Abstract
Purpose
To compare the efficacy, safety and stability of standard epithelium‐off cross‐linking (SCXL) versus accelerated epithelium‐off cross‐linking (ACXL) and transepithelial epithelium‐on cross‐linking (TCXL) in the treatment of progressive keratoconus (KC) in children.
Methods
This prospective multicentre controlled trial included 271 eyes (136 children) with grade 1–3 progressive KC who were randomized to undergo SCXL (n = 91, as a control group), ACXL (n = 92) or TCXL (n = 88). Uncorrected and corrected distance visual acuity, subjective refraction, pachymetry, keratometry and corneal topography measurements were recorded preoperatively and 6, 12 and 24 months postoperatively.
Results
At 1 year, there was no significant difference in uncorrected distance visual acuity, refractive sphere, cylinder, spherical equivalent or Kmax between the ACXL and SCXL groups; however, during year 2, ACXL regressed while SCXL continued to improve. After 2 years, there were significant differences in all visual, refractive and keratometric components between SCXL and both ACXL and TCXL (p < 0.0001) and between ACXL and TCXL (p < 0.0001). KC progressed in 5.4% of patients who had ACXL and 28.4% of those who had TCXL but in none of those who had SCXL. Vernal keratoconjunctivitis was documented in 43.3% of eyes that progressed postoperatively.
Conclusion
SCXL was more effective for paediatric KC and achieved greater stability than either ACXL or TCXL, and ACXL was superior to TCXL. SCXL also achieved marked improvement in both myopia and spherical equivalent; however, these refractive outcomes were unpredictable and uncontrollable. TCXL had a 28.4% failure rate within 2 years. SCXL is preferable for management of paediatric KC.
Keywords: accelerated CXL, keratoconus progression, paediatric keratoconus, standard cross‐linking, transepithelial CXL, vernal keratoconjunctivitis
Introduction
Keratoconus (KC) is a corneal ectatic disease characterized by progressive myopia and irregular astigmatism that leads to visual deterioration because of progressive irregular stromal thinning with cone formation. Keratoconus typically starts in the teenage years but is also known to affect children as young as 4 years of age (Sabti et al. 2015).
Keratoconus is usually more aggressive in children than in adults. It progresses rapidly and can be missed or misdiagnosed until in the advanced stages. Paediatric KC is sometimes associated with eye allergy, vernal keratoconjunctivitis (VKC), chronic eye rubbing and limbal stem cell deficiency (Leoni‐Mesplie et al. 2012). The management is the same as in adults; however, more active progression of KC with rapid loss of vision remains the challenge facing corneal surgeons. In the Middle East, the reported prevalence of KC is 0.9%–3.3% (Mukhtar & Ambati 2018). Keratoconus is three times more prevalent in Arabs than in Persians (Hashemi et al. 2013) and is also more prevalent in Arabs than in Caucasians (Kok et al. 2012). Furthermore, the prevalence rates of KC in certain areas of Egypt and the Gulf states may be higher than previously thought, given reports from these areas that show a prevalence ranging from 17.5% in Egypt to 24% in Saudi Arabia in patients seeking refractive surgery (El Rami et al. 2015; Saro et al. 2018).
Corneal cross‐linking of progressive keratoconus was introduced by Wollensak et al. (2003) in Germany. It is the first successful treatment modality to stop the progression of keratoconus by increasing the intrinsic corneal biomechanical stability. Their original standard cross‐linking (SCXL) procedure, sometimes being called the Dresden protocol, includes 30 min of ultraviolet‐A (UVA) irradiation of 3 mW/cm² surface irradiance for 30 min with the epithelium‐off. SCXL is an evidence‐based treatment with well‐documented long‐term efficacy in KC treatment (Caporossi et al. 2010; Raiskup et al. 2015).
In the last decade, a new generation of cross‐linking (CXL) techniques has been developed and undergone several modifications. Modified epithelium‐off CXL procedures, known as accelerated CXL (ACXL), have been devised to shorten the CXL time by increasing the irradiance intensity (Mita et al. 2014; Medeiros et al. 2016). Transepithelial accelerated CXL (TCXL) has also been developed to avoid removal of epithelium, shorten the procedure time and decrease the risk of postoperative complications (Caporossi et al. 2012a; Hersh et al. 2018).
The outcomes of ACXL and TCXL are not fully established, especially in paediatric KC. The primary aim of this study was to compare the efficacy and long‐term stability of ACXL and TCXL with that of SCXL in the treatment of paediatric KC by documenting the development of corneal ectasia (Kmax > 1D) after cross‐linking. The secondary aim was to assess visual and refractive changes and potential side effects.
Patients and Methods
The protocol for this prospective comparative multicentre randomized controlled trial was approved by the ethics committee of Sohag Faculty of Medicine, Sohag University, Egypt and conducted in accordance with the tenets of the Declaration of Helsinki. The study is registered at the Pan African Clinical Trial Registry (PACTR201804003282725). All surgeries were performed at three major private Egyptian eye centres in Sohag, Zagazig and Cairo.
The study included 136 paediatric patients (271 eyes) with KC. The progressive nature of the disease, its manifestations and treatment options were carefully explained to all parents. All parents were made aware of the type of CXL their child would receive and its possible consequences. All parents provided signed informed consent before surgery.
The inclusion criteria were age < 18 years, documented progressive KC (grade 1, 2 or 3 according to the Amsler‐Krumeich classification) and a corneal thickness at the thinnest location of > 400 μm. The exclusion criteria were advanced KC, severe dry eye disease, preoperative corneal opacity and concomitant ocular infection or pathology. Progression of KC was confirmed by an increase in Kmax readings of ˃1 D.
The patients were examined preoperatively by history‐taking and screening for VKC and chronic eye rubbing. All included eyes underwent a comprehensive ophthalmic examination consisting of assessment of uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), subjective refraction, slit‐lamp evaluation and Scheimpflug corneal topography for pachymetry and keratometry. The study outcomes were UDVA, CDVA, the refractive parameters (spherical, astigmatic and SE measures) and topographic parameters (Kmax and corneal thickness at the thinnest location).
The devices used to obtain the measurements were the Pentacam HR corneal topographer (Pentacam HR; Oculus Inc., Wetzlar, Germany), Opto XLink corneal crosslinking system (Opto Global Pty Ltd., Adelaide, Australia) and Avedro KXL system (Avedro Inc., Burlington, MA, USA).
Surgical procedures
The enrolled eyes were randomized in approximately equal numbers to an SCXL (control) group (n = 91, 46 patients), an ACXL group (n = 92, 46 patients) or a TCXL group (n = 88, 44 patients). Both eyes in each patient were cross‐linked using the same CXL procedure (e.g. SCXL) after previous random assignment to a CXL method. All patients received topical anaesthesia, that is benoxinate hydrochloride 0.4% eye drops (Benox 4%; Eipico Inc., Cairo, Egypt), instilled every 5 minutes for half an hour preoperatively.
SCXL group
An 8‐mm marker zone was used to delineate the central 8‐mm corneal zone. The corneal epithelium within the 8‐mm central zone was removed manually using a blunt hockey knife. Riboflavin 0.1% solution with dextran (Ricrolin; Sooft Italia S.p.A., Montegiorgio FM, Italy) was applied to the cornea every 3 min for 30 min. The cornea was then irradiated by continuous UVA irradiation (total surface irradiance, 5.4 J/cm2; power, 3 mW/cm2; irradiance, 2.984 mW/cm2) for 30 min using the Opto XLink crosslinking system. Riboflavin solution was applied at 2‐minute intervals during irradiation. At the end of the procedure, the corneas were irrigated. Contact lenses were then worn until re‐epithelialization was complete.
ACXL group
The corneal epithelium was scraped as in the SCXL group. Riboflavin ophthalmic solution 0.1% with hydroxypropyl methylcellulose (VibeX Rapid; Avedro Inc.) was applied at 2‐min intervals to complete a total of 10 min of soaking time. Next, the corneas were irradiated with UVA using the Avedro KXL system. The Avedro nomogram was used with a power of 30 mW/cm2 to deliver 7.2 J/cm2 of total energy using the pulsed mode (1 second on, 1 second off) for a total treatment time of 8 min to achieve 4 min of UVA time. VibeX Rapid solution was applied to the corneal surface at 2‐min intervals during irradiation. The next steps were completed as for SCXL.
TCXL group
Riboflavin 0.25%, benzalkonium chloride and hydroxypropyl methylcellulose (ParaCel, Avedro Inc.) were applied to the intact, epithelium‐on cornea for 4.5 min, with riboflavin applied at a rate of 1 drop every 90 seconds. Dextran‐free isotonic riboflavin 0.22% (VibeX Xtra, Avedro Inc.) was then applied for 6 min at a rate of 1 drop every 90 seconds, followed by UVA irradiation of the cornea using the Avedro KXL system. The Avedro nomogram was used at 45 mW/cm2 power to deliver a total energy of 7.2 J/cm2 using the pulsed mode (1 second, 1 second off) for a total treatment time of 5:20 minutes to achieve 2:40 minutes of UVA time.
Ophthalmic medication and follow‐up
All eyes in the SCXL and ACXL groups received the same topical and oral therapy postoperatively. The topical therapy comprised gatifloxacin 0.3% eye drops (Zymar; Allergan, Madison, NJ, USA), prednisolone acetate 1% eye drops (Pred Forte; Allergan) and lubricant eye drops (Systane Ultra, Alcon Laboratories, Fort Worth, TX, USA) every 2 hr on postoperative day 1 and then five times daily for the rest of the first postoperative week. The contact lenses were removed when corneal re‐epithelialization was complete. The antibiotic eye drops were discontinued in the second postoperative week, and the steroid and lubricant eye drops were tapered gradually in the second and third postoperative weeks. In the TCXL group, the only postoperative treatment was topical gatifloxacin 0.3% five times daily for 1 week, prednisolone acetate 1% and lubricant eye drops five times, three times and twice daily in postoperative weeks 1, 2 and 3, respectively.
Follow‐up Pentacam corneal topography was scheduled at 6, 12 and 24 months postoperatively. The postoperative data collected included UDVA, CDVA, myopic and astigmatic values, SE, Kmax and corneal thickness at the thinnest location. Slit‐lamp examination was performed at all follow‐up visits to check for complications, such as corneal haze and opacification. Corneal haze was graded on a 6‐point scale (0, none; 0.5, faint; 1, mild; 2, moderate; 3, severe or opacity obscuring details of the iris; 4, marked, seen without slit‐lamp examination) (Kim et al. 2004).
In case that any of the study eyes became complicated with postoperative KC progression (Kmax > 1D) due to primary treatment failure; retreatment with CXL will be performed for these eyes as soon as possible even during the study period to avoid further deterioration of the children's corneas. In such cases, we planned to statistically analyse the data of these eyes by using the last observation carried forward (LOCF) principle; before being retreated with CXL, via conserving the data of our last observation of each case in the subsequent study time points.
Statistical analysis
Quantitative data are shown as the mean ± standard deviation and were compared between the three groups using analysis of variance (anova) and the Bonferroni post hoc test. Three groups were compared using the Kruskal–Wallis test and two groups by the Mann–Whitney test. Qualitative data are shown as the number (percentage) and were compared using the chi‐square test. The data obtained preoperatively and at 6, 12 and 24 months were compared using repeated‐measures anova. Sphericity was examined using Mauchly's test of sphericity. The Bonferroni post hoc test was used to compare the differences at each time point. Kaplan–Meier curves were constructed using the Excel program and differences examined for statistical significance using the log‐rank test. The data were analysed using SPSS for Windows (version 16; IBM Corp., Armonk, NY, USA). A p‐value < 0.05 was considered statistically significant.
Results
The study included 271 eyes with progressive KC in 136 patients (67 male, 69 female; mean age 14.36 ± 2.11 [9–17] years). The eyes were divided into an SCXL group (n = 91), an ACXL group (n = 92), and a TCXL group (n = 88). All children were screened for VKC and chronic eye rubbing, which are known to be risk factors for progression of KC. The demographic and descriptive statistics are shown in Table 1. There were no significant between‐group differences.
Table 1.
Characteristics of the study population
| Variable |
SCXL N = 91 eyes |
ACXL N = 92 eyes |
TCXL N = 88 eyes |
P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| Age/years | |||||||
| Mean ± SD | 14.13 ± 2.18 | 14.4 ± 2.09 | 14.57 ± 2.03 | 0.72 | 1.00 | 1.00 | 1.00 |
| Median (range) | 15 (9:17) | 15 (9:17) | 15 (10:17) | ||||
| Gender | |||||||
| Patients (136) | 46 | 46 | 44 | 0.56 | 0.84 | 0.60 | 0.79 |
| Male (67) | 23 (50%) | 24 (52.17%) | 20 (45.45%) | ||||
| Female (69) | 23 (50%) | 22 (47.83%) | 24 (54.55%) | ||||
| Preoperative history of VKC/RUBBING | |||||||
| Patients (136) | 46 | 46 | 44 | 0.44 | 0.83 | 0.58 | 0.86 |
| No (98) | 31 (67.39%) | 34 (73.91%) | 33 (75.00%) | ||||
| Yes (38) | 15 (32.61%) | 12 (26.09%) | 11 (25.00%) | ||||
| Preoperative KC grading | |||||||
| Eyes (271) | 91 | 92 | 88 | 0.24 | 0.32 | 0.10 | 0.38 |
| Grade 1 (58) | 15 (16.48%) | 19 (20.65%) | 22 (25.00%) | ||||
| Grade 2 (128) | 47 (51.65%) | 42 (45.65%) | 41 (46.59%) | ||||
| Grade 3 (85) | 29 (31.87%) | 31 (33.70%) | 25 (28.41%) | ||||
P compared the 3 groups, P1 compared SCXL & ACXL, P2 compared SCXL & TCXL, P3 compared ACXL & TCXL.
Visual, refractive and topographic outcomes
In the SCXL group, significant improvements in UDVA and CDVA were found at all postoperative assessments (p < 0.0001, Table 2). By month 24, the respective mean UDVA and CDVA values had improved from 1.11 ± 0.43 and 0.47 ± 0.40 logMAR to 0.85 ± 0.34 and 0.23 ± 0.25 logMAR, respectively (p < 0.0001). Improvements in UDVA and CDVA were stable and steady during follow‐up (Table 2). All refractive measures, that is sphere, cylinder and SE, in the SCXL group showed significant improvements of 1.08 ± 1.11 D, 0.31 ± 0.19 D and 1.24 ± 1.12 D, respectively, by month 24 (p < 0.0001). The improvements in the refractive values remained stable during follow‐up (Table 2). There was also a significant number of eyes, 64 eyes (70.3%), experiencing a reduction of Kmax > 1 D (p < 0.0001) whereas 27 (29.7%) showed more or less a stability of Kmax during the 24‐month follow‐up. There was a significant decrease in corneal thickness of 8.9 ± 14.95 μm (p = 0.003) at the end of follow‐up (Table 2).
Table 2.
Preoperative and postoperative visual, refractive, topographic and tomographic data analysis in the standard epithelium‐off cross‐linking (SCXL) group
| Variable |
Preoperative Mean ± SD |
Postoperative 6th month Mean ± SD |
Postoperative 12th month Mean ± SD |
Postoperative 24th month Mean ± SD |
Difference (post–pre) Mean ± SD (95% CI) |
|---|---|---|---|---|---|
| Visual outcomes | |||||
| UDVA | 1.11 ± 0.43 | 0.97 ± 0.8 | 0.93 ± 0.35 | 0.85 ± 0.34 | −0.26 ± 0.12 (−0.30: −0.21) |
| P1 < 0.0001, P2 < 0.0001, P3 < 0.0001, P4 = 0.047, P5 < 0.0001, P6 < 0.0001 | |||||
| CDVA | 0.47 ± 0.40 | 0.40 ± 0.37 | 0.27 ± 0.29 | 0.23 ± 0.25 | −0.24 ± 0.18 (−0.32: −0.18) |
| P1 < 0.0001, P2 < 0.0001, P3 < 0.0001, P4 < 0.0001, P5 < 0.0001, P6 = 0.001 | |||||
| Refractive outcomes | |||||
| Sphere | −3.88 ± 2.65 | −3.51 ± 2.53 | −3.28 ± 2.45 | −2.79 ± 2.12 | 1.09 ± 1.11 (0.67:1.49) |
| P1 < 0.0001, P2 < 0.0001, P3 < 0.0001, P4 = 0.002, P5 = 0.005, P6 = 0.06 | |||||
| Cylinder | −3.32 ± 1.37 | −3.25 ± 1.36 | −3.11 ± 1.42 | −3.01 ± 1.40 | 0.31 ± 0.19 (0.24:0.38) |
| P1 = 0.08, P2 < 0.0001, P3 < 0.0001, P4 = 0.03, P5 < 0.0001, P6 = 0.02 | |||||
| SE | −5.53 ± 2.99 | −5.13 ± 2.85 | −4.84 ± 2.80 | −4.30 ± 2.50 | 1.23 ± 1.12 (0.82:1.65) |
| P1 < 0.0001, P2 < 0.0001, P3 < 0.0001, P4 < 0.0001, P5 = 0.001, P6 = 0.04 | |||||
| Topographic outcomes: | |||||
| Kmax | 50.78 ± 3.82 | 50.35 ± 3.70 | 50.18 ± 3.62 | 49.61 ± 3.67 | −1.17 ± 1.01 (−1.54: −0.79) |
| P1 = 0.003, P2 < 0.0001, P3 < 0.0001, P4 = 0.04, P5 < 0.0001, P6 < 0.0001 | |||||
| Pachymetry | 453.07 ± 30.55 | 449.53 ± 30.79 | 448.47 ± 30.56 | 444.17 ± 33.25 | −8.9 ± 14.95 (−14.48: −3.32) |
| P1 < 0.0001, P2 = 0.04, P3 = 0.003, P4 = 1.00, P5 = 1.00, P6 = 1.00 | |||||
P1 compared pre and post 6 ms, P2 compared pre and post 12 ms, P3 compared pre and post 24 ms, P4 compared. post 6 ms and post 12 ms, P5 compared post 6 ms and post 24 ms, P6 compared post 12 ms and post 24 ms, and CI is confidence of interval
In the ACXL group, there was a significant improvement in mean UDVA and CDVA at months 6 (p = 0.01 and p = 0.03, respectively) and 12 (p = 0.007 and p = 0.01) but significant regression had occurred by month 24 (p = 0.009 and p = 0.01; Table 3). By the end of the study, the mean overall improvement in UDVA (0.04 ± 0.22 logMAR) and CDVA (0.03 ± 0.21 logMAR) was not significant (p = 0.48 and p = 0.09, respectively). There was neither improvement in mean sphere or SE values at month 24 (p = 0.40 and p = 0.82, respectively) after an initial significant improvement at months 6 (p = 0.04 and p = 0.03) and 12 (p = 0.01 and p = 0.01; Table 3). However, the mean postoperative cylinder remained stable throughout the study, albeit with no significant improvement (p = 1.00). The mean postoperative Kmax also remained stable but the final improvement at 2 years was not significant (p = 1.00) despite significant improvements at postoperative months 6 and 12 (p = 0.03 and p = 0.01, respectively). In this group, 47 eyes (51.1%) showed improvement in Kmax readings up to 1 D at month 12; however, only 28 eyes (30.4%) maintained this improvement at month 24. The postoperative changes in corneal thickness were not significant at the end of follow‐up (p = 1.00).
Table 3.
Preoperative and postoperative visual, refractive, topographic and tomographic data analysis in the accelerated epithelium‐off cross‐linking (ACXL) group
| Variable |
Preoperative Mean ± SD |
Postoperative 6th month Mean ± SD |
Postoperative 12th month Mean ± SD |
Postoperative 24th month Mean ± SD |
Difference (post–pre) Mean ± SD (95% CI) |
|---|---|---|---|---|---|
| Visual outcomes: | |||||
| UDVA | 0.97 ± 0.26 | 0.88 ± 0.24 | 0.81 ± 0.25 | 0.93 ± 0.28 | −0.04 ± 0.22 (−0.11:0.06) |
| P1 = 0.01, P2 = 0.007, P3 = 0.48, P4 = 0.02, P5 = .04, P6 = 0.009 | |||||
| CDVA | 0.41 ± 0.20 | 0.35 ± 0.19 | 0.30 ± 0.21 | 0.38 ± 0.28 | −0.03 ± 0.21 (−0.08:0.08) |
| P1 = 0.03, P2 = 0.01, P3 = 0.09, P4 = 0.03, P5 = 0.54, P6 = 0.01 | |||||
| Refractive outcomes: | |||||
| Sphere | −3.46 ± 2.28 | −3.21 ± 2.29 | −2.97 ± 2.36 | −3.26 ± 2.63 | 0.20 ± 0.74 (−0.21:0.38) |
| P1 = 0.04, P2 = 0.01, P3 = 0.40, P4 = 0.03, P5 = 1.00, P6 = 0.04 | |||||
| Cylinder | −3.53 ± 1.15 | −3.44 ± 1.14 | −3.32 ± 1.18 | −3.52 ± 1.24 | 0.01 ± 0.45 (−0.17:0.20) |
| P1 = 0.52 P2 = 0.09, P3 = 1.00, P4 = 1.00, P5 = 1.00, P6 = 0.86 | |||||
| SE | −5.23 ± 2.24 | −4.93 ± 2.25 | −4.67 ± 2.32 | −5.03 ± 2.64 | 0.20 ± 0.92 (−0.25:0.45) |
| P1 = 0.03, P2 = 0.01, P3 = 0.82, P4 = 0.04, P5 = 1.00, P6 = 0.06 | |||||
| Topographic outcomes: | |||||
| Kmax | 50.70 ± 3.51 | 50.38 ± 3.49 | 50.12 ± 3.53 | 50.47 ± 3.72 | −0.23 ± 1.17 (−0.60:0.72) |
| P1 = 0.03, P2 = 0.01, P3 = 1.00, P4 = 0.02, P5 = 0.85, P6 = 1.00 | |||||
| Pachymetry | 453.47 ± 41.67 | 452.33 ± 41.45 | 449.97 ± 41.54 | 449.76 ± 45.80 | −3.71 ± 7.76 (−12.24:4.79) |
| P1 = 0.74, P2 = 0.01, P3 = 1.00, P4 = 0.08, P5 = 0.04, P6 = 1.00 | |||||
P1 compared pre and post 6 ms, P2 compared pre and post 12 ms, P3 compared pre and post 24 ms, P4 compared post 6 ms and post 12 ms, P5 compared post 6 ms and post 24 ms, P6 compared post 12 ms and post 24 ms, and CI is confidence of interval
In the TCXL group, there was worsening of both UDVA and CDVA during the study period (Table 4). The mean preoperative UDVA and CDVA values were 0.99 ± 0.23 logMAR and 0.46 ± 0.13 logMAR, respectively, but worsened to 1.16 ± 0.30 logMAR and 0.60 ± 0.21 logMAR at month 24 (p < 0.0001). The deterioration in these components became significant by month 6. Similarly, the mean refractive components showed deterioration that became significant at month 12 (Table 4). Topographic readings revealed a significant progression in mean Kmax from 50.69 ± 1.51 D preoperatively to 51.61 ± 1.86 D at month 24 (p < 0.0001). Kmax showed significant progression (p = 0.02 at month 12; Table 4). The mean pachymetry value had decreased by 6.75 ± 9.02 μm (p < 0.0001) after 2 years (Table 4).
Table 4.
Preoperative and postoperative visual, refractive, topographic and tomographic data analysis in the accelerated epithelium‐on cross‐linking (TCXL) group
| Variable |
Preoperative Mean ± SD |
Postoperative 6th month Mean ± SD |
Postoperative 12th month Mean ± SD |
Postoperative 24th month Mean ± SD |
Difference (post–pre) Mean ± SD (95% CI) |
|---|---|---|---|---|---|
| Visual outcomes: | |||||
| UDVA | 0.99 ± 0.23 | 1.02 ± 0.23 | 1.03 ± 0.26 | 1.16 ± 0.30 | 0.17 ± 0.09 (0.08:0.21) |
| P1 = 0.001, P2 = 0.02, P3 < 0.0001, P4 = 1.00, P5 < 0.0001, P6 = 0.001 | |||||
| CDVA | 0.46 ± 0.13 | 0.50 ± 0.15 | 0.50 ± 0.18 | 0.60 ± 0.21 | 0.14 ± 0.08 (0.07:0.18) |
| P1 = 0.001, P2 = 0.02, P3 < 0.0001, P4 = 01.00, P5 < 0.0001, P6 = 0.003 | |||||
| Refractive outcomes: | |||||
| Sphere | −3.56 ± 1.37 | −3.59 ± 1.42 | −3.79 ± 1.48 | −4.13 ± 1.51 | −0.57 ± 0.63 (−0.82: −0.15) |
| P1 = 1.00, P2 = 0.10, P3 < 0.0001, P4 = 0.30, P5 = 0.006, P6 = 0.01 | |||||
| Cylinder | −3.14 ± 1.48 | −3.21 ± 1.48 | −3.46 ± 1.60 | −3.67 ± 1.66 | −0.53 ± 0.58 (−0.74: −0.26) |
| P1 = 0.18, P2 = 0.005, P3 = 0.0006, P4 = 0.03, P5 = 0.002, P6 = 0.05 | |||||
| SE | −5.13 ± 1.57 | −5.20 ± 1.60 | −5.52 ± 1.72 | −5.97 ± 1.81 | −0.84 ± 0.92 (−1.23: −0.30) |
| P1 = 0.79, P2 = 0.04, P3 < 0.0001, P4 = 0.10, P5 < 0.0001, P6 = 0.007 | |||||
| Topographic outcomes: | |||||
| Kmax | 50.69 ± 1.51 | 50.71 ± 1.48 | 51.03 ± 1.59 | 51.61 ± 1.86 | 0.92 ± 1.15 (0.19:1.46) |
| P1 = 1.00, P2 = 0.02, P3 < 0.0001, P4 = 0.008, P5 = 0.04, P6 = 0.03 | |||||
| Pachymetry | 454.13 ± 26.89 | 453.57 ± 26.65 | 451.99 ± 26.78 | 447.38 ± 32.62 | −6.75 ± 9.02 (−15.61: −3.00) |
| P1 = 0.43, P2 = 0.002, P3 < 0.0001, P4 = 0.03, P5 < 0.0001, P6 < 0.0001 | |||||
P1 compared pre and post 6 ms, P2 compared pre and post 12 ms, P3 compared pre and post 24 ms, P4 compared post 6 ms and post 12 ms, P5 compared post 6 ms and post 24 ms, P6 compared post 12 ms and post 24 ms, and CI is confidence of interval
Between‐group comparisons
There were no significant between‐group differences in the mean values for the study parameters at baseline. However, there were significant differences in the mean UDVA and CDVA values between the three groups throughout the study (p < 0.0001) in favour of SCXL followed by ACXL. Similarly, the SE, sphere and cylinder refractions showed significant differences at all time points in favour of SCXL (Table 5). Figure 1 shows the differences in postoperative changes at each time point in the three groups.
Table 5.
Analysis of visual and refractive changes between the three treatment groups.
| Change | SCXL N = 91 |
ACXL N = 92 |
TCXL N = 88 |
P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| UDVA | |||||||
| Preoperative, Mean ± SD | 1.11 ± 0.43 | 0.97 ± 0.26 | 0.99 ± 0.23 | 0.34 | 0.17 | 0.26 | 0.78 |
| Post‐6 ms–Preoperative, Mean ± SD | −0.14 ± 0.07 | −0.09 ± 0.05 | 0.03 ± 0.05 | 0.0001 | 0.007 | 0.0001 | 0.0001 |
| Post‐12 ms–Preoperative, Mean ± SD | −0.18 ± 0.11 | −0.16 ± 0.05 | 0.04 ± 0.08 | 0.0001 | 0.09 | 0.0001 | 0.0001 |
| Post‐24 ms–Preoperative, Mean ± SD | −0.26 ± 0.12 | −0.04 ± 0.22 | 0.17 ± 0.09 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| CDVA | |||||||
| Preoperative, Mean ± SD | 0.47 ± 0.40 | 0.41 ± 0.20 | 0.46 ± 0.13 | 0.58 | 0.87 | 0.33 | 0.42 |
| Post‐6 ms–Preoperative, Mean ± SD | −0.07 ± 0.07 | −0.06 ± 0.04 | 0.04 ± 0.04 | 0.0001 | 0.08 | 0.0001 | 0.0005 |
| Post‐12 ms–Preoperative, Mean ± SD | −0.20 ± 0.16 | −0.11 ± 0.07 | −0.04 ± 0.06 | 0.0001 | 0.004 | 0.0001 | 0.0003 |
| Post‐24 ms–Preoperative, Mean ± SD | −0.24 ± 0.18 | −0.03 ± 0.21 | 0.14 ± 0.08 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Sphere | |||||||
| Preoperative, Mean ± SD | −3.88 ± 2.65 | −3.46 ± 2.28 | −3.56 ± 1.37 | 0.67 | 0.50 | 0.51 | 0.50 |
| Post‐6 ms–Preoperative, Mean ± SD | 0.37 ± 0.28 | 0.25 ± 0.12 | −0.03 ± 0.23 | 0.0001 | 0.02 | 0.0001 | 0.004 |
| Post‐12 ms–Preoperative, Mean ± SD | 0.60 ± 0.35 | 0.49 ± 0.24 | −0.23 ± 0.50 | 0.0001 | 0.06 | 0.0001 | 0.0001 |
| Post‐24 ms–Preoperative, Mean ± SD | 1.09 ± 1.11 | 0.20 ± 0.74 | −0.57 ± 0.63 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Cylinder | |||||||
| Preoperative, Mean ± SD | −3.32 ± 1.37 | −3.53 ± 1.15 | −3.14 ± 1.48 | 0.32 | 0.28 | 0.54 | 0.17 |
| Post‐6 ms–Preoperative, Mean ± SD | 0.07 ± 0.20 | 0.09 ± 0.18 | −0.07 ± 0.16 | 0.003 | 0.87 | 0.008 | 0.006 |
| Post‐12 ms–Preoperative, Mean ± SD | 0.21 ± 0.16 | 0.21 ± 0.23 | −0.32 ± 0.44 | 0.0001 | 0.94 | 0.0001 | 0.0001 |
| Post‐24 ms–Preoperative, Mean ± SD | 0.31 ± 0.19 | 0.01 ± 0.45 | −0.53 ± 0.58 | <0.0001 | 0.001 | 0.0001 | 0.0001 |
| SE | |||||||
| Preoperative, Mean ± SD | −5.53 ± 2.99 | −5.23 ± 2.24 | −5.13 ± 1.57 | 0.95 | 0.94 | 0.68 | 0.88 |
| Post‐6 ms–Preoperative, Mean ± SD | 0.40 ± 0.28 | 0.30 ± 0.16 | −0.07 ± 0.23 | 0.0001 | 0.08 | 0.0001 | 0.0001 |
| Post‐12 ms–Preoperative, Mean ± SD | 0.69 ± 0.35 | 0.65 ± 0.27 | −0.39 ± 0.65 | 0.0001 | 0.71 | 0.0001 | 0.0001 |
| Post‐24 ms–Preoperative, Mean ± SD | 1.23 ± 0.12 | 0.20 ± 0.92 | −0.84 ± 0.92 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
P compared the 3 groups, P1 compared SCXL & ACXL, P2 compared SCXL & TCXL, P3 compared ACXL & TCXL.
Figure 1.
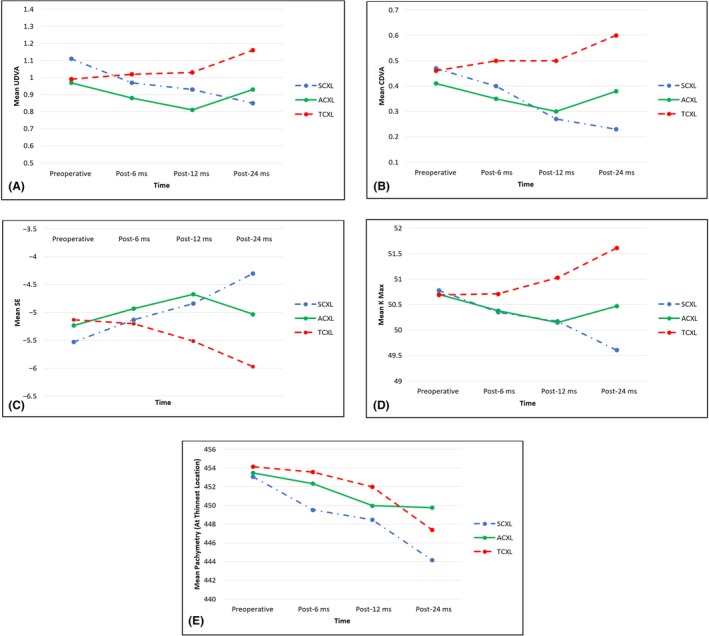
Comparison of visual, refractive and topographic pathways in paediatric patients during 24 months of follow‐up after three different cross‐linking procedures. A, UDVA pathway; B, CDVA pathway; C, spherical equivalent pathway; D, Kmax pathway; E, corneal thickness at the thinnest location pathway. ACXL, accelerated epithelium‐off cross‐linking; CDVA, corrected distance visual acuity; SCXL, standard epithelium‐off cross‐linking; TCXL, accelerated epithelium‐on cross‐linking; UDVA, uncorrected distance visual acuity
Topographic changes up to 12 months
The differences in Kmax between the SCXL and ACXL groups were not significant at month 6 (p = 0.38) or month 12 (p = 1.00). However, there were statistically significant differences in all the mean postoperative Kmax readings in favour of the SCXL and ACXL groups when compared with the TCXL group at months 6 and 12 (both p < 0.0001; Table 6, Figs 1, 2A,B). Furthermore, there were statistically significant between‐group differences in mean corneal thickness at the thinnest location at months 6 (p = 0.01) and 12 (p = 0.02; Table 6).
Table 6.
Analysis of topographic and tomographic changes between the three treatment groups.
| Variable | SCXL N = 91 |
ACXL N = 92 |
TCXL N = 88 |
P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| K max | |||||||
| Preoperative, Mean ± SD | 50.78 ± 3.82 | 50.70 ± 3.51 | 50.69 ± 1.51 | 0.99 | 1.00 | 1.00 | 1.00 |
| Post‐6 ms–Preoperative, Mean ± SD | −0.43 ± 0.65 | −0.32 ± 0.53 | 0.02 ± 0.10 | 0.0001 | 0.38 | 0.0001 | 0.0001 |
| Post‐12 ms–Preoperative Mean ± S | −0.60 ± 0.85 | −0.58 ± 0.93 | 0.34 ± 0.45 | <0.0001 | 1.00 | <0.0001 | <0.0001 |
| Post‐24 ms–Preoperative, Mean ± SD | −1.17 ± 1.01 | −0.23 ± 1.17 | 0.92 ± 1.15 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Pachymetry | |||||||
| Preoperative, Mean ± SD | 453.07 ± 30.55 | 453.47 ± 41.67 | 454.13 ± 26.89 | 0.99 | 1.00 | 1.00 | 1.00 |
| Post‐6 ms–Preoperative, Mean ± SD | −3.54 ± 2.54 | −1.14 ± 3.34 | −0.56 ± 1.63 | 0.01 | 0.02 | 0.0001 | 0.60 |
| Post‐12 ms–Preoperative, Mean ± SD | −4.6 ± 8.83 | −3.5 ± 7.85 | −2.14 ± 3.94 | 0.02 | 0.04 | 0.009 | 0.08 |
| Post‐24 ms–Preoperative, Mean ± SD | −8.9 ± 14.95 | −3.71 ± 7.76 | −6.75 ± 9.02 | 0.03 | 0.01 | 0.06 | 0.04 |
P compared the 3 groups, P1 compared SCXL & ACXL, P2 compared SCXL & TCXL, P3 compared ACXL & TCXL.
Figure 2.
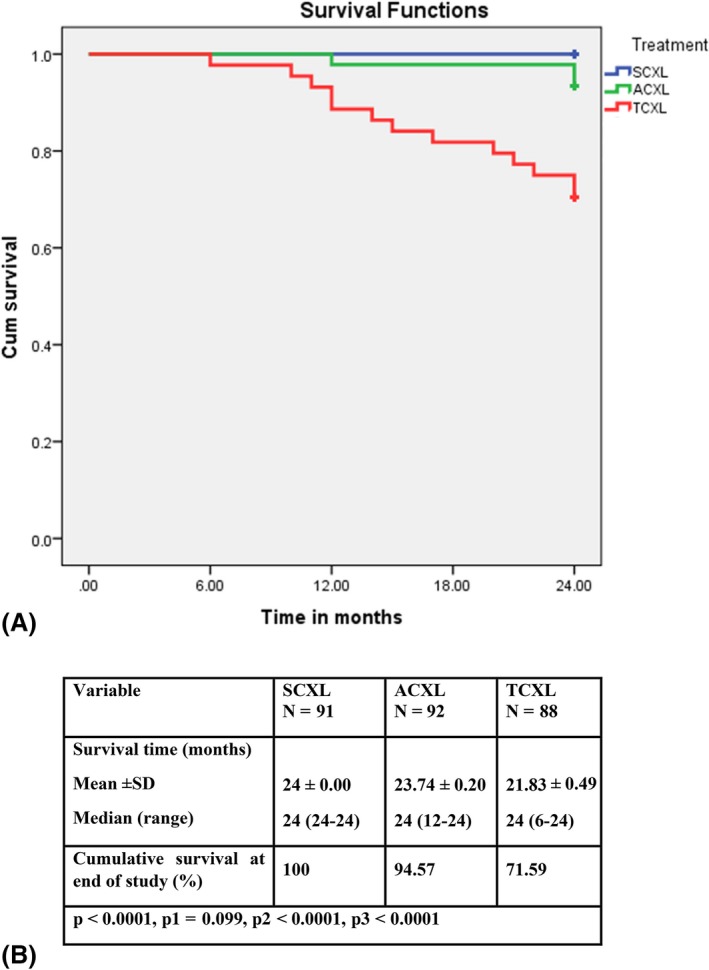
Kaplan–Meier analysis; A, Kaplan–Meier survival curves (showing ability of the cornea to survive functionally after halting of progression of keratoconus by CXL) for the three study groups depending on postoperative Kmax progression (˃1 D); B, Mean postoperative survival time (months during which CXL successfully halted progression of keratoconus and after which CXL failure was documented or the study ended) in the three study groups. Log‐rank test. P, difference between the three groups; P1, difference between SCXL and ACXL; P2, difference between SCXL and TCXL; P3, difference between ACXL and TCXL; ACXL, accelerated epithelium‐off cross‐linking; SCXL, standard epithelium‐off cross‐linking; TCXL, accelerated epithelium‐on cross‐linking
Topographic changes at 24 months
There were significant differences in the postoperative Kmax values between the SCXL group and the other two groups (p < 0.0001). At month 24, the improvement in corneal flattening was significantly greater in the SCXL group than in the ACXL group, with respective mean reductions in Kmax of 1.17 ± 1.01 D and 0.23 ± 1.17 D (p = 0.0001); in contrast, there was a significant increase in Kmax of 0.92 ± 1.15 D in the TCXL group (p < 0.0001), indicating marked deterioration. All K readings were significantly better after ACXL than after TCXL month 24 (p=<0.0001; Table 6, Figs 1, 2A,B). There were also significant differences in the mean postoperative changes in corneal thickness between the three groups (p = 0.03; Fig. 1).
Complications
The management and outcomes of both early and late postoperative complications in the three groups are shown in Table 7.
Table 7.
Early and late postoperative complications in the three study groups
| Complications |
SCXL N = 91 |
ACXL N = 92 |
TCXL N = 88 |
Management | Fate |
|---|---|---|---|---|---|
| Photophobia, pain and watery eyes | 37 (40.7%) | 28 (30.4%) | 2 (2.3%) |
|
Resolved in 24–48 hours |
| Delay in epithelial healing | 17 (18.7%) | 4 (4.3%) | zero (0.0%) |
|
All eyes showed complete re‐epithelization in the 2nd week except 2 eyes in SCXL group |
| Persistent epithelial defect (PED) | 2 (2.2%) | zero (0.0%) | zero (0.0%) |
|
One eye showed complete re‐epithelization with haze in the 3rd month. The other eye herald with remaining corneal oedema, haze and opacification |
| Corneal stromal opacity | 1 (1.1%) | zero (0.0%) | zero (0.0%) |
|
The condition ended in permanent central corneal stromal opacity after 6 months with final CDVA 1.2 logMAR (preoperative CDVA 0.4 logMAR) |
| Corneal haze |
57 (62.6%):
|
21 (22.8%):
|
zero (0.0%) |
|
All eyes showed complete recovery within 6 months except one eye in SCXL group that ended in permanent stromal opacity |
| KC progression | zero (0.0%) | 5 (5.4%) | 25 (28.4%) |
|
The retreated eyes showed good stability till the end of the study |
Progression of KC
Progression of KC was documented after ACXL and TCXL but not after SCXL (Table 7). Progression was documented in 5 eyes (5.4%) in the ACXL group (2 at month 12 and 3 at month 24) and in 25 eyes (28.4%) in the TCXL group (1 each at months 11 and 22; 2 each at months 6, 10, 15, 17, 20 and 21; 3 each at months 12 and 14; and 5 at month 24) during the study (Fig. 2). Keratoconus (KC) progressed in many children in the TCXL group, and their parents made early unscheduled visits because of visual deterioration and/or VKC. There was a positive postoperative association between recurrent active VKC and progression of KC in 13 (43.3%) of the 30 eyes with documented progression after surgery in the ACXL and TCXL groups.
The total success rates (percentage of eyes with no deterioration of Kmax) were 100%, 94.6% and 71.6% in the SCXL, ACXL and TCXL groups, respectively, at month 24. The between‐group differences were significant (p < 0.0001). The Kaplan–Meier curve analysis for the probability of success in the three groups is shown in Fig. 2. We used Kmax (progression ˃1 D) as the main parameter for assessment of postoperative progression of KC by Kaplan–Meier curve analysis. The mean postoperative survival time (months during which CXL successfully halted progression of KC and after which CXL failure was documented or the study ended) was 24 ± 0.00 months, 23.74 ± 0.20 months and 21.83 ± 0.49 months in the SCXL, ACXL and TCXL groups, respectively (Fig. 2B). Treatment failures were noted as early as 6 months postop in TCXL and 12 months postop in the ACXL group (Fig 2A+B). Some treatment failure underwent repeat treatment with SCXL during the study period. In these cases, the consecutive data were excluded from the data analysis.
From a total of 30 eyes documented with postoperative KC progression in both ACXL and TCXL groups, LOCF principle was used for conserving the data of 22 eyes retreated with SCXL during the study period; 2 eyes in ACXL (retreated at month 12) and 20 eyes in TCXL groups (2 each retreated at months 6 and 18; 6 eyes at month 12; 5 each at months 15 and 22). The data of these 22 eyes at last observation; before retreatment with SCXL, were recorded and maintained till the end of the study. However, the remaining 8 eyes (3 eyes from ACXL and 5 eyes from TCXL groups) were retreated with SCXL after end of the study with no impact on the study outcomes.
Discussion
This study compared the long‐term efficacy and stability of three different CXL protocols used in the treatment of paediatric KC and found the SCXL (Dresden) protocol to be superior to ACXL and TCXL in terms of halting postoperative progression of KC in children. Furthermore, SCXL was associated with good improvement in the visual and refractive outcomes of KC, in particular sphere and SE; however, this improvement varied from one eye to another. There were no cases of progression during 24 months of follow‐up after SCXL; however, 5.4% progressed after ACXL, as did 28.4% after TCXL. Our study confirmed that epithelial‐off CXL was more effective in stabilizing and improving corneal topography and visual outcomes in children with KC, so should be the first choice for halting progression of KC in this population. Transepithelial accelerated CXL (TCXL) had the worst outcomes, possibly because of poor penetration of riboflavin into the intact corneal epithelium. The high‐intensity UV fluence protocol used might also have an impact on the outcomes of TCXL.
Paediatric KC differs from adult KC in that paediatric KC is often more advanced at the time of diagnosis, behaves more aggressively, progresses more rapidly in terms of visual and refractive deterioration, has less favourable postoperative outcomes with no guarantee of long‐term post‐CXL stability and has higher failure rates, with a greater likelihood of needing repeat CXL or other interventions, mainly corneal transplantation (Chatzis & Hafezi 2012; Leoni‐Mesplie et al. 2012; Kodavoor et al. 2014; Soeters et al. 2014; Mukhtar & Ambati 2018). In concordance with the results in our SCXL group, Caporossi et al. (2012b) reported significant improvements in UDVA, CDVA and topographic outcomes after 36 months of follow‐up in 152 children who underwent SCXL. Furthermore, Also many other studies have reported a halt of progression of paediatric keratoconus using SCXL (Arora et al. 2012; Vinciguerra et al. 2012; Zotta et al. 2012; Hafez 2014; Uçakhan et al. 2016; Iqbal et al. 2019a,b). However, Godefrooij et al. (2016) reported progression of KC in 22% of paediatric patients who underwent SCXL despite significant improvement in Kmax during 5 years of follow‐up and attributed this to decentralization of cones. Similarly, Mazzotta et al. (2018) observed a 24% failure long‐term progression rate in paediatric keratoconus after SCXL. (Mazzotta et al. 2018). Several studies have evaluated ACXL in paediatric KC (Ozgurhan et al. 2014; Shetty et al. 2014; Badawi 2017) and reported results similar to ours.
Caporossi et al. (2013) compared SCXL and TCXL (3 mW/cm2 for 30 minutes) in paediatric patients who underwent TCXL and detected progression of KC and marked deterioration in visual and topographic parameters in 50% of cases at postoperative month 12. Their paediatric KC progression rate (50% at 12 months) was much higher than that in our study (28.4% at 24 months). In line with our results, Olivo‐Payne et al. (2017) recorded a KC progression rate of 8.6% by 18 months after TCXL (3 minutes with 30 mW/cm2 power) in children while Soeters et al. (2015) reported a 23% progression rate following TCXL. In contrast, Salman (2013) confirmed the efficiency of transepithelial CXL with no progression of KC and Magli et al. (2013) concluded that TCXL has the same efficacy as epithelium‐off CXL.
Many authors had studied the demarcation line (DL) as a substitute indicator for the impact of CXL and treatment depth. Most researchers agree that the depth of the DL is shallower after ACXL than after SCXL, possibly because of the shorter soaking time with ACXL (Seiler & Hafezi 2006; Bouheraoua et al. 2014; Ng et al. 2015). Mazzotta et al. (2019) confirmed a strong relationship between DL and improvement in corneal biomechanical efficacy, thus achieving more efficient functioning of CXL with better stability. They considered that CXL involves limited amounts of collagen residues with the ability to attract free radicals. Finally, they concluded that effective cross‐linking cannot be increased infinitely in a very thin layer because of limited cross‐linking intensity with a saturation effect but can be maximized by increasing the volume and depth of the corneal tissue being cross‐linked. Therefore, the shallower DL after ACXL and TCXL than after SCXL is clear proof of its lesser biomechanical efficacy. We did not investigate the DL but did document a KC progression rate of 5.4% after ACXL and conclude that SCXL is more biomechanically efficient than ACXL.
Moreover, CXL is less efficient in paediatric patients than in adults in terms of long‐term morphological, functional and visual improvements because of the dynamic nature of ectatic corneas in children (Vinciguerra et al. 2012; Mukhtar & Ambati 2018). Furthermore, natural cross‐linking occurs with ageing, which may explain the good stability following CXL in adults (El Rami et al. 2015). Therefore, many authors recommend that children with ectatic corneas should undergo CXL as early as possible to avoid progression of KC (Chatzis & Hafezi 2012; Kankariya et al. 2013; Mukhtar & Ambati 2018). Because of the more aggressive behaviour of paediatric KC and the low rate of complications we think KC in children should be treated early after its first diagnosis and regardless of its stage. The opportunity for early diagnosis had been missed in most of our children; at the time of intervention, 21.4% of cases had grade I, 47.2% had grade II, and 31.4% had grade III KC, again underscoring the need for early diagnosis to avoid delayed intervention and improve postoperative outcomes. In patients with continued progression of keratoconus after ACXL and TCXL retreatment with SCXL might be an option. Therefore, the possibility of retreatment should be discussed with parents before the first CXL treatment.
Many factors have been linked with development of KC in the teenage years, particularly hormonal changes during puberty and active VKC with chronic eye rubbing (Mukhtar & Ambati 2018; Sharif et al. 2018). The mechanical stress of eye rubbing has been identified as a possibly decisive cofactor in the development and progression of KC especially in children (Lindsay et al. 2000) and a cause of treatment failure and postoperative complications, which points to a need for appropriate preoperative treatment of VKC (Shetty et al. 2014; Mukhtar & Ambati 2018; Iqbal et al. 2019b). At the end of our study, we documented progression of KC in 3 (25%) of 12 eyes with VKC after ACXL. This rate is higher than the progression rate of 17.7% reported by Shetty et al. (2014). Both studies agreed in terms of aggressive medical control of active VKC and chronic eye rubbing postoperatively. Recently, Mazzotta et al. (2018) analysed the ten‐year outcomes of the Siena protocol (a modification of the Dresden protocol) in paediatric patients with progressive KC and reported a 24% Kmax progression rate of > 1 D in children aged ≤ 15 years at the time of their first CXL treatment and in those with severe VKC and eye rubbing. They concluded that postoperative progression of KC is more aggressive and rapid in children (progression rate, 24%) than in adults (5%–6%) and more likely to require repeat CXL. Consistent with that study, we recorded a postoperative KC progression (Kmax progression > 1 D) rate of 28.4% after TCXL group and 5.4% after ACXL. However, in agreement with their study, we found an association of active VKC and eye rubbing in 43.3% of patients with postoperative progression.
The precise collagen turnover time in the adult cornea is still unclear (Paik et al. 2018); however, there is evidence that age affects corneal changes and impacts its metabolic behaviour, which would explain the more rapid progression of KC in children (Chatzis & Hafezi 2012; Mazzotta 2018). Wollensak et al. (2003) published the first CXL study and highlighted the importance of corneal collagen turnover time, which has since been estimated to be 2–3 years (Mazzotta 2018). This collagen turnover time is at least doubled after CXL, reaching 6–7 years and potentially even 8 years (Mazzotta 2018) because of apoptosis of keratocytes following CXL, as evidenced by in vivo confocal microscopy (Mazzotta et al. 2015). Furthermore, the turnover rate may be significantly decreased after CXL due to additional chemical bonds and loss of collagen‐producing keratocytes by apoptosis.
Corneal haze was recorded in significantly more eyes after SCXL than after ACXL in our study (62.6% versus 22.8%). Wollensak & Herbst (2010) reported anterior stromal corneal haze following SCXL that they attributed to lacunar oedema in the keratocytes postoperatively. They also reported that this corneal haze following CXL is usually transient, resolves within the early postoperative months, and is a sign of successful SCXL. Our findings are consistent with theirs in that the corneal haze resolved slowly within the first 6 postoperative months, and our best outcomes were in patients who underwent SCXL, 62.6% of whom initially had postoperative corneal haze.
Although we used two different systems for UVA irradiation, which could be considered a potential source of bias, our finding of a high failure rate of TCXL is not unique. However, we do acknowledge that our study has some limitations because two different UVA irradiation systems were used (OptoXlink and Averdro KXL). In addition, two different accelerated treatment protocols were used for ACXL (30mW for 8 minutes, pulsed mode) compared with TCXL (45 mW for 5:20 minutes, pulsed mode). We are not sure whether or not the high fluence TCXL protocol contributed to the higher failure rate of epithelium‐on CXL when compared with the better outcomes achieved by lower fluence ACXL protocol despite using the same device. Use of a consistent UVA delivery system in future studies will undoubtedly yield more conclusive data. Furthermore, we do acknowledge that treatment of both eyes in the same individual may introduce a degree of bias. We also do acknowledge that the need to retreat the postoperative KC progression in the children's eyes with SCXL during the study period was an ethical and unavoidable issue just to prevent further KC progression which, however, could be a potential source of bias despite using the LOCF principle.
In conclusion, our findings reinforce the premise that SCXL is an efficient protocol for improving KC and halting its progression in paediatric patients and the overall success rate of SCXL was 100% during 2 years of follow‐up. Accelerated CXL (ACXL) appears to be equivalent to SCXL in terms of visual improvement and corneal flattening in the first year after surgery; however, SCXL was superior after 2 years, with a total success rate of 94.6%. In contrast, TCXL did not result in significant improvement in vision or Kmax and had a success rate of only 71.6%. Therefore, TCXL is not recommended for progressive KC in children. VKC and eye rubbing were identified as risk factors for postoperative progression of KC. Given the lack of severe side effects despite some treatment failures and the more aggressive course of paediatric KC, we recommend early treatment of KC even in children without documented progression and at an early Amsler stage. Our personal opinion is based on our study outcomes in children (9–17 years old) and our clinical experience.
The authors are grateful for the assistance of Mr. Hamza Mohammed, Mr. Seif Mohammed and Ms, Lina Mohammed with this research. They also appreciate the help and support of Drs. Mona Abo‐Ali, Ashraf Soliman, Mohamed Omar, Hosam Elzembely, Tarek Tawfik, Islam Hosny and Foad Yousef, as well as that of the EPK Group.
References
- Arora R, Gupta D, Goyal JL & Jain P (2012): Results of corneal collagen cross‐linking in pediatric patients. J Refract Surg 28: 759–762. [DOI] [PubMed] [Google Scholar]
- Badawi AE (2017): Accelerated corneal collagen cross‐linking in pediatric keratoconus: one year study. Saudi J Ophthalmol 31: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouheraoua N, Jouve L, El Sanharawi M et al. (2014): Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen‐crosslinking in keratoconus. Invest Ophthalmol Vis Sci 55: 7601–7609. [DOI] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Baiocchi S & Caporossi T (2010): Long‐term results of riboflavin ultraviolet a corneal collagen cross‐linking for keratoconus in Italy: the Siena Eye Cross Study. Am J Ophthalmol 149: 585–593. [DOI] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T & Paradiso AL (2012a): Transepithelial corneal collagen crosslinking for keratoconus: qualitative investigation by in vivo HRT II confocal analysis. Eur J Ophthalmol 22(Suppl 7): S81–S88. [DOI] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R & Balestrazzi A (2012b): Riboflavin‐UVA‐induced corneal collagen cross‐linking in pediatric patients. Cornea 31: 227–231. [DOI] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D & Caporossi T (2013): Transepithelial corneal collagen crosslinking for progressive keratoconus: 24‐month clinical results. J Cataract Refract Surg 39: 1157–1163. [DOI] [PubMed] [Google Scholar]
- Chatzis N & Hafezi F (2012): Progression of keratoconus and efficacy of pediatric corneal collagen cross‐linking in children and adolescents. J Refract Surg 28: 753–758. [DOI] [PubMed] [Google Scholar]
- El Rami H, Chelala E, Dirani A, Fadlallah A, Fakhoury H, Cherfan C, Cherfan G & Jarade E (2015): An update on the safety and efficacy of corneal collagen cross‐linking in pediatric keratoconus. Biomed Res Int 2015: 25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefrooij DA, Soeters N, Imhof SM & Wisse RP (2016): Corneal cross‐linking for pediatric keratoconus: long‐term results. Cornea 35: 954–958. [DOI] [PubMed] [Google Scholar]
- Hafez MI (2014): Comparison of epithelium‐off and transepithelial corneal collagen cross‐linking for treatment of keratoconus. J Egypt Ophthalmol Soc 107: 181–186. [Google Scholar]
- Hashemi H, Khabazkhoob M & Fotouhi A (2013): Topographic keratoconus is not rare in an Iranian population: the Tehran eye study. Ophthalmic Epidemiol 20: 385–391. [DOI] [PubMed] [Google Scholar]
- Hersh PS, Lai MJ, Gelles JD & Lesniak SP (2018): Transepithelial corneal crosslinking for keratoconus. J Cataract Refract Surg 44: 313–322. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Elmassry A, Tawfik A et al. (2019a): Standard cross‐linking versus photorefractive keratectomy combined with accelerated cross‐linking for keratoconus management: a comparative study. Acta Ophthalmol 97: e623–e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Elmassry A, Tawfik A et al. (2019b): Analysis of the outcomes of combined cross‐linking with intracorneal ring segment implantation for the treatment of pediatric keratoconus. Curr Eye Res 44: 125–134. [DOI] [PubMed] [Google Scholar]
- Kankariya VP, Kymionis GD, Diakonis VF & Yoo SH (2013): Management of pediatric keratoconus ‐ evolving role of corneal collagen cross‐linking: an update. Ind J Ophthalmol 61: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Kim JC, Park WC, Seo JS & Chang HR (2004): Effect of thermal preconditioning before excimer laser photoablation. J Korean Med Sci 19: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavoor SK, Arsiwala AZ & Ramamurthy D (2014): One‐year clinical study on efficacy of corneal cross‐linking in Indian children with progressive keratoconus. Cornea 33: 919–922. [DOI] [PubMed] [Google Scholar]
- Kok YO, Tam GF & Loon SC (2012): Review: keratoconus in Asia. Cornea 31: 581–593. [DOI] [PubMed] [Google Scholar]
- Leoni‐Mesplie S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplie N & Colin J (2012): Scalability and severity of keratoconus in children. Am J Ophthalmol 154: 56–62. [DOI] [PubMed] [Google Scholar]
- Lindsay RG, Bruce AS & Gutteridge IF (2000): Keratoconus associated with continual eye rubbing due to punctual agenesis. Cornea 19: 567–569. [DOI] [PubMed] [Google Scholar]
- Magli A, Forte R, Tortori A, Capasso L, Marsico G & Piozzi E (2013): Epithelium‐off corneal collagen cross‐linking versus transepithelial cross‐linking for pediatric keratoconus. Cornea 32: 597–601. [DOI] [PubMed] [Google Scholar]
- Mazzotta C (2018): Reply: Letters to the Editor. Cornea 37: e51–e52. [DOI] [PubMed] [Google Scholar]
- Mazzotta C, Hafezi F, Kymionis G, Caragiuli S, Jacob S, Traversi C, Barabino S & Randleman JB (2015): In vivo confocal microscopy after corneal collagen crosslinking. Ocul Surf 13: 298–314. [DOI] [PubMed] [Google Scholar]
- Mazzotta C, Traversi C, Baiocchi S, Bagaglia S, Caporossi O, Villano A & Caporossi A (2018): Corneal collagen cross‐linking with riboflavin and ultraviolet A light for pediatric keratoconus: ten‐year results. Cornea 37: 560–566. [DOI] [PubMed] [Google Scholar]
- Mazzotta C, Wollensak G, Raiskup F, Pandolfi A & Spoerl E (2019): The meaning of the demarcation line after riboflavin‐UVA corneal collagen crosslinking. J Expert Rev Ophthalmol 14: 115–131. [Google Scholar]
- Medeiros CS, Giacomin NT, Bueno RL, Ghanem RC, Moraes HV Jr & Santhiago MR (2016): Accelerated corneal collagen crosslinking: technique, efficacy, safety, and applications. J Cataract Refract Surg 42: 1826–1835. [DOI] [PubMed] [Google Scholar]
- Mita M, Waring GO IV & Tomita M (2014): High‐irradiance accelerated collagen crosslinking for the treatment of keratoconus: six‐month results. J Cataract Refract Surg 40: 1032–1040. [DOI] [PubMed] [Google Scholar]
- Mukhtar S & Ambati BK (2018): Pediatric keratoconus: a review of the literature. Int Ophthalmol 38: 2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AL, Chan TC, Lai JS & Cheng AC (2015): Comparison of the central and peripheral corneal stromal demarcation line depth in conventional versus accelerated collagen cross‐linking. Cornea 34: 1432–1436. [DOI] [PubMed] [Google Scholar]
- Olivo‐Payne A, Serna‐Ojeda JC, Hernandez‐Bogantes E et al. (2017): Trans‐epithelial accelerated corneal cross‐linking for keratoconus in children. Int J Ophthalmol 10: 1919–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgurhan EB, Kara N, Cankaya KI, Kurt T & Demirok A (2014): Accelerated corneal cross‐linking in pediatric patients with keratoconus: 24‐month outcomes. J Refract Surg 30: 843–849. [DOI] [PubMed] [Google Scholar]
- Paik DC & Trokel SL & Suh LH (2018): Just what do we know about corneal collagen turnover? Cornea 37: e49–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiskup F, Theuring A, Pillunat LE & Spoerl E (2015): Corneal collagen crosslinking with riboflavin and ultraviolet‐A light in progressive keratoconus: ten‐year results. J Cataract Refract Surg 41: 41–46. [DOI] [PubMed] [Google Scholar]
- Sabti S, Tappeiner C & Frueh BE (2015): Corneal cross‐linking in a 4‐year‐old child with keratoconus and Down syndrome. Cornea 34: 1157–1160. [DOI] [PubMed] [Google Scholar]
- Salman AG (2013): Transepithelial corneal collagen crosslinking for progressive keratoconus in a pediatric age group. J Cataract Refract Surg 39: 1164–1170. [DOI] [PubMed] [Google Scholar]
- Saro AS, Radwan GA, Mohammed UA & Abozaid MA (2018): Screening for keratoconus in a refractive surgery population of Upper Egypt. Delta J Ophthalmol 19: 19–23. [Google Scholar]
- Seiler T & Hafezi F (2006): Corneal cross‐linking‐induced stromal demarcation line. Cornea 25: 1057–1059. [DOI] [PubMed] [Google Scholar]
- Sharif R, Bak‐Nielsen S, Hjortdal J & Karamichos D (2018): Pathogenesis of keratoconus: the intriguing therapeutic potential of prolactin‐inducible protein. Prog Retin Eye Res 67: 150–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty R, Nagaraja H, Jayadev C, Pahuja NK, Kurian Kummelil M & Nuijts R (2014): Accelerated corneal collagen cross‐linking in pediatric patients: two‐year follow‐up results. Biomed Res Int 2014: 894095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeters N, van der Valk R & Tahzib NG (2014): Corneal cross‐linking for treatment of progressive keratoconus in various age groups. J Refract Surg 30: 454–460. [DOI] [PubMed] [Google Scholar]
- Soeters N, Wisse RP, Godefrooij DA, Imhof SM & Tahzib NG (2015): Transepithelial versus epithelium‐off corneal cross‐linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol 159: 821–828. [DOI] [PubMed] [Google Scholar]
- Uçakhan ÖÖ, Bayraktutar BN & Saglik A (2016): Pediatric corneal collagen cross‐linking: long‐term follow‐up of visual, refractive, and topographic outcomes. Cornea 35: 162–168. [DOI] [PubMed] [Google Scholar]
- Vinciguerra P, Albé E, Frueh BE, Trazza S & Epstein D (2012): Two‐year corneal cross‐linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol 154: 520–526. [DOI] [PubMed] [Google Scholar]
- Wollensak G & Herbst H (2010): Significance of the lacunar hydration pattern after corneal cross‐linking. Cornea 29: 899–903. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E & Seiler T (2003): Riboflavin/ultraviolet A‐induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135: 620–627. [DOI] [PubMed] [Google Scholar]
- Zotta PG, Moschou KA, Diakonis VF, Kymionis GD, Almaliotis DD, Karamitsos AP & Karampatakis VE (2012): Corneal collagen cross‐linking for progressive keratoconus in pediatric patients: a feasibility study. J Refract Surg 28: 793–799. [DOI] [PubMed] [Google Scholar]


