Abstract
Berberine (BBR), a small alkaloid, is used as a hypoglycemic agent in China. Stachyose (Sta), a Rehmannia glutinosa oligosaccharide, acts as a prebiotic. This study aimed to evaluate whether BBR combined with Sta produced better glycometabolism than BBR alone, and explored the effects on gut microbiota and metabolomics. Type‐2 diabetic db/db mice were administered BBR (100 mg/kg), Sta (200 mg/kg), or both by gavage once daily. Glucose metabolism, the balance of α‐ and β‐cells, and mucin‐2 expression were ameliorated by combined treatment of BBR and Sta, with stronger effects than upon treatment with BBR alone. The microbial diversity and richness were altered after combined treatment and after treatment with BBR alone. The abundance of Akkermansia muciniphila was increased by combined treatment compared to treatment with BBR alone, while the levels of the metabolite all‐trans‐heptaprenyl diphosphate were decreased and the levels of fumaric acid were increased, which both showed a strong correlation with A. muciniphila. In summary, BBR combined with Sta produced better glycometabolism than BBR alone through modulating gut microbiota and fecal metabolomics, and may aid in the development of a novel pharmaceutical strategy for treating Type 2 diabetes mellitus.
Keywords: berberine, metabolomics, microbiota, stachyose, type 2 diabetes
1. INTRODUCTION
Chinese medicine has provided people with useful preparations that have been a resource for drug discovery. Berberine, a small alkaloid isolated from medicinal plants, such as Coptis chinensis Franch. (Ranunculaceae), Platycladus orientalis (Linn.) Franco (Rutaceae), and Berberis thunbergii DC. (Berberidaceae), has been reported to exert many therapeutic effects, including anti‐diabetes, lipid‐lowering, and anti‐cancer. Currently, berberine is primarily obtained by chemical synthesis and is used in China in the treatment of diarrhea for its effects on intestines and its antibacterial effects (Imenshahidi & Hosseinzadeh, 2019; More, Kharat, & Kharat, 2017). Although berberine is also used in obese diabetic patients, its poor intestinal absorption may induce different degrees of diarrhea in some patients (Imenshahidi & Hosseinzadeh, 2019) and therefore prevents long‐term and wide application in the treatment of Type 2 diabetes mellitus (T2DM). Moreover, studies revealed that berberine exerts antidiabetic effects by modulating gut microbiota (Han, Lin, & Huang, 2011). We hypothesized that the use of a prebiotic during berberine treatment may improve glycometabolism.
Stachyose, an abundant Rehmannia glutinosa oligosaccharide isolated from the water extract of fresh R. glutinosa (Scrophulariaceae), has been reported to (a) act as a prebiotic to enhance the growth and activity of beneficial bacteria, (b) exhibit a hypoglycemic effect, and (c) improve inflammation through modulating gut microbiota in vivo (Liu et al., 2018; Pacifici et al., 2017; Zhang et al., 2004). Furthermore, stachyose increases the absorption of tea polyphenols (Li, Huang, Gao, & Yang, 2016), which has led us to hypothesize that stachyose may improve the hypoglycemic action of berberine.
This study aimed to evaluate the influence of berberine combined with stachyose on glucose metabolism and explored the effects on gut microbiota and fecal metabolomics in diabetic db/db mice.
2. MATERIALS AND METHODS
2.1. Chemical compounds
Berberine hydrochloride (purity 98%) was obtained from the Northeast General Pharmaceutical Factory (Shenyang, China). Stachyose (purity >80%) was obtained from the laboratory of Professor Dequan Yu (Beijing, China).
2.2. Ethics statement
All animal experiments adhered to the Chinese standards and guidelines for the use of laboratory animals (GB14925‐2001 and MOST 2006a), and were carried out with the approval of the Experimental Animal Welfare Ethics Committee of the Institute of Materia Medica (Chinese Academy of Medical Sciences and Peking Union Medical College) under No. 00000814. Male BKS‐Leprem2Cd479/Nju (Lepr KO/KO, db/db) mice and wild‐type mice (Lepr wt/wt) aged 4–8 weeks were obtained from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Animals were kept in a temperature‐ and humidity‐controlled environment with a 12/12‐hr light/dark cycle and water ad libitum. All mice were fed special feed consisting of crude protein (≥220 g/kg), crude fat (≥40 g/kg), crude fiber (≤50 g/kg), crude ash (≤80 g/kg), calcium (10–18 g/kg), total phosphorus (6–12 g/kg), lysine (≥13.2 g/kg), and methionine and cystine (≥7.8 g/kg) (XieTong Organism, Nanjing, China).
2.3. Experimental design
The db/db mice were divided into four groups (n = 11): the diabetic‐control group (Con), stachyose‐treated group (Sta, 200 mg/kg), berberine‐treated group (BBR, 100 mg/kg), and berberine with stachyose‐treated group (Sta + BBR, Sta: 40 mg/ml, 200 mg/kg; BBR: 20 mg/ml, 100 mg/kg). Wild‐type mice were used as healthy controls (Nor, n = 12). All mice were administered drugs or an equivalent volume of water once daily (0.05 ml/10 g body weight) by gavage for 55 days.
2.4. Fasting blood glucose, non‐fasting blood glucose, and HbA1c assays
Blood was collected from tail tips, and fasting blood glucose (FBG, after fasting from 8:00 a.m. until 12:00 a.m. with water ad libitum) and non‐fasting blood glucose (NFBG) were monitored by the glucose‐oxidase method (Biosino Bio‐Technology & Science Inc., Beijing, China). After 45 days of treatment, HbA1c levels were measured (Homa Biological Beijing, China).
2.5. Oral glucose‐tolerance test and insulin‐tolerance test
The oral glucose tolerance test was performed after 16 and 37 days of treatment following glucose loading by gavage (2 g/kg, 0.05 ml/10 g body weight). The insulin tolerance test was performed after 48 days of treatment after subcutaneous injection of insulin (0.4 U/kg, 0.05 ml/10 g body weight) (Peng et al., 2014).
2.6. Immunofluorescent assay
All mice were sacrificed through cervical dislocation and the pancreas was dissected to prepare 5‐μm paraffin slides, which were stained against insulin and glucagon and analyzed (n = 6; Li et al., 2017).
2.7. Immunohistochemistry assay
About 4 cm of ileum was fixed in 4% paraformaldehyde to prepare 5‐μm paraffin slides, followed by antibody staining against mucin‐2 (Abcam, Cambridge, United Kingdom) (n = 4; Zhong et al., 2019). Images were captured with a Mirax scanner (3DHISTECH, Hungary), and the area of positive points was calculated with Image Pro (MediaCybernetics, Rockville, MD).
2.8. Illumina sequencing
Feces were collected after 44 days of treatment and subjected to bacterial DNA isolation. The V3–V4 hypervariable region of the 16S rRNA gene was amplified with primers 338F (5′‐ACTCCTACGGGAGGCAGCAG‐3′) and 806R (5′‐GGACTACHVGGGTWTCTAAT‐3′) and TransStart FastPfu DNA polymerase (TRANSGEN BIOTECH, Beijing, China) with the following PCR program: 3 min at 95°C; 27 cycles of 30 s at 95°C; 30 s at 55°C; 45 s at 72°C; and 10 min at 72°C. The products were extracted, purified, quantified, and sequenced with an Illumina MiSeq platform following the standard PE300‐sequencing protocol (Major Bio‐Pharm Technology, Shanghai, China). The sequencing data were processed (Lv, Peng, Liu, Xu, & Su, 2017), and valid data were analyzed on the free online Majorbio I‐Sanger Cloud Platform (http://www.i-sanger.com).
2.9. Metabolomic analysis
Feces were mixed with L‐2‐chlorophenylalanine and homogenized in methanol–water (4:1, vol/vol), followed by sonication on ice and incubation at −20°C for 30 min. After centrifugation for 10 min at 10,000 rpm at 4°C, the supernatant was analyzed by high‐performance liquid chromatography‐mass spectrometry (LC–MS) with a UPLC‐Xevo G2‐XS QTof (Waters Corporation, Milford, MA). Briefly, an ACQUITY BEH C18 column (100 mm × 2.1 mm i.d. × 1.7 μm) was used. Aqueous formic acid (0.1%, vol/vol) and acetonitrile containing 0.1% (vol/vol) formic acid separately served as mobile phases A and B. The mass spectrometric data ranging from 50 to 1,000 m/z were collected. A mixture of all samples at an equal volume ratio was prepared as quality control, and six quality controls were analyzed.
Raw data were processed with Progenesis QI software (Waters Corporation). The positive and negative data were combined and imported into the SIMCA‐P+ 14.0 software package (Umetrics, Umeå, Sweden). Unsupervised principal component analysis (PCA) was used to analyze the distributional profile of all samples, and supervised orthogonal partial least‐squares discriminant analysis (OPLS‐DA) was performed to visualize the differential metabolites according to the standards of variable importance in the projection (VIP) > 1.0 and p < .05 in the multidimensional analysis and Student's t test, respectively. Response‐permutation testing (RPT) was performed to guard against overfitting and validate the reliability of each mathematical model. The differential metabolites were mapped to pathways according to the KEGG database.
2.10. Statistical analysis
Data are expressed as mean ± SE. Data were analyzed using one‐way ANOVA with Bonferroni corrections and two‐tailed Student's t tests and plotted by GraphPad Prism 7.01 (GraphPad Software, San Diego, CA), except the results of immunofluorescence and immunohistochemistry assays, gut microbial analysis, and fecal metabolomics. p < .05 was considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. Effect of berberine with stachyose on glucose metabolism
In comparison to the Con group, FBG levels in the BBR group were reduced during the initial period (p < .05) and were continually reduced in the Sta + BBR group (p < .05; Figure 1a). NFBG levels were lowered after 10 days of treatment in the BBR and Sta + BBR groups, but the effect was gradually weakened (Figure 1b). HbA1c levels were decreased by only 0.29% in the BBR group and by 0.65% in the Sta + BBR group (p = .180; Figure 1c). The FBG and NFBG levels were not altered in the Sta group.
Figure 1.
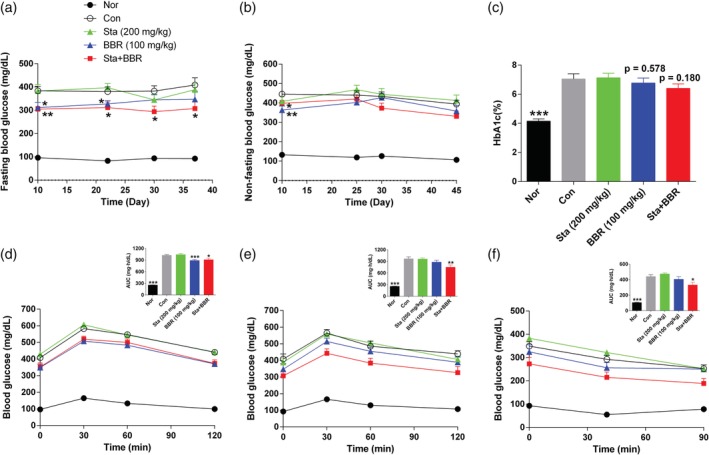
Effects of berberine combined with stachyose on glucose metabolism. (a) Fasting blood glucose levels. (b) Non‐fasting blood glucose levels. (c) HbA1c levels. (d, e) Oral glucose tolerance test performed after (d) 16 days and (e) 37 days of treatment. (f) Insulin tolerance test performed after 48 days of treatment. Data are expressed as mean ± SE; n = 10–12 for (a), (d), and (e); n = 9–12 for (b) and (f); n = 8–12 for (c). ***p < .001, **p < .01, *p < .05 versus Con. BBR, berberine; Con, diabetic control; Nor, healthy control; Sta, stachyose
The blood glucose levels and the area under the blood glucose curve (AUC) following oral glucose loading were decreased in the BBR and Sta + BBR groups after 16 days of treatment (p < .05), but were decreased only in the Sta + BBR group (p < .05) after 37 days (Figure 1d,e). Although the blood glucose levels and AUC following subcutaneous injection of insulin were significantly decreased in the Sta + BBR group after 48 days of treatment, the percentage decrease in blood glucose levels in the insulin tolerance test (i.e., 40 min after insulin injection) was not altered (Figure 1f), suggesting that improvement of glycometabolism resulted from the amelioration of β‐cell function.
Although various studies about berberine and stachyose have been published (Imenshahidi & Hosseinzadeh, 2019; Li et al., 2016), to the best of our knowledge, this is the first report about their combined effects on glucose metabolism. As is well known, the concentrations and ratios of components used in combined treatment greatly influence the effects. In order to find the optimal ratio of berberine and stachyose, berberine (100 mg/kg) was combined with 50, 100, and 200 mg/kg stachyose, and 50 mg/kg stachyose was combined with 50, 100, and 200 mg/kg berberine. The agents were administered in alloxan‐induced diabetic mice by gavage, and hypoglycemic effects were studied. Berberine (100 mg/kg) with stachyose (200 mg/kg) produced better hypoglycemic effects than berberine alone (100 mg/kg), (File S1). Thus, berberine (100 mg/kg) and stachyose (200 mg/kg) were used in the combination treatment, consistent with our previous study (Han et al., 2016).
Considering the fact that the low oral bioavailability and poor intestinal absorption of berberine are key constraints of its clinical use, we speculated that the beneficial effects of stachyose on gut microbiota and on the intestinal balance may contribute to the improvements in glycometabolism. Nevertheless, as opposed to Zhang et al.'s results (Zhang et al., 2004), stachyose did not decrease the glycaemia of diabetic db/db mice; differences in mechanisms by which hyperglycemia is induced in animal models may explain this discrepancy.
3.2. Effect of berberine with stachyose on the balance of α‐ and β‐cells
In comparison to the Nor group, glucagon‐positive points were no longer observed on the border of islets, and the ratio of glucagon‐positive area to total islet area was significantly increased in the Con group, while the ratio of insulin‐positive area to total islet area and the ratio of insulin‐positive area to glucagon‐positive area were significantly reduced, suggesting an imbalance between α‐ and β‐cells. In comparison to the Con group, although no significant improvement in the distribution of α‐ and β‐cells was found (Figure 2a), the ratio of glucagon‐positive area to total islet area was significantly decreased in all treatment groups (Figure 2b), the ratio of insulin‐positive area to total islet area was reduced in the BBR group (p < .05, Figure 2c), and the ratio of insulin‐positive area to glucagon‐positive area was increased in the Sta + BBR group (p < .05, Figure 2d).
Figure 2.
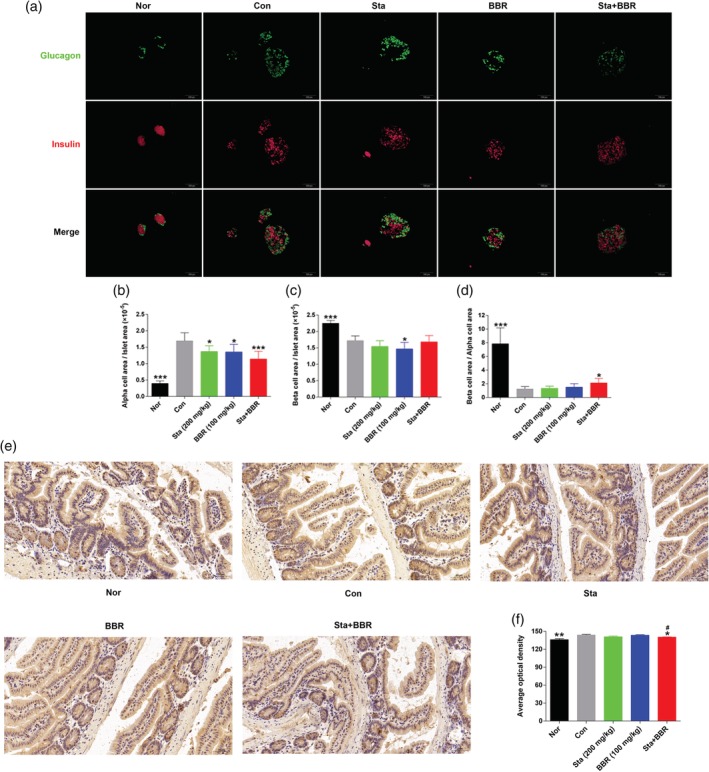
Effects of berberine combined with stachyose on the balance of α‐ and β‐cells and expression of mucin‐2 in the ileum. (a) Immunofluorescent staining of glucagon and insulin. Glucagon is shown in green, and insulin in red. (b–d) Statistical analysis of the ratio of (b) glucagon‐positive area to total islet area, (c) insulin‐positive area to total islet area, and (d) insulin‐positive area to glucagon‐positive area. (e) Immunohistochemical staining of mucin‐2. Mucin‐2 is shown in brown, and the cell nucleus in blue. (f) Statistical analysis of mucin‐2. All representative images were taken at ×200 magnification. Data are expressed as mean ± SE; n = 6 for (a–d), n = 4 for (e) and (f). ***p < .01, *p < .05 versus Con, # p < .05 versus BBR. BBR, berberine; Con, diabetic control; Nor, healthy control; Sta, stachyose
It is well known that the insulin‐producing β‐cells and glucagon‐producing α‐cells are two cell subtypes that are important in maintaining balance in glucose metabolism. The β‐cells have a long lifespan, and in case of physiological demand, such as insulin resistance, they divide in a compensatory manner to secrete more insulin and maintain euglycemia. If the β‐cell mass reduces about 90%, hyperglycemia occurs. The α‐cell mass increases in diabetes and aggravates the hyperglycemia, though it plays a crucial role in reducing hypoglycemic attack through secreting glucagon. Furthermore, insulin could modulate glucagon secretion, and α‐cells can convert to β‐cells (Kawamori et al., 2009; Thorel et al., 2010). Thus, evaluating the proportional change of α‐ and β‐cell mass helps to analyze the functional state of islets. Immunofluorescent staining showed that berberine combined with stachyose increased the ratio of insulin‐positive area to glucagon‐positive area, that is, the ratio of β‐cell to α‐cell mass, suggesting amelioration of the imbalance between α‐ and β‐cells.
3.3. Effect of berberine with stachyose on the expression of mucin‐2
The expression of mucin‐2 in ileum was increased in the Con group compared to the Nor group (p < .05). In comparison to the Con group, the expression of mucin‐2 was decreased in the Sta + BBR and Sta groups, with a decrease of 2.4% (p < .05) and 2.1% (p > .05), respectively; no change was found in the BBR group (Figure 2e,f).
Mucin‐2, a large glycoprotein that plays an important role in maintaining intestinal balance through forming a physical barrier between intestinal content and the epithelium, is produced by goblet cells and released constitutively or in a stimulated manner (Ambort et al., 2012). One report found that tumors with chronic inflammation contained higher levels of mucin‐2 than tumors with acute inflammation (Mejías‐Luque et al., 2010), indicating that the expression of mucin‐2 is elevated upon chronic inflammation. Furthermore, high mucin‐2 expression induces endoplasmic‐reticulum (ER) stress and apoptosis in goblet cells, leading to a dysfunctional epithelial barrier (Tawiah et al., 2018). T2DM is a chronic metabolic disease accompanied by chronic inflammation. In the present study, we show that stachyose may help berberine to ameliorate ER stress and reduce apoptosis of goblet cells by decreasing the expression of mucin‐2.
3.4. Effect of berberine with stachyose on the diversity and richness of gut microbiota
A total of 994,760 valid sequences were obtained and clustered into 654 OTUs according to the minimum sample‐sequence number (Table S1). The numbers of OTUs were not different between the Con and Nor groups, but were decreased in the BBR and the Sta + BBR groups in comparison to the Con group (p < .001); there was no significant difference between the BBR and Sta + BBR groups in terms of OTU number (Figure 3a). The Shannon and Chao indices, which separately reflect the diversity and richness of gut microbiota, exhibited trends similar to the OTU numbers (Figure 3b,c).
Figure 3.
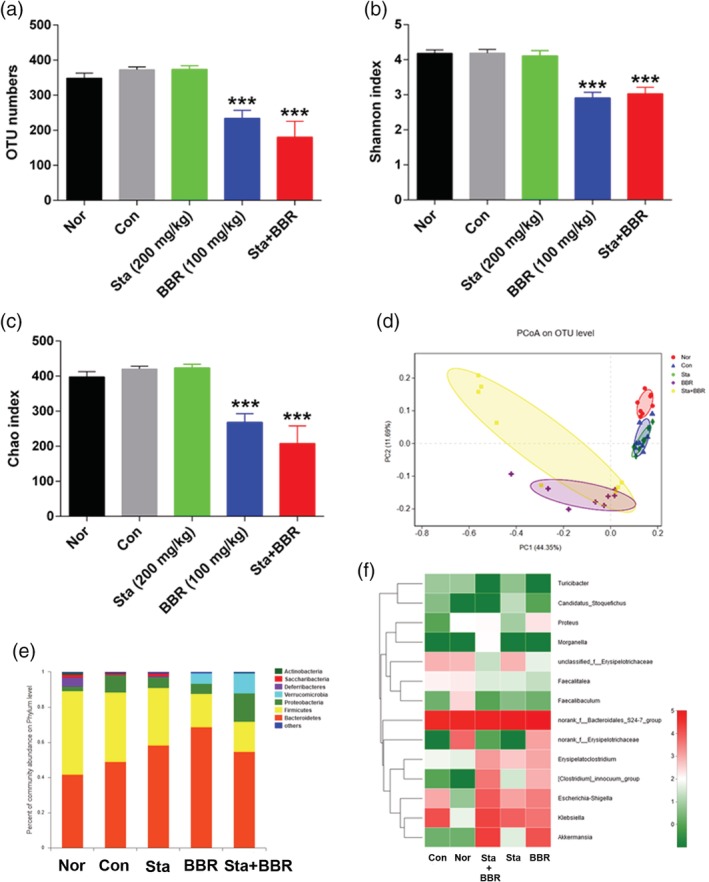
Effects of berberine combined with stachyose on the structure of microbiota. (a) Total OTU numbers. (b) Shannon index. (c) Chao index. (d) Principal coordinate analysis (PCoA). (e) Relative abundance of bacteria at the phylum level by taxon‐based analysis. (f) Heat map of relative abundance of some bacteria at the genus level. Data are expressed as mean ± SE for (a–c); n = 8. ***p < .001 versus Con. BBR, berberine; Con, diabetic control; Nor, healthy control; Sta, stachyose
Unweighted Unifrac PCoA based on OTUs revealed that the bacterial community was significantly changed in the Con group along the first principal coordinate (PC1) in comparison to the Nor group, and was remarkably altered both along PC1 and the second principal coordinate (PC2) in the BBR and Sta + BBR groups in comparison to the Con group. Furthermore, the bacterial community was driven away from the Nor group along PC1 and was closer to the Nor group along PC2 in the Sta + BBR group in comparison to the BBR group (Figure 3d). Thus, both berberine alone and berberine with stachyose reduced the diversity and richness of microbiota, which may mainly result from the broad antibacterial action of berberine (More et al., 2017), which is only minimally reversed by the prebiotic stachyose. No significant differences were found between the BBR and Sta + BBR groups.
3.5. Effect of berberine with stachyose on the composition of microbiota
At the phylum level, the dominant microbial communities in all mice were Bacteroidetes and Firmicutes. In comparison to the Con group, the abundances of Saccharibacteria, Deferribacteres, Actinobacteria, and Firmicutes were reduced, and the abundance of Verrucomicrobia was increased in the BBR and Sta + BBR groups. Furthermore, the abundance of Verrucomicrobia was significantly increased in the Sta + BBR group in comparison to the BBR group (Figures 3e and S1). Additionally, OTU654, which was classified into Akkermansia at the genus level and A. muciniphila at the species level, was increased by 91.4% in the Sta + BBR group in comparison to the BBR group. Treatment with stachyose had no influence on OTU654 (Figure 3f and Table 1).
Table 1.
Effects of berberine combined with stachyose on the abundance of OTU654
| Group | Sequence number | Relative abundance (%) |
|---|---|---|
| Nor | 2 | 0.01 |
| Con | 2 | 0.01 |
| Sta | 44 | 0.13 |
| BBR | 12,004 | 34.26 |
| Sta + BBR | 22,985 | 65.60 |
Abbreviations: BBR, berberine; Con, diabetic control; Nor, healthy control; Sta, stachyose.
Verrucomicrobia is a phylum that includes phylogenetically related bacteria. One report showed that Verrucomicrobia is highly abundant in healthy Chilean subjects (Fujio‐Vejar et al., 2017). A. muciniphila, a strictly anaerobic, Gram‐negative, and mucus‐inhabiting bacterium, is one of the few identified Verrucomicrobia species. A previous study showed that abundance of A. muciniphila is decreased in obese humans (Everard et al., 2013), and treatment with metformin significantly increased abundance of Akkermansia spp. in diet‐induced obese mice, suggesting a relationship with the improvement in glucose metabolism (Shin et al., 2014). Thus, the increased abundance of the phylum Verrucomicrobia and the species A. muciniphila contributed to the improvement in glycometabolism induced by berberine with stachyose.
Additionally, being a highly specialized bacterium, A. muciniphila produces mucin degrading enzymes, uses mucin as the sole source of carbon and nitrogen, and stimulates mucin and mucus secretion in a positive feedback loop (Ottman et al., 2017). Thus, the reduction in mucin‐2 expression in ileum induced by berberine with stachyose may result from the increased abundance of A. muciniphila.
3.6. Effect of berberine with stachyose on fecal metabolomic profile
A total of 2,614 metabolites were found after removing the peaks representing internal standards and the unidentified peaks (Table S2). PCA showed that there was one outlier among all samples, but none were found in the comparisons between two groups (Figure S2). RPT demonstrated that each OPLS‐DA model was reliable, and OPLS‐DA revealed significant differences between the two groups (Figures 4a and S3). A total of 184, 210, 200, and 39 metabolites were changed in the comparisons of Nor versus Con, Con versus BBR, Con versus Sta + BBR, and BBR versus Sta + BBR, respectively, according to the standards of VIP > 1.0 and p < .05 (Table S3). Differential metabolites with KEGG numbers were annotated with the HMDB, KEGG COMPOUND, and LIPID MAPS databases, and 48 metabolites were screened; 41 of them were categorized into 14 major classes and mapped to different biochemical pathways (Figure 4b; Table S4).
Figure 4.
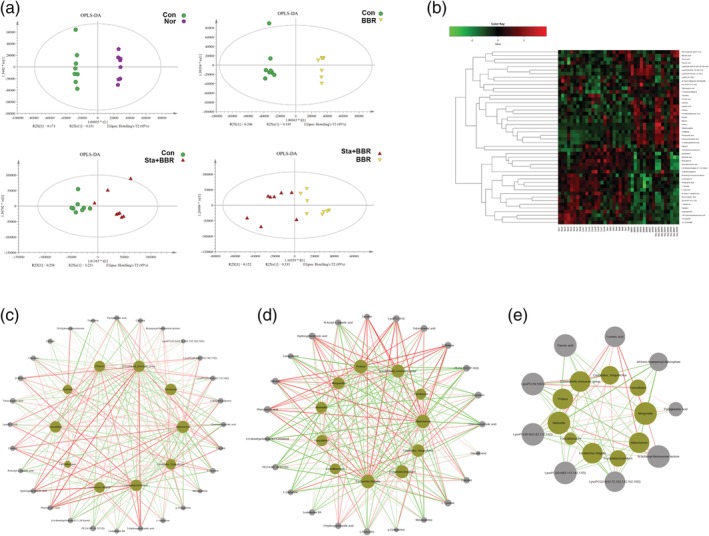
Characteristics of metabolites. (a) Orthogonal partial least‐squares discriminant analysis (OPLS‐DA). (b) Color‐coded heat map of 48 metabolites. The color scale represents the scaled abundance of each metabolite, with red and green separately indicating increased and decreased abundance, respectively. (c–e) Correlation network analysis of bacteria as in Figure 3f (green nodes) and differential metabolites in the comparison of (c) BBR versus Con, (d) Sta + BBR versus Con, and (e) Sta + BBR versus BBR (gray nodes). The red and green lines represent positive and negative correlations, respectively. BBR, berberine; Con, diabetic control; Nor, healthy control; Sta, stachyose
Levels of glyceric acid, which is involved in glycerolipid metabolism, and PC (14:0/P‐18:1[11Z]), which is involved in arachidonic acid metabolism and glycerophospholipid metabolism, were significantly elevated in the Sta + BBR group in comparison to the Con group, while levels of l‐isoleucine, l‐tyrosine, leukotriene B4, and capsaicin, which are involved in inflammatory pathways or biosynthesis of antibiotics, were significantly decreased in the Sta + BBR and the BBR groups. Furthermore, levels of pyruvic acid, a crucial substrate of gluconeogenesis, were significantly decreased in the Sta + BBR group in comparison to the BBR group, as were all‐trans‐heptaprenyl diphosphate and LysoPC(18:1[9Z]); in contrast, levels of fumaric acid, were significantly increased (Table S4).
3.7. Correlations between gut microbial structure and the metabolome
A total of 238 correlations were reported between 10 genera and 28 metabolites in the comparison of BBR versus Con, and 228 correlations were reported between 11 genera and 23 metabolites in the comparison of Sta + BBR versus Con (Figure 4c,d). l‐tyrosine, a ketogenic amino acid and the precursor of epinephrine that is involved in the biosynthesis of antibiotics, was negatively correlated with Akkermansia (p < .05) in the comparisons of BBR versus Con and Sta + BBR versus Con. Furthermore, a total of 73 correlations were reported between 10 genera and 9 metabolites in the comparison of BBR versus Sta + BBR, and all‐trans‐heptaprenyl diphosphate was negatively correlated with Akkermansia (p < .05), while fumaric acid was positively correlated with Akkermansia (p < .05; Figure 4e). These results provide insight in the molecular mechanisms underlying the improvements in glycometabolism induced by berberine with stachyose.
Additionally, the benefits of berberine combined with stachyose on glycometabolism are reminiscent of an ancient Chinese formula for treating diabetes, the Qianjin Huanglian pill, which is comprised of C. chinensis and fresh R. glutinosa. Berberine and stachyose, the main components of C. chinensis and fresh R. glutinosa, may provide further information about the functional mechanism of the Qianjin Huanglian pill.
In summary, berberine combined with stachyose continually decreased the FBG levels, improved the impaired oral glucose tolerance, and ameliorated the balance of α‐ and β‐cells and intestinal ER stress in diabetic db/db mice. The beneficial effects were stronger than those of berberine alone. Furthermore, in comparison to berberine, berberbine combined with stachyose significantly increased the abundance of bacteria of the phylum Verrucomicrobia, in particular A. muciniphila, and changed the levels of fumaric acid and all‐trans‐heptaprenyl diphosphate, which both showed a significant correlation with A. muciniphila. Hence, berberine combined with stachyose may be a novel pharmaceutical strategy for treating T2DM.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
C.L. designed the research and performed most of the research. X.W., L.L., M.L., R.L., and S.S. participated in the in vivo experiment. S.L., Y.H., T.Z., Q.L., H.C., G.B. took part in collecting tissue samples at the end of the experiment. Y.H. mainly performed the studies in alloxan mice. C.L. analyzed the data and wrote the manuscript. Z.S. supplied academic and technical support.
Supporting information
Appendix S1: Supporting Information
Figure S1 Pie chart showing the relative abundance of bacteria at the phylum level by taxon‐based analysis.
Figure S2 Principal component analysis (PCA) of metabolites.
Figure S3 The response permutation testing (RPT) of each OPLS‐DA model.
Table S1 Supporting Information
Table S2 Supporting Information
Table S3 Supporting Information
Table S4 Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81773962); the Drug Innovation Major Project (Nos. 2018ZX09711001‐003‐009 and 2018ZX09711001‐009‐014); and the CAMS Initiative for Innovative Medicine (CAMS‐I2M) (Nos. 2016‐I2M‐2‐006 and 2017‐I2M‐1‐010). We thank Professor Dequan Yu for supplying stachyose and Majorbio for analyzing the data. We thank Accdon (http://www.accdon.com) for providing linguistic assistance.
Li C‐N, Wang X, Lei L, et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phytotherapy Research. 2020;34:1166–1174. 10.1002/ptr.6588
Funding information CAMS Initiative for Innovative Medicine, Grant/Award Numbers: 2016‐I2M‐2‐006, 2017‐I2M‐1‐010; Drug Innovation Major Project, Grant/Award Numbers: 2018ZX09711001‐003‐009, 2018ZX09711001‐009‐014; National Natural Science Foundation of China, Grant/Award Number: 81773962
REFERENCES
- Ambort, D. , Johansson, M. E. , Gustafsson, J. K. , Nilsson, H. E. , Ermund, A. , Johansson, B. R. , … Hansson, G. C. (2012). Calcium and pH‐dependent packing and release of the gel‐forming MUC2 mucin. Proceedings of the National Academy of Sciences of the United States of America, 109(15), 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard, A. , Belzer, C. , Geurts, L. , Ouwerkerk, J. P. , Druart, C. , Bindels, L. B. , … Cani, P. D. (2013). Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proceedings of the National Academy of Sciences of the United States of America, 110(22), 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio‐Vejar, S. , Vasquez, Y. , Morales, P. , Magne, F. , Vera‐Wolf, P. , Ugalde, J. A. , … Gotteland, M. (2017). The gut microbiota of healthy Chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Frontiers in Microbiology, 8(6), 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Lin, H. , & Huang, W. (2011). Modulating gut microbiota as an anti‐diabetic mechanism of berberine. Medical Science Monitor, 17(7), RA164–RA167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. , Li, C. , Huan, Y. , Sun, S. , Mu, Y. , & Shen, Z. (2016). Effects of berberine compatible with stachyose on glucolipid metabolism and gut microbiota in diabetic mice. Chinese Journal of Clinical Pharmacology, 32(12), 11211124. [Google Scholar]
- Imenshahidi, M. , & Hosseinzadeh, H. (2019). Berberine and barberry (Berberis vulgaris): A clinical review. Phytotherapy Research, 33(3), 504–523. [DOI] [PubMed] [Google Scholar]
- Kawamori, D. , Kurpad, A. J. , Hu, J. , Liew, C. W. , Shih, J. L. , Ford, E. L. , … Kulkami, R. N. (2009). Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metabolism, 9(4), 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Yang, M. , Hou, G. , Liu, S. , Huan, Y. , Yu, D. , … Shen, Z. (2017). A human glucagon‐like peptide‐1‐albumin recombinant protein with prolonged hypoglycemic effect provides efficient and beneficial control of glucose metabolism in diabetic mice. Biological & Pharmaceutical Bulletin, 40(9), 1399–1408. [DOI] [PubMed] [Google Scholar]
- Li, W. , Huang, D. , Gao, A. , & Yang, X. (2016). Stachyose increases absorption and hepatoprotective effect of tea polyphenols in high fructose‐fed mice. Molecular Nutrition & Food Research, 60(3), 502–510. [DOI] [PubMed] [Google Scholar]
- Liu, G. , Bei, J. , Liang, L. , Yu, G. , Li, L. , & Li, Q. (2018). Stachyose improves inflammation through modulating gut microbiota of high‐fat diet/streptozotocin‐induced type 2 diabetes in rats. Molecular Nutrition & Food Research, 62(6), e1700954–e1700961. [DOI] [PubMed] [Google Scholar]
- Lv, Z. , Peng, G. , Liu, W. , Xu, H. , & Su, J. (2017). Berberine blocks the relapse of Clostridium difficile infection in C57BL/6 mice after standard vancomycin treatment. Antimicrobial Agents and Chemotherapy, 59(7), 3726–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejías‐Luque, R. , Lindén, S. K. , Garrido, M. , Tye, H. , Najdovska, M. , Jenkins, B. J. , … de Bolós, C. (2010). Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene, 29(12), 1753–1762. [DOI] [PubMed] [Google Scholar]
- More, N. V. , Kharat, K. R. , & Kharat, A. S. (2017). Berberine from Argemone mexicana L exhibits a broadspectrum antibacterial activity. Acta Biochimica Polonica, 64(4), 653–660. [DOI] [PubMed] [Google Scholar]
- Ottman, N. , Davids, M. , Suarez‐Diez, M. , Boeren, S. , Schaap, P. J. , Martins Dos Santos, V. A. P. , … de Vos, W. M. (2017). Genome‐scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin‐degrading lifestyle. Applied and Environmental Microbiology, 83(18), e01014–e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici, S. , Song, J. , Zhang, C. , Wang, Q. , Glahn, R. P. , Kolba, N. , & Tako, E. (2017). Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut microflora populations. Nutrients, 9(3), 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J. , Huan, Y. , Jiang, Q. , Sun, S. , Jia, C. , & Shen, Z. (2014). Effects and potential mechanisms of pioglitazone on lipid metabolism in obese diabetic KKAy mice. PPAR Research, 2014, 538183–538196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, N. R. , Lee, J. C. , Lee, H. Y. , Kim, M. S. , Whon, T. W. , Lee, M. S. , & Bae, J. W. (2014). An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet‐induced obese mice. Gut, 63(5), 727–735. [DOI] [PubMed] [Google Scholar]
- Tawiah, A. , Cornick, S. , Moreau, F. , Gorman, H. , Kumar, M. , Tiwari, S. , & Chadee, K. (2018). High MUC2 mucin expression and misfolding induce cellular stress, reactive oxygen production, and apoptosis in goblet cells. The American Journal of Pathology, 188(6), 1354–1373. [DOI] [PubMed] [Google Scholar]
- Thorel, F. , Népote, V. , Avril, I. , Kohno, K. , Desqraz, R. , Chera, S. , & Herrera, P. L. (2010). Conversion of adult pancreatic alpha‐cells to beta‐cells after extreme beta‐cell loss. Nature, 464(7292), 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Jia, Z. , Kong, L. , Ma, H. , Ren, J. , Li, M. , & Ge, X. (2004). Stachyose extract from Rehmannia glutinosa Libosch. To lower plasma glucose in normal and diabetic rats by oral administration. Pharmazie, 59(7), 552–556. [PubMed] [Google Scholar]
- Zhong, C. , Qiu, S. , Li, J. , Shen, J. , Zu, Y. , Shi, J. , & Sui, G. (2019). Ellagic acid synergistically potentiates inhibitory activities of chemotherapeutic agents to human hepatocellular carcinoma. Phytomedicine, 59(4), 152921–152931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Figure S1 Pie chart showing the relative abundance of bacteria at the phylum level by taxon‐based analysis.
Figure S2 Principal component analysis (PCA) of metabolites.
Figure S3 The response permutation testing (RPT) of each OPLS‐DA model.
Table S1 Supporting Information
Table S2 Supporting Information
Table S3 Supporting Information
Table S4 Supporting Information


