Abstract
Objective
As part of the value‐based healthcare programme in our hospital, a set of patient‐reported outcome measures was developed together with patients and implemented in the dedicated Turner Syndrome (TS) outpatient clinic. This study aims to investigate different aspects of health‐related quality of life (HR‐QoL) and psychosocial functioning in women with TS in order to establish new possible targets for therapy.
Design/Participants
A comprehensive set of questionnaires (EQ‐5D, PSS‐10, CIS‐20, Ferti‐QoL, FSFI) was developed and used to capture different aspects of HR‐QoL and psychosocial functioning in a large cohort of adult women with Turner syndrome. All consecutive women, ≥18 years, who visited the outpatient clinic of our tertiary centre were eligible for inclusion.
Results
Of the eligible 201 women who were invited to participate, 177 women (age 34 ± 12 years, mean ± SD) completed at least one of the validated questionnaires (88%). Women with TS reported a lower health‐related quality of life (EQ‐5D: 0.857 vs 0.892, P = .003), perceived more stress (PSS‐10:14.7 vs 13.3; P = .012) and experienced increased fatigue (CIS‐20: P < .001) compared to the general Dutch population. A relationship between noncardiac comorbidities (eg diabetes, orthopaedic complaints) and HR‐QoL was found (R = .508).
Conclusions
We showed that TS women suffer from impaired HR‐QoL, more perceived stress and increased fatigue compared to healthy controls. A relationship between noncardiac comorbidities and HR‐QoL was found. Especially perceived stress and increased fatigue can be considered targets for improvement of HR‐QoL in TS women.
Keywords: congenital heart defects, hypogonadism‐stress, patient‐reported outcome measures, physiological‐fatigue, quality of life, Turner syndrome
1. INTRODUCTION
Turner syndrome (TS), first described by Henry Turner in 1938, is caused by a partial or complete monosomy of the X‐chromosome and occurs in 1 per 2500 live born females.1, 2 Classic characteristics of TS include: short stature, webbing of the neck, oestrogen deficiency and decreased fertility.3, 4, 5 Moreover, significant morbidity in these patients is caused by the cardiovascular abnormalities of heart and thoracic vessels, which affect an estimated 50% of women with TS.6 Of these congenital defects the bicuspid aortic valve (BAV: 15%‐30%), coarctation of the aorta (CoA 12%‐17%) and partial anomalous pulmonary venous return (PAPVR: 18%‐25%) are most prevalent.2, 6, 7, 8 Additionally diabetes, obesity and hypertension are often seen in these women, adding to their cardiovascular risk.9 Although cardiovascular and other physical morbidities of women with TS have already been described in detail, literature is still inconclusive or contradictory on the impact of TS on HR‐QoL.10 In addition to the physical factors, HR‐QoL in these women is also affected by the mild neuropsychological deficits these patients may encounter, such as the relative weakness in visual‐spatial, executive and social cognitive domains.11, 12, 13 Additionally, other factors such as lower socioeconomic status or impaired ability to work may affect QoL. Finally, fertility issues may also have impact on QoL in TS women. Since the phenotype is so multi‐dimensional and heterogeneous, traditional psychometric tools, such as the SF‐36, cannot completely capture the broad range of psychosocial problems in these patients.14 Moreover, the importance of psychometrically sound disease‐specific tools has been stressed in other chronic illnesses, where more generic tools often fail to capture loss of quality of life on disease‐specific domains.15 In this study, we describe the development and first results of a set of clinician and patient‐reported outcome set in Turner syndrome as part of the value‐based healthcare programmes in our hospital.16 We aim to provide a comprehensive evaluation of HR‐QoL and different domains of psychosocial functioning in women with TS in order to identify individual and disease‐specific targets for effective improvement of their HR‐QoL.
2. METHODS
2.1. Development of the outcome set
For the development of such a set, out organization works according to a so called blueprint which is adapted to the specific situation of a disease team. This way of working is described in detail elsewhere.16 In brief, a disease team is formed consisting of the most important disciplines involved in the treatment, in this case the departments of internal medicine, gynaecology and cardiology. A representation of patients is invite to participate in this process. In our situation, we had a 24‐year‐old patient and the mother of a patient being also member of the national patient organization; they are volunteers for the Turner patients federation and have been in contact with and of support to many Turner women. With this group and technically supervised by a value‐based healthcare expert, the team discussed in four sessions the following items: current care path, possible outcome measures from literature and complemented with topics from the patients that really matter to them. Consensus based this long list of outcome topics was decreased to a more discrete based on frequency and impact. Finally, the outcome topics were translated into validated outcome measures and built into our data capture tool in order to measure them electronically. Before every outpatient clinic visit, patients receive an email with a web link to the questions, so they were able to answer them at home before the visit. During the visit, the result was discussed with them. Our tertiary care centre has developed a 5‐year VBHC‐strategy to transform the institute into a true value innovator. An integrated, multidisciplinary Turner syndrome‐care unit was already in place within our institute. The Turner team is composed of a cardiologist, endocrinologist, gynaecologist, physiotherapist, psychologist and ENT specialist, and other specialists are consulted upon indication.
For women with Turner syndrome, navigating the complex healthcare pathway involving many specialties can be daunting. Therefore, this pathway was redesigned analogous to the breast cancer care pathway described earlier (Ref 16). A critical step within VBHC is defining an outcomes set, and this was done with the described multidisciplinary team. After both a literature survey and from experience, all participants worked on a long list of outcome domains and ranked based on prevalence, impact for the patient and relevance for quality of care. For sake of feasibility, the final list was discussed and selected using a modified Delphi method, see figure. The PROMS selected are described below under ‘instruments’ and in more detail in Appendix S1.
As described earlier, PROMS in the VBHC‐pathways in our institution are captured using an in‐house developed open source electronical data collection tool, which allows the construction of data collection‐forms and automatic distribution of PROMs (Ref. 16). Emails are sent to the patients in order to activate the distribution of PROMs. After the right treatment, pathway is selected by the physician, and all the following PROMs will be sent automatically by the tool at the right time‐point. The tool was linked to the EHR enabling the review of the collected data for individual patients at the (outpatient) clinic. The secure platform is build‐up by two software programs, LimeSurvey17 and GemsTracker.18 The development team simultaneously developed a user‐friendly interface to display the collected data. Longitudinal PRO data and data from the caregivers are collected in these pathways.
2.2. Setting and study design
All patients in our centre of expertise are seen according to a new value‐based healthcare clinical pathway which includes annual visits to the dedicated Turner outpatient clinic.5, 19 Adult women with Turner syndrome who visited the outpatient clinic between December 2015 and October 2018 were prospectively included. Cardiovascular examination was performed including electrocardiography (ECG) and transthoracic echocardiogram (TTE) and advanced cardiac imaging (CT or CMR). Karyotype was taken from the clinical genetics report. The study complied with the Declaration of Helsinki and was approved by the medical ethical committee of the Erasmus Medical Centre. Written informed consent was waived.
2.3. Instruments
As a part of a hospital wide value‐based healthcare programme patients were asked to complete 12 questionnaires: 5 standardized questionnaires: the EuroQol‐5D (EQ‐5D), Hospital Anxiety and Depression Scale (HADS), Checklist Individual Strength (CIS‐20), Perceived Stress Scale (PSS‐10), Fertility Quality of Life (Ferti‐QoL), 4 questionnaires on sexual functioning (sexual activity question, brief sexual symptom checklist for women (BSSC‐W), female sexual functioning index (FSFI) and the female sexual distress scale‐revised (FSFD‐R)) and 3 questionnaires on body image and the perceived burden questionnaires.20, 21, 22 The questionnaires have been described in more detail in supplementary methods. The sexuality questionnaires were offered to the women with TS aged 20 and 45 years because sexuality starts later in women with TS 23, 24, 25, 26 and end earlier because of hormonal changes.
2.4. Statistics
Continuous variables were presented as mean ± standard deviation (SD) or as median with an interquartile range. Categorical variables were presented as frequencies and percentages. We examined distributions by visually assessing histograms of the data by calculating Z‐values of skewness and kurtosis, and by tested for normality using the Shapiro‐Wilk test. For comparison of normally distributed continuous variables between two groups, the Student's t test was used. Univariate linear regression analysis was performed. Subsequently, multivariate linear regression analysis was performed to identify patient characteristics that were significantly associated with different measures of HR‐QoL or other psychosocial outcomes. In case of collinearity, we entered the variable with the strongest correlation with the outcome into the multivariate linear regression analysis. As previously described the tri‐modal distribution of the EQ‐5D outcome measure hampers ordinary least‐squares (OLS) regression, three‐part regression methods seem to have better prediction power than OLS with EQ‐5D data, although OLS seems quite robust.27 Therefore, we conducted separate linear regression for the .5‐.99 range as an additional ‘sensitivity analysis’. The statistical tests were two sided, and a P‐value below .05 was considered significant. The IBM spss ® statistics 21.0 software was used to analyse the data.
3. RESULTS
The questionnaires were offered to all 201 women with TS who visited our outpatient clinic between December 2015 and September 2018, of which 177 women completed at least one of the questionnaires (response rate: 88%) and were included in this study. Baseline characteristics of the 177 women are presented in Table 1. The mean time to complete the questionnaires was 15 minutes. All women seen in our outpatient clinic between the age of 18 and 52 use HRT.
Table 1.
Baseline characteristics
| Baseline characteristics |
Women with TS Median [IQR] |
|---|---|
| Age, y | 33 [18] |
| Height, cm | 157 [11] |
| Weight, kg | 64 [19] |
| Systolic BP, mm Hg | 125 [21] |
| Diastolic BP, mm Hg | 76 [17] |
| Body surface area, m2 | 1.65 [0.22] |
| Body mass index, kg/m2 | 25.4 [6.4] |
| Sinus rhythm, n = 167 | 167 (100) |
| Heart rate, bpm | 75 (15) |
| Karyotype | 171 (97) |
| Monosomy X | 93 (52.5) |
| Mosaic | 25 (14) |
| Isochromosomes | 25 (14) |
| Deletions | 6 (3.4) |
| Polyploidy | 9 (5.1) |
| Ring chromosomes | 5 (2.8) |
| Y material | 8 (4.5) |
Continuous data are presented as mean ± SD. Categorical data are presented as n (%).
3.1. Demographics and morbidity
Many women indicated living with a partner (Table 2; n = 50, 28%). Of the women living with parents or family (n = 51), 15 (29%) were older than 25 years of age. In total, 29 women (16%) raised a total of 30 children. The highest level of educational attainment was known in 123 (69%) participants; most of who had finished intermediate vocational education in 51 (29%). Women were often employed, either fulltime (n = 23, 13%) or part‐time (n = 26, 20%). Of the 90 participants, less than half indicated to practice sports regularly, on average 3 hours (median, IQR 3 hours) per week. Noticeably, all but 1 participant practiced individual sports. Data on morbidity is presented in Table 3. Most frequently encountered problems included: ear and/or hearing problems (n = 57; 32%) and cardiovascular disease (n = 56; 32%). In the 56 women with cardiac disease, several different structural heart defects were identified, of which a bicuspid aortic valve (BAV) was the most frequent (19%). Aortic dilatation was found in 10 patients (6%), and 1 patient had suffered from dissection (0.6%).
Table 2.
Demographics
| Demographics (N = 177) | n | % |
|---|---|---|
| Living situation | 174 | 98 |
| With a partner | 50 | 29 |
| Alone | 47 | 27 |
| Living with parents | 44 | 25 |
| Family | 7 | 4 |
| Friends | 2 | 1 |
| Children | 30 | 16 |
| Biological children | 12 | 40 |
| Foster or adopted children | 11 | 37 |
| After oocyte donation | 7 | 23 |
| Highest level of educational attainment | 123 | 69 |
| Prevocational secondary | 14 | 11 |
| Senior general secondary/preuniversity | 17 | 14 |
| Intermediate vocational | 51 | 41 |
| Higher vocational | 19 | 15 |
| University education | 13 | 11 |
| Current occupation | 90 | 51 |
| Fulltime employed | 23 | 26 |
| Part‐time employed | 36 | 40 |
| Still attend educational services | 16 | 18 |
| Unemployed | 12 | 13 |
| Sports participation | 73 | 41 |
| Jogging | 19 | 26 |
| Cycling | 17 | 23 |
| Fitness | 16 | 22 |
| Team sports | 1 | 1 |
Completeness per domain (eg living situation) is presented as n (%) of the total cohort (n = 177). Data per item is presented as n, % of the corresponding domain.
Table 3.
Associated morbidity
| Morbidity (n = 177) | n | % |
|---|---|---|
| Otological problems | 57 | 32 |
| Cardiovascular disease | 56 | 32 |
| BAV | 34 | 19 |
| PAPVR | 12 | 7 |
| CoA | 9 | 5 |
| bovine aortic arch | 2 | 1 |
| Persistent duct | 2 | 1 |
| PLSCV | 2 | 1 |
| ASD | 1 | 0.6 |
| VSD | 1 | 0.6 |
| Hypothyroidism | 30 | 17 |
| Dento‐facial malformations | 26 | 15 |
| Orthopaedic problems | 21 | 12 |
| Liver dysfunction | 20 | 11 |
| Renal malformations | 13 | 7 |
| Diabetes | 6 | 3 |
| Coeliac disease | 3 | 2 |
| Hyperthyroidism | 1 | 1 |
Data per item are presented as n, %.
3.2. Primary outcome
3.2.1. EQ‐5D
The EQ‐5D was completed by nearly all patients (n = 175, 99%). Women with TS scored lower compared to the general Dutch population (0.857 vs 0.892; P = .003) as shown in Figure 1.28 Women with TS scored significantly higher on the ‘problems with daily activity’ (P < .001) and the ‘fear and anxiety’ (P < .001) compared to healthy controls (Figure S1). Finally, the EQ‐5D included a subjective ‘health score’ (EQ‐VAS; Figure S2), where the women with TS (n = 176) scored significantly lower than healthy women (76.8 vs 82, P < .001).
Figure 1.
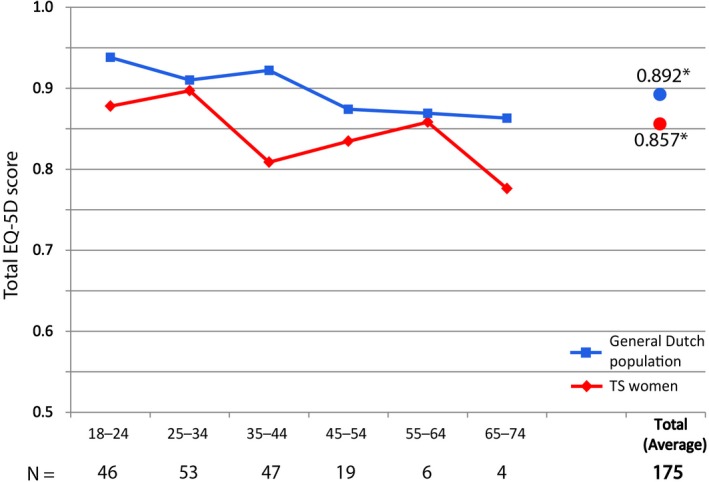
Total EQ‐5D (HR‐QoL) scores per age group in women with TS vs the general Dutch population. EQ‐5D scores per decade of life. The red line depicts scores in women with TS. The blue line depicts scores in a cohort of healthy Dutch non‐ women with TS. *: average over all age groups (P < .003) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Secondary outcomes
3.3.1. PSS‐10
Of the 176 women (99%) who completed the PSS‐10 questionnaire more than half (n = 90, 51%) indicated low or average stress (<13 points), while 23% (n = 41) experienced stress levels above average (between 13‐20 points), and 25% (n = 45) reported high stress levels (>20 points). Women with TS scored significantly higher than their non‐TS peers, indicating a higher level of perceived stress. Especially, women in the age group 45‐59 years scored higher compared to their peers (n = 45:16.18 vs 12.99, P‐value: .002), whereas higher but nonstatically significant values where observed in the other age groups (18‐39 years, n = 124:14.2 vs 13.34, P‐value: .084) (≥60 years, n = 7:13.71 vs 12.82, P‐value: .403).
3.3.2. CIS‐20
The CIS‐20 was completed by 175 participants (99%). In our cohort, 32% of patients (n = 56) scored 76 or higher indicating increased fatigue. In Figure 2, we compare our cohort of women with TS to reference populations from literature, showing that women with TS are significantly more fatigued than healthy controls, indicated by a higher total score (67 vs 55; P < .001). They also score significantly worse on the subscores of ‘fatigue’ and ‘concentration’ subscales compared to controls (29 vs 23; P < .001 and 17 vs 12; P < .001 respectively, Figure 2).
Figure 2.
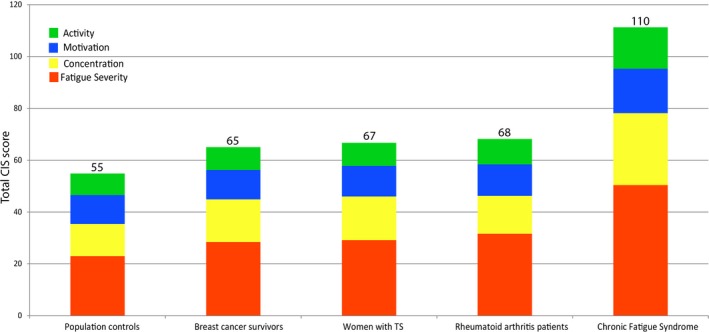
Total CIS‐20 (fatigue) scores in TS women vs different non‐Turner syndrome reference populations from literature. Total CIS‐score per patient group colors indicates different subdomains, total CIS‐20 scores are depicted on top of bars. Higher scores indicate more fatigue [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3.3. HADS
The HADS measures fear and depression in patients under medical treatment. This questionnaire was offered to 27 who showed indications of emotional suffering on the EQ‐5D and was completed by 20/27 eligible participants (74%). One of whom scored between 8 and 10 points, indicating a ‘possible depression or anxiety disorder’, while 19 women (95%) scored above 10 points, indicative of a ‘probable depression or anxiety disorder’. Since this questionnaire was offered only to a subset of patients, no comparison to a reference population could be made.
3.3.4. Fertility and sexuality
The fertility‐related quality of life (Ferti‐QoL) was completed by 57 of the 140 eligible patients (41%). Women with TS scored significantly lower on the relational subscale (78 vs 71; P = .009) indicating more impact of fertility problems on the marriage or partnership (Figure S3) compared to a reference population of women who underwent medically assisted reproduction.29 Conversely, they scored better on the mind‐body (90 vs 71; P < .001), social (82 vs 74; P < .001) and emotional subscores (81 vs 60; P < .001), indicating less impact of negative emotions on quality of life and impact of fertility problems, cognitions and behaviour on physical health. On the total Ferti‐QoL women with TS scored higher (82 vs 71; P = .1467) indicating better fertility‐related quality of life.
Additionally, the four questionnaires on sexual activity and sexual functioning in women (sexual activity question, BSSC‐W, FSFI and FSFD‐R) were completed by 57/140 (41%), 40/140 (29%), 8/9 (89%) and 2/5 (40%) participants, respectively. On the ‘sexual activity question’ (mean age: 37.5 ± 8.4years), 32 women (56%) indicated that they were sexually active. On the BSSC‐W (mean age: 38 ± 8.9years), 33 of the 40 respondents (82.5%) indicated that they were content with their sex life, 7 were not (17.5%). Causes for dissatisfaction with sex life included a lack of libido (n = 3; 7%), reduced genital sensations (n = 1; 2.4%), vaginal dryness (n = 1; 2.4%) or pain during intercourse (n = 2; 5%). As a part of the value‐based healthcare programme, these women were then referred to a sexologist.
3.3.5. Body image and disease burden
The BCS questionnaire on body image was answered by 175 (99%) women, of who 66 (37%) indicated to be discontent with their appearance. Scores of the body‐image questionnaire on the 52 areas of physique are shown in Figure S4. Women with TS were least content with their waist, build, belly, weight and figure. Also, reflected in this questionnaire is the discontent with their energy level. The burden questionnaire was completed by 177 women (100%). Self‐reported burden scored on six domains is shown in Table S1. The question which asked how much these issues on the different domains affected the participant on a scale from 0‐10 was answered (n = 161; 91%) with a mean score of 4.6 (SD ± 2.6). Of the physical complaints, fatigue was especially frequently reported by women in our cohort. Other physical problems (12%) often concerned joint and muscle pain (4%). The most frequently reported emotional complaint in the ‘other emotional complaints’ category (4%) was work‐related stress (2%). Problems in social interaction concerned the connection with peers (15%) or engaging in a romantic relationship (16%). In the ‘work‐related category’, issues reported often included mild neuropsychological problems such as memory (19%), and concentration (20%) issues. The majority of other complaints in the work category (5%) specifically described the feeling of being overburdened by work (4%). Women with TS also frequently reported concerns about the future, often related to their education or work (30%).
3.4. Determinants of quality of life
Univariate linear regression showed age, diabetes, liver dysfunction, orthopaedic problem, fatigue (CIS‐20) and stress (PSS) to be associated (P < .2) with worse quality of life (EQ‐5D). Subsequent backward multivariate linear regression analysis revealed that higher fatigue (β=−.004, SE < .001, P < .001), orthopaedic complaints, defined as physical problems (eg scoliosis, leg length discrepancy or Madelung's deformity (β=−.049, SE = .027, P=.072) and diabetes (β=−.090, SE = .047, P = .056) were associated with worse EQ‐5D score (r 2: .508). Moreover, both PSS‐10 score and CIS‐20 score were independently associated with a lower EQ‐5D outcome (β=−.014, P < .001 and β=−.005, P < .001, respectively). However, the PSS score was collinear with CIS‐20 score (β=−.2454, P < .001, R 2: .498) and since CIS‐20 had the strongest correlation PSS was removed from the analysis. Additional piece‐wise separate linear regression for the 0.5‐0.99 EQ‐5D range improved the correlation, but did not change the outcome. Notably, the presence of cardiac disease or hearing impairment did not influence quality of life (EQ‐5D). No significant difference was found between the different levels of educational attainment for EQ‐5D (P = .9156), CIS‐20 (P = .759) or PSS‐10 (P = .380) or for partnership status on EQ‐5D (P = .488), CIS‐20 (P = .055) or PSS‐10 (P = .265). A summary of the questionnaires and their results has been provided in Figure S5.
4. DISCUSSION
In order to analyse the added value of our care for patients with TS, we developed together with a small patients representation a set of clinician and patient‐reported outcome variables related to items that really matter to them in daily life. Subsequently, we measured these outcome variables in a group of TS patients. This study describes the results of these measurements. In this large cohort of women with TS, we found an impaired health‐related quality of life (HR‐QoL), correlated to higher stress and more fatigue compared to control populations. When considering determinants for these results, we found that especially having comorbidities such as diabetes and orthopaedic complaints were related to lower HR‐QoL. This impaired quality of life was reported before, in which at least some domains of QoL were found to be decreased in women with TS.26, 30, 31, 32, 33, 34, 35, 36 However, three studies found QoL in women with TS to be comparable to non‐TS controls,37, 38, 39 or found even better scores by women with TS on some aspects such as social and emotional functioning.38 Our study further determined in which domains Turner patients are especially experiencing difficulties and which factors contributed to their reduced mental health.
Moreover, in our cohort ‘fatigue’ as measured by the CIS‐20 was strongly correlated with a lower quality of life. Fatigue is a complaint frequently encountered in the care for women with TS, often in absence of physical disease, with no clear cause for this debilitating complaint. We also found the increased score for fatigue to be correlated with high stress scores, and this was not sufficiently explained by any physical comorbidities. In literature, it has been suggested that women with TS exhibit greater anaerobic stress during exercise, leading to increased muscle fatigue.40 Also, menopausal symptom may affect fatigue in TS women. However, such an effect cannot be determined from the results in the current study.
Another remarkable finding was that almost all women with TS engaged in sports chose an individual sports activity (eg running or fitness). It might be that a team sport is too demanding for these women as they have impaired performance across both verbal and visual‐spatial domains,41 or perhaps that they may feel more comfortable in an individual setting because of their lower satisfaction with their body image. This clearly warrants further study.
We also consulted a ‘Turner life coach’ involved in the value‐based healthcare programme, for the interpretation of our results. Turner life coaches are women with TS who ‘coach’ Turner women in day‐to‐day problems. She suggested that the extreme fatigue may partially be explained by the mismatch that women with TS experience between what is expected of them by themselves and their environment and what they are capable of doing. This may result from the impaired concentration and attention leading to impaired executive function and subsequent fatigue. This is something that is also clearly reflected in the work domain of the burden questionnaire. Another potential explanation for these findings in women with TS is offered by Tancredi et al, who suggest that TS patients might have an overactive sympathetic nervous system (SNS).42 Zuckerman et al43 showed that TS patients have an increased resting norepinephrine level. These TS women also had a lower response of catecholamines to exercise, which may be reflected in an increased basal tone of the SNS, resulting in high blood pressure, relative tachycardia, shorter conduction times and a decreased VO2max. This may culminate in the increased fatigue often experienced by these women.
Stress in women with TS has been investigated by Fjermestad et al32 (n = 57, mean age 40.6 ± 11.1 years), showing that they experience more stress than healthy controls. We also found an increased stress level in our cohort of Turner patients. In addition, we found that stress also negatively influenced HR‐QoL. However, causality cannot be established based on this cross‐sectional study, but stress reduction might be a tool for clinicians to improve QoL in women with TS.
From literature it is well‐known that fertility issues play an important role in women with TS.10, 36 Indeed, the questionnaire on fertility was not well‐responded to, showing that this issue might by a difficult topic for TS women. The results showed a lower relational quality of life but a higher score on the mind‐body and emotional subscores compared to a retrospective cohort of Dutch women who underwent medically assisted reproduction. The questionnaires used in this study are well validated in other populations and have been used in clinical setting. We have not evaluated the burden of filling out the questionnaire itself, but it takes approximately 15 minutes and we feel this is a relatively small effort. We do aim to evaluate the acceptability in future studies. Conversely, clinicians are often time‐pressed and the time it takes to fill in a questionnaire cannot be spent elsewhere. Therefore, the set‐up of automated sent questionnaires, providing so much important information is an important asset in the assessment for the clinician. In our study, the feasibility was good as 177 out of the 201 women did fill in the questionnaires. An important hurdle is that some specific questionnaires such as the one on sexuality were only marginally filled in. This hurdle needs attention. Of the women that did fill in these questionnaires just over half were sexually active and of these around 80% were content with their sex life. There might be a selection bias of women feeling more comfortable completing these questionnaires being more positive on their sex life, compared with women who feel uncomfortable, it may have been confronting or women may have considered questions not relevant.44 Also, these questionnaires have not yet been validated specifically for the TS population which limits their interpretation. Therefore, future research should focus on developing disease‐specific tools, as previous studies show that disease‐specific HR‐QoL instruments can contribute to more effective treatment interventions as they have more power to detect small differences and changes over time.15, 45, 46, 47
4.1. Clinical implications
We want to underline the need for screening with Turner specific questionnaires covering a broader range of HR‐QoL and varying aspects of psychosocial functioning in women with TS. Reis et al10 described 18 different instruments in a recent review, mostly the SF‐36, used to assess QoL and related domains. TS, however, is a very heterogeneous syndrome that is not easily covered in a limited set of questions. However, a single validated TS specific questionnaire would in our opinion allow to more easily score QoL and act timely and appropriately. Considering our results, such a questionnaire would need to cover the HR‐QoL aspects of the EQ‐5D, stress, fatigue and sexuality. Indeed, if all women with TS would fill in this questionnaire, problems could be identified timely and possible therapy can be offered and initiated. Once a common methodology has been established to investigate QOL in these women, other possible sources of bias, such as ascertainment bias due to the tendency of TS women to give expected responses, could be studied further. For some women group, sessions may provide useful support, while others clearly need individual coaching or psychological therapy. In addition, a well‐structured exercise programme may be useful in selected women with TS. Finally, attention for and information on sexual functioning and fertility should be organized for all women with TS.
The main limitation of our study is that not all questionnaires were completed by all participants, which possibly introduced bias. Patient who experienced higher stress, more fatigue or lower QoL could have been less motivated to participate in this comprehensive set of questionnaires. A second limitation lies in the retrospective manner in which electronic patient records were searched for patient‐specific clinical information, which is sensitive to incompleteness. All women with TS are seen in our centre and the current cohort should therefore be representative of the general TS population. Furthermore, we had no data on the women who did not respond to the questionnaires, we were therefore unable to assess a potential difference between these groups. A final limitation is that there was no reference data on educational attainment and partnership status and therefore no comparison on these aspects could be made. This would be an important aspect to take into account in designing future studies.
5. CONCLUSION
Value‐based healthcare means aiming at improving outcomes that really matter to patients. In order to do this, we should first measure these outcomes. By doing so, we showed that women with TS scored lower on HR‐QoL and reported more stress and fatigue compared to healthy controls. Determinants which were found to affect HR‐QoL were physical factors, such as diabetes and orthopaedic complaints, but also stress and fatigue were clearly associated with lower HR‐QoL.
CONFLICT OF INTEREST
Nothing to declare.
Supporting information
ACKNOWLEDGEMENTS
We would like to thank Remko de Vries for his hard work and dedication in the day‐to‐day care for women with TS at the outpatient clinic in the past years
van den Hoven AT, Bons LR, Dykgraaf RHM, et al. A value‐based healthcare approach: Health‐related quality of life and psychosocial functioning in women with Turner syndrome. Clin Endocrinol (Oxf). 2020;92:434–442. 10.1111/cen.14166
Funding information
We gratefully acknowledge funding by the Dutch Heart Foundation (grant number: 2013T093).
DATA AVAILABILITY STATEMENT
Data available on reasonable request from the authors.
REFERENCES
- 1. Turner H. A syndrome of Infantilism, congenital webbed neck and cubitus valgus. Endocrinology. 1938;23:556‐574. [PubMed] [Google Scholar]
- 2. Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91(10):3897‐3902. [DOI] [PubMed] [Google Scholar]
- 3. Bondy CA, Matura LA, Wooten N, Troendle J, Zinn AR, Bakalov VK. The physical phenotype of girls and women with Turner syndrome is not X‐imprinted. Hum Genet. 2007;121(3–4):469‐474. [DOI] [PubMed] [Google Scholar]
- 4. Gravholt CH. Epidemiological, endocrine and metabolic features in Turner syndrome. Arq Bras Endocrinol Metabol. 2005;49(1):145‐156. [DOI] [PubMed] [Google Scholar]
- 5. Gravholt CH, Andersen NH, Conway GS, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177(3):G1‐G70. [DOI] [PubMed] [Google Scholar]
- 6. Ho VB, Bakalov VK, Cooley M, et al. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004;110(12):1694‐1700. [DOI] [PubMed] [Google Scholar]
- 7. van den Hoven AT, Chelu RG, Duijnhouwer AL, et al. Partial anomalous pulmonary venous return in Turner syndrome. Eur J Radiol. 2017;95:141‐146. [DOI] [PubMed] [Google Scholar]
- 8. Gutmark‐Little I, Hor KN, Cnota J, Gottliebson WM, Backeljauw PF. Partial anomalous pulmonary venous return is common in Turner syndrome. J Pediatr Endocrinol Metab. 2012;25(5–6):435‐440. [DOI] [PubMed] [Google Scholar]
- 9. Calcaterra V, Brambilla P, Maffe GC, et al. Metabolic syndrome in Turner syndrome and relation between body composition and clinical, genetic, and ultrasonographic characteristics. Metab Syndr Relat Disord. 2014;12(3):159‐164. [DOI] [PubMed] [Google Scholar]
- 10. Reis CT, de Assumpcao MS, Guerra‐Junior G, de Lemos‐Marini SHV. Systematic review of quality of life in Turner syndrome. Qual Life Res. 2018;27(8):1985‐2006. [DOI] [PubMed] [Google Scholar]
- 11. Rovet JF. The psychoeducational characteristics of children with Turner syndrome. J Learn Disabil. 1993;26(5):333‐341. [DOI] [PubMed] [Google Scholar]
- 12. McCauley E, Ito J, Kay T. Psychosocial functioning in girls with Turner's syndrome and short stature: social skills, behavior problems, and self‐concept. J Am Acad Child Psychiatry. 1986;25(1):105‐112. [DOI] [PubMed] [Google Scholar]
- 13. Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 2009;15(4):270‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldfinger JZ, Preiss LR, Devereux RB, et al. Marfan syndrome and quality of life in the GenTAC registry. J Am Coll Cardiol. 2017;69(23):2821‐2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quittner AL, Modi A, Cruz I. Systematic review of health‐related quality of life measures for children with respiratory conditions. Paediatr Respir Rev. 2008;9(3):220‐232. [DOI] [PubMed] [Google Scholar]
- 16. van Egdom LSE, Lagendijk M, van der Kemp MH, et al. Implementation of value based breast cancer care. Eur J Surg Oncol. 2019;45(7):1163‐1170. [DOI] [PubMed] [Google Scholar]
- 17. LimeSurvey GmbH , Survey Services & Consulting, Hamburg, Germany. https://www.limesurvey.org/
- 18. Gemstracker . https://gemstracker.org/
- 19. Mortensen KH, Young L, De Backer J, et al. Cardiovascular imaging in Turner syndrome: state‐of‐the‐art practice across the lifespan. Heart. 2018;104(22):1823‐1831. [DOI] [PubMed] [Google Scholar]
- 20. Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress in women. J Sex Marital Ther. 2002;28(4):317‐330. [DOI] [PubMed] [Google Scholar]
- 21. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self‐report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191‐208. [DOI] [PubMed] [Google Scholar]
- 22. ter Kuile MM, Brauer M, Laan E. The Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS): psychometric properties within a Dutch population. J Sex Marital Ther. 2006;32(4):289‐304. [DOI] [PubMed] [Google Scholar]
- 23. Sylven L, Magnusson C, Hagenfeldt K, von Schoultz B. Life with Turner's syndrome—A psychosocial report from 22 middle‐aged women. Acta Endocrinol (Copenh). 1993;129(3):188‐194. [DOI] [PubMed] [Google Scholar]
- 24. Carel JC, Elie C, Ecosse E, et al. Self‐esteem and social adjustment in young women with Turner syndrome–influence of pubertal management and sexuality: population‐based cohort study. J Clin Endocrinol Metab. 2006;91(8):2972‐2979. [DOI] [PubMed] [Google Scholar]
- 25. Pavlidis K, McCauley E, Sybert VP. Psychosocial and sexual functioning in women with Turner syndrome. Clin Genet. 1995;47(2):85‐89. [DOI] [PubMed] [Google Scholar]
- 26. Naess EE, Bahr D, Gravholt CH. Health status in women with Turner syndrome: a questionnaire study on health status, education, work participation and aspects of sexual functioning. Clin Endocrinol (Oxf). 2010;72(5):678‐684. [DOI] [PubMed] [Google Scholar]
- 27. Crott R. Direct mapping of the QLQ‐C30 to EQ‐5D preferences: a comparison of regression methods. Pharmacoecon Open. 2018;2(2):165‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janssen B, Szende A. Population norms for the EQ‐5D (pp. 19‐30). Dordrecht, NL: Springer; 2014. [PubMed] [Google Scholar]
- 29. Aarts JW, van Empel IW, Boivin J, Nelen WL, Kremer JA, Verhaak CM. Relationship between quality of life and distress in infertility: a validation study of the Dutch FertiQoL. Hum Reprod. 2011;26(5):1112‐1118. [DOI] [PubMed] [Google Scholar]
- 30. Lasaite L, Kriksciuniene R, Zilaitiene B, Verkauskiene R. Emotional state, cognitive functioning and quality of life of adult women with Turner syndrome in Lithuania. Growth Horm IGF Res. 2019;45:37‐42. [DOI] [PubMed] [Google Scholar]
- 31. Krantz E, Landin‐Wilhelmsen K, Trimpou P, Bryman I, Wide U. Health‐related quality of life in Turner syndrome and the influence of growth hormone therapy: a 20‐year follow‐up. J Clin Endocrinol Metab. 2019;104(11):5073‐5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fjermestad KW, Naess EE, Bahr D, Gravholt CH. A 6‐year follow‐up survey of health status in middle‐aged women with Turner syndrome. Clin Endocrinol (Oxf). 2016;85(3):423‐429. [DOI] [PubMed] [Google Scholar]
- 33. Nadeem M, Roche EF. Health‐related quality of life in Turner syndrome and the influence of key features. J Pediatr Endocrinol Metab. 2014;27(3–4):283‐289. [DOI] [PubMed] [Google Scholar]
- 34. Lasaite L, Lasiene D, Lasas L. Cognition, emotions and quality of life in Lithuanian girls with Turner syndrome after growth hormone therapy discontinuation. J Pediatr Endocrinol Metab. 2010;23(5):443‐450. [PubMed] [Google Scholar]
- 35. Amundson E, Boman UW, Barrenas ML, Bryman I, Landin‐Wilhelmsen K. Impact of growth hormone therapy on quality of life in adults with turner syndrome. J Clin Endocrinol Metab. 2010;95(3):1355‐1359. [DOI] [PubMed] [Google Scholar]
- 36. Boman UW, Bryman I, Moller A. Psychological well‐being in women with Turner syndrome: somatic and social correlates. J Psychosom Obstet Gynaecol. 2004;25(3–4):211‐219. [DOI] [PubMed] [Google Scholar]
- 37. Taback SP, Van Vliet G. Health‐related quality of life of young adults with Turner syndrome following a long‐term randomized controlled trial of recombinant human growth hormone. BMC Pediatr. 2011;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bannink EM, Raat H, Mulder PG, de Muinck Keizer‐Schrama SM. Quality of life after growth hormone therapy and induced puberty in women with Turner syndrome. J Pediatr. 2006;148(1):95‐101. [DOI] [PubMed] [Google Scholar]
- 39. Carel JC, Ecosse E, Bastie‐Sigeac I, et al. Quality of life determinants in young women with Turner's syndrome after growth hormone treatment: results of the StaTur population‐based cohort study. J Clin Endocrinol Metab. 2005;90(4):1992‐1997. [DOI] [PubMed] [Google Scholar]
- 40. Wells GD, O'Gorman CS, Rayner T, et al. Skeletal muscle abnormalities in girls and adolescents with Turner syndrome. J Clin Endocrinol Metab. 2013;98(6):2521‐2527. [DOI] [PubMed] [Google Scholar]
- 41. Hart SJ, Davenport ML, Hooper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006;129(Pt 5):1125‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tancredi G, Versacci P, Pasquino AM, et al. Cardiopulmonary response to exercise and cardiac assessment in patients with turner syndrome. Am J Cardiol. 2011;107(7):1076‐1082. [DOI] [PubMed] [Google Scholar]
- 43. Zuckerman‐Levin N, Zinder O, Greenberg A, Levin M, Jacob G, Hochberg Z. Physiological and catecholamine response to sympathetic stimulation in turner syndrome. Clin Endocrinol (Oxf). 2006;64(4):410‐415. [DOI] [PubMed] [Google Scholar]
- 44. Althof SE, Rosen RC, Perelman MA, Rubio‐Aurioles E. Standard operating procedures for taking a sexual history. J Sex Med. 2013;10(1):26‐35. [DOI] [PubMed] [Google Scholar]
- 45. Quittner AL, Goldbeck L, Abbott J, et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: results of The International Depression Epidemiological Study across nine countries. Thorax. 2014;69(12):1090‐1097. [DOI] [PubMed] [Google Scholar]
- 46. Goldbeck L, Fidika A, Herle M, Quittner AL. Psychological interventions for individuals with cystic fibrosis and their families. Cochrane Database Syst Rev. 2014;6:CD003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Neve‐Enthoven NG, Callens N, van Kuyk M, et al. Psychosocial well‐being in Dutch adults with disorders of sex development. J Psychosom Res. 2016;83:57‐64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on reasonable request from the authors.


