Abstract
Objective
This study aimed to examine the associations of maternal early‐pregnancy glucose and insulin concentrations with offspring cardiometabolic risk factors and fat distribution.
Methods
In a population‐based prospective cohort study among 3,737 mothers and their children, random maternal glucose and insulin concentrations were measured at a median gestational age of 13.2 (95% range 10.5‐17.1) weeks. Childhood fat, blood pressure, and blood concentrations of lipids, glucose, and insulin at the age of 10 years were measured.
Results
Higher maternal early‐pregnancy glucose and insulin concentrations were associated with a higher risk of childhood overweight, and higher maternal early‐pregnancy insulin concentrations were associated with an increased childhood risk of clustering of cardiometabolic risk factors (all P < 0.05). These associations were explained by maternal prepregnancy BMI. Independent of maternal prepregnancy BMI, one SD score (SDS) higher maternal early‐pregnancy glucose and insulin concentrations were associated with higher childhood glucose (0.08 SDS, 95% CI: 0.04‐0.11) and insulin concentrations (0.07 SDS, 95% CI: 0.03‐0.10), but not with childhood blood pressure, lipids, and fat measures.
Conclusions
These results suggest that maternal early‐pregnancy random glucose and insulin concentrations are associated with childhood glucose and insulin concentrations but not with other childhood cardiometabolic risk factors.
Study Importance.
What is already known?
-
►
Gestational diabetes is associated with increased risks of offspring obesity, type 2 diabetes, and metabolic syndrome.
-
►
Maternal glucose concentrations in mid‐ and late pregnancy below the diagnostic threshold of gestational diabetes may already be associated with childhood cardiometabolic risk factors.
What does this study add?
-
►
Higher maternal early‐pregnancy glucose and insulin concentrations are associated with higher childhood glucose and insulin concentrations, independent of maternal socioeconomic and lifestyle factors and birth, infant, and childhood characteristics.
-
►
Associations of maternal early‐pregnancy glucose and insulin concentrations with childhood overweight, clustering of cardiometabolic risk factors, blood pressure, lipids, and detailed measures of fat are explained by maternal prepregnancy BMI.
How might these results change the focus of clinical practice?
-
►
Children with an increased risk of glucose intolerance may be already identified by a suboptimal maternal glucose metabolism in early pregnancy.
-
►
Preventive strategies aiming to improve childhood cardiometabolic health may be more effective when optimizing maternal weight status before pregnancy than maternal glucose metabolism.
Introduction
Gestational diabetes is associated with increased risks of offspring obesity, type 2 diabetes, and metabolic syndrome (1, 2, 3, 4, 5). Increasing evidence has suggested that these risks might not be confined to women diagnosed with gestational diabetes but that they may already exist in offspring exposed to maternal glucose concentrations below diagnostic thresholds (6, 7). Previous studies have reported associations of maternal glucose concentrations in mid‐ and late pregnancy with offspring cardiometabolic risk factors (6, 7). However, as fetal cardiovascular and metabolic development already starts in the first trimester, early pregnancy may already be a critical period for the adverse influence of a suboptimal maternal glucose metabolism on the development of the fetal cardiometabolic system. Increases of maternal glucose and insulin concentrations from early pregnancy onward may directly affect placental development and increase nutrient transfer to the developing fetus. This may subsequently lead to increased fetal growth as well as adaptations in adipogenesis and pancreatic and vascular development. These adaptations may increase the susceptibility to cardiometabolic disease in later life (4, 8, 9, 10, 11, 12). Altered childhood body fat development may especially be involved in the associations of maternal glycemia with offspring cardiometabolic risk factors (9). A few studies have shown an association of maternal fasting glucose concentrations in pregnancy with increased childhood sum of skinfolds and waist circumference (6, 7, 13). However, it is not clear whether this includes overall fat or more specifically visceral fat accumulation, which is known to be more strongly related with cardiometabolic disease (14, 15). We hypothesized that higher maternal early‐pregnancy glucose concentrations are associated with an unfavorable offspring cardiometabolic risk profile and suboptimal body fat distribution.
Therefore, in a population‐based prospective cohort from early pregnancy onward among 3,737 mothers and their children, we assessed the associations of maternal early‐pregnancy glucose and insulin concentrations across the full range with cardiometabolic risk factors and detailed measurements of general and abdominal fat in childhood. We additionally explored whether these associations are independent of maternal lifestyle factors and birth, infant, or childhood characteristics.
Methods
Study design and participants
This study was embedded in the Generation R Study, a population‐based prospective cohort study from early pregnancy onward in Rotterdam, The Netherlands (16). Approval for the study was obtained from the Medical Ethical Committee of Erasmus University Medical Center, Rotterdam. Written consent was obtained from the parents of all participants. In total, 8,879 pregnant women were enrolled between 2001 and 2005. Of these, 6,117 mothers had early‐pregnancy information on glucose and insulin concentrations available and had singleton live‐born children. Cardiometabolic follow‐up measurements at the age of 10 years were available for 3,737 of their children (Figure 1). Main reasons for missing data were participants lost to follow‐up and no consent or failure of venous punctures (16).
Figure 1.
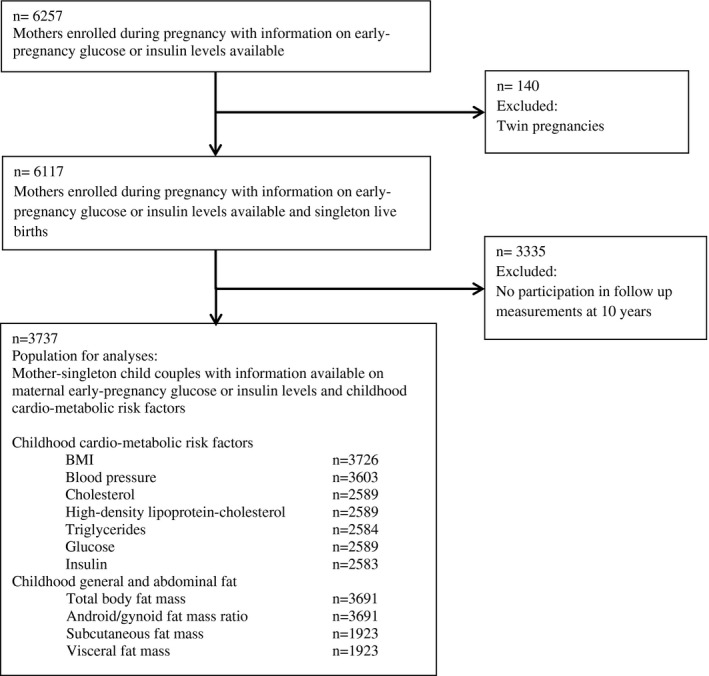
Flowchart of the study participants.
Maternal early‐pregnancy glucose and insulin concentrations
Nonfasting blood samples were collected at enrollment in the study before 18 weeks of gestation (median: 13.2 weeks; 95% range: 10.5‐17.1). Glucose concentration (millimoles per liter) is an enzymatic quantity and was measured with c702 module on the Cobas 8000 analyzer (Roche, Almere, the Netherlands). Insulin concentration (picomoles per liter) was measured with electrochemiluminescence immunoassay on the Cobas e411 analyzer (Roche).
Childhood cardiometabolic risk factors and general and abdominal fat measurements
At the age of 10 years, we measured height and weight without shoes and heavy clothing and calculated BMI (kilograms per meter squared). Childhood BMI standard deviation scores (SDS) adjusted for sex and age were constructed based on Dutch reference growth charts (Growth Analyzer 4.0; Dutch Growth Research Foundation, Rotterdam, Netherlands) (17). We defined childhood overweight and underweight by categorizing childhood weight status according to the International Obesity Task Force cutoffs (18). Overweight and obesity were combined into one category, and children with underweight were excluded only in this variable (n = 266). We observed similar results when children with underweight were included in the analyses (results not shown). Systolic and diastolic blood pressures (millimeters of mercury) were measured at the right brachial artery, four times with 1‐minute intervals, using the validated automatic sphygmanometer Datascope Accutorr Plus (Paramus, New Jersey) (19). Mean systolic and diastolic blood pressure values were calculated using the last three blood pressure measurements. We obtained nonfasting venous blood samples and measured total cholesterol (millimoles per liter), high‐density lipoprotein (HDL) cholesterol (millimoles per liter), triglycerides (millimoles per liter), glucose (millimoles per liter), and insulin (picomoles per liter) concentrations.
We measured total, android, and gynoid body fat mass by dual‐energy x‐ray absorptiometry (Lunar iDXA; GE Healthcare, Madison, Wisconsin) and calculated android/gynoid fat mass ratio (20). Abdominal subcutaneous and visceral fat measures were obtained from magnetic resonance imaging (MRI) scans using a 3.0‐T MRI (Discovery MR750w; GE Healthcare, Milwaukee, Wisconsin) as described previously (16, 21). Childhood body fat mass is strongly influenced by height of the child (22). To enable assessment of the associations of maternal glucose metabolism with childhood adiposity measures independent of childhood size, we constructed childhood fat mass measures independent of height of the child. Using log‐log regressions, we estimated the optimal adjustment for childhood height needed to construct height‐independent fat mass measures (details in online Supporting Information Methods 1) (22, 23, 24). We calculated total fat mass and subcutaneous fat mass indices (total and subcutaneous fat mass/height4) and visceral fat mass index (visceral fat mass/height3).
Clustering of cardiometabolic risk factors was defined as having three or more of the following components: visceral fat mass index ≥ 75th percentile, systolic or diastolic blood pressure ≥ 75th percentile, triglycerides ≥ 75th percentile, or HDL cholesterol ≤ 25th percentile; and insulin ≥ 75th percentile (25). Because waist circumference was not available, we used visceral fat mass index as a proxy for waist circumference.
Covariates
Information on maternal educational level, ethnicity, parity, weight just before pregnancy, maximum weight during pregnancy, smoking, and total daily energy intake (in kilojoules) during pregnancy was obtained through questionnaires (16). Maternal height was measured at intake without shoes and BMI was calculated (16). We obtained information about diagnosis of gestational diabetes and child’s sex, gestational age at birth, and birth weight from medical records (16). Preterm birth was defined as a gestational age at birth < 37 weeks. We created gestational age‐ and sex‐adjusted SDS of birth weight using North‐European reference growth charts (26). We defined small for gestational age and large for gestational age at birth as the lowest and the highest 10 percentiles of gestational‐age–adjusted birth weight, respectively. We obtained information on breastfeeding in infancy by questionnaire (16).
Statistical analysis
First, we performed a nonresponse analysis to compare children with and without follow‐up measurements at the age of 10 years. Second, we assessed the associations of maternal early‐pregnancy glucose and insulin concentrations across the full range with the risks of childhood overweight and clustering of cardiometabolic risk factors using multiple logistic regression models. Third, we used multiple linear regression models to assess the associations of maternal early‐pregnancy glucose and insulin concentrations with childhood BMI, blood pressure, lipids, and glucose and insulin concentrations across the full range separately and with detailed childhood general and abdominal fat measurements. We used three different models for the analyses. The first was the basic model, which was adjusted for gestational age at enrollment and child’s age and sex at follow‐up measurements. The second was the confounder model, which was the basic model additionally adjusted for confounding covariates and was considered as the main model. Based on literature, maternal ethnicity, educational level, parity, smoking, and daily total caloric intake were considered as potential confounders. Only maternal ethnicity and educational level were selected in the model based on their association with exposures and outcomes and change in effect estimates of > 10% in our study sample. The third model was the maternal BMI model, which was the confounder model additionally adjusted for maternal prepregnancy BMI. Because previous studies have suggested that associations between gestational diabetes and childhood BMI are largely explained by maternal prepregnancy BMI, we constructed this separate maternal prepregnancy BMI model (12). Correlation coefficients for correlation between maternal glucose and insulin concentrations and prepregnancy BMI were 0.16 and 0.20 for maternal glucose and insulin concentrations, respectively. For associations that persisted after adjustment for maternal prepregnancy BMI, we further explored whether these associations were mediated by gestational weight gain, birth weight, infant breastfeeding, or childhood BMI by adding these variables separately to the maternal BMI model. We tested for interactions of maternal glucose and insulin with maternal BMI, maternal ethnicity, and child’s sex, but none was significant and no further stratified analyses were performed (27, 28, 29). We performed the following sensitivity analyses: (1) we excluded women with a diagnosis of gestational diabetes (n = 34) because we were interested in the associations of maternal glucose and insulin concentrations within a nondiabetic population; (2) we repeated the analyses excluding children born preterm, small for gestational age at birth, or large for gestational age at birth to explore whether these adverse birth outcomes explained potential associations.
Not normally distributed exposure and outcome measures were log transformed. To enable comparison of effect estimates, we constructed SDS of exposures and outcomes. To reduce selection bias because of missing data, multiple imputations of covariates (pooled results of five imputed data sets) were performed (30). We applied Bonferroni correction to take multiple testing into account. As outcomes were strongly correlated, we divided the α of 0.05 by four categories (fat measures, blood pressure, lipid concentrations, and glucose/insulin concentrations), resulting in P < 0.013. All analyses were performed using SPSS Statistics version 24.0 for Windows (IBM Corp., Armonk, New York).
Results
Characteristics of study participants
Table 1 shows the population characteristics. In early pregnancy, the mean maternal glucose concentration was 4.4 mmol/L (SD 0.9) and the median insulin concentration was 114.0 pmol/L (95% range: 24.1‐491.8). Nonresponse analyses showed that mothers of children included in the analyses compared with mothers lost to follow‐up were, on average, older, more frequently European, and more highly educated and that they had a higher prepregnancy weight and had children with a higher birth weight. No differences in early‐pregnancy glucose and insulin concentrations were present (Supporting Information Table S1).
Table 1.
Characteristics of study population
| Total group (n = 3,737) | |
|---|---|
| Maternal characteristics | |
| Age at enrollment, mean (SD), y | 30.7 (4.7) |
| Height, mean (SD), cm | 168.2 (7.4) |
| Prepregnancy weight, median (95% range), kg | 65.0 (50.3‐90.0) |
| Prepregnancy BMI, median (95% range), kg/m2 | 22.6 (18.8‐31.9) |
| Ethnicity, n (%) | |
| Dutch | 2,193 (58.7) |
| European | 299 (8.0) |
| Cape Verdean | 153 (4.1) |
| Dutch Antillean | 66 (1.8) |
| Moroccan | 169 (4.5) |
| Surinamese | 272 (7.3) |
| Turkish | 218 (5.8) |
| Education, n high (%) | 1,855 (49.6) |
| Parity, n nulliparous (%) | 2,230 (59.7) |
| Smoking during pregnancy, n yes (%) | 853 (22.8) |
| Gestational weight gain, mean (SD), kg | 15.1 (5.7) |
| Daily energy intake, mean (SD), kJ | 8,581 (2,294) |
| Gestational age at intake, median (95% range), wk | 13.2 (10.5‐17.1) |
| Glucose concentration, mean (SD), mmol/L | 4.4 (0.9) |
| Insulin concentration, median (95% range), pmol/L | 114.0 (24.1‐491.8) |
| Gestational diabetes, n (%) | 34 (0.9) |
| Infant characteristics | |
| Sex, n female (%) | 1,894 (50.7) |
| Gestational age at birth, median (95% range), wk | 40.3 (37.1‐42.1) |
| Birth weight, mean (SD), g | 3,437 (550) |
| Small for gestational age, n (%) | 373 (10.0) |
| Large for gestational age, n (%) | 373 (10.0) |
| Preterm birth, n (%) | 155 (4.1) |
| Ever breastfed, n yes (%) | 2,878 (77.0) |
| Childhood characteristics | |
| Age, mean (SD), y | 9.8 (0.4) |
| Height, mean (SD), cm | 141.6 (6.7) |
| Weight, median (95% range), kg | 33.8 (26.4‐49.7) |
| BMI, median (95% range), kg/m2 | 16.9 (14.4‐23.3) |
| Fat | |
| Total fat mass, median (95% range) | 8,417 (4,905‐19,116) |
| Android/gynoid fat mass ratio, median (95% range) | 0.24 (0.16‐0.44) |
| Subcutaneous fat mass, median (95% frange), g | 1,294 (642‐4,271) |
| Visceral fat mass, median (95% range), g | 369 (187‐853) |
| Blood pressure | |
| Systolic, mean (SD), mmHg | 103.1 (7.9) |
| Diastolic, mean (SD), mmHg | 58.5 (6.4) |
| Lipid concentrations | |
| Total cholesterol, mean (SD), mmol/L | 4.31 (0.66) |
| High‐density lipoprotein cholesterol, mean (SD), mmol/L | 1.48 (0.34) |
| Triglycerides, median (95% range), mmol/L | 0.98 (0.47‐2.28) |
| Glucose, mean (SD), mmol/L | 5.20 (0.94) |
| Insulin, median (95% range), pmol/L | 174.60 (45.87‐512.40) |
| Overweight/obesity, n (%) | 643 (17.2) |
| Clustering of cardiometabolic risk factors, n (%) | 261 (7.1) |
Childhood cardiometabolic risk factors
Figure 2 shows that, in the confounder model, 1‐SDS higher maternal early‐pregnancy glucose and insulin concentrations were associated with an increased risk of childhood overweight (odds ratio [OR] 1.14, 95% CI: 1.04‐1.24 and OR 1.20, 95% CI: 1.10‐1.32 per SDS increase in maternal glucose and insulin concentrations, respectively). A 1‐SDS higher maternal early‐pregnancy insulin concentration, but not glucose concentration, was associated with clustering of cardiometabolic risk factors in childhood (OR 1.20, 95% CI: 1.04‐1.38 per SDS increase in maternal insulin concentration). All of these associations attenuated to nonsignificance after adjustment for maternal prepregnancy BMI.
Figure 2.
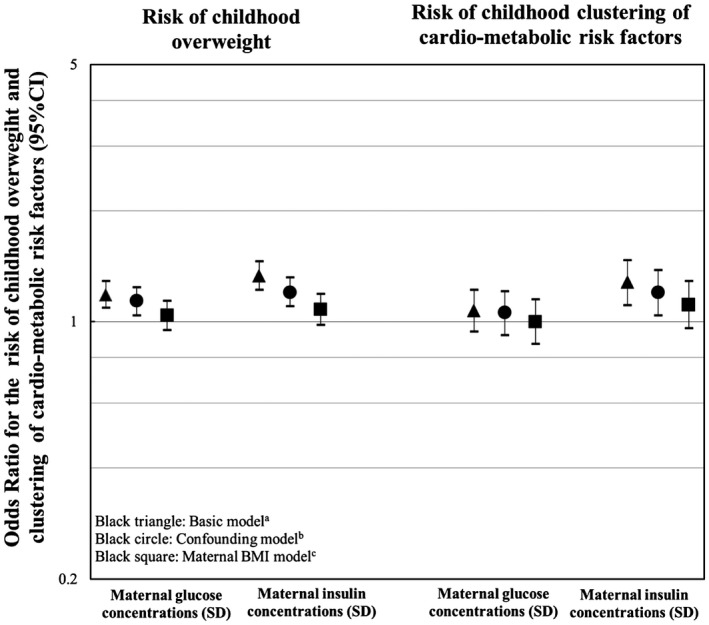
Associations of maternal early‐pregnancy glucose and insulin concentrations and childhood risks of overweight and clustering of cardiometabolic risk factors. Values represent odds ratios (95% CI) from logistic regression models that reflect the risks of childhood overweight for SDS change in maternal glucose and insulin concentrations. aBasic model includes gestational age at enrollment and child’s age and sex at follow‐up measurements. bConfounder model includes the basic model additionally adjusted for ethnicity and maternal educational level. cMaternal BMI model includes the confounder model additionally adjusted for maternal prepregnancy BMI.
Table 2 shows the associations of maternal glucose and insulin concentrations with each of the childhood cardiometabolic risk factors separately. In the confounder model, a 1‐SDS higher maternal glucose concentration was associated with lower HDL cholesterol (−0.04 SDS, 95% CI: −0.08 to −0.01 per SDS increase in glucose concentration). A 1‐SDS higher maternal insulin concentration was associated with higher childhood BMI (0.05 SDS, 95% CI: 0.02 to 0.08 per SDS increase in insulin concentration) and systolic blood pressure (0.04 SDS, 95% CI: 0.01 to 0.07 per SDS increase in insulin concentration). These associations attenuated to nonsignificance after adjustment for maternal prepregnancy BMI. A 1‐SDS higher maternal early‐pregnancy glucose concentration was associated with higher glucose concentration in childhood (0.08 SDS, 95% CI: 0.04‐0.11 per SDS increase in maternal glucose concentration), whereas a 1‐SDS higher maternal early‐pregnancy insulin concentration was associated with higher childhood insulin concentration (0.07 SDS, 95% CI: 0.03‐0.10 per SDS increase in maternal insulin concentration). The association of maternal glucose concentration with childhood glucose concentration was not affected by additional adjustment for maternal prepregnancy BMI, whereas the association of maternal early‐pregnancy insulin concentration with childhood insulin concentration only slightly attenuated after adjustment for maternal prepregnancy BMI. Further adjustment for gestational weight gain, birth weight, infant breastfeeding, and childhood BMI did not materially affect the associations (Supporting Information Table S2).
Table 2.
Associations of maternal early‐pregnancy glucose and insulin concentrations with childhood cardiometabolic risk factors
| Model | BMI (SDS) (n = 3,726) | Systolic blood pressure (SDS) (n = 3,603) | Diastolic blood pressure (SDS) (n = 3,603) | Total cholesterol concentrations (SDS) (n = 2,589) | HDL cholesterol concentrations (SDS) (n = 2,589) | Triglyceride concentrations (SDS) (n = 2,584) | Glucose concentrations (SDS) (n = 2,589) | Insulin concentrations (SDS) (n = 2,583) |
|---|---|---|---|---|---|---|---|---|
| Maternal glucose concentrations (SDS) | ||||||||
| Basic model a | 0.04 (0.00 to 0.07) | 0.03 (0.00 to 0.06) | 0.04 (0.01 to 0.07)* | −0.01 (−0.05 to 0.03) | −0.05 (−0.08 to −0.01)* | −0.02 (−0.06 to 0.02) | 0.08 (0.04 to 0.11)* | 0.04 (0.00 to 0.08) |
| Confounder model b | 0.02 (−0.01 to 0.06) | 0.02 (−0.01 to 0.06) | 0.03 (0.00 to 0.07) | −0.01 (−0.05 to 0.03) | −0.04 (−0.08 to −0.01)* | −0.03 (−0.06 to 0.01) | 0.08 (0.04 to 0.11)* | 0.04 (0.00 to 0.07) |
| Maternal BMI model c | NA | NA | 0.02 (−0.01 to 0.06) | NA | −0.03 (−0.07 to 0.01) | NA | 0.08 (0.04 to 0.12)* | 0.03 (−0.01 to 0.06) |
| Maternal insulin concentrations (SDS) | ||||||||
| Basic model a | 0.08 (0.05 to 0.12)* | 0.06 (0.03 to 0.09)* | 0.05 (0.01 to 0.08)* | 0.00 (−0.04 to 0.04) | −0.06 (−0.10 to −0.02)* | 0.01 (−0.03 to 0.05) | 0.02 (−0.02 to 0.06) | 0.08 (0.04 to 0.12)* |
| Confounder model b | 0.05 (0.02 to 0.08)* | 0.04 (0.01 to 0.07)* | 0.03 (−0.01 to 0.06) | −0.01 (−0.04 to 0.03) | −0.05 (−0.09 to −0.01) | 0.00 (−0.04 to 0.04) | 0.02 (−0.02 to 0.06) | 0.07 (0.03 to 0.10)* |
| Maternal BMI model c | −0.01 (−0.05 to 0.02) | 0.01 (−0.02 to 0.05) | NA | NA | NA | NA | NA | 0.05 (0.02 to 0.09)* |
Values represent regression coefficients (95% CI) from linear regression models that reflect differences in childhood outcomes in SDS per SDS change in maternal glucose and insulin concentrations. Estimates based on multiple imputed data.
Basic model includes gestational age at enrollment and child’s age and sex at follow‐up measurements.
Confounder model includes basic model additionally adjusted for ethnicity and maternal educational level.
Maternal BMI model includes confounder model additionally adjusted for maternal prepregnancy BMI.
P < 0.013 (Bonferroni corrected P value for multiple testing).
SDS, standard deviation score; HDL, high‐density lipoprotein; NA, not applicable.
Childhood general and abdominal fat
Table 3 shows that in the confounder model, a 1‐SDS higher maternal early‐pregnancy insulin concentration, but not glucose concentration, was associated with higher childhood total fat mass index (0.06 SDS, 95% CI: 0.03‐0.09 per SDS increase in insulin concentration), android/gynoid fat mass ratio (0.05 SDS, 95% CI: 0.02‐0.08 per SDS increase in insulin concentration), and subcutaneous fat mass index (0.07 SDS, 95% CI: 0.03‐0.11 per SDS increase in insulin concentration). All of these associations of maternal insulin concentration with childhood total fat mass index, android/gynoid fat mass ratio, and abdominal subcutaneous fat mass index attenuated to nonsignificance after adjustment for maternal prepregnancy BMI. No associations of maternal glucose or insulin concentrations with childhood visceral fat mass index were present.
Table 3.
Associations of maternal early‐pregnancy glucose and insulin concentrations with childhood general and abdominal fat
| Model | Total fat mass index (SDS) (n = 3,684) | Android/gynoid fat mass ratio (SDS) (n = 3,691) | Subcutaneous fat mass index (SDS) (n = 1,919) a | Visceral fat mass index (SDS) (n = 1,919) a |
|---|---|---|---|---|
| Maternal glucose concentrations (SDS) | ||||
| Basic model b | 0.05 (0.02 to 0.08)* | 0.04 (0.00 to 0.07) | 0.04 (−0.01 to 0.08) | −0.01 (−0.05 to 0.04) |
| Confounder model c | 0.03 (0.00 to 0.06) | 0.02 (−0.01 to 0.05) | 0.03 (−0.02 to 0.07) | −0.01 (−0.06 to 0.03) |
| Maternal BMI model d | NA | NA | NA | NA |
| Maternal insulin concentrations (SDS) | ||||
| Basic model b | 0.11 (0.08 to 0.14)* | 0.09 (0.06 to 0.12)* | 0.11 (0.06 to 0.15)* | 0.03 (−0.01 to 0.08) |
| Confounder model c | 0.06 (0.03 to 0.09)* | 0.05 (0.02 to 0.08)* | 0.07 (0.02 to 0.11)* | 0.02 (−0.02 to 0.07) |
| Maternal BMI model d | 0.01 (−0.02 to 0.04) | 0.01 (−0.02 to 0.04) | 0.02 (−0.02 to 0.06) | NA |
Values represent regression coefficients (95% CI) from linear regression models that reflect differences in childhood outcomes in SDS per SDS change in maternal glucose and insulin concentrations. Estimates based on multiple imputed data.
Magnetic resonance imaging follow‐up measurements performed in subgroup of children.
Basic model includes gestational age at enrollment and child’s age and sex at follow‐up measurements.
Confounder model includes basic model additionally adjusted for ethnicity and maternal educational level.
Maternal BMI model includes confounder model additionally adjusted for maternal prepregnancy BMI.
P < 0.013 (Bonferroni corrected P value for multiple testing).
SDS, standard deviation score; NA, not applicable.
Sensitivity analyses
No differences in findings were present when mothers with gestational diabetes were excluded from the analyses (data not shown). We observed largely similar results when children with adverse birth outcomes were excluded from the analyses (Supporting Information Tables S3‐S6).
Discussion
In this prospective cohort study, we observed that higher maternal early‐pregnancy glucose and insulin concentrations were associated with higher childhood glucose and insulin concentrations at the age of 10 years. The associations of maternal early‐pregnancy glucose and insulin concentrations with other childhood cardiometabolic risk factors and detailed measurements of general and abdominal fat were explained by maternal prepregnancy BMI.
Interpretation of main findings
A high number of pregnancies are complicated by gestational diabetes. Next to an increased risk of maternal complications, intrauterine exposure to gestational diabetes is associated with adverse cardiometabolic outcomes in the offspring (4). Previous studies have already reported associations between higher late‐pregnancy maternal glucose concentrations already below the clinical threshold of gestational diabetes with offspring cardiometabolic risk factors (6, 31, 32). A study among 970 Chinese mother‐child pairs reported that third‐trimester maternal fasting glucose concentrations were associated with a higher risk for obesity, higher systolic blood pressure, and abnormal glucose tolerance at the age of 7 years, independent of maternal prepregnancy BMI (6). A cohort study in the United Kingdom including 2,563 women and their offspring showed that, independent of maternal prepregnancy BMI, glycosuria in midpregnancy was associated with higher offspring BMI and fasting insulin concentrations but not with blood pressure and lipid concentrations(31). It is likely that women who develop gestational diabetes or hyperglycemia later in pregnancy already have a suboptimal glucose metabolism in early pregnancy, a critical period for placental and fetal cardiometabolic development (9, 33). Suboptimal maternal glucose and insulin concentrations in early pregnancy may adversely affect placental development, predisposing to alterations in fetal nutrient supply, growth, and development (34). In addition, suboptimal maternal early‐pregnancy glucose concentrations may have direct adverse influences on fetal cardiometabolic development (9).
In the current study, we observed that higher maternal glucose and insulin concentrations in early pregnancy were associated with higher childhood risks of overweight and clustering of cardiometabolic risk factors. However, these associations attenuated after adjustment for maternal prepregnancy BMI. These findings suggest that maternal prepregnancy BMI, a known risk factor for insulin resistance in pregnancy and cardiometabolic risk factors in childhood, explains the associations of maternal early‐pregnancy glucose and insulin concentrations with childhood overweight and cardiometabolic risk factors (9). When we further explored the associations of maternal early‐pregnancy glucose and insulin concentrations with individual cardiometabolic risk factors, we observed that higher maternal glucose and insulin concentrations were associated with higher offspring glucose and insulin concentrations, respectively. These associations were independent of maternal prepregnancy BMI, gestational weight gain, birth weight, infant breastfeeding, and childhood BMI. Findings were also similar when we excluded children with adverse birth outcomes from the analyses. Thus, these factors do not seem to explain the associations of maternal glucose and insulin concentrations with childhood glucose metabolism. This suggests that at least part of the association may be due to an intrauterine effect of maternal glucose and insulin concentrations on offspring glucose metabolism. Similar to previous studies performed later in pregnancy using fasting glucose samples, we did not find an association of maternal early‐pregnancy glucose and insulin concentrations with childhood BMI, blood pressure, and lipid concentrations, independent of maternal prepregnancy BMI (31). Thus, our results suggest that maternal glucose and insulin concentrations, as soon as early pregnancy, are related to higher childhood glucose and insulin concentrations, irrespective of maternal, birth, and childhood characteristics, but not to other cardiometabolic outcomes. Whether maternal factors other than impaired glucose metabolism as a consequence of higher maternal BMI, such as altered maternal hormone status, play a role in the association of maternal prepregnancy BMI with childhood BMI, blood pressure, and lipids should be further studied.
Animal and mechanistic studies proposed that offspring fat accumulation and adverse fat distribution might be involved in the associations of maternal hyperglycemia with offspring cardiometabolic risk factors. Observational studies have confirmed this hypothesis and reported associations of maternal fasting glucose concentrations in pregnancy with adverse offspring body fat composition, measured by sum of skinfolds and waist circumference (6, 7, 31, 35, 36). However, these measures are suboptimal, as waist circumference does not distinguish subcutaneous from visceral fat, whereas visceral abdominal fat is much more closely related to risk of cardiometabolic disease in later life (14). In the present study, we observed that higher maternal early‐pregnancy insulin concentrations but not glucose concentrations were associated with childhood total body fat mass, android/gynoid fat mass ratio, and subcutaneous abdominal fat mass. In line with the associations of maternal glucose and insulin concentrations with childhood BMI, blood pressure, and lipids, all associations of maternal glucose and insulin concentrations with detailed measurements of childhood general and abdominal fat in the present study were fully explained by maternal prepregnancy BMI. Contrary to our hypothesis, no specific associations with childhood visceral fat mass were present. It might be that associations with childhood visceral fat are more apparent among higher risk populations or at older ages. Further studies are needed to explore the detailed role of a suboptimal offspring body fat distribution in response to impaired maternal glucose metabolism during pregnancy within different populations and using advanced imaging techniques. Based on our results, it seems that maternal early‐pregnancy glucose and insulin concentrations are associated with childhood subcutaneous fat accumulation, but these associations are explained by maternal prepregnancy BMI.
Within this study, we only observed independent associations of maternal early‐pregnancy glucose and insulin concentrations with childhood glucose and insulin concentrations. These associations provide insight into potential underlying mechanisms, and they may be explained through several pathways. First, shared genetic factors are expected to have a contribution in the association between maternal glucose and insulin concentrations with offspring glucose and insulin concentrations (37). Second, higher maternal early‐pregnancy glucose concentrations lead to fetal hyperinsulinemia, whereas higher maternal early‐pregnancy insulin concentrations are involved in protein, lipolysis, and early placental development. Together, this could cause alternations in fetal nutrient supply, affecting fetal pancreatic beta‐cell development and increasing fetal insulin secretion. These irreversible alterations may subsequently lead to increased glucose and insulin concentrations in childhood (9, 38, 39). Furthermore, higher maternal glucose concentrations may also be involved in gene expression through DNA methylation, leading to altered insulin secretion in the offspring (40). Further studies are needed to disentangle the complex mechanisms underlying the association of maternal glucose and insulin concentrations with childhood glucose metabolism.
The observed effect estimates for the associations of maternal early‐pregnancy glucose and insulin concentrations with childhood glucose and insulin concentrations were relatively small but they may be important on a population level. Previous studies have shown that childhood glucose and insulin concentrations tend to track into adulthood. A study among 1,766 children showed that children with higher fasting glucose concentrations at the age of 10 years had a higher risk of developing type 2 diabetes in adolescence (6). Similarly, a study among 1,723 children reported that children with higher fasting glucose concentrations within the normal range had a higher risk of prediabetes and type 2 diabetes in adulthood (7). A study among 4,857 American Indian children without diabetes showed that children with higher glucose concentrations after a glucose tolerance test had a higher risk of premature death, but this effect was not independent of concurrent childhood BMI (41). Together, these findings suggest that even subclinical differences in childhood glucose and insulin concentrations may be related to the development of type 2 diabetes in later life (42). Maternal prepregnancy BMI seems to explain the associations of maternal glucose and insulin concentrations with other childhood cardiometabolic risk factors and childhood body fat development. This suggests that preventive strategies, aimed at improving offspring cardiometabolic health, might be more effective when focusing on optimizing maternal prepregnancy BMI than on optimizing maternal glucose concentrations from early pregnancy onward.
Methodological considerations
Strengths of this study are the prospective design, large sample size, and the use of detailed fat measures obtained through MRI. Although only 61% of children from mothers with information on glucose and insulin concentrations in pregnancy participated in follow‐up measurements, we do not expect that nonresponse affected our effect estimates, as maternal insulin and glucose concentrations did not differ between these groups. The generalizability of our results may be affected by a selection toward a relatively healthy, high‐educated study population. We obtained nonfasting glucose and insulin concentrations, sampled on nonfixed times throughout the day. This may have led to nondifferential misclassification, causing an underestimation of our associations. Although we simultaneously measured insulin concentrations to substantiate our findings, random glucose concentrations cannot directly assess insulin resistance. However, random glucose concentrations are useful for identifying women at risk for gestational diabetes and they are used in clinical practice as a screening method in early pregnancy (43, 44). In addition, we measured maternal glucose and insulin concentrations once during early pregnancy. Impaired glucose tolerance in early pregnancy has been suggested to persist throughout pregnancy (33). Further studies are needed with multiple, more detailed maternal glucose measurements, including fasting glucose concentrations and detailed postprandial glucose measurements throughout pregnancy. These studies also need to use more advanced statistical methods to provide further insight into critical periods for potential adverse effects of impaired maternal glucose metabolism on offspring glucose metabolism. We did not have information available on clinical diagnosis of type 2 diabetes in the offspring. However, we expect the percentage of childhood type 2 diabetes according to clinical diagnosis within our cohort to be low, as the average age of the children in our cohort is 9.8 years, whereas the onset of type 2 diabetes mostly occurs at later childhood ages (45). Further studies are needed to assess whether maternal early‐pregnancy glucose and insulin concentrations are also associated with the risk of type 2 diabetes in the offspring during adolescence. Finally, although we had detailed information on maternal and childhood sociodemographic and lifestyle factors available, because of the observational study design, residual confounding by, for example, childhood dietary factors and physical activity may have influenced our results.
Conclusion
Maternal early‐pregnancy random glucose and insulin concentrations were associated with higher childhood glucose and insulin concentrations, independent of maternal and childhood characteristics. When taking maternal prepregnancy BMI into account, no associations of maternal glucose and insulin concentrations with other childhood cardiometabolic risk factors were present.
Funding agencies
The Generation R Study is financially supported by the Erasmus University Medical Center, Rotterdam, the Erasmus University Rotterdam, and the Netherlands Organization for Health Research and Development. RG received funding from the Dutch Heart Foundation (grant number 2017T013), the Dutch Diabetes Foundation (grant number 2017.81.002), and the Netherlands Organization for Health Research and Development (NWO, ZonMw, grant number 543003109). PJ received funding from the Dutch Diabetes Foundation (grant number 2013.81.1664). VJ received a grant from the Netherlands Organization for Health Research and Development (NWO, ZonMw‐VIDI 016.136.361) and a European Research Council Consolidator Grant (ERC‐2014‐CoG‐648916).
Disclosure
The authors declared no conflict of interest.
Author contributions
RW, EV, and RG designed and constructed the research, wrote the paper, and had primary responsibility for the final content. RW and EV carried out the statistical analysis. VJ, ES, PJ, and EO coordinated data acquisition and critically reviewed and revised the manuscript. All authors approved the final manuscript and agree to be accountable for all aspects of the work.
Supporting information
Supinfo
Acknowledgments
The Generation R Study is conducted by the Erasmus University Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam, and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam, The Netherlands. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
References
- 1. Damm P, Houshmand‐Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long‐term consequences for mother and offspring: a view from Denmark. Diabetologia 2016;59:1396‐1399. [DOI] [PubMed] [Google Scholar]
- 2. Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diet‐treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009;94:2464‐2470. [DOI] [PubMed] [Google Scholar]
- 3. Kawasaki M, Arata N, Miyazaki C, et al. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: a systematic review and meta‐analysis. PLoS One 2018;13:e0190676. doi: 10.1371/journal.pone.0190676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowe WL Jr, Scholtens DM, Lowe LP, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 2018;320:1005‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patro Golab B, Santos S, Voerman E, et al. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: an individual participant data meta‐analysis. Lancet Child Adolesc Health 2018;2:812‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tam WH, Ma RCW, Ozaki R, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017;40:679‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowe WL Jr, Lowe LP, Kuang A, et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow‐up Study. Diabetologia 2019;62:598‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol 2007;92:287‐298. [DOI] [PubMed] [Google Scholar]
- 9. Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010;16:255‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harmon KA, Gerard L, Jensen DR, et al. Continuous glucose profiles in obese and normal‐weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198‐2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta‐analysis. Diabetologia 2012;55:3114‐3127. [DOI] [PubMed] [Google Scholar]
- 12. Philipps LH, Santhakumaran S, Gale C, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta‐analysis. Diabetologia 2011;54:1957‐1966. [DOI] [PubMed] [Google Scholar]
- 13. Gingras V, Rifas‐Shiman SL, Derks IPM, Aris IM, Oken E, Hivert MF. Associations of gestational glucose tolerance with offspring body composition and estimated insulin resistance in early adolescence. Diabetes Care 2018;41:e164‐e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation 2011;124:e837‐e841. [DOI] [PubMed] [Google Scholar]
- 15. Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301‐1313. [DOI] [PubMed] [Google Scholar]
- 16. Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update. Eur J Epidemiol 2016;31:1243‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fredriks AM, van Buuren S, Wit JM, Verloove‐Vanhorick SP. Body index measurements in 1996‐7 compared with 1980. Arch Dis Child 2000;82:107‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong SN, Tz Sung RY, Leung LC. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Press Monit 2006;11:281‐291. [DOI] [PubMed] [Google Scholar]
- 20. Helba M, Binkovitz LA. Pediatric body composition analysis with dual‐energy X‐ray absorptiometry. Pediatr Radiol 2009;39:647‐656. [DOI] [PubMed] [Google Scholar]
- 21. Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev 2011;12:e504‐e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wells JC, Cole TJ. Adjustment of fat‐free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord 2002;26:947‐952. [DOI] [PubMed] [Google Scholar]
- 23. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16:621‐638. [DOI] [PubMed] [Google Scholar]
- 24. VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height‐normalized indices of the body's fat‐free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr 1990;52:953‐959. [DOI] [PubMed] [Google Scholar]
- 25. Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2009;119:628‐647. [DOI] [PubMed] [Google Scholar]
- 26. Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977‐1981). Acta Paediatr Scand 1991;80:756‐762. [DOI] [PubMed] [Google Scholar]
- 27. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290‐e296. [DOI] [PubMed] [Google Scholar]
- 28. Schwartz N, Nachum Z, Green MS. The prevalence of gestational diabetes mellitus recurrence–effect of ethnicity and parity: a metaanalysis. Am J Obstet Gynecol 2015;213:310‐317. [DOI] [PubMed] [Google Scholar]
- 29. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988‐1994. Arch Intern Med 2003;163:427‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. doi: 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel S, Fraser A, Davey Smith G, et al. Associations of gestational diabetes, existing diabetes, and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes Care 2012;35:63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scholtens DM, Kuang A, Lowe LP, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow‐Up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care 2019;42:381‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smirnakis KV, Martinez A, Blatman KH, Wolf M, Ecker JL, Thadhani R. Early pregnancy insulin resistance and subsequent gestational diabetes mellitus. Diabetes Care 2005;28:1207‐1208. [DOI] [PubMed] [Google Scholar]
- 34. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017;356:j1. doi: 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lowe WL Jr, Scholtens DM, Kuang A, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow‐up Study (HAPO FUS): maternal gestational diabetes and childhood glucose metabolism. Diabetes Care 2019;42:372‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao P, Liu E, Qiao Y, et al. Maternal gestational diabetes and childhood obesity at age 9–11: results of a multinational study. Diabetologia 2016;59:2339‐2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho YM, Kim TH, Lim S, et al. Type 2 diabetes‐associated genetic variants discovered in the recent genome‐wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia 2009;52:253‐261. [DOI] [PubMed] [Google Scholar]
- 38. O'Tierney‐Ginn P, Presley L, Myers S, Catalano P. Placental growth response to maternal insulin in early pregnancy. J Clin Endocrinol Metab 2015;100:159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monteiro LJ, Norman JE, Rice GE, Illanes SE. Fetal programming and gestational diabetes mellitus. Placenta 2016;48(Suppl 1):S54‐S60. [DOI] [PubMed] [Google Scholar]
- 40. Ruchat SM, Hivert MF, Bouchard L. Epigenetic programming of obesity and diabetes by in utero exposure to gestational diabetes mellitus. Nutr Rev 2013;71(Suppl 1):S88‐S94. [DOI] [PubMed] [Google Scholar]
- 41. Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362:485‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiss R, Santoro N, Giannini C, Galderisi A, Umano GR, Caprio S. Prediabetes in youth ‐ mechanisms and biomarkers. Lancet Child Adolesc Health 2017;1:240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nederlandse Vereniging voor Obstetrie en Gynaecologie . Diabetes Mellitus en Zwangerschap. 2018. https://www.nvog.nl/wp-content/uploads/2018/10/NVOG-richtlijn-Diabetes-mellitus-en-zwangerschap-v3.0-2018.pdf [Google Scholar]
- 44. Meek CL, Murphy HR, Simmons D. Random plasma glucose in early pregnancy is a better predictor of gestational diabetes diagnosis than maternal obesity. Diabetologia 2016;59:445‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dabelea D, Mayer‐Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo


