Abstract
Objective
Autoimmune disease is an emerging condition among persons exposed to the September 11, 2001 attack on the World Trade Center (WTC). Components of the dust cloud resulting from the collapse of the WTC have been associated with development of a systemic autoimmune disease, as has posttraumatic stress disorder (PTSD). We undertook this study to determine whether dust exposure and PTSD were associated with an increased risk of systemic autoimmune disease in a 9/11‐exposed cohort.
Methods
Among 43,133 WTC Health Registry enrollees, 2,786 self‐reported having a post‐9/11 systemic autoimmune disease. We obtained informed consent to review medical records to validate systemic autoimmune disease diagnoses for 1,041 enrollees. Diagnoses of systemic autoimmune diseases were confirmed by classification criteria, rheumatologist diagnosis, or having been prescribed systemic autoimmune disease medication. Controls were enrollees who denied having an autoimmune disease diagnosis (n = 37,017). We used multivariable log‐binomial regression to examine the association between multiple 9/11 exposures and risk of post‐9/11 systemic autoimmune disease, stratifying by responders (rescue, recovery, and clean‐up workers) and community members (e.g., residents, area workers).
Results
We identified 118 persons with systemic autoimmune disease. Rheumatoid arthritis was most frequent (n = 71), followed by Sjӧgren's syndrome (n = 22), systemic lupus erythematosus (n = 20), myositis (n = 9), mixed connective tissue disease (n = 7), and scleroderma (n = 4). Among 9/11 responders, those with intense dust cloud exposure had almost twice the risk of systemic autoimmune disease (adjusted risk ratio 1.86 [95% confidence interval 1.02–3.40]). Community members with PTSD had a nearly 3‐fold increased risk of systemic autoimmune disease.
Conclusion
Intense dust cloud exposure among responders and PTSD among community members were associated with a statistically significant increased risk of new‐onset systemic autoimmune disease. Clinicians treating 9/11 survivors should be aware of the potential increased risk of systemic autoimmune disease in this population.
Introduction
Systemic autoimmune diseases are an emerging health concern among individuals who were exposed to the September 11, 2001 terrorist attack on the World Trade Center (WTC) in New York City. The collapse of the WTC towers after the attack resulted in the release of a cloud of dust and debris that covered parts of Lower Manhattan, New York. Further exposure occurred subsequently through the resuspension of dust particles during recovery and clean‐up activities 1. Crystalline silica, a known risk factor for autoimmune diseases 2, 3, 4, 5, 6, 7, 8, was a major component of the dust cloud 1, 9. Additional components of the dust cloud and the air at the site during the clean‐up period 1, 10 have been previously associated with autoimmune diseases, including organic hydrocarbon solvents 11, 12, 13, fine particulate matter (PM2.5) 14, 15, 16, and asbestos 8, 17.
In addition to the physical exposures, many people witnessed or experienced traumatic events on 9/11 as well as continued occupational or personal reminders of the attacks. Posttraumatic stress disorder (PTSD), one of the most common post‐9/11 mental health disorders 18, has been associated with the subsequent onset of rheumatoid arthritis (RA) and other autoimmune disorders in both veteran and civilian populations 19, 20, 21.
Studies conducted among New York City firefighters and emergency medical services personnel have identified an exposure‐response relationship between length of time worked at the WTC site, level of exposure to the dust and debris following the attacks, and systemic autoimmune disease 22, 23. Currently, there have been no published studies on autoimmune diseases among community members who were exposed to the attack or among responders and clean‐up workers other than those with the Fire Department of the City of New York (FDNY).
This study aimed to identify systemic autoimmune diseases among a cohort of 9/11‐exposed adults. We also sought to determine whether high levels of 9/11 dust exposure or PTSD were associated with an increased risk of systemic autoimmune disease and whether this association differed between 9/11 responders and community members.
Subjects and Methods
WTC Health Registry
The WTC Health Registry (referred to as the Registry) is a longitudinal, prospective cohort of 71,426 enrollees who were exposed to the events of September 11, 2001 in New York City, or who were involved in the subsequent recovery and clean‐up effort, which lasted until July 2002. The Registry comprises individuals who were part of the rescue, recovery, and clean‐up response (responders) (43%) and those who worked, resided, attended school, or were in transit in Lower Manhattan the morning of the attack (community members) (57%), and has been estimated to include 17% of eligible exposed individuals 24, 25. Approximately 10% of responders in the Registry were FDNY firefighters or emergency medical services personnel on 9/11 25. Potential enrollees were identified and recruited using employer, organizational, and building occupant lists and an outreach and advertising campaign 18. A more detailed description of the Registry's recruitment methods has been published elsewhere 18. Enrollment occurred in 2003–2004 via telephone interviews (95%) or in‐person interviews (5%) (wave 1), at which time interviewers collected demographic, exposure, and physical and mental health data. All enrollees provided verbal informed consent at Registry enrollment. There have been subsequent Registry‐wide follow‐up surveys, including wave 2 (2006–2007, 68% response rate) and wave 3 (2011–2012, 63% response rate), which was the first Registry survey that included questions on autoimmune diseases. The study was approved by the Institutional Review Board of the New York City Department of Health and Mental Hygiene.
Autoimmune disease study participants
Eligible enrollees included all those age ≥18 years who reported on the wave 3 survey that they had ever been told by a physician or other health professional that they had RA or “(an)other autoimmune disorders (e.g., lupus, scleroderma, polymyositis).” Participants were eligible if they indicated that the year they were first told was 2001 or later. We excluded 8 enrollees who died between their wave 3 survey and the launch of the autoimmune disease study in 2014, leaving 2,786 potential cases of post‐9/11 autoimmune disease for further examination. A detailed flow chart of the study population is shown in Figure 1.
Figure 1.
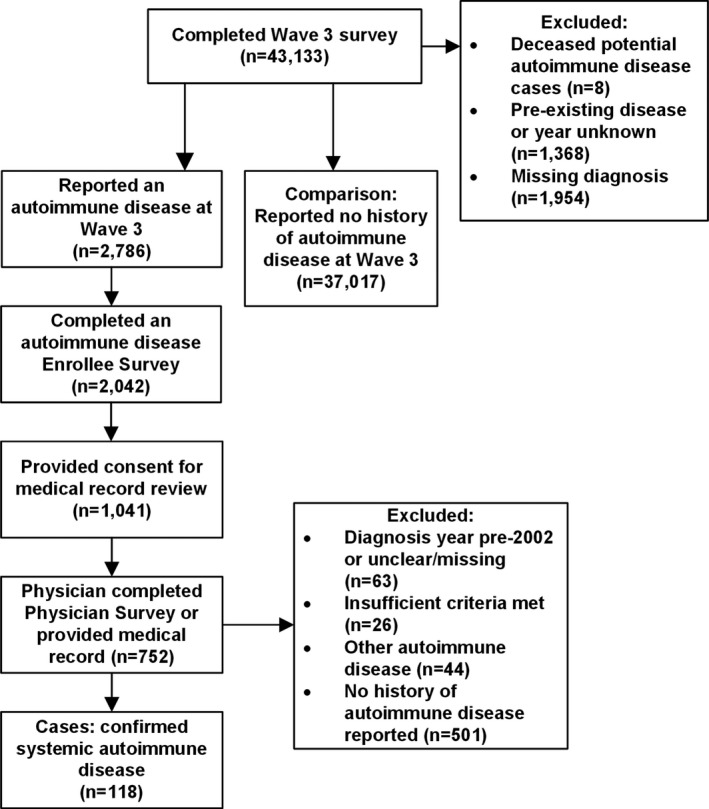
Flow chart of the World Trade Center Health Registry systemic autoimmune disease study population. Systemic autoimmune diseases (rheumatoid arthritis, Sjӧgren's syndrome, systemic lupus erythematosus, polymyositis/dermatomyositis, mixed connective tissue disease, and scleroderma) were diagnosed in 2002 or later. Cases had to either meet disease‐specific classification criteria, have been diagnosed by a board‐certified rheumatologist, or have been prescribed a medication commonly used to treat systemic autoimmune disease.
Phase I (autoimmune disease enrollee survey)
An in‐depth autoimmune disease follow‐up survey or survey link (enrollee survey) was mailed or e‐mailed to all eligible Registry enrollees in May 2014. The enrollee survey asked participants whether they had been told by a clinician that they had RA or other type of arthritis, systemic lupus erythematosus (SLE), scleroderma, polymyositis (PM)/dermatomyositis (DM), mixed connective tissue disease (MCTD), Sjӧgren's syndrome (SS), or other autoimmune disease, and whether they were taking medication for their condition. The survey included the CTD Screening Questionnaire (CSQ) 26, 27, which is a screening tool for the systemic autoimmune diseases listed above, and includes questions on symptoms and blood tests. Additional questions covered topics including general health, symptoms of other systemic autoimmune conditions, and family history of autoimmune disease. We created versions of the paper survey in English, Spanish, and Chinese; the online version was only available in English.
Enrollees who did not respond to the initial e‐mail invitation or mailed paper survey were sent up to 1–2 reminder postcards, 6 reminder e‐mails, and 2 additional mailings. Registry and New York City Department of Health and Mental Hygiene call center staff then telephoned enrollees who had not yet completed the survey, with up to 10 phone call attempts and 2 telephone messages left on their home or mobile phone.
Phase II (consent for release of medical records and physician survey)
Most enrollee survey respondents had some indication of possible post‐9/11 autoimmune disease, either because they screened positive via the CSQ, they reported an autoimmune disease diagnosis, or they reported taking a medication consistent with treatment for autoimmune disease (data not shown). Therefore, all enrollees who completed the survey (n = 2,042; response rate 73%) were sent a letter with a consent form authorizing the release of their medical records to the Registry. The consent form requested permission to obtain copies of enrollee medical records and to send a survey (physician survey) to their rheumatologist or to the physician who could provide information regarding their autoimmune disease diagnosis. Letters and consent forms were written in English, Spanish, and Chinese. Of those registrants who completed the enrollee survey, 1,041 (51%) provided the Registry with their informed consent. We used methods similar to those used for the enrollee survey to increase participation.
The physician survey, first mailed in May 2015, included checklists adapted from the American College of Rheumatology classification criteria for RA 28, SLE 29, and scleroderma 30; Bohan and Peter's criteria for PM/DM 31, 32; Kahn's criteria for MCTD 33; and American–European Consensus Group criteria for SS 34. The survey also queried the enrollee's year of diagnosis for each condition, any medications prescribed to treat the autoimmune disease, and whether the physician was a board‐certified rheumatologist.
In response to feedback received from physicians, we also offered the option of having medical records sent directly to the Registry for abstraction. Physicians who did not respond to the initial request were sent additional requests via mail, fax, and office telephone. Medical records retrieval contractors then followed up with physicians who had not responded to multiple requests from the Registry. Of the registrants who submitted consent forms, ~10% listed more than one physician, and attempts were made to contact multiple physicians when listed. Our report is presented based on the medical records and physician surveys obtained as of November 2017.
Cases
Documentation of an autoimmune disease and the year of diagnosis were abstracted either from the completed physician survey or the enrollee's medical records and reviewed independently by 2 Registry research staff, including the Registry's Medical Director (SM‐A, JEC). Medical records with unclear diagnoses were reviewed independently by 2 board‐certified rheumatologists (PMI, JRB). If the rheumatologists disagreed, they met and discussed the cases until agreement was reached. Agreement was achieved for all records.
Participants’ conditions were classified as RA, SLE, scleroderma, SS, MCTD, and PM/DM based on ≥1 of the following 3 criteria: 1) met classification criteria for a specific systemic autoimmune disease; 2) diagnosis determined by a board‐certified rheumatologist; and 3) medication prescribed commonly for autoimmune disease.
We only included participants who received their first systemic autoimmune disease diagnosis in 2002 or later. Enrollees for whom we were unable to verify a diagnosis, including those who did not participate in the enrollee survey (n = 744), those who did not provide informed consent (n = 1,001), those for whom a physician survey or medical record was not submitted by November 2017 (n = 289), and those whose survey did not indicate the presence of a systemic autoimmune disease (n = 545) or where the timing or classification criteria were unclear, incomplete, or prior to 2002 (n = 89), were excluded from our analysis.
Comparison group
The comparison study population included all enrollees age ≥18 years who completed the wave 3 survey and who denied having ever been diagnosed with RA or another autoimmune disease.
9/11 exposure
We used 4 metrics of 9/11‐related hazards. The first was a measure of intensity of exposure to the cloud of dust and debris on September 11, using responses to the wave 1 survey and the wave 2 survey. Intense dust cloud exposure was defined as having been caught in the dust cloud the morning of 9/11 and also experiencing an additional marker of intense exposure, including the inability to see a few feet ahead, trouble walking or navigating due to dust cloud thickness, taking shelter from the dust cloud, being covered in dust and debris, and the inability to hear anything while in the dust cloud.
The second and third measures were composite dust exposure scores, with one specific to responders and the other specific to community members. These indices are based on those developed by Li et al 35. Briefly, the responder index included the date of arrival to work at the WTC site, duration of work, and exposure to the dust cloud and to the events of the morning of 9/11, and its scoring system was derived using the Delphi method. For this analysis, we defined very high exposure among responders as a score of ≥29.5 (the 75th percentile). The community member index score, dichotomized into categories of “high” and “low” (with “low” defined as low, intermediate, or moderately high), was based on a hierarchy of exposure intensity and duration, including dust cloud exposure and exposures specific to whether the person was a resident (home evacuation and date of return), area worker (date returned to work), or present in Lower Manhattan on 9/11 (for school students/staff members and passersby).
The fourth measure of exposure was the number of months worked at the WTC site. This was calculated based on the work start and end dates provided at wave 1 and was limited to responders.
9/11‐related PTSD
PTSD was assessed at wave 1 using the 9/11‐specific PTSD checklist and was defined as a score of ≥44 on the checklist. The PTSD checklist is a 17‐item questionnaire that asks respondents how much they have been bothered by various symptoms in the past 30 days. The PTSD checklist and, in particular, the use of a cutoff score of 44 have been shown to have high diagnostic efficiency 36. Persons with missing items were included in the appropriate category if their PTSD status could be definitively identified based on their completed items; otherwise, they were categorized as missing.
Covariates
All covariates included in our analysis were self‐reported at wave 1, including age, sex, race/ethnicity, educational attainment (high school diploma or less, some college, or college degree and higher), and history of smoking. For our models, we used age at wave 3, which was derived based on date of birth and date of wave 3 completion.
Statistical analysis
This analysis focused on the 6 systemic autoimmune diseases that were specifically queried on the enrollee survey and physician survey. Diagnoses of RA, SLE, PM/DM, scleroderma, SS, and MCTD were aggregated into one outcome, referred to as systemic autoimmune disease. On both surveys, we also included an “other” category, from which we identified additional cases of autoimmune disease. However, these were either organ‐specific disorders (e.g., Hashimoto thyroiditis) or conditions for which we were unable to collect sufficient classification criteria for validation.
We conducted a bivariate analysis to compare cases and noncases using chi‐square test for independence, Fisher's exact test for sparse cell sizes, t‐test, and Wilcoxon's rank sum test, as appropriate. Due to inherent differences in characteristics and exposures between responders and community members, we stratified our bivariate analysis and multivariable log‐binomial regression to examine each group separately. In these models, we analyzed the association between 9/11 exposure, PTSD, and the risk of systemic autoimmune disease. Each 9/11 exposure was modeled separately, as was PTSD, and all models were adjusted for sex, age, race/ethnicity, and smoking history. We included educational attainment in our PTSD models to account for potential confounding. Variables were selected for inclusion based on known risk factors for autoimmune disease.
We performed 4 sensitivity analyses. First, we limited our cases to those who met classification criteria, i.e., a more conservative and specific case definition. Second, we excluded diagnoses obtained prior to 2005 to account for possible disease latency effects. We conducted a third analysis examining only RA, the most frequently reported autoimmune disease in this population, as the outcome measure. Finally, we stratified the main analytic exposure models by PTSD status at wave 1. All analyses were performed using SAS Enterprise Guide 7.13.
Results
We identified 118 persons with post‐9/11 systemic autoimmune disease in our population, of whom 62 were responders and 56 were community members. Sixty‐six cases met disease classification criteria, 43 only met the case criterion of having been diagnosed by a board‐certified rheumatologist, and 9 only met the criterion of having been prescribed medication for an autoimmune disease. The most commonly reported diagnosis was RA (n = 71), followed by SS (n = 22), SLE (n = 20), PM/DM (n = 9), MCTD (n = 7), and scleroderma (n = 4) (Table 1). Twelve percent of cases had >1 post‐9/11 systemic autoimmune disease. Of enrollees who screened positive on the CSQ, 20% were subsequently verified as having an autoimmune disease, compared with only 4% among those who did not screen positive (P < 0.0001).
Table 1.
Specific systemic autoimmune disease diagnoses among World Trade Center Health Registry enrollees (n = 118)a
| RA | 71 |
| SS | 22 |
| SLE | 20 |
| Myositis (PM or DM) | 9 |
| MCTDb | 7 |
| Scleroderma | 4 |
Total number of enrollees with a systemic autoimmune disease will be >118, due to individuals with >1 systemic autoimmune disease diagnosis: rheumatoid arthritis (RA) and Sjögren's syndrome (SS) (n = 4); systemic lupus erythematosus (SLE) and mixed connective tissue disease (MCTD) (n = 3); RA and MCTD (n = 2); RA and SLE (n = 2); SLE and SS (n = 1); RA and myositis (n = 1); RA and myositis, and MCTD (n = 1). PM = polymyositis; DM = dermatomyositis.
Diagnoses for 6 persons with MCTD, all of whom also had another systemic autoimmune disease, were made based on the opinion of the treating physician, though independent confirmation of classification criteria could not be made.
In both the responder group and the community member group, those who developed autoimmune disease included a higher percentage of females (48% of responders who developed autoimmune disease were female versus 21% of responders who did not, and 86% of community members who developed autoimmune disease were female versus 53% of community members who did not; P < 0.0001) and were on average slightly older (age 55 years versus 51 years and age 54 years versus 51 years; each P < 0.05) (Table 2). A higher proportion of community members with systemic autoimmune disease had PTSD than those in the comparison group (40% versus 15%; P < 0.0001). There were no significant differences in race/ethnicity, 9/11‐related exposures, or smoking history at the bivariate level.
Table 2.
Characteristics of the WTC Health Registry study population stratified by systemic autoimmune disease case statusa
| Responders | Community members | |||
|---|---|---|---|---|
| Comparison group (n = 17,284) | Systemic autoimmune disease cases (n = 62) | Comparison group (n = 19,733) | Systemic autoimmune disease cases (n = 56) | |
| Sex | ||||
| Female | 3,676 (21.3) | 30 (48.4)b | 10,384 (52.6) | 48 (85.7)b |
| Male | 13,608 (78.7) | 32 (51.6)b | 9,349 (47.4) | 8 (14.3)b |
| Age at wave 3, mean ± SD years | 51.3 ± 10.3 | 55.1 ± 9.5b | 51.1 ± 12.6 | 53.8 ± 10.1b |
| Race/ethnicity | ||||
| Non‐Latino white | 13,338 (77.2) | 45 (72.6) | 12,899 (65.4) | 32 (57.1) |
| Non‐Latino African Americanc | 1,133 (6.6) | – | 2,410 (12.2) | 11 (19.6) |
| Latinoc | 1,896 (11.0) | 8 (12.9) | 2,134 (10.8) | – |
| Asian or otherc | 917 (5.3) | – | 2,290 (11.6) | – |
| Educational attainment | ||||
| High school or less | 4,391 (25.6) | 21 (33.9) | 3,348 (17.1) | 13 (23.6) |
| Some college | 5,391 (31.4) | 22 (35.5) | 3,631 (18.6) | 13 (23.6) |
| College graduate and higher | 7,395 (43.1) | 19 (30.7) | 12,592 (64.3) | 29 (52.7) |
| PTSD | ||||
| Yes | 1,885 (11.0) | 11 (17.7) | 2,940 (15.3) | 22 (40.0)b |
| No | 15,299 (89.0) | 51 (82.3) | 16,268 (84.7) | 33 (60.0)b |
| Smoking history | ||||
| Ever | 7,332 (42.7) | 28 (45.2) | 8,006 (41.0) | 29 (52.7) |
| Never | 9,861 (57.4) | 34 (54.8) | 11,535 (59.0) | 26 (47.3) |
| 9/11‐related exposures | ||||
| Dust intensity | ||||
| Intense | 3,208 (19.6) | 15 (26.3) | 5,991 (33.9) | 23 (43.4) |
| None/some | 13,154 (80.4) | 42 (73.7) | 11,691 (66.1) | 30 (56.6) |
| Dust composite score | ||||
| High | 4,204 (25.1) | 15 (24.6) | 1,916 (9.8) | 6 (10.7) |
| Low | 12,539 (74.9) | 46 (75.4) | 17,683 (90.2) | 50 (89.3) |
| Worked at WTC site, median (IQR) months | 0.6 (0.2–1.7) | 0.8 (0.2–1.8) | – | – |
Except where indicated otherwise, values are the number (%). PTSD = posttraumatic stress disorder; WTC = World Trade Center; IQR = interquartile range.
P < 0.05 versus comparison group.
Data were missing for some subjects/parameters. Values shown are based on the totals with data available.
Our multivariable models revealed that women had a 3–5 times greater risk of systemic autoimmune disease compared with men (Tables 3 and 4). This result was consistent and significant in all models for both responders and community members. Older age, included as a continuous variable, was significant among responders. The adjusted risk ratios (RRs) for Non‐Latino African American enrollees and Latino enrollees were consistently elevated compared with Non‐Latino white enrollees, and the adjusted RRs for Asians and persons of other race/ethnicities were consistently lower compared with Non‐Latino white enrollees, though none of the differences reached statistical significance. Lower levels of educational attainment were associated with an increased risk of systemic autoimmune disease only among responders. Community members with a history of smoking had an elevated, though nonsignificant, risk of systemic autoimmune disease.
Table 3.
Risk of post‐9/11 systemic autoimmune disease among responders enrolled in the WTC Health Registrya
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| adjusted RR (95% CI) | adjusted RR (95% CI) | adjusted RR (95% CI) | adjusted RR (95% CI) | |
| 9/11‐related exposure | ||||
| Dust intensity | – | – | – | – |
| Intense | 1.86 (1.02–3.40)b | – | – | – |
| None/some | Referent | – | – | – |
| Dust composite score | ||||
| High | – | 1.86 (0.98–3.53) | – | – |
| Low | – | Referent | – | – |
| Months worked at WTC site (continuous) | – | – | 1.10 (0.97–1.24) | – |
| PTSD | ||||
| Yes | – | – | – | 1.42 (0.72–2.80) |
| No | – | – | – | Referent |
| Demographics | ||||
| Age at wave 3 (continuous) | 1.03 (1.01–1.06)b | 1.04 (1.01–1.06)b | 1.04 (1.01–1.06)b | 1.03 (1.01–1.06)b |
| Sex | ||||
| Female | 3.96 (2.34–6.70)b | 4.10 (2.39–7.06)b | 3.41 (2.04–5.73)b | 3.75 (2.25–6.25)b |
| Male | Referent | Referent | Referent | Referent |
| Race/ethnicity | ||||
| Non‐Latino white | Referent | Referent | Referent | Referent |
| Non‐Latino African American | 1.24 (0.49–3.13) | 1.70 (0.77–3.79) | 1.39 (0.59–3.29) | 1.39 (0.62–3.12) |
| Latino | 1.39 (0.65–2.99) | 1.37 (0.64–2.93) | 1.31 (0.61–2.83) | 1.07 (0.49–2.33) |
| Asian and other | 0.70 (0.17–2.91) | 0.68 (0.17–2.82) | 0.68 (0.17–2.82) | 0.70 (0.17–2.90) |
| Educational attainment | ||||
| High school or less | – | – | – | 2.21 (1.16–4.21)b |
| Some college | – | – | – | 1.91 (1.03–3.56)b |
| College graduate and higher | – | – | – | Referent |
| Smoking history | ||||
| Ever | 1.01 (0.59–1.70) | 1.03 (0.62–1.71) | 0.98 (0.59–1.65) | 0.98 (0.59–1.63) |
| Never | Referent | Referent | Referent | Referent |
WTC = World Trade Center; RR = risk ratio; 95% CI = 95% confidence interval; PTSD = posttraumatic stress disorder.
P < 0.05.
Table 4.
Risk of post‐9/11 systemic autoimmune disease among community members enrolled in the WTC Health Registrya
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| adjusted RR (95% CI) | adjusted RR (95% CI) | adjusted RR (95% CI) | |
| 9/11‐related exposures | |||
| Dust intensity | – | – | – |
| Intense | 1.50 (0.87–2.60) | – | – |
| None/some | Referent | – | – |
| Dust composite score | |||
| High | – | 1.06 (0.45–2.48) | – |
| Low | – | Referent | – |
| PTSD | |||
| Yes | – | – | 2.80 (1.60–4.90)b |
| No | – | – | Referent |
| Demographics | |||
| Age at wave 3 (continuous) | 1.02 (1.00–1.04) | 1.02 (1.00–1.04) | 1.01 (0.99–1.04) |
| Sex | |||
| Female | 5.04 (2.35–10.79)b | 5.18 (2.43–11.05)b | 4.69 (2.20–10.04)b |
| Male | Referent | Referent | Referent |
| Race/ethnicity | |||
| Non‐Latino white | Referent | Referent | Referent |
| Non‐Latino African American | 1.18 (0.55–2.50) | 1.36 (0.68–2.73) | 1.12 (0.55–2.30) |
| Latino | 1.44 (0.66–3.15) | 1.52 (0.73–3.19) | 1.15 (0.53–2.49) |
| Asian and other | 0.61 (0.19–2.01) | 0.57 (0.17–1.87) | 0.56 (0.17–1.83) |
| Educational attainment | |||
| High school or less | – | – | 1.23 (0.62–2.45) |
| Some college | – | – | 1.08 (0.55–2.12) |
| College graduate and higher | – | – | Referent |
| Smoking history | |||
| Ever | 1.47 (0.84–2.56) | 1.56 (0.90–2.68) | 1.43 (0.83–2.46) |
| Never | Referent | Referent | Referent |
WTC = World Trade Center; RR = risk ratio; 95% CI = 95% confidence interval; PTSD = posttraumatic stress disorder.
P < 0.05.
Among responders, those who experienced intense dust cloud exposure were at increased risk of systemic autoimmune disease (adjusted RR 1.86 [95% CI 1.02–3.40]). Though the adjusted RRs for dust composite score, PTSD, and for each month worked at the WTC site were elevated, none were statistically significant (Table 3).
Among community members, neither dust cloud intensity nor dust composite score was significantly associated with systemic autoimmune disease (Table 4). However, PTSD was associated with a nearly 3‐fold increased risk of systemic autoimmune disease (adjusted RR 2.80 [95% CI 1.60–4.90]).
The results of our sensitivity analyses showed that, when cases were limited to those who met classification criteria (35 responders and 31 community members), there were associations, though not significant, between 9/11 exposures and systemic autoimmune disease among responders. Intense dust cloud exposure and PTSD were both associated with a statistically significant increased risk (>2‐fold) for systemic autoimmune disease among community members. When our analysis was restricted to cases diagnosed in 2005 or later, the initial analysis was unchanged, though the magnitude of the association between PTSD and systemic autoimmune disease among community members increased (Table 5).
Table 5.
Results of sensitivity analyses of risk of systemic autoimmune disease among WTC Health Registry enrolleesa
| Cases that met classification criteria, adjusted RR (95% CI) | Cases diagnosed in 2005 or later, adjusted RR (95% CI) | RA only, adjusted RR (95% CI) | With PTSD, adjusted RR (95% CI) | Without PTSD, adjusted RR (95% CI) | |
|---|---|---|---|---|---|
| Responders | |||||
| Dust intensity | |||||
| Intense | 1.57 (0.66–3.69) | 1.85 (0.99–3.45) | 1.84 (0.85–3.99) | 1.28 (0.37–4.42) | 1.88 (0.94–3.75) |
| None/some | Referent | Referent | Referent | Referent | Referent |
| Dust composite score | |||||
| High | 2.03 (0.84‐4.93) | 1.82 (0.94‐3.53) | 1.81 (0.79‐4.11) | 0.60 (0.12‐3.07) | 2.28 (1.13‐4.61)b |
| Low | Referent | Referent | Referent | Referent | Referent |
| Months worked at WTC site (continuous) | 1.03 (0.86–1.23) | 1.10 (0.98–1.25) | 0.99 (0.83–1.18) | 0.95 (0.73–1.26) | 1.13 (0.99–1.29) |
| PTSD | |||||
| Yes | 1.84 (0.77–4.38) | 1.39 (0.68–2.82) | 1.71 (0.76–3.85) | – | – |
| No | Referent | Referent | Referent | – | – |
| Community members | |||||
| Dust intensity | |||||
| Intense | 2.12 (1.03–4.35)b | 1.45 (0.81–2.61) | 1.33 (0.66–2.69) | 0.46 (0.18–1.21) | 2.40 (1.21–4.75)b |
| None/some | Referent | Referent | Referent | Referent | Referent |
| Dust composite score | |||||
| High | 0.96 (0.29–3.17) | 1.00 (0.39–2.53) | 0.55 (0.13–2.31) | 0.46 (0.06–3.43) | 1.51 (0.58–3.93) |
| Low | Referent | Referent | Referent | Referent | Referent |
| PTSD | |||||
| Yes | 2.63 (1.24–5.56)b | 3.40 (1.90–6.10)b | 3.94 (1.92–8.07)b | – | – |
| No | Referent | Referent | Referent | – | – |
All models adjusted for age, sex, race/ethnicity, and smoking history. Models with posttraumatic stress disorder (PTSD) were also adjusted for educational attainment. Every 9/11 exposure or characteristic received its own model. WTC = World Trade Center; RR = risk ratio; 95% CI = 95% confidence interval; RA = rheumatoid arthritis.
P = < 0.05.
The results of our analysis examining only RA as an outcome among responders were similar in magnitude to the findings in our analysis of those with the combined outcome, though the association with dust intensity lost significance. Among community members, neither dust exposure variable was significant. However, we observed an elevated association between PTSD and RA (adjusted RR 3.94 [95% CI 1.92–8.07]) (Table 5).
After stratifying by PTSD status, we observed that among responders without PTSD, the association between dust composite score and systemic autoimmune disease became statistically significant and increased in magnitude (adjusted RR 2.28 [95% CI 1.13–4.61]) compared with the main results. There were associations, though not significant, between intense dust exposure and systemic autoimmune disease among responders regardless of PTSD status. We also observed effect modification between intense dust cloud exposure and PTSD among community members, with an elevated and significant adjusted RR among those without PTSD and a nonsignificant adjusted RR of <1 among those with PTSD. There was a similar pattern for the dust composite score, though neither association was significant.
Discussion
Intense dust cloud exposure and 9/11‐related PTSD were associated with a greater risk of systemic autoimmune disease among Registry enrollees. Among responders, the present analysis found that intense dust cloud exposure was associated with a higher risk of systemic autoimmune disease. Among community members, there was a strong association between PTSD and new‐onset systemic autoimmune disease. The responder group and community member group had inherently different demographic compositions and different types of 9/11 exposures 9. In the responder group, 79% were male, and only 47% were male in the community member group. Since autoimmune diseases are generally more prevalent among women 37, 38, 39, it is important to identify an elevated risk of autoimmune diseases among mostly male responders.
Our results are similar to 2 FDNY studies of predominantly male firefighters, which showed that increased duration (number of months) and levels of WTC rescue and recovery work were linked to increased odds and incidence of systemic autoimmune disease, respectively 22, 23. FDNY researchers found that each additional month worked at the WTC site yielded a 13% increase in the odds of developing a systemic autoimmune disease 23. In our analysis of responders, there was a 10% increased risk of systemic autoimmune disease for each month worked at the WTC site, though this result was not significant. All those who responded to the 9/11 attack were involved in rescue, recovery, and/or clean‐up work. Some responders arrived at the site on 9/11 and may have been caught in the cloud of dust and debris, while others arrived days or weeks later and may have been exposed to resuspended 9/11 dust as they sifted through or moved debris 1. Responders were not always able to wear appropriate respiratory protection 40, which may have resulted in increased inhalation of fine particulate matter, crystalline silica, asbestos, and organic hydrocarbon solvents 1, 9, all of which have a documented association with autoimmune disease 2, 3, 4, 5, 6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17.
Our analysis used multiple measures of exposure, and mixed results were obtained. Duration of work at the WTC site and an exposure index comprising a dust composite score both had an elevated association, though not statistically significant, with systemic autoimmune disease. However, intense dust cloud exposure was significantly associated with new‐onset systemic autoimmune disease among responders. Those who experienced intense dust cloud exposure were also likely exposed to many of the other traumatic events the morning of 9/11, and the definition of intense dust cloud exposure relies on subjective measures that may be associated with trauma or fear (e.g., inability to see or hear, needing to take cover). Given the significant association between PTSD and systemic autoimmune disease identified in this study (possible association between 9/11 exposures and PTSD, and the strong association between dust exposure and systemic autoimmune disease among those without PTSD), the potential mediating or modifying role that PTSD may have had in the development of autoimmune diseases due to 9/11 exposure cannot be discounted. Prior studies conducted among US veterans of the wars in Vietnam, Iraq, and Afghanistan have identified temporal associations between PTSD and subsequent autoimmune diseases 19, 20, as has an analysis conducted among women enrolled in the Nurses’ Health Study II 21. Future analyses should examine whether PTSD or other mental health conditions play a mediating role in the association between 9/11 exposure and subsequent autoimmune disease.
Autoimmune disorders result from interactions between the environment, genetics, and the immune system. Inhalation of dust containing crystalline silica, solvents, diesel exhaust, particulate air pollution, and cigarette smoke have been hypothesized to induce systemic autoimmune diseases via inflammatory pathways, dysregulation of the immune response, and increased peptide citrullination 41, 42, 43, 44, 45, 46. Environmental risk factors have been associated with many epigenetic DNA methylation changes 47. Evidence from animal studies and other experimental research suggests that reduced methylation is present in immune cell types in persons with SLE and in those with other autoimmune disease, including reduced methylation of the X chromosome, resulting in overexpression of genes specifically among women and increased susceptibility to these diseases 47. Differences in methylation patterns have also been associated with disease severity among persons with SLE 48.
This study has several limitations. Informed consent for medical record release was not obtained from approximately half of respondents who completed the enrollee survey. Enrollees who provided informed consent to review their medical records were more likely to be male and a 9/11 responder compared with those who did not provide consent (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41175/abstract). Given the higher proportion of women who developed autoimmune disease compared with men, a number of cases may have been undetected. Because women in our population were less likely to have experienced high levels of 9/11 exposure, differential availability of data between men and women may have inflated our results. However, after stratifying by sex, the association between 9/11 exposures and autoimmune disease was qualitatively similar in men and women (data not shown). In addition, among responders who were mostly male, there was no difference in the proportion exposed to intense dust among those who provided informed consent and those who did not. This further suggests that selection bias did not greatly impact our results. Enrollees who provided informed consent were more likely to have screened positive for autoimmune disease on the CSQ (73% versus 63%; P < 0.0001) (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41175/abstract). However, given that there were no significant differences in exposure or PTSD among those who provided informed consent and those who did not, this did not likely bias our results.
Another limitation of the study is that due to the different potential 9/11 exposures experienced by responders and community members, we were unable to compare the dust composite scores between the 2 groups. Additionally, while the index for responders relies on a score that was divided into quartiles, the index for community members is based on an ordinal hierarchy of what are likely more intense/chronic dust exposures, which in turn are based on whether a community member was a resident, area worker, passerby, or school student/staff. Therefore, the magnitude of differences between categories are qualitative rather than quantitative. Additionally, the prior occupational exposure history for our responders was not collected, and therefore we were unable to control for potential pre‐9/11 exposures to crystalline silica or other components.
Next, due to resource constraints, medical record reviews to confirm the absence of autoimmune disease among our control population were not conducted. However, due to the rarity of autoimmune diseases, it is likely that the number of false negatives in our comparison population is small, and any misclassification among the comparisons would likely have little effect on our findings.
Finally, our study sample included enrollees who completed a wave 3 survey in 2011–2012; therefore, we likely missed autoimmune disease cases among enrollees who did not complete the wave 3 survey, whether due to death, illness, or lack of participation for other reasons. A smaller proportion of wave 3 survey participants had PTSD at baseline compared with those who did not participate (15% versus 19%; P < 0.0001). However, it does not appear that persons with PTSD were more likely to overreport or underreport a diagnosis compared with those without PTSD, as there was no significant difference in the likelihood that a self‐reported diagnosis would be verified as a case based on PTSD status at wave 1 (P = 0.51). Those with PTSD also comprised a similar proportion of both self‐reported cases and verified cases (31% and 28%). A prior evaluation of Registry nonresponse bias found that there was no association between wave 3 survey participation status and either chronic health at wave 1 or having been caught in a dust cloud on 9/11 49.
Numerous studies, including this one, have found that autoimmune diseases are often overreported by study participants. For example, the positive predictive value of self‐reported RA is low, ranging between 21% and 34% 50, 51, 52. In 2 large population‐based studies that investigated risk factors for RA (the Nurse's Health Study and the Iowa Women's Health Study), only 6–7% of self‐reported RA diagnoses could be confirmed by medical record review 53, 54.
To address the issue of overreporting, only study participants with autoimmune disease verified by a physician or by medical record review were considered. Of the 2,161 enrollees who reported an RA diagnosis after 9/11 on their wave 3 survey, 71 cases (3%) were confirmed. When limited to those who provided permission to have their medical records reviewed (n = 558), our validation rate for enrollees with RA rose to 13%. The reasons for these low rates may include confusion over whether their disease is autoimmune in nature (e.g., mistaking osteoarthritis for RA), having autoimmune‐like symptoms but no physician diagnosis, and lack of access to a rheumatologist. The difficulty in diagnosing an autoimmune disease and length of time between symptom onset and diagnosis add to this complexity 55.
In addition to using medically verified diagnoses and classification criteria, strengths of this study included a high response rate to the enrollee survey and the physician survey, a relatively large sample size, and prospective design. Our study identified similar associations, though not significant, between 9/11 exposures and systemic autoimmune disease in enrollees who met classification criteria, which strengthens our findings and highlights the importance of using rigorous diagnostic criteria. The similar findings of our sensitivity analysis limited to cases diagnosed in 2005 or later also helped confirm the main results of this study.
Systemic autoimmune diseases are associated with exposure to 9/11‐related dust and debris and PTSD. Given that they are difficult to diagnose, it is not surprising that a pattern of increased risk by level of 9/11 exposure has only emerged in recent years. It also demonstrates the need to monitor the health of populations affected by a disaster over the long term.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Ms Miller‐Archie had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Miller‐Archie, Izmirly, Walker, Dasilva, Petrsoric, Cone.
Acquisition of data
Miller‐Archie, Izmirly, Walker, Dasilva, Petrsoric, Cone.
Analysis and interpretation of data
Miller‐Archie, Izmirly, Berman, Brite, Walker, Cone.
Supporting information
Acknowledgments
We thank the WTC Health Registry enrollees and the physicians who participated in this study. In addition, we would like to thank those who contributed to this study's success including Hilary Parton, Mark Farfel, Robert Brackbill, Monique Fairclough, Melanie Jacobson, Jiehui Li, Cassandra Stanton, Sherwet Rashed, Alice Welch, Hannah Jordan, Donna Eisenhower, Michael Sanderson, Ingrid Edshteyn, Sukhminder Osahan, Mayris Webber, David Prezant, Nadia Jaber, Curtis Noonan, Jean Pfau, and Pui Ying Chan for content, methodologic, and analytic expertise; Jorge Guengue, David Wu, Sameh Sertial, and Sherry Li for data management, web services, and IT systems design; Lennon Turner, Felix Ortega, Saimone Walker, Talytha Utley, Raymond Jimenez, Tanya Fareira, Carlos Espada, Lucius Yao, Danielle Covarrubias, Sean Locke, Ho Ki Mok, Lydia Leon, and Lisa Ianotto for participant outreach, survey intake and review, and vendor management; and Charon Gwynn, James Hadler, Sharon Perlman, and Sandhya George for manuscript review.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention (CDC), or the Department of Health and Human Services.
Supported by the National Institute for Occupational Safety and Health of the CDC (cooperative agreements 2U50/OH009739 and 5U50/OH009739), the Agency for Toxic Substances and Disease Registry, CDC (cooperative agreement U50/ATU272750, which included support from the National Center for Environmental Health, CDC), and the New York City Department of Health and Mental Hygiene.
1Sara A. Miller‐Archie, MPH, Jennifer Brite, DrPH, Deborah J. Walker, PhD, Renato C. Dasilva, MPA, Lysa J. Petrsoric, MPH, LMSW, James E. Cone, MD, MPH: New York City Department of Health and Mental Hygiene, New York, New York; 2Peter M. Izmirly, MD: New York University School of Medicine, New York, New York; 3Jessica R. Berman, MD: Hospital for Special Surgery and Weill Cornell Medical College, New York, New York.
No potential conflicts of interest relevant to this article were reported.
References
- 1. Lioy PJ, Weisel CP, Millette JR, Eisenreich S, Vallero D, Offenberg J, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect 2002;110:703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol 2016;28:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller FW, Pollard KM, Parks CG, Germolec DR, Leung PS, Selmi C, et al. Criteria for environmentally associated autoimmune diseases. J Autoimmun 2012;39:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finckh A, Cooper GS, Chibnik LB, Costenbader KH, Watts J, Pankey H, et al. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis Rheum 2006;54:3648–54. [DOI] [PubMed] [Google Scholar]
- 5. Stolt P, Källberg H, Lundberg I, Sjögren B, Klareskog L, Alfredsson L, et al. Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis 2005;64:582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parks CG, Cooper GS, Nylander‐French LA, Sanderson WT, Dement JM, Cohen PL, et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population‐based, case–control study in the southeastern United States. Arthritis Rheum 2002;46:1840–50. [DOI] [PubMed] [Google Scholar]
- 7. Haustein U, Ziegler V, Hermann K, Mehlhorn J, Schmidt C. Silica‐induced scleroderma. J Am Acad Dermatol 1990;22:444–8. [DOI] [PubMed] [Google Scholar]
- 8. Ilar A, Klareskog L, Saevarsdottir S, Wiebert P, Askling J, Gustavsson P, et al. Occupational exposure to asbestos and silica and risk of developing rheumatoid arthritis: findings from a Swedish population‐based case‐control study. RMD Open 2019;5:e000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landrigan PJ, Lioy PJ, Thurston G, Berkowitz G, Chen LC, Chillrud SN, et al. Health and environmental consequences of the world trade center disaster. Environ Health Perspect 2004;112:731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pleil JD, Vette AF, Johnson BA, Rappaport SM. Air levels of carcinogenic polycyclic aromatic hydrocarbons after the World Trade Center disaster. Proc Natl Acad Sci U S A 2004;101:11685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barragán‐Martínez C, Speck‐Hernández CA, Montoya‐Ortiz G, Mantilla RD, Anaya JM, Rojas‐Villarraga A. Organic solvents as risk factor for autoimmune diseases: a systematic review and meta‐analysis. PLoS One 2012;7:e51506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nietert PJ, Sutherland SE, Silver RM, Pandey JP, Knapp RG, Hoel DG, et al. Is occupational organic solvent exposure a risk factor for scleroderma? Arthritis Rheum 1998;41:1111–8. [DOI] [PubMed] [Google Scholar]
- 13. Diot E, Lesire V, Guilmot J, Metzger M, Pilore R, Rogier S, et al. Systemic sclerosis and occupational risk factors: a case‐control study. Occup Environ Med 2002;59:545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernatsky S, Smargiassi A, Johnson M, Kaplan GG, Barnabe C, Svenson L, et al. Fine particulate air pollution, nitrogen dioxide, and systemic autoimmune rheumatic disease in Calgary, Alberta. Environ Res 2015;140:474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hart JE, Laden F, Puett R, Costenbader KH, Karlson EW. Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ Health Perspect 2009;117:1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Roos AJ, Koehoorn M, Tamburic L, Davies HW, Brauer M. Proximity to traffic, ambient air pollution, and community noise in relation to incident rheumatoid arthritis. Environ Health Perspect 2014;122:1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noonan CW, Pfau JC, Larson TC, Spence MR. Nested case–control study of autoimmune disease in an asbestos‐exposed population. Environ Health Perspect 2006;114:1243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farfel M, DiGrande L, Brackbill R, Prann A, Cone J, Friedman S, et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health 2008;85:880–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boscarino JA, Forsberg C, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med 2010;72:481–6. [DOI] [PubMed] [Google Scholar]
- 20. O'Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry 2015;77:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee YC, Agnew‐Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, et al. Post‐traumatic stress disorder and risk of incident rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Webber MP, Moir W, Crowson CS, Cohen HW, Zeig‐Owens R, Hall CB, et al. Post‐September 11, 2001, incidence of systemic autoimmune diseases in World Trade Center‐exposed firefighters and emergency medical service workers. Mayo Clin Proc 2016;91:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Webber MP, Moir W, Zeig‐Owens R, Glaser MS, Jaber N, Hall C, et al. Nested case–control study of selected systemic autoimmune diseases in World Trade Center rescue/recovery workers. Arthritis Rheumatol 2015;67:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy J, Brackbill RM, Thalji L, Dolan M, Pulliam P, Walker DJ. Measuring and maximizing coverage in the World Trade Center Health Registry. Stat Med 2007;26:1688–701. [DOI] [PubMed] [Google Scholar]
- 25. Brackbill R, DiGrande L, Perrin M, Walker D, Wu D, Pulliam P, et al. New York City Department of Health and Mental Hygiene Agency for Toxic Substances and Disease Registry: World Trade Center Health Registry: data file user's manual. New York: RTI International, New York City Department of Health and Mental Hygiene, and the Agency for Toxic Substances and Disease Registry; 2006. URL: https://www1.nyc.gov/assets/911health/downloads/pdf/wtc/wtc-datafile-manual.pdf. [Google Scholar]
- 26. Karlson EW, Sanchez‐Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5:297–302. [DOI] [PubMed] [Google Scholar]
- 27. Karlson EW, Costenbader KH, McAlindon TE, Massarotti EM, Fitzgerald LM, Jajoo R, et al. High sensitivity, specificity, and predictive value of the Connective Tissue Disease Screening Questionnaire among urban African‐American women. Lupus 2005;14:832–6. [DOI] [PubMed] [Google Scholar]
- 28. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 29. Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 30. Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- 32. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. [DOI] [PubMed] [Google Scholar]
- 33. Bennett R. Overlap syndromes In: Firestein GS, Budd RC, Harris ED, Jr, McInnes IB, Ruddy S, Sergent JS, editors. Kelley's textbook of rheumatology. 8th ed Philadelphia: Saunders; 2008. [Google Scholar]
- 34. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Brackbill RM, Liao TS, Qiao B, Cone JE, Farfel MR, et al. Ten‐year cancer incidence in rescue/recovery workers and civilians exposed to the September 11, 2001 terrorist attacks on the World Trade Center. Am J Ind Med 2016;59:709–21. [DOI] [PubMed] [Google Scholar]
- 36. Blanchard EB, Jones‐Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther 1996;34:669–73. [DOI] [PubMed] [Google Scholar]
- 37. Izmirly P, Buyon J, Wan I, Belmont HM, Sahl S, Salmon JE, et al. The incidence and prevalence of adult primary Sjӧgren's syndrome in New York County. Arthritis Care Res (Hoboken) 2019;71:949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Izmirly PM, Wan I, Sahl S, Puyon JP, Belmont HM, Salmon JE, et al. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York: the Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017;69:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davidson A, Diamond B. General features of autoimmune disease In: Rose NR, Mackay IR, editors. The autoimmune diseases. 5th ed San Diego: Elsevier; 2014. [Google Scholar]
- 40. Antao VC, Pallos LL, Shim YK, Sapp JH II, Brackbill RM, Cone JE, et al. Respiratory protective equipment, mask use, and respiratory outcomes among World Trade Center rescue and recovery workers. Am J Ind Med 2011;54:897–905. [DOI] [PubMed] [Google Scholar]
- 41. Pollard KM, Christy JM, Cauvi DM, Kono DH. Environmental xenobiotic exposure and autoimmunity. Curr Opin Toxicol 2018;10:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Povey A, Guppy MJ, Wood M, Knight C, Black CM, Silman AJ. Cytochrome P2 polymorphisms and susceptibility to scleroderma following exposure to organic solvents. Arthritis Rheum 2001;44:662–5. [DOI] [PubMed] [Google Scholar]
- 43. Essouma M, Noubiap JJ. Is air pollution a risk factor for rheumatoid arthritis? [review]. J Inflamm (Lond) 2015;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mastrofrancesco A, Alfè M, Rosato E, Gargiulo V, Beatrice C, Di Blasio G, et al. Proinflammatory effects of diesel exhaust nanoparticles on scleroderma skin cells. J Immunol Res 2014;2014:138751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yin G, Wang Y, Cen XM, Yang M, Liang Y, Xie QB. Lipid peroxidation‐mediated inflammation promotes cell apoptosis through activation of NF‐κB pathway in rheumatoid arthritis synovial cells. Mediators Inflamm 2015;2015:460310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus 2006;15:737–45. [DOI] [PubMed] [Google Scholar]
- 47. Carnero‐Montoro E, Alarcón‐Riquelme ME. Epigenome‐wide association studies for systemic autoimmune diseases: the road behind and the road ahead. Clin Immunol 2018;196:21–33. [DOI] [PubMed] [Google Scholar]
- 48. Lanata CM, Paranjpe I, Nititham J, Taylor KE, Gianfrancesco M, Paranjpe M, et al. A phenotypic and genomics approach in a multi‐ethnic cohort to subtype systemic lupus erythematosus. Nat Commun 2019;10:3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu S, Brackbill RM, Stellman SD, Ghuman S, Farfel M. Evaluation of non‐response bias in a cohort study of World Trade Center terrorist attack survivors. BMC Res Notes 2015;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kvien TK, Glennås A, Knudsrød OG, Smedstad LM. The validity of self‐reported diagnosis of rheumatoid arthritis: results from a population survey followed by clinical examinations. J Rheumatol 1996;23:1866–71. [PubMed] [Google Scholar]
- 51. Ling S, Fried LP, Garrett E, Hirsch R, Guralanik JM, Hochberg MC. The accuracy of self‐report of physician diagnosed rheumatoid arthritis in moderately to severely disabled older women. J Rheumatol 2000;27:1390–4. [PubMed] [Google Scholar]
- 52. Star VL, Scott JC, Sherwin R, Lane N, Nevitt MC, Hochberg MC. Validity of self‐reported rheumatoid arthritis in elderly women. J Rheumatol 1996;23:1862–5. [PubMed] [Google Scholar]
- 53. Karlson E, Mandl LA, Hankinson SE, Grodstein F. Do breast‐feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum 2004;50:3458–67. [DOI] [PubMed] [Google Scholar]
- 54. Mikuls TR, Cerhan JR, Criswell LA, Merlino L, Mudano AS, Burma M, et al. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 2002;46:83–91. [DOI] [PubMed] [Google Scholar]
- 55. Rose NR, Mackay IR. Autoimmune disease: the consequence of disturbed homeostasis In: Mackay I, Rose NR, editors. The autoimmune diseases. 5th ed San Diego: Elsevier; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


