Abstract
We conducted this study for the purpose of evaluating the protective mechanisms of curcumin against oxidative stress in asthenozoospermic individuals. Asthenozoospermic individuals were grouped into AS group, curcumin treatment group and brusatol + curcumin treatment group. The sperm motility was measured by computer‐aided sperm analysis. We conducted flow cytometry and spectrophotometry to assess the levels of reactive oxygen species (ROS) and malondialdehyde (MDA). Chlortetracycline (CTC) was used to examine the acrosomal reaction of spermatozoa. Also, Western blotting was carried to measure antioxidant gene Nrf2 (nuclear factor erythroid 2‑related factor) expression level. As our results shown, treatment with curcumin significantly decreased ROS formation and MDA production, compared with spermatozoa of AS group; however, Nrf2 inhibitor, Brusatol, inhibited Nrf2 expression and sperm function. Our results have shown that curcumin might protect spermatozoa by regulating Nrf2 level.
Keywords: asthenozoospermia, curcumin, motility, nuclear factor erythroid 2‑related factor, reactive oxygen species
1. INTRODUCTION
Affecting approximately 10% to 15% of reproductive‐age couples worldwide, infertility has been a global problem, and of these, male infertility accounts for 50% (Kobori, 2019). Male infertility is a disorder induced by multiple factors, in which defective sperm function is a major cause (Hull et al., 1986). Defined as total motility <40% or progressive motility <32%, asthenozoospermia can be caused by genetic or environmental factors, such as oxidative stress, which can account for 18% of male subfertility and infertility cases (Curi et al., 2003; Hunault et al., 2004).
Oxidative stress harmfully affects sperm motility through overproduction of the reactive oxygen species (ROS) including hydrogen peroxide and superoxide anions, inducing asthenozoospermia (Sikka, Rajasekaran, & Hellstrom, 2008). Accordingly, high ROS levels are considered to be involved in spermatozoa DNA fragmentation, lipid peroxidation damage, as well as apoptosis (Menkveld, 2010). However, spermatozoa possess a well‐developed antioxidant defence network, including antioxidant genes and enzymes which perform a crucial role in protecting sperm motility and viability (Lamond et al., 2003). For example, the antioxidant gene, Nrf2 (nuclear factor erythroid 2‐related factor 2) is reported to bind promoters of antioxidant genes to promote antioxidant enzymes expression (Cheng, Kalabus, Zhang, & Blanco, 2012). In mice with deficiency of Nrf2, it was elaborated that sperm concentration and motility were impaired (Nakamura et al., 2010). Further, evidence showed that compared with Nrf2 expression in spermatozoa from fertile men, the spermatozoa of asthenozoospermic individuals possessed lower level of Nrf2 expression (Kang, Zixin, Yulin, Xingcheng, & Bolan, 2012). Thus, Nrf2, as an antioxidant gene, is very important for maintaining spermatozoa motility through suppressing oxidative stress (Prabagaran, Hansen, Drury, & Moley, 2014).
Currently, much attention has been devoted to antioxidants, such as vitamin C, coenzyme Q10, human‐growth hormone, carnitine and glutathione for the prevention of ROS overproduction in males (Giacone et al., 2017; Ross et al., 2010; Saeednia, Nashtaei, Bahadoran, Aleyasin, & Amidi, 2016,but there are limits due to inefficiency (Greco et al., 2005). Hence, it is necessary to seek effective therapies for asthenozoospermia.
The property of plants possessing a series of constituents which can prevent oxidative stress (Shah, Khan, Naz, & Khan, 2014) provides some ideas of seeking effective therapies for asthenozoospermia. Curcumin, a natural polyphenolic compound which is classified as diferuloylmethane (yellow in colour), is constituent of the rhizome of Curcuma longa Lin and possess the characteristic of anti‐oxidative, anti‐inflammatory, free radical scavenging etc (Dai et al., 2018; Sahin, Orhan, Tuzcu, Tuzcu, & Sahin, 2012) . Ithas been reported that curcumin could improve bull spermatozoa and rat testis subjected to induced oxidative stress or could improve cryopreserved boar spermatozoa (Chanapiwat & Kaeoket, 2015; Sahoo, Roy, & Chainy, 2008; Tvrda et al., 2016). Meanwhile, curcumin could improve sperm motility from the patients with leucocytosperm and infertile men (Alizadeh et al., 2017; Zhang, Diao, Duan, Yi, & Cai, 2017), on the contrary, curcumin could also serve as a potential nonsteroidal contraceptive by blocking sperm motility (Naz & Lough, 2014). With the above contradiction and unknown mechanism, we aimed to further determine the influence of curcumin on spermatozoa motility and gain deep insight into the mechanism how curcumin plays its roles.
In our study, we used ejaculated spermatozoa from asthenozoospermic individuals as our model and examined sperm motility, mitochondria function, lipid peroxidation, acrosome reaction and apoptosis level to evaluate the function of curcumin.
2. MATERIALS AND METHODS
2.1. Donors of semen samples and ethical approval
Semen samples of normozoospermic and asthenozoospermic individuals who were volunteers enrolled from the Department of Andrology of Obstetrics and Gynecology Hospital Affiliated to Nanjing Medical University. The study was approved by the Ethics Committee of Obstetrics and Gynecology Hospital Affiliated to Nanjing Medical University. All participants were given informed consent before recruitment into the study.
2.2. Semen preparation
After sexual abstinence for 3 days, semen samples were collected through masturbation and were liquefied for 30 min and delivered to laboratory in <60 min after ejaculation according to World Health Organization (WHO) guidelines (Menkveld, 2010). Asthenozoospermia was defined by the following criteria: progressive motility <32%; sperm concentration >15 million/ml; total motility (progressive motility + nonprogressive motility) <40%; and normal forms of sperm morphology >4%. 10% (w/v) bovine serum albumin (Sigma) was added to modified human tubular fluid (irvinesci.lnc.) medium to wash semen samples. Subsequently, the semen samples were applied to density gradient centrifugation with 90% (v/v) and 50% (v/v) Percol (GE Healthcare) at 300 g for 20 min. Spermatozoa were incubated with curcumin or dimethyl sulfoxide for 30 min at 37°C. The spermatozoa motility was measured through a computer‐assisted semen analyser (Hamilton Thorne, lnc.).
2.3. Reagent
Brusatol was soluble in dimethyl sulfoxide (DMSO) and the final concentration of brusatol incubated with spermatozoa was 40 nM. The final concentration of DMSO mixed with spermatozoa was 2%.
2.4. Mitochondrial membrane potentials assay
Spermatozoa were assayed for loss of mitochondrial inner transmembrane potential by JC‐1 probe (Beyotime). Spermatozoa were mixed with JC‐1 staining solution (5 μg/ml). The spermatozoa were washed twice in PBS and examined by flow cytometry.
2.5. Measurement of spermatozoa ATP
We examined spermatozoa ATP production by Enhanced ATP Assay Kit (Beyotime). The spermatozoa were placed into detecting solution. Afterwards, we calculated ATP levels through the luminescence signals.
2.6. Measurement of ROS level
ROS of spermatozoa was detected according to the method of reactive oxygen species assay kit (Beyotime). We resuspended spermatozoa in 10 μM dihydrodichlorofluorescein diacetate (DCFH‐DA) added with serum‐free medium for 20 min, the fluorescence intensity was examined by flow cytometer (BD,NYC, China).
2.7. Malondialdehyde (MDA) assay
MDA was measured using the Lipid Peroxidation MDA Assay Kit (Beyotime) and was detected by at 532 nm.
2.8. Chlortetracycline fluorescence assessment of sperm
We performed chlortetracycline (CTC) fluorescence analysis as reported by Ying et al. (2006). The spermatozoa were resuspended in culture medium (irvinesci.lnc.), mixed with liquid paraffin (37°C, 5% CO2). Afterwards, spermatozoa were added in formalin and incubated for 300 min. We collected spermatozoa suspensions for fluorescence microscopy analysis to evaluate CTC staining.
2.9. TUNEL Assay
Apoptosis of cells was measured by TUNEL Apoptosis Detection Kit (Vazyme Biotech Co., Ltd.). Human spermatozoa were added in 4% PFA for 25 min at room temperature. After three washes, Proteinase K was also placed into the spermatozoa and mixed with 1 × Equilibration Buffer. Then, the spermatozoa were stained with DAPI and analysed by a fluorescence microscope.
2.10. Western blot analysis
Western blot was conducted as previous description (Castaneda et al., 2017). Proteins were then separated through SDS/PAGE and subsequently transferred onto a polyvinylidene difluoride membrane. Next, the proteins were blocked with 5% milk for 2 hr and incubated with primary antibodies overnight at 4°C. After three washes, the membranes were incubated with secondary antibodies for 2 hr. The signals from the detected proteins were visualised.
2.11. Statistical analysis
We repeated all trials at least three times. The differences between groups were analysed using one‐way analysis of variance (ANOVA). All data were represented with the mean ± the standard error (SE). Additionally, the analyses were conducted using the statistical package SPSS (Inc.) for Windows.
3. RESULTS
3.1. One hundred nanomolar curcumin increased the motility of spermatozoa of asthenozoospermia
To address the effects of curcumin upon asthenozoospermia, we initially incubated spermatozoa of asthenozoospermia with various concentrations of curcumin (1 nM, 100 nM, 1 mM, 1 M). Spermatozoa incubated with 100 nM curcumin showed most significantly increased motility compared to AS group, rather unexpectedly, the spermatozoa incubated with curcumin at concentrations of 1 mM and 1 M resulted in a decreased total and progressive motility than AS group (Figure 1a,b). Meanwhile, we observed that motility of spermatozoa from normozoospermic individuals was unaffected by 100 nM curcumin treatment (Figure 1c).
Figure 1.
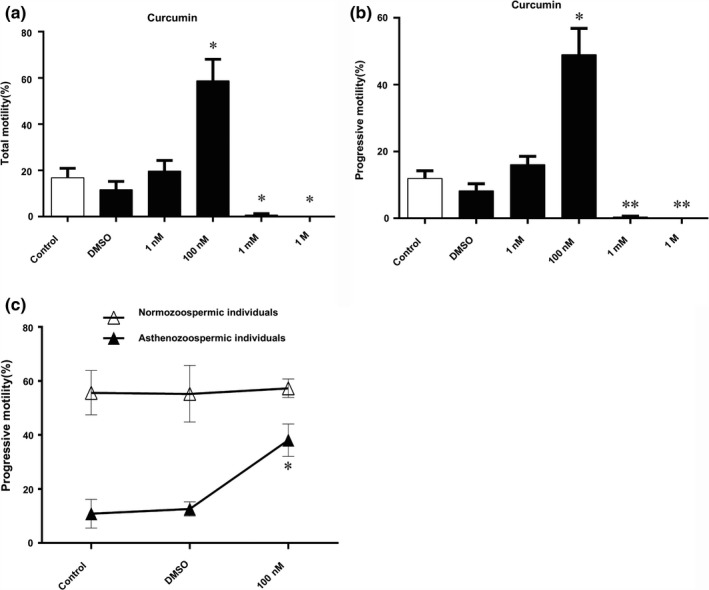
Effect of curcumin on spermatozoa motility. (a, b) Effect of various concentrations of curcumin (1 nM, 100 nM, 1 mM, 1 M) on motility (PR + NP) and PR of spermatozoa of asthenozoospermia. (c) Effect of 100 nM curcumin on spermatozoa motility (PR + NP) and PR of normozoospermic and asthenozoospermia individuals. Results are showed as the mean ± SE. **p ≤ .01 compared with control
3.2. Curcumin rescued mitochondrial function of spermatozoa of asthenozoospermia
Spermatozoa membrane potential and ATP levels were tested for the purposes of evaluating mitochondrial function. Our study revealed more spermatozoa of asthenozoospermia individuals yielding low membrane potential, which might lead to less ATP synthesis than that of normozoospermic individuals (Figure 2). However, 100 nM curcumin could rescue the membrane potential of spermatozoa from asthenozoospermia (Figure 2a–e) and ATP production (Figure 2f), indicating that curcumin could improve mitochondrial function.
Figure 2.
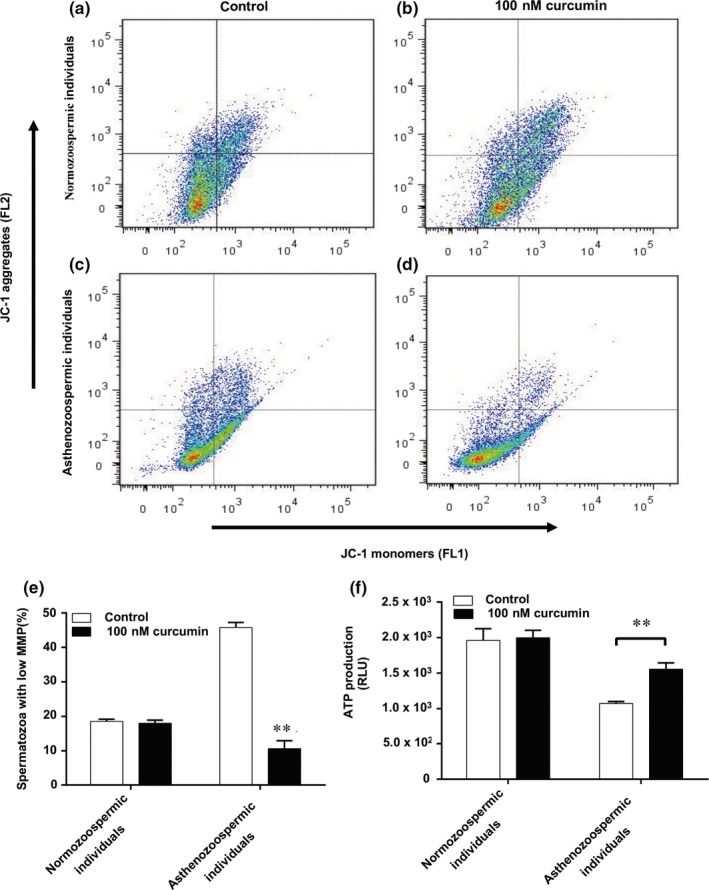
Effect of curcumin on spermatozoa mitochondrial function. (a‐d) Representative image showing the percentage of spermatozoa with polarised and depolarised mitochondrial membrane. (e) Average percentage of spermatozoa with low MMP ± standard error (SE) (n = 3). (f) Effect of curcumin on ATP production. Results are presented as the mean ± SD. **p < .01 compared with control
3.3. Curcumin reduced the ROS production, level of MDA, apoptosis and increased sperm acrosome reaction of spermatozoa of asthenozoospermia
As expected, the mitochondrial function of spermatozoa was impaired in cases presenting asthenozoospermia. Considering that the mitochondria were the major source of ROS production, we further analysed the ROS level through flow. Compared to normozoospermic individuals, spermatozoa of asthenozoospermia had higher ROS levels which were reduced by 100 nM curcumin treatment (Figure 3a–e). To further examine the effect of ROS on spermatozoa, we measured MDA levels for testing lipid peroxidation. As shown in Figure 3f, we presented that curcumin caused significant decrease in lipid peroxidation in spermatozoa of asthenozoospermia. We also showed that there were more TUNEL‐positive spermatozoa in asthenozoospermia individuals compared to that of normozoospermic individuals (Figure 3g,i). To further investigate the effect of curcumin on apoptosis of spermatozoa, we incubated the spermatozoa of asthenozoospermia individuals and normozoospermic individuals with 100 nM curcumin, and we measured the apoptosis level. We found that 100 nM curcumin treatment reduced the level of apoptosis in asthenozoospermia individuals compared to that of normozoospermic individuals (Figure 3h,j,k). As demonstrated in the literature, sperm motility is associated with acrosome reaction (Ying et al., 2006). Thus, we examined whether curcumin could improve sperm acrosome reaction induced by the calcium ionophore A23187. The results showed increased acrosome‐reacted spermatozoa induced by A23187 in curcumin group compared with that of AS group (Figure 3l).
Figure 3.
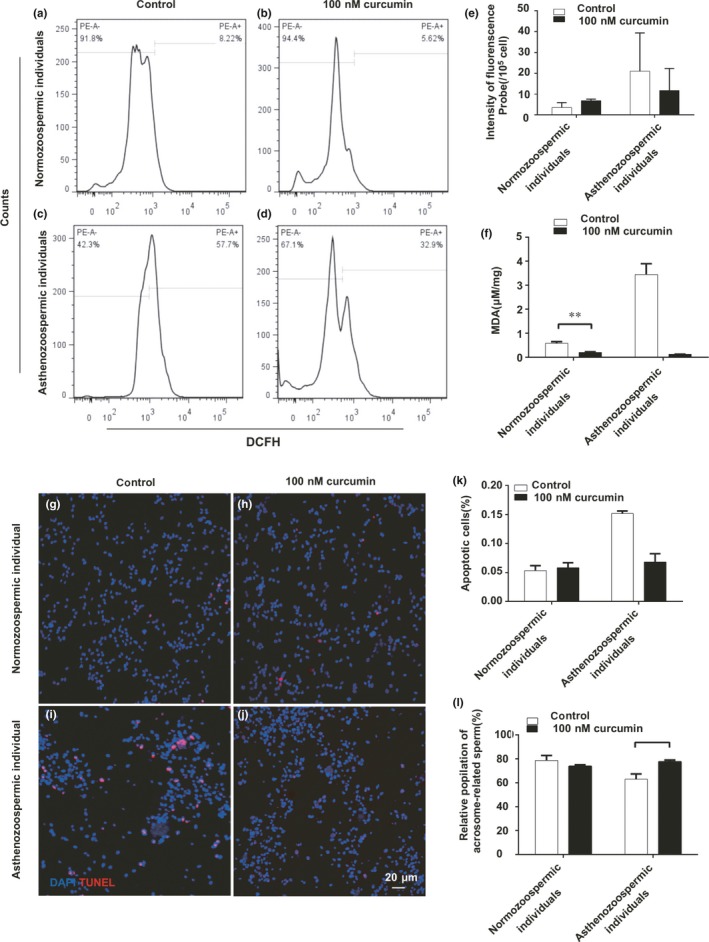
Effect of curcumin on spermatozoa ROS levels, MDA production and acrosome reaction (AR). (a‐d) Representative image showing spermatozoa ROS generation by DCFH‐DA oxidation‐based fluorescence using a flow cytometry. (e) Mean fluorescence units in each treated groups. Results are presented as the mean ± SE. (f) Column chart representing MDA levels. The results are expressed as the mean ± SE. *p < .05, **p < .01 compared with control. (g–j) Terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick end labelling (TUNEL) staining images of spermatozoa from normozoospermic and asthenozoospermia individuals, bar = 20 μm. (k) Quantification of TUNEL‐positive staining spermatozoa (n = 200). Results are showed as the mean ± SE. **p < .01 compared with control. (l) Chlortetracycline fluorescence data presented as percentage of cells expressing the AR pattern. *p < .05 compared with control
3.4. Brusatol inhibited the motility of spermatozoa improved by curcumin
Accordingly, curcumin plays an important part in protecting cells against oxidative stress through Nrf2 (Ganan‐Gomez, Wei, Yang, Boyano‐Adanez, & Garcia‐Manero, 2013; Olayanju et al., 2015). Considering Nrf2 involved in spermatogenesis, we examined whether curcumin improved asthenozoospermia through Nrf2, and we used brusatol, as the inhibitor of Nrf2, to inhibit Nrf2 expression and study the effect of curcumin on spermatozoa. After treatment with curcumin and brusatol, we showed that Nrf2 protein level was reduced compared with the curcumin group (Figure 4a). As we expected, the total and progressive motility of spermatozoa from asthenozoospermia individuals were unchanged compared to AS group but lower than that of the curcumin group (Figure 4b,c). These observations demonstrated that curcumin might function as an antioxidant through Nrf2 to improve the sperm motility.
Figure 4.
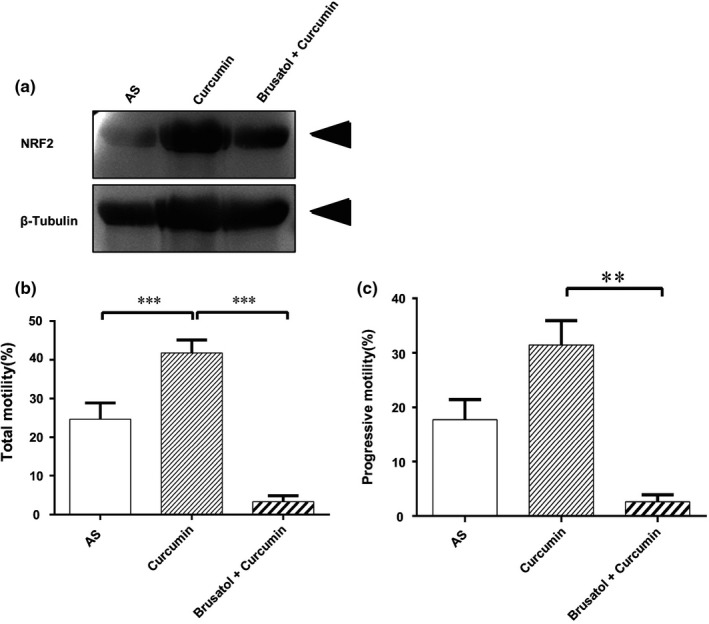
Effect of NRF2‐inhibitory Brusatol on sperm motility. (a) Confirmation of NRF2 protein expression by Western blotting. (b) Effect of Brusatol on spermatozoa motility (PR + NP) and (c) PR of asthenozoospermia individuals. Results are presented as the mean ± SE. **p ≤ .01, ***p ≤ .01 compared with control
3.5. Brusatol impaired efficacy of curcumin
Additionally, we observed that apoptosis levels, mitochondrial function, ROS production, MDA levels and acrosome reaction of spermatozoa from asthenozoospermia individuals in the group of cotreatment with brusatol/curcumin revealed no significant change compared to spermatozoa in AS group (Figures 5 and 6).
Figure 5.
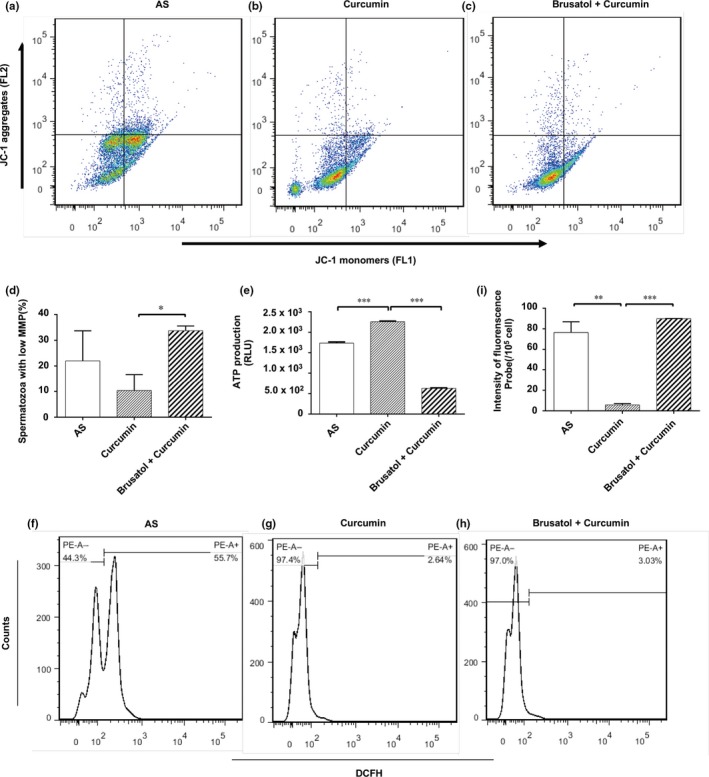
Effect of Brusatol on spermatozoa mitochondrial function, ROS levels. (a–c) Representative image showing the percentage of spermatozoa with polarised and depolarised mitochondrial membrane. (d) Average percentage of spermatozoa with low MMP ± standard error (SE) (n = 3). (e) Effects of curcumin on ATP production. Results are showed as the mean ± SE. *p < .05, ***p < .001. (f–h) Representative image showing spermatozoa ROS generation by DCFH‐DA oxidation‐based fluorescence using a flow cytometry. (i) Mean fluorescence units in each treated groups. Results are presented as the mean ± SE. **p < .01, ***p < .001
Figure 6.
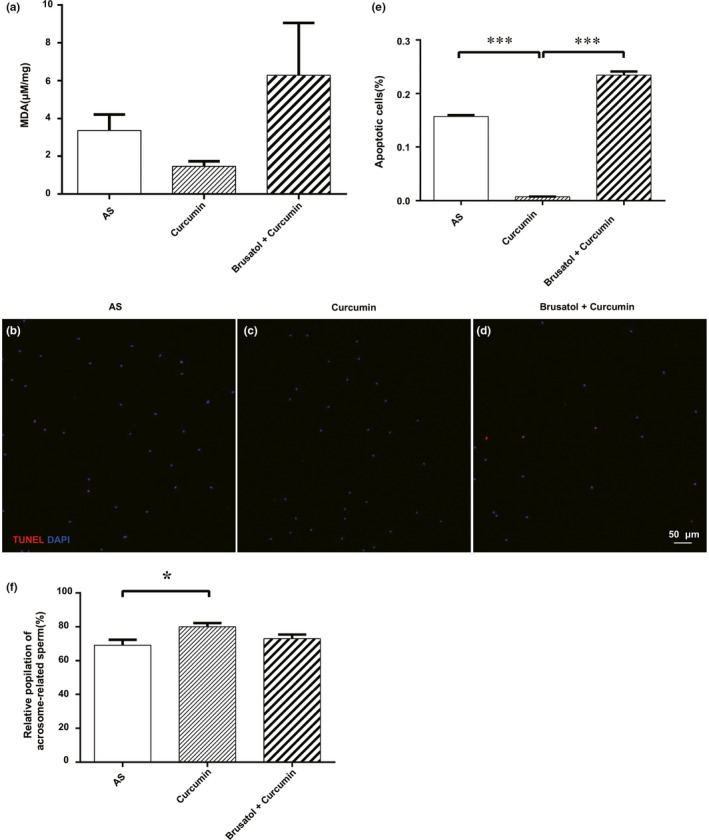
Effect of Brusatol on spermatozoa MDA production and the acrosome reaction (AR) and apoptosis. (a) The results are expressed as the mean ± SD. (b‐d) Terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick end labelling (TUNEL) staining images of spermatozoa three groups, bar = 50 μm. (e) Quantification of TUNEL‐positive staining spermatozoa (n = 200). (f) Chlortetracycline fluorescence data are presented as percentage of spermatozoa expressing the AR pattern. *p < .05 compared with control. Results are presented as the mean ± SE (n = 3). ***p < .001
4. DISCUSSION
Asthenospermia, described as reduced sperm viability and forward motility, is reported to be a risk factor of human male infertility (Shen, Wang, Liang, & He, 2013; Zhang et al., 2016). Our study explored the effects of various curcumin doses on sperm motility of infertile men suffering asthenozoospermia. Based on this information, 100 nM of curcumin significantly increased the motility of spermatozoa in asthenozoospermic individuals. The present study suggests that curcumin can ameliorate sperm motility in a dose‐independent manner and could induce toxicity to sperm motility when it is applied beyond a certain concentration.
It is clear that spermatozoa acquire amounts of energy provided by mitochondria surrounding their flagella through oxidative phosphorylation and glycolysis, to support the ability and activity during fertilisation, which means asthenozoospermia might be relevant with mitochondrial dysfunction (Piomboni, Focarelli, Stendardi, Ferramosca, & Zara, 2012). Mitochondrial membrane potential (MMP), utilised as a gauge of mitochondrial function, whose decrease is an indicator of the destruction of the mitochondrial electron transport chain, subsequent reduction in the production of ATP, cell dysfunction and even death (Evenson, Darzynkiewicz, & Melamed, 1982; Li et al., 2017). Our results showed reduced MMP in the spermatozoa of asthenozoospermia individuals compared to that of normozoospermic individuals. Also, several other researchers reported defects in mitochondrial respiratory activity of asthenozoospermia (Ferramosca, Focarelli, Piomboni, Coppola, & Zara, 2008; Pelliccione et al., 2011). Additionally, it has been pointed out that reduced motile spermatozoa of infertile patients were shown to exhibit lower MMP and increasing of spermatozoa MMP is positively correlated with sperm motility and fertility potential (Chan & Chan, 2015; Li et al., 2012). Our present research presented that curcumin‐treated human spermatozoa showed increased mitochondrial activity compared to that in the AS group, indicating that curcumin might increase the motility of spermatozoa through improving mitochondrial function.
It is well known that intracellular ROS formation are primarily produced by cellular mitochondria (Chan, 2006). Small amounts of ROS are necessary for regulating normal spermatozoa function; however, ROS will damage the spermatozoa when beyond some certain extent (Griveau, & Le Lannou, 2010). Disruption of the balance between ROS generation and antioxidant scavenging could induce the development of oxidative stress. As we have shown in Figure 3, ROS reproduction increased in spermatozoa from asthenozoospermic individuals but decreased in curcumin treatment group. These findings suggest that curcumin may improve asthenozoospermia by reducing ROS reproduction. Furthermore, the sperm plasma membrane with the property of possessing rich polyunsaturated fatty acids makes it susceptible to lipid peroxidation, resulting in membrane integrity loss, such as sperm–oocyte fusion and the ability to undergo acrosomal exocytosis (Aitken, Harkiss, & Buckingham, 1993). In the present study, the spermatozoa exposed to 100 nM curcumin for 30 min had significantly decreased level of MDA and increased acrosome reaction than that in the control group. These suggested that curcumin might improve asthenozoospermia by reducing ROS reproduction.
Previous studies have shown that curcumin could uncouple the keap1‐Nrf2 complex, an event which leads to the stabilisation of Nrf2 followed by its subsequent transport into cell nuclei, all of which leading to the transcription of several antioxidant genes (HO‐1, NQO‐1, GSH‐Px, CAT and SOD1) involved in antioxidant responses (Xie, Wu, Shen, Li, & Wu, 2018). But intriguingly, the spermatozoa only have nuclei with little cytoplasm surrounding the flagellum, making it difficult to observe the translocation of Nrf2 and explain the relation between Nrf2 and expression level of antioxidant genes. As a result, our study had certain limitations, but we demonstrated that curcumin upregulated Nrf2 expression of asthenozoospermia, indicating that curcumin improved asthenospermia through Nrf2. However, the detailed mechanism of this phenomenon remains to be further elucidated.
In short, our study demonstrated upregulation of Nrf2 induced by curcumin might protect spermatozoa of asthenozoospermic individuals through decreasing ROS production and apoptosis level. Our study broadens potential application of curcumin in clinical asthenozoospermia.
Zhou Q, Wu X, Liu Y, et al. Curcumin improves asthenozoospermia by inhibiting reactive oxygen species reproduction through nuclear factor erythroid 2‐related factor 2 activation. Andrologia. 2020;52:e13491 10.1111/and.13491
Qiao Zhou and Xun Wu are contributed equally.
Contributor Information
Hongshan Ge, Email: ge_hongshan@126.com.
Junqiang Zhang, Email: zhangjunqiang_njmu@126.com.
REFERENCES
- Aitken, R. J. , Harkiss, D. , & Buckingham, D. W. (1993). Analysis of lipid peroxidation mechanisms in human spermatozoa. Molecular Reproduction & Development, 35(3), 302 10.1002/mrd.1080350313 [DOI] [PubMed] [Google Scholar]
- Alizadeh, F. , Javadi, M. , Karami, A. A. , Gholaminejad, F. , Kavianpour, M. , & Haghighian, H. K. (2017). Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytotherapy Research Ptr, 32(4). [DOI] [PubMed] [Google Scholar]
- Castaneda, J. M. , Hua, R. , Miyata, H. , Oji, A. , Guo, Y. , Cheng, Y. , … Liu, M. (2017). TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proceedings of the National Academy of Sciences of the United States of America, 114(27), E5370–E5378. 10.1073/pnas.1621279114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, D. C. (2006). Mitochondria: Dynamic organelles in disease, aging, and development. Cell, 125(7), 1241–1252. 10.1016/j.cell.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Chan, J. Y. , & Chan, S. H. (2015). Activation of endogenous antioxidants as a common therapeutic strategy against cancer, neurodegeneration and cardiovascular diseases: A lesson learnt from DJ‐1. Pharmacology & Therapeutics, 156, 69–74. 10.1016/j.pharmthera.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Chanapiwat, P. , & Kaeoket, K. (2015). The effect of Curcuma longa extracted (curcumin) on the quality of cryopreserved boar semen. Animal Science Journal, 86(9), 863–868. 10.1111/asj.12395 [DOI] [PubMed] [Google Scholar]
- Cheng, Q. , Kalabus, J. L. , Zhang, J. , & Blanco, J. G. (2012). A conserved antioxidant response element (ARE) in the promoter of human carbonyl reductase 3 (CBR3) mediates induction by the master redox switch Nrf2. Biochemical Pharmacology, 83(1), 139–148. 10.1016/j.bcp.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curi, S. M. , Ariagno, J. I. , Chenlo, P. H. , Mendeluk, G. R. , Pugliese, M. N. , Sardi segovia, L. M. , … Blanco, A. M. (2003). Asthenozoospermia: Analysis of a large population. Archives of Andrology, 49(5), 343–349. 10.1080/01485010390219656 [DOI] [PubMed] [Google Scholar]
- Dai, C. , Ciccotosto, G. D. , Cappai, R. , Tang, S. , Li, D. , Xie, S. , … Velkov, T. (2018). Curcumin attenuates colistin‐induced neurotoxicity in N2a cells via anti‐inflammatory activity, suppression of oxidative stress, and apoptosis. Molecular Neurobiology, 55(1), 421–434. 10.1007/s12035-016-0276-6 [DOI] [PubMed] [Google Scholar]
- Evenson, D. P. , Darzynkiewicz, Z. , & Melamed, M. R. (1982). Simultaneous measurement by flow cytometry of sperm cell viability and mitochondrial membrane potential related to cell motility. Journal of Histochemistry and Cytochemistry, 30(3), 279–280. 10.1177/30.3.6174566 [DOI] [PubMed] [Google Scholar]
- Ferramosca, A. , Focarelli, R. , Piomboni, P. , Coppola, L. , & Zara, V. (2008). Oxygen uptake by mitochondria in demembranated human spermatozoa: A reliable tool for the evaluation of sperm respiratory efficiency. International Journal of Andrology, 31(3), 337–345. 10.1111/j.1365-2605.2007.00775.x [DOI] [PubMed] [Google Scholar]
- Ganan‐Gomez, I. , Wei, Y. , Yang, H. , Boyano‐Adanez, M. C. , & Garcia‐Manero, G. (2013). Oncogenic functions of the transcription factor Nrf2. Free Radical Biology and Medicine, 65, 750–764. 10.1016/j.freeradbiomed.2013.06.041 [DOI] [PubMed] [Google Scholar]
- Giacone, F. , Condorelli, R. A. , Mongioi, L. M. , Bullara, V. , La Vignera, S. , & Calogero, A. E. (2017). In vitro effects of zinc, D‐aspartic acid, and coenzyme‐Q10 on sperm function. Endocrine, 56(2), 408–415. 10.1007/s12020-016-1013-7 [DOI] [PubMed] [Google Scholar]
- Greco, E. , Iacobelli, M. , Rienzi, L. , Ubaldi, F. , Ferrero, S. , & Tesarik, J. (2005). Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. Journal of Andrology, 26(3), 349–353. 10.2164/jandrol.04146 [DOI] [PubMed] [Google Scholar]
- Griveau, J. F. , Le Lannou, D. (2010). Reactive oxygen species and human spermatozoa: Physiology and pathology. International Journal of Andrology, 20(2), 61–69. 10.1046/j.1365-2605.1997.00044.x [DOI] [PubMed] [Google Scholar]
- Hull, M. G. , Glazener, C. M. , Kelly, N. J. , Conway, D. I. , Foster, P. A. , Hinton, R. A. , … Desai, K. M. (1986). Population study of causes, treatment, and outcome of infertility. British Medical Journal, 292(6515), 272 10.1136/bmj.292.6515.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault, C. C. , Habbema, J. D. F. , Eijkemans, M. J. C. , Collins, J. A. , Evers, J. L. H. , & te Velde, E. R. (2004). Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Human Reproduction, 19(9), 2019–2026. 10.1093/humrep/deh365 [DOI] [PubMed] [Google Scholar]
- Kang, C. , Zixin, M. , Yulin, Z. , Xingcheng, G. , & Bolan, Y. (2012). Low NRF2 mRNA expression in spermatozoa from men with low sperm motility. Tohoku Journal of Experimental Medicine, 228(3), 259–266. 10.1620/tjem.228.259 [DOI] [PubMed] [Google Scholar]
- Kobori, Y. (2019). Home testing for male factor infertility: A review of current options. Fertility and Sterility, 111(5), 864–870. 10.1016/j.fertnstert.2019.01.032 [DOI] [PubMed] [Google Scholar]
- Lamond, S. , Watkinson, M. , Rutherford, T. , Laing, K. , Whiting, A. , Smallwood, A. , … Banerjee, S. (2003). Gene‐specific chromatin damage in human spermatozoa can be blocked by antioxidants that target mitochondria. Reproductive BioMedicine Online, 7(4), 407–418. 10.1016/S1472-6483(10)61884-6 [DOI] [PubMed] [Google Scholar]
- Li, X. , Tian, M. , Zhang, G. E. , Zhang, R. , Feng, R. , Guo, L. , … He, X. (2017). Spatially dependent fluorescent probe for detecting different situations of mitochondrial membrane potential conveniently and efficiently. Analytical Chemistry, 89(6), 3335–3344. 10.1021/acs.analchem.6b03842 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Zhou, Y. , Liu, R. , Lin, H. , Liu, W. , Xiao, W. , & Lin, Q. (2012). Effects of semen processing on the generation of reactive oxygen species and mitochondrial membrane potential of human spermatozoa. Andrologia, 44(3), 157–163. 10.1111/j.1439-0272.2010.01123.x [DOI] [PubMed] [Google Scholar]
- Menkveld, R. . (2010). Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian Journal of Andrology, 12(1), 47–58. 10.1038/aja.2009.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, B. N. , Lawson, G. , Chan, J. Y. , Banuelos, J. , Cortés, M. M. , Hoang, Y. D. , … Luderer, U. (2010). Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age‐dependent manner. Free Radical Biology & Medicine, 49(9), 1368–1379. 10.1016/j.freeradbiomed.2010.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz, R. K. , & Lough, M. L. (2014). Curcumin as a potential non‐steroidal contraceptive with spermicidal and microbicidal properties. European Journal of Obstetrics & Gynecology, 176(5), 142–148. 10.1016/j.ejogrb.2014.01.024 [DOI] [PubMed] [Google Scholar]
- Olayanju, A. , Copple, I. M. , Bryan, H. K. , Edge, G. T. , Sison, R. L. , Wong, M. W. , … Park, B. K. (2015). Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity‐implications for therapeutic targeting of Nrf2. Free Radical Biology and Medicine, 78, 202–212. 10.1016/j.freeradbiomed.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliccione, F. , Micillo, A. , Cordeschi, G. , D'Angeli, A. , Necozione, S. , Gandini, L. , … Francavilla, S. (2011). Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertility and Sterility, 95(2), 641–646. 10.1016/j.fertnstert.2010.07.1086 [DOI] [PubMed] [Google Scholar]
- Piomboni, P. , Focarelli, R. , Stendardi, A. , Ferramosca, A. , & Zara, V. (2012). The role of mitochondria in energy production for human sperm motility. International Journal of Andrology, 35(2), 109–124. 10.1111/j.1365-2605.2011.01218.x [DOI] [PubMed] [Google Scholar]
- Prabagaran, E. , Hansen, D. A. , Drury, A. M. , & Moley, K. H. (2014). Modulation of cell cycle progression in the spermatocyte cell line [GC‐2spd(ts) Cell‐Line] by cigarette smoke condensate (CSC) via arylhydrocarbon receptor‐nuclear factor erythroid 2‐related factor 2 (Ahr‐Nrf2) pathway. Biology of Reproduction, 90(1), 188–195. [DOI] [PubMed] [Google Scholar]
- Ross, C. , Morriss, A. , Khairy, M. , Khalaf, Y. , Braude, P. , Coomarasamy, A. , & El‐Toukhy, T. (2010). A systematic review of the effect of oral antioxidants on male infertility. Reproductive Biomedicine Online, 20(6), 711–723. 10.1016/j.rbmo.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Saeednia, S. , Nashtaei, M. S. , Bahadoran, H. , Aleyasin, A. , & Amidi, F. (2016). Effect of nerve growth factor on sperm quality in asthenozoosprmic men during cryopreservation. Reproductive Biology & Endocrinology, 14(1), 29 10.1186/s12958-016-0163-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin, K. , Orhan, C. , Tuzcu, Z. , Tuzcu, M. , & Sahin, N. (2012). Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO‐1 pathway in quail. Food and Chemical Toxicology, 50(11), 4035–4041. 10.1016/j.fct.2012.08.029 [DOI] [PubMed] [Google Scholar]
- Sahoo, D. K. , Roy, A. , & Chainy, G. B. (2008). Protective effects of vitamin E and curcumin on L‐thyroxine‐induced rat testicular oxidative stress. Chemico‐Biological Interactions, 176(2–3), 121–128. 10.1016/j.cbi.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Shah, N. A. , Khan, M. R. , Naz, K. , & Khan, M. A. (2014). Antioxidant potential, DNA protection, and HPLC‐DAD analysis of neglected medicinal Jurinea dolomiaea roots. Biomed Research International, 2014(726241), 726241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, S. , Wang, J. , Liang, J. , & He, D. (2013). Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World Journal of Urology, 31(6), 1395–1401. 10.1007/s00345-013-1023-5 [DOI] [PubMed] [Google Scholar]
- Sikka, S. C. , Rajasekaran, M. , & Hellstrom, W. J. (2008). Role of oxidative stress and antioxidants in male infertility. Toxicology Letters, 180(6), S204–S204. [PubMed] [Google Scholar]
- Tvrda, E. , Tusimova, E. , Kovacik, A. , Paal, D. , Greifova, H. , Abdramanov, A. , & Lukac, N. (2016). Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Animal Reproduction Science, 172, 10–20. 10.1016/j.anireprosci.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Xie, Z. , Wu, B. , Shen, G. , Li, X. , & Wu, Q. (2018). Curcumin alleviates liver oxidative stress in type 1 diabetic rats. Molecular Medicine Reports, 17(1), 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, L. , Ran, H. , Yuying, Y. , Jianmin, L. , Qixian, S. , & Jiahao, S. (2006). Human testicular protein NYD‐SP16 is involved in sperm capacitation and the acrosome reaction. Fertility & Sterility, 86(4 Suppl), 1228–1234. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Diao, R. Y. , Duan, Y. G. , Yi, T. H. , & Cai, Z. M. (2017). In vitro antioxidant effect of curcumin on human sperm quality in leucocytospermia. Andrologia, 49(10), e12760. [DOI] [PubMed] [Google Scholar]
- Zhang, S. H. , Zhang, J. H. , Ding, X. P. , Zhang, S. , Chen, H. H. , & Jing, Y. L. (2016). Association of polymorphisms in tektin‐t gene with idiopathic asthenozoospermia in Sichuan. China Journal of Assisted Reproduction and Genetics, 33(2), 181–187. 10.1007/s10815-015-0617-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


