FlowJo software (FlowJo).
2.8. In vitro whole blood culture and cytokine measurement
Cytokine measurement in supernatants collected from in vitro whole blood cultures was performed as previously described. 15 In brief, heparinized whole blood was cultured in 200 μl of RPMI 1640 medium containing the final 1 µg HA/ml of inactivated whole‐virion influenza A(H5N1) or A(H3N2) vaccine. Supernatant was collected after 72 hr of incubation and kept at −30°C until analysis. Levels of 10 cytokines (IL‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, IFN‐γ, TNF‐α, and granulocyte/macrophage‐colony stimulating factor) in the supernatants were determined using a Human Cytokine Magnetic 10‐Plex Panel (Thermo Fisher Scientific) and the Luminex 100 system (Hitachi Solutions, Shinagawa, Tokyo, Japan) according to the manufacturer's instructions. Samples with concentrations below the lower detection limit were assigned the relevant threshold value. To determine the influenza‐specific cytokine response, the background cytokine production from blood samples cultured with medium alone was subtracted from the cytokine production after stimulation.
2.9. Statistical analysis
Statistical analysis was performed using the GraphPad Prism statistical software package (version 6.0h; Graph Pad Software, San Diego, CA, USA). Two‐way anovas followed by Sidak's multiple comparisons tests were used to compare each data set except for the plasma cell numbers, which were compared by Kruskal–Wallis tests followed by Dunn's multiple comparisons tests. Correlation analyses were performed using the Spearman rank correlation coefficient. The threshold for statistical significance was set at 5% (P < 0.05).
3. RESULTS
3.1. Intranasal administration of an inactivated whole‐virion influenza A(H5N1) vaccine successfully induced serum and nasal antibody responses
In our previous studies, we demonstrated that intranasal vaccination of healthy adults against seasonal influenza strains, which involved spraying inactivated whole seasonal influenza virion antigens (45 µg HA) into the nasal cavity, could significantly induce serum and mucosal antibody responses. 10 It has previously been shown that an inactivated vaccine generated from influenza A(H5N1) virus was less immunogenic in healthy adults, 16 and two doses of injectable inactivated H5 influenza vaccines combined with adjuvant have been prepared as prepandemic vaccines. 17 Therefore, we determined that the H5 influenza vaccine would require at least three doses for the induction of sufficient immune responses by intranasal administration. Thus, in this study, we evaluated the immune response following three intranasal doses of an inactivated whole‐virion influenza A(H5N1) vaccine containing 45 µg HA. In addition, the impact of the addition of CVP (a mucoadhesive excipient that can increase the retention of vaccine antigens at the mucosal surface) 18 on the vaccine‐induced immune response was evaluated. Serum and nasal wash samples were collected at the time of each vaccination (days 0, 21, and 280) and 3 weeks after the second and third vaccinations (days 42 and 301), and the neutralizing antibody titers in the samples were measured. A moderate serum neutralizing antibody response and a weak mucosal neutralizing antibody response against the virus strain A/Indonesia/5/05, which is antigenically homologous to the vaccine virus, could be observed at 3 weeks after the second vaccination (day 42) in about half of the participants. However, no significant difference was observed between antibody responses induced by vaccines with or without CVP (Figure 1a,b). The serum antibody titers against A/Indonesia/5/05 virus remained consistent from day 42 to day 280, whereas the nasal wash antibody titers decreased significantly from day 42 to day 280 (Figure 1a,b). Thus, the duration of the local antibody response at the nasal mucosa was shorter than that of the serum antibody response. However, at 3 weeks after the third vaccination (day 301), prominent antibody responses could be observed in both the serum and nasal mucosa; 88.0% (with CVP) or 83.3% (without CVP) of vaccinated patients showed a greater than fourfold increase in the serum antibody response, whereas 76.0% (with CVP) or 58.3% (without CVP) showed a greater than fourfold increase in the mucosal antibody response. In addition, the geometric mean titer (GMT) of neutralizing antibodies increased 32.9‐fold (with CVP) or 16.9‐fold (without CVP) in the serum, and 10.56‐fold (with CVP) or 4.62‐fold (without CVP) in the nasal wash, compared with GMTs of neutralizing antibodies on day 0 in the serum and nasal wash, respectively (Figure 1a,b). Increased serum and mucosal antibody responses were seen in patients who received vaccines with CVP on day 301. The increase in mucosal antibody titers was greater than the increase in serum antibody titers, suggesting that CVP may enhance local antibody responses by increasing the viscosity of the vaccine. Next, antibody responses against virus strains A/Vietnam/1194/04 and A/Laos/JP127/07, which are antigenically heterologous to the vaccine virus, were evaluated. A relatively subtle neutralizing antibody response against these viruses could be observed both in the serum and in the nasal mucosa on day 301, and antibody responses were higher in patients that were administered vaccines containing CVP in both cases (Figure 1c‐f). These results suggest that three intranasal doses of a vaccine containing inactivated whole A(H5N1) virus (45 µg HA) and CVP could induce sufficient systemic and mucosal immune responses.
Figure 1.
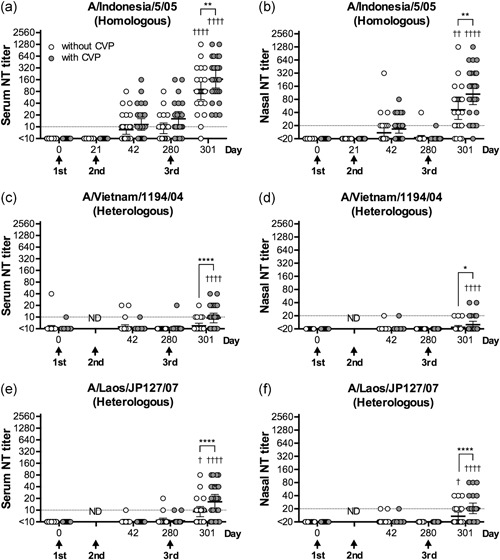
NT assays against influenza A(H5N1) virus strains. Neutralizing antibody titers against three A(H5N1) virus strains, (a,b) A/Indonesia/5/05 (homologous to the vaccine strain); (c,d) A/Vietnam/1194/04 (heterologous to the vaccine strain); and (e,f) A/Laos/JP127/07 (heterologous to the vaccine strain), in serum (panels a, c, and e) and nasal wash (panels b,d, and f) samples collected from healthy adult volunteers administered three doses of intranasal inactivated whole‐virion H5 influenza vaccine in the presence or absence of CVP before (0) or 21, 42, 280, and 301 days after the first vaccination. Each circle represents the NT titer of an individual sample. White circles represent samples from individuals administered vaccines without CVP. Gray circles represent samples from individuals administered vaccines with CVP. The data are shown on scatter plots as the geometric mean with the 95% confidence interval. *P < 0.05, **P < 0.01, ****P < 0.0001, as determined by two‐way anova followed by Sidak's multiple comparisons test between patients with and without CVP at each time point. † P < 0.05, †† P < 0.01, †††† P < 0.0001, as determined by two‐way anova followed by Sidak's multiple comparisons test for titers in each group at each time point, compared with day 0
3.2. The local neutralizing antibody response induced by intranasal vaccination with inactivated H5 influenza is due to IgA antibodies
An HA‐specific ELISA was performed to determine the isotype of the neutralizing antibodies detected in the serum and nasal mucosa of vaccinated patients. Both HA‐specific IgG and IgA antibodies were induced in the serum (Figure 2a,b). However, only HA‐specific IgA antibodies, not IgG antibodies, could be detected in the nasal mucosa (Figure 2c,d). These results suggest that both IgG and IgA antibodies contribute to the neutralizing antibody response in the serum, whereas only IgA antibodies are involved in the neutralizing antibody response in the nasal mucosa. Furthermore, the addition of CVP to the vaccine significantly increased the HA‐specific IgA antibody titers in nasal wash samples, indicating that the increase in neutralizing activity in patients administered CVP‐containing vaccines was due to an increase in HA‐specific IgA antibody induction at the nasal mucosa.
Figure 2.
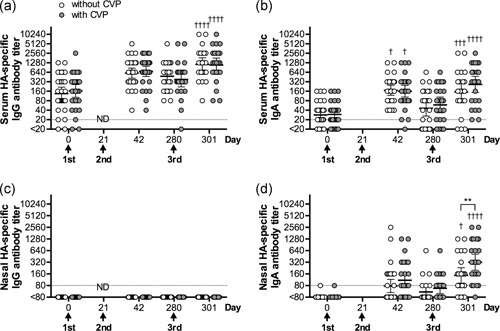
HA‐specific antibody titers in serum and nasal wash samples. Measurement of A/Indonesia/5/05 virus HA‐specific (a) serum IgG, (b) serum IgA, (c) nasal IgG, and (d) nasal IgA titers before (day 0) or 21, 42, 280, and 301 days after the first vaccination by ELISA. White circles represent samples from individuals administered vaccines without CVP. Gray circles represent samples from individuals administered vaccines with CVP. The data are shown on scatter plots as the geometric mean with the 95% confidence interval. **P < 0.01, as determined by two‐way anova followed by Sidak's multiple comparisons test between patients with and without CVP at each time point. † P < 0.05, ††† P < 0.001, †††† P < 0.0001, as determined by two‐way anova followed by Sidak's multiple comparisons test for titers in each group at each time point, compared with day 0. ND, not done
3.3. The HA‐specific IgA antibody response at the nasal mucosa strongly correlates with the nasal neutralizing antibody titer
It was previously reported that neutralizing antibody titers in the nasal wash, in addition to serum antibody titers and neutralizing antibody titers, could be used as a surrogate for immune responses induced by intranasal inactivated vaccines. 9 , 10 Measurement of the nasal neutralizing antibody titer is therefore especially useful for evaluating the mucosal immune response induced by intranasal vaccines. However, collection of a large enough volume of nasal wash is challenging, as measurement of the neutralizing antibody titer requires more than 100 ml nasal wash per individual, and nasal washes are usually done by the study participants themselves. By contrast, measurement of HA‐specific antibodies by ELISA is much easier and requires a smaller volume of nasal wash sample (30 and 15 µl of each standardized nasal wash were used for neutralization assay and ELISA, respectively). Therefore, to evaluate whether HA‐specific antibody titers could serve as a surrogate for neutralizing antibody titers, the relationship between HA‐specific IgG and IgA antibody titers and neutralizing antibody titers was analyzed. Serum neutralization titers were well correlated with HA‐specific serum IgG rather than IgA titers (Figure 3a,b). A clear correlation could be observed between the HA‐specific nasal IgA titer and the nasal neutralizing antibody titer (Figure 3d); by contrast, HA‐specific nasal IgG titer was not detected at all (Figure 3c). In addition, HA‐specific IgA antibody titers correlated with nasal neutralizing antibody titers more strongly, compared with the correlation between HA‐specific IgG antibody titers and serum neutralizing antibody titers (Figure 3a,d). Therefore, the HA‐specific nasal IgA antibody titer could be used as a surrogate for the mucosal immune response.
Figure 3.
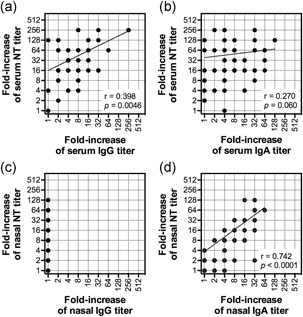
Correaltion between netralizing antibody titers and HA‐specific antibody titers. (a,b) Correlation between increased serum neutralizing antibody titers and serum (a) IgG or (b) IgA titers (n = 49). Serum neutralizing antibody titers showed a weak positive correlation with serum IgG titers (Spearman r = 0.398; P = 0.0046). (c,d) Correlation between increased nasal neutralizing antibody titers and nasal (c) IgG or (d) IgA titers (n = 49). Titers were measured on day 0 (before vaccination) and day 301. Nasal neutralizing antibody titers strongly correlated with nasal IgA titers (Spearman r = 0.742; P < 0.0001) but not with nasal IgG titers
3.4. Evaluation of peripheral blood immune cell responses induced by intranasal vaccination with inactivated H5 influenza
We have shown that the neutralizing antibody titer and the HA‐specific IgA antibody titer in the nasal mucosa could serve as surrogates for the local immune response induced by intranasal vaccination. However, nasal wash sample collection is not a standard procedure at medical facilities and is usually performed by the study participants themselves. Therefore, a large‐scale study requiring continuous collection of nasal wash samples would be nearly impossible to conduct. Analysis of the immune response in PBMCs was previously used to evaluate the efficacy of various vaccines, with inconsistent results. It was shown that high numbers of vaccine‐specific plasmablasts are transiently induced in the human peripheral blood at 1 week postvaccination, and characterization of vaccine‐induced antibodies was done by analyzing these plasmablasts. 19 Therefore, we examined whether PBMCs collected from recipients that received the intranasal inactivated H5 influenza vaccine could be used to evaluate the vaccine‐induced immune response. PBMCs were collected before vaccination (day 0), 1 week after the first vaccination (day 7), and 1 week after the second vaccination (day 28), and the proportion of plasma cells among total lymphocytes was determined. The number of plasma cells was significantly increased at 1 week after the second vaccination (day 28), suggesting that plasma cells are transiently induced in the peripheral blood by intranasal vaccination with the inactivated H5 influenza vaccine (Figure 4a). Next, heparinized peripheral whole blood collected before vaccination (day 0) and 3 weeks after the second vaccination (day 42) was incubated with either A(H5N1) or A(H3N2) inactivated whole virus antigen for 6 hr, and the numbers of activated T cells were determined. The proportion of activated CD154+ CD4+ T cells among total CD4+ T cells (Figure 4b) and the proportion of IL‐2‐ or TNF‐α‐, but not INF‐γ‐, expressing cells among activated CD154+ CD4+ Th cells (Figure 4c–e) was significantly higher in peripheral blood collected after vaccination (day 42) than in blood collected before vaccination (day 0). This response was specific and only occurred when peripheral blood was incubated with A(H5N1) antigen. This was further confirmed by measuring cytokines in supernatants of whole blood cultures, in which peripheral blood collected before vaccination (day 0) and after vaccination (day 42) was incubated with either A(H5N1) or A(H3N2) antigen for 3 days (Figure 4f–k). IL‐1β and IL‐8 induction was significantly higher in postvaccinated samples regardless of antigen type used, and IL‐6 was induced by stimulation with A(H3N2) rather than A(H5N1) antigen (Figure 4i–k). As for typical cytokines produced from Th cells, IFN‐γ, and IL‐4 tended to increase without significant differences between prevaccination and postvaccination levels (Figure 4f–k). Of note, IL‐2 induction was significantly higher in PBMCs obtained after vaccination (day 42) by stimulation with H5N1 antigen. These observations suggest that Th1 cells that recognize H5N1 antigens were presumably induced in the peripheral blood 3 weeks after intranasal administration of inactivated H5 influenza vaccine. Furthermore, these results indicate that evaluation of the immune cell response induced by intranasal inactivated influenza vaccination could be done by analysis of peripheral blood collected at either 1 week or 3 weeks postvaccination.
Figure 4.
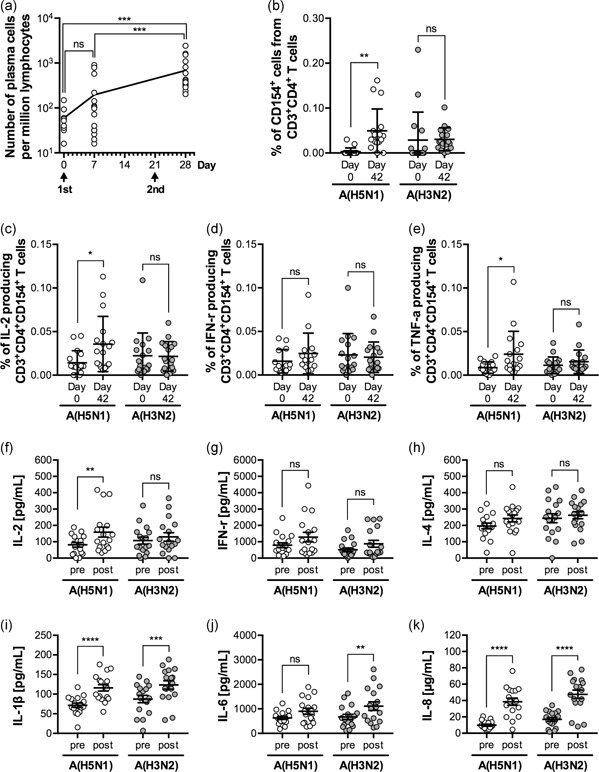
Immune cell responses in patients receiving intranasal inactivated influenza vaccines. (a) The number of plasma cells (CD19+, CD38++, CD27++, CD2–, CD3–, CD4–, CD10–, IgD–, and CD20–) among peripheral blood lymphocytes collected before (day 0; n = 9) and after vaccination (day 7; n = 17 and day 28; n = 17) was measured by flow cytometry. The data are shown on scatter plots as the geometric mean with the 95% confidence interval. The P‐values were calculated by Kruskal–Wallis tests followed by Dunn's multiple comparisons tests (***P < 0.001). (b–e) Heparinized whole blood collected before (day 0) and after vaccination (day 42) were stimulated with either A(H5N1) antigen (vaccine antigen; white circles) or A(H3N2) antigen (negative control; gray circles). Following incubation, the proportion of (b) CD154+ (activated) cells among CD4+ Th cells, and of (c) IL‐2‐, (d) IFN‐γ‐, or (e) TNF‐α‐producing cells among CD154+ CD4+ Th cells was measured by flow cytometry. Activated Th cells, IL‐2‐ or TNF‐α‐producing activated Th cells significantly increased after incubation of whole blood with vaccine antigen, suggesting an increase in vaccine antigen‐specific Th cells in the peripheral blood. Scatter plots show the mean ± SD (n = 17). The P‐values were calculated by two‐way anova followed by Sidak's multiple comparisons test (*P < 0.05, **P < 0.01). (f–k) Peripheral whole blood collected before (day 0) and after vaccination (day 42) was incubated in the presence of either A(H5N1) antigen (vaccine antigen; white circles) or A(H3N2) antigen (negative control; gray circles). The amount of (f) IL‐2, (g) IFN‐γ, (h) IL‐4, (i) IL‐1β, (j) IL‐6, and (k) IL‐8 in the culture supernatant measured by multiplex ELISA was shown. IL‐2 production was significantly enhanced by incubation with the vaccine antigen. Scatter plots show the mean ± SD (n = 17). The P‐values were calculated by two‐way anova followed by Sidak's multiple comparisons test (*P < 0.05). ns, not significant
3.5. Peripheral blood immune cell responses correlate with the serum neutralizing antibody titer but not with the neutralizing antibody titer at the nasal mucosa
It could be assumed that the plasma cells and vaccine antigen‐specific Th1 cells detected in the peripheral blood after the second vaccination were specifically induced by intranasal vaccination. However, the relationship between these immune cell responses and the titers of neutralizing antibodies in the serum and nasal mucosa, which directly contributes to antiviral protection, is not yet known. Thus, we examined the correlation between neutralizing antibody titers in serum and nasal wash samples collected after the third vaccination (day 301) and the immune cell response after the second vaccination (day 28 or 42). Peripheral blood plasma cell numbers on day 28 and the proportion of vaccine antigen‐specific Th1 cells on day 42 positively correlated with serum neutralizing antibody titers (Figure 5a,b). By contrast, these peripheral immune cell responses did not show any correlation with neutralizing antibody titers at the nasal mucosa (Figure 5c,d). This suggests that the serum neutralizing antibody response can be evaluated by analyzing peripheral blood immune cells collected at a relatively early time point after vaccination, while peripheral immune cell analysis is insufficient to evaluate the mucosal immune response.
Figure 5.
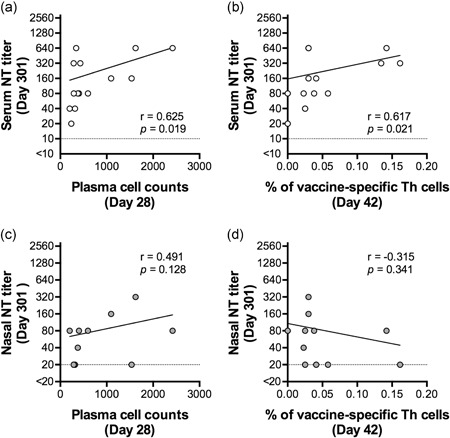
Correlation between neutralizing antibody responses and immne cell responses. (a,b) Correlation between serum neutralizing antibody titers after vaccination (day 301) and peripheral plasma cell numbers after vaccination (day 28; panel a) or the proportion of peripheral vaccine antigen‐specific Th1 cells after vaccination (day 42; panel b). The proportion of vaccine antigen‐specific Th1 cells was determined by calculating the ratio of CD154+ cells activated by inactivated whole‐virion influenza A(H5N1) virus antigen among total CD4+ T cells. Immune cell responses in the peripheral blood (plasma cell numbers at 28 days postvaccination and proportion of vaccine antigen‐specific Th1 cells at 42 days postvaccination) preceded the serum antibody response and positively correlated with antibody responses at 301 days postvaccination (n = 14; Spearman r = 0.625; P = 0.019 and n = 14; Spearman r = 0.617; P = 0.021, respectively). (c,d) Correlation between mucosal neutralizing antibody titers after vaccination (day 301) and peripheral plasma cell numbers after vaccination (day 28; panel c) or the proportion of peripheral vaccine antigen‐specific Th1 cells after vaccination (day 42; panel d). Neither peripheral immune cell response correlated with the mucosal antibody titer (n = 11; Spearman r = 0.491; P = 0.128 and n = 11; Spearman r = −0.315; P = 0.341, respectively)
4. DISCUSSION
In this study, PBMCs, in addition to serum and nasal wash samples, were collected from human patients immunized with an intranasal inactivated whole‐virion vaccine of influenza A(H5N1) virus for the characterization of vaccine‐induced immune responses. The study explored the feasibility of intranasal inactivated vaccine development for nonseasonal influenza and characterized the immune response induced by an intranasal inactivated vaccine of influenza A(H5N1) virus.
In previous intranasal inactivated whole influenza vaccine studies, intranasal inactivated vaccines containing monovalent or trivalent vaccine antigens could successfully induce neutralizing antibodies in the serum and nasal mucosa. 8 , 9 , 10 The neutralizing antibodies in the nasal mucosa were mainly S‐IgA antibodies that form multimeric structures. In fact, trimeric and tetrameric S‐IgA antibodies showed higher virus‐neutralizing activity than smaller IgA antibody molecules. 8 , 9 It was assumed that the induction of neutralizing antibodies at the nasal mucosa observed in these studies resulted from a boosting effect on baseline immunity by intranasal vaccination, due to the fact that the vaccine antigens used were seasonal influenza viruses, and the majority of study participants possessed preexisting immunity against these viruses prior to vaccination. This is the case in injected vaccines as well: the current split vaccine can induce a sufficient antibody response in healthy adults with a single injection without the addition of vaccine adjuvants. 20 , 21 Because intranasal inactivated vaccines result in less immune induction than injected vaccines in general, the use of highly immunogenic vaccine antigens or the addition of mucosal vaccine adjuvants is required to induce a sufficient antibody response. 22 In the case of intranasal inactivated whole‐virion seasonal influenza vaccines, two doses were needed to induce a sufficient immune response in individuals possessing baseline immunity. 9 , 10 Thus, additional measures need to be taken to achieve a successful immune response against virus antigens for which patients would not be expected to have baseline immunity, such as the avian influenza A(H5N1) virus. In this study, we demonstrated that virus‐neutralizing antibodies could be induced in the serum and nasal mucosa by three intranasal administrations of inactivated whole‐virion vaccine of influenza A(H5N1) virus. In addition, the induction of mucosal antibodies could be increased (as demonstrated by the increase in HA‐specific IgA levels in nasal wash samples) by the addition of CVP, a mucoadhesive excipient, to the vaccine. 18 In a previous intranasal inactivated influenza vaccine study in monkeys, 18 although CVP increased the retention of vaccine antigens at the mucosal surface, its potential to enhance the mucosal antibody response in humans remained unclear. The current study demonstrates that the human mucosal antibody response can be enhanced by increasing the mucosal retention of vaccine antigens, and it highlights the importance of exploring vaccine additives that can enhance the mucosal immune response by increasing the efficiency of antigen uptake rather than by stimulating the innate immune system, as is the case for the majority of vaccine adjuvants. It is known that CVP is highly safe for human use, and it has already been used as an additive with various topical agents, including a collunarium. 18 Safety is a strong consideration in the development of intranasal inactivated vaccines because a severe adverse effect (facial paralysis) was previously reported in individuals that received intranasal administration of an inactivated vaccine containing a bacterial toxin‐derived adjuvant. 23 , 24 Thus, attempts to improve vaccine efficacy with substances that are known to be safe (e.g. CVP) are highly advantageous.
In the development of intranasal inactivated vaccines, for which the mechanism of action is different from that of injected vaccines, it will be important to establish appropriate methods to evaluate the vaccine‐induced immune response, as a surrogate for vaccine effectiveness. In our prior studies, we demonstrated that the antibody response induced by an intranasal vaccine could be estimated by measuring the mucosal neutralizing antibody titer using nasal wash specimens. 9 , 10 , 11 However, measurement of nasal neutralizing antibody titers requires large volumes of nasal wash samples (100 ml per individual), and collection of nasal wash samples must be done by the study participants themselves, which makes nasal wash sample collection extremely difficult to implement in large‐scale trials. Therefore, a simpler method to evaluate the mucosal immune response is needed. In this study, nasal HA‐specific IgA antibody responses clearly correlated with mucosal neutralizing antibody titers, indicating that measurement of HA‐specific IgA antibody titers will enable evaluation of the mucosal antibody response using a much smaller sample (approximately one‐tenth of the amount used for measuring neutralizing antibody titers). The feasibility of PBMC‐based methods for the evaluation of intranasal vaccine‐induced immune responses was also explored in this study. Consistent with observations in individuals administered injected vaccines, 19 the number of peripheral plasma cells increased 1 week after administration of the intranasal inactivated vaccine, indicating the transient induction of vaccine antigen‐specific plasma cells in the peripheral blood. In addition, an increase in Th1 cells that specifically recognize vaccine antigens was observed at 3 weeks postvaccination, suggesting that peripheral blood cell samples may be utilized for the evaluation of intranasal vaccine‐induced immune responses. Future in‐depth analyses of antibody clones produced by these plasma cells induced by vaccination in the periphery, including characterization of intranasal vaccine‐derived antibody clones at the monoclonal level, may deepen our understanding of the mechanism of action of intranasal inactivated vaccines. 22 , 25 However, it must be noted that the induction of peripheral blood immune responses positively correlated with neutralizing antibody titers in the serum but not in the nasal wash samples. Thus, analysis of the peripheral blood immune cell response may act as a surrogate for the systemic immune response to intranasal vaccination but not for the mucosal immune response. For accurate evaluation of the efficacy of intranasal vaccines, the immune response at the vaccination site must not be neglected. Therefore, further studies are required to identify an immune cell response that is predictive of the mucosal immune response.
In summary, three intranasal doses of an inactivated whole‐virion influenza vaccine containing CVP could induce systemic and local immune responses even against an influenza A(H5N1) virus strain to which the study participants did not have baseline immunity. This confirms the potential of intranasal inactivated influenza vaccines, not only for seasonal influenza strains, but also for pandemic influenza strains. To support the development of these intranasal inactivated vaccines, detailed analyses of vaccine‐induced mucosal immune responses and further development of methods to evaluate the mucosal immune response are needed.
DISCLOSURE
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
We thank Mr T. Tanimoto, Dr Y. Gomi, Dr S. Manabe, Mr T. Ishikawa, and Dr Y. Okuno at BIKEN for supplying the inactivated whole virus influenza vaccines; Mr T. Miyazaki and Mr T. Kamishita (Toko Yakuhin Kogyo Co., Ltd.) for their valuable suggestions about intranasal vaccination procedures; and Ms Kaori Sano (National Institute of Infectious Diseases) for critical reading of the manuscript and her valuable suggestions. The work was supported in part by a Grant‐in‐Aid for Research on Emerging and Reemerging Infectious Diseases from the Japanese Ministry of Health, Labor, and Welfare (MHLW) (Grant numbers: H25‐Shinkou‐Ippan‐018) and, a Grant‐in‐Aid for Research on Emerging and Reemerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED) (Grant numbers: JP18fk0108012, JP19fk0108051, JP19fk0108082 and JP19fk0108083). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ainai A, van Riet E, Ito R, et al. Human immune responses elicited by an intranasal inactivated H5 influenza vaccine. Microbiology and Immunology. 2020;64:313–325. 10.1111/1348-0421.12775
Akira Ainai and Elly van Riet contributed equally to this study.
REFERENCES
- 1. Murphy BR. Mucosal immunity to viruses In: Ogra PL, Mestecky J, Lamm ME, Strober W, Mcgee JR, Bienenstock J, editors. Handbook of mucosal immunology. San Diego: Academic Press; 1994. [Google Scholar]
- 2. Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr Top Microbiol Immunol. 1989;146:107‐16. [DOI] [PubMed] [Google Scholar]
- 3. Ito R, Ozaki YA, Yoshikawa T, et al. Roles of anti‐hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine. 2003;21:2362‐71. [DOI] [PubMed] [Google Scholar]
- 4. Renegar KB, Small PA Jr., Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978‐86. [DOI] [PubMed] [Google Scholar]
- 5. Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26:476‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology. 5th ed Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 7. Ichinohe T, Kawaguchi A, Tamura S, et al. Intranasal immunization with H5N1 vaccine plus Poly I:Poly C12U, a Toll‐like receptor agonist, protects mice against homologous and heterologous virus challenge. Microbes Infect. 2007;9:1333‐40. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki T, Kawaguchi A, Ainai A, et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc Natl Acad Sci USA. 2015;112:7809‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terauchi Y, Sano K, Ainai A, et al. IgA polymerization contributes to efficient virus neutralization on human upper respiratory mucosa after intranasal inactivated influenza vaccine administration. Hum Vaccin Immunother. 2018;14:1351‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ainai A, Tamura SI, Suzuki T, et al. Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults. Hum Vaccin Immunother. 2013;9:1962‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ainai A, Tamura S, Suzuki T, et al. Characterization of neutralizing antibodies in adults after intranasal vaccination with an inactivated influenza vaccine. J Med Virol. 2012;84:336‐44. [DOI] [PubMed] [Google Scholar]
- 12. Davenport FM, Hennessy AV, Brandon FM, Webster RG, Barrett CD Jr., Lease GO. Comparisons of serologic and febrile responses in humans to vaccination with influenza A viruses or their hemagglutinins. J Lab Clin Med. 1964;63:5‐13. [PubMed] [Google Scholar]
- 13. Phelan MA, Mayner RE, Bucher DJ, Ennis FA. Purification of influenza virus glycoproteins for the preparation and standardization of immunological potency testing reagents. J Biol Stand. 1980;8:233‐42. [DOI] [PubMed] [Google Scholar]
- 14. Fink K. Origin and function of circulating plasmablasts during acute viral infections. Front Immunol. 2012;3:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Riet E, Adegnika AA, Retra K, et al. Cellular and humoral responses to influenza in gabonese children living in rural and semi‐urban areas. J Infect Dis. 2007;196:1671‐78. [DOI] [PubMed] [Google Scholar]
- 16. Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343‐51. [DOI] [PubMed] [Google Scholar]
- 17. Rockman S, Brown L. Pre‐pandemic and pandemic influenza vaccines. Hum Vaccin. 2010;6:792‐801. [DOI] [PubMed] [Google Scholar]
- 18. Saito S, Ainai A, Suzuki T, et al. The effect of mucoadhesive excipient on the nasal retention time of and the antibody responses induced by an intranasal influenza vaccine. Vaccine. 2016;34:1201‐7. [DOI] [PubMed] [Google Scholar]
- 19. Lanzavecchia A. Dissecting human antibody responses: useful, basic and surprising findings. EMBO Mol Med. 2018;10:e8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015‐2016 Season. N Engl J Med. 2017;377:534‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seki Y, Onose A, Sugaya N. Influenza vaccine effectiveness in adults based on the rapid influenza diagnostic test results, during the 2015/16 season. J Infect Chemother. 2017;23:615‐20. [DOI] [PubMed] [Google Scholar]
- 22. Sano K, Ainai A, Suzuki T, Hasegawa H. Intranasal inactivated influenza vaccines for the prevention of seasonal influenza epidemics. Expert Rev Vaccines. 2018;17:687‐96. [DOI] [PubMed] [Google Scholar]
- 23. Mutsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350:896‐903. [DOI] [PubMed] [Google Scholar]
- 24. Sendi P, Locher R, Bucheli B, Battegay M. Intranasal influenza vaccine in a working population. Clin Infect Dis. 2004;38:974‐80. [DOI] [PubMed] [Google Scholar]
- 25. Sano K, Ainai A, Suzuki T, Hasegawa H. The road to a more effective influenza vaccine: up to date studies and future prospects. Vaccine. 2017;35:5388‐95. [DOI] [PubMed] [Google Scholar]


