Abstract
3,4‐Methylenedioxymethamphetamine (MDMA)–assisted psychotherapy for posttraumatic stress disorder (PTSD) has been shown to significantly reduce clinical symptomatology, but posttraumatic growth (PTG), which consists of positive changes in self‐perception, interpersonal relationships, or philosophy of life, has not been studied with this treatment. Participant data (n = 60) were pooled from three Phase 2 clinical studies employing triple‐blind crossover designs. Participants were required to meet DSM‐IV‐R criteria for PTSD with a score higher than 50 on the Clinician‐Administered PTSD Scale (CAPS‐IV) as well as previous inadequate response to pharmacological and/or psychotherapeutic treatment. Data were aggregated into two groups: an active MDMA dose group (75–125 mg of MDMA; n = 45) or placebo/active control (0–40 mg of MDMA; n = 15). Measures included the Posttraumatic Growth Inventory (PTGI) and the CAPS‐IV, which were administered at baseline, primary endpoint, treatment exit, and 12‐month follow‐up. At primary endpoint, the MDMA group demonstrated more PTG, Hedges’ g = 1.14, 95% CI [0.49, 1.78], p < .001; and a larger reduction in PTSD symptom severity, Hedges’ g = 0.88, 95% CI [−0.28, 1.50], p < .001, relative to the control group. Relative to baseline, at the 12‐month follow‐up, within‐subject PTG was higher, p < .001; PTSD symptom severity scores were lower, p < .001; and two‐thirds of participants (67.2%) no longer met criteria for PTSD. MDMA‐assisted psychotherapy for PTSD resulted in PTG and clinical symptom reductions of large‐magnitude effect sizes. Results suggest that PTG may provide a new mechanism of action warranting further study.
A novel treatment for posttraumatic stress disorder (PTSD) utilizing 3,4‐methylenedioxymethamphetamine (MDMA) to enhance psychotherapy first entered clinical trials in 2000 (Bouso, Doblin, Farré, Alcázar, & Gómez‐Jarabo, 2008), and six randomized controlled studies had been completed as of 2017 (Feduccia, Holland & Mithoefer, 2017). Results from these trials demonstrate that MDMA‐assisted psychotherapy produces significant reductions in PTSD symptoms (Mithoefer et al., 2018; Mithoefer, Wagner, Mithoefer, Jerome, & Doblin, 2011; Oehen, Traber, Widmer, & Schnyder, 2013; Ot'alora et al., 2018) and that these reductions endure (Mithoefer et al., 2013). Results of a pooled analysis of six Phase 2 studies (Mithoefer et al., 2019) demonstrated that two psychotherapy sessions with active doses of MDMA more than doubled PTSD symptom reduction compared to psychotherapy with placebo controls (estimated mean difference between groups = −22.0, SE = 5.17), d = 0.8. The Food and Drug Administration (FDA) designated MDMA‐assisted psychotherapy as a Breakthrough Therapy in 2017, indicating that the treatment addresses a serious or life‐threatening condition and may have preliminary evidence demonstrating substantial improvement over existing therapies for PTSD. However, posttraumatic growth (PTG), defined as positive posttrauma changes in self‐perception, interpersonal relationships, or philosophy of life, has not yet been studied as an independent outcome or a mechanism of symptom reduction with MDMA‐assisted psychotherapy.
Given the high incidence and prevalence of PTSD, it is critical to pursue promising treatments and explore and model possible mechanisms of action (Kessler et al., 2005). The reported lifetime prevalence of PTSD in the general population of the United States is approximately 6.8%, and the disorder affects about 7.7 million U.S. adults in any given year (Kessler et al., 2005). According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐V; American Psychiatric Association [APA], 2013), individuals diagnosed with PTSD will persistently reexperience aspects of the traumatic event, avoid trauma‐related stimuli, and experience negative thoughts or feelings as well as increased hyperarousal and reactivity related to the trauma. Existing PTSD research has tended to focus on symptom severity as a primary outcome while neglecting positive life changes that may accompany an engagement with trauma in a psychotherapeutic intervention, independent of symptom reduction. Although one criterion for a clinical diagnosis according to DSM‐5 is for the ailment to cause “clinically significant distress or impairment in social, occupational, or other important areas of functioning” (APA, 2013), and although functional impairment has been closely linked to the severity of PTSD symptomatology, few intervention PTSD trials have evaluated whether functioning in these domains improves (Rodriguez, Holowka, & Marx, 2012).
Posttraumatic growth has been defined as “positive psychological change experienced as a result of the struggle with highly challenging life circumstances” (Calhoun & Tedeschi, 1999; Tedeschi & Calhoun, 2004). Tedeschi and Calhoun (1996) grouped these positive changes into three main categories: changes in self‐perception, changes in interpersonal relationships, and changes in philosophy of life. The authors of the Posttraumatic Growth Inventory (Tedeschi & Calhoun, 1996; PTGI), a widely used and validated measure of PTG, identified five factors underlying the construct: relating to others, new possibilities, personal strengths, spiritual change, and appreciation of life (Taku, Cann, Calhoun, & Tedeschi, 2008). Research using the PTGI has concentrated on how the struggle with adversity affects these factors (Shakespeare‐Finch & Lurie‐Beck, 2014). Calhoun and Tedeschi (1998) theorized that psychotherapy may facilitate PTG and that this growth may play a role in the consolidation of symptom reduction. The results of a meta‐analysis of 42 studies on PTSD that assessed both PTSD symptoms and PTG found that “both positive and negative posttrauma outcomes can co‐occur” and that evidence exists for a significant relation between them, moderated by age and trauma type (Roepke, 2015).
Roepke (2015) identified 12 randomized controlled trials that used a measure to assess PTG before and after a clinical intervention. Only five of the studies found that the intervention achieved statistically significant growth as compared to a control condition; these included group cognitive behavioral stress management (Antoni et al., 2001; Penedo et al., 2006), an online cognitive behavioral therapy intervention for PTSD symptoms (Knaevelsrud, Liedl, & Maercker, 2010), an online intervention for complicated grief (Wagner, Knaevelsrud, & Maercker, 2007), and written or spoken disclosure about adversity (Slavin‐Spenny, Cohen, Oberleitner, & Lumley, 2011). For these five studies, Cohen's d effect sizes ranged from small to large, with none exceeding an effect size value of 1.0. A review of the extant literature revealed no studies in which PTG was assessed in either a trial of psychotropic medication or medication‐assisted psychotherapy.
The peer‐reviewed literature on the PTG construct is not without criticism. Self‐report of PTG may not necessarily reflect actual growth, evidenced by observable changes, but may rather indicate perceived change. This was supported by Frazier and colleagues (2009), who found no correlation between perceived growth and actual growth except for an association between perceived growth and increased distress from pre‐ to posttrauma. As an alternative, PTG could be understood as a motivated positive illusion that serves a protective function (McFarland & Alvaro, 2000). However, evidence contrary to this perspective has also been reported—high PTG prior to or soon after traumatic event exposure has been associated with significantly higher levels of posttraumatic stress at 15 months postevent or later (Blix, Birkeland, Hansen & Heir, 2016; Engelhard, Lommen, & Sijbrandij, 2014).
It is possible that MDMA‐assisted psychotherapy may promote PTG among individuals with PTSD. The drug is associated with complex pharmacodynamics and pharmacokinetics (de la Torre et al., 2004; Hysek et al., 2012; Simmler et al., 2013) and with beneficial subjective effects, including improved self‐reported well‐being, enhanced interpersonal closeness, more empathy for self and others, reduced distress in response to social exclusion and prosociality (Bershad, Miller, Baggott, & de Wit, 2016; Kamilar‐Britt & Bedi, 2015), enhanced introspection, and emotional openness (Bedi, Van Dam, & Redman, 2010; Greer & Tolbert, 1986, Bershad et al., 2016). However, little has been published regarding the effect of this MDMA treatment on participants, beyond the primary outcome of PTSD symptom reduction. Could MDMA‐assisted psychotherapy improve participants’ lives in other ways? Do participants report having better relationships, increased appreciation for life, or an enhanced sense of personal efficacy after engaging with their trauma in therapy? Such changes can be understood as PTG and positive psychological changes as a result of dealing with extremely negative experiences, such as military combat, life‐threatening illness, or sexual assault (Tedeschi & Calhoun, 2004).
In this exploratory secondary analysis of data pooled from three Phase 2 trials, we hypothesized that MDMA‐assisted psychotherapy would influence PTG positively while also reducing trauma symptoms. The examination of PTG in a clinical‐trial context represents an advance in evaluation psychotherapy research, as it has yet to be studied as an outcome in medication‐assisted psychotherapy. Our secondary objective was to evaluate whether reduction of PTSD symptoms was correlated with changes in PTG.
Method
Participants
Of the six completed Phase 2 studies of MDMA‐assisted psychotherapy for PTSD, only three studies included the PTGI; these studies are included in the current analysis. Data were pooled from three Phase 2 clinical studies (MP‐4, MP‐8, MP‐12) with similar study designs; two of the studies, MP‐8 (Mithoefer et al., 2018) and MP‐12 (Ot'alora et al., 2018), have been published. Study sites were in the United States (MP‐8, MP‐12) and Canada (MP‐4). Protocols and amendments were approved by independent review boards (MP‐8, MP‐12: Western Copernicus Group Independent Review Board, Research Triangle Park, NC; MP‐4: IRB Services/Chesapeake, Aurora ON). Written informed consent was obtained from all participants.
Recruitment for studies was done through referrals by health care professionals, internet advertisements, and word of mouth. Participants were individuals 18 years of age and older who had a PTSD diagnosis that persisted for over 6 months. Candidates were required to have a CAPS‐IV total severity score of 50 or higher for the MP‐8 and MP‐12 studies and 60 or higher for the MP‐4 study, with PTSD symptoms present for at least 6 months. To determine if potential participants had excluded comorbid psychiatric diagnoses, the Structured Clinical Interview for DSM‐IV Axis I Diagnosis (SCID; First, Spitzer, Gibbon, & Williams, 1997) was administered during screening. Exclusion criteria included presence of major medical conditions, past or current psychotic disorder, pregnancy or lactation, and weight under 48 kg. Participants with active substance use disorders within 60 days of screening for the MP‐8 and MP‐12 studies or within 6 months of screening in MP‐4 study were excluded. The CONSORT flow diagrams are available for two of the three studies (Mithoefer et al., 2018; Ot'alora et al., 2018).
Procedure
The three studies tested different active doses of MDMA (75 mg, n = 7; 100 mg, n = 9; 125 mg, n = 29) compared to either active control doses of MDMA (30 mg, n = 7; 40 mg, n = 6) or placebo (0 mg, n = 2). Procedures are described for pooled data from the active MDMA dose group (MDMA group: 75–125 mg of MDMA, n = 45) or the placebo/active control group (control group: 0–40 mg of MDMA, n = 15). Participants were randomized (control or active MDMA doses) to receive 8 hours of manualized psychotherapy in two blinded experimental sessions scheduled 3–5 weeks apart. Approximately 1.5–2.5 hr after the initial dose, an optional supplemental dose equal to half the initial was available. Three nondrug 90‐min therapy sessions preceded the first experimental session, and three nondrug 90‐min therapy sessions followed each experimental session.
A therapy team, consisting of a male therapist and female therapist, was present for all therapy sessions. The psychotherapeutic method is a manualized nondirective approach described in the MDMA‐assisted Psychotherapy Treatment Manual (Mithoefer, 2017). Participants stayed overnight after experimental sessions, with an attendant at the study site.
After unblinding, all participants randomized to the control group (0–40 mg) and 75 mg dosage groups had three open‐label sessions with active doses of MDMA (100–125 mg) in a crossover segment; participants in the active dose (100–125 mg) arm underwent one additional open‐label session with MDMA. Due to the crossover design of the study, all participants who completed the 12‐month follow‐up received MDMA‐assisted Tpsychotherapy; thus, no comparison group is available for this time point. The MDMA was synthesized by David Nichols at Purdue University (MP‐8, MP‐12), or Lipomed AG, Arlesheim, Switzerland (MP‐4), and encapsulated with lactose to make equivalent‐weight gelatin capsules across dose groups.
All measures were administered at baseline, prior to the first session, when participants received MDMA or placebo. The primary endpoint was 1 month after the second blinded MDMA or placebo session. Outcomes were assessed again for the active‐dose group (100–125 mg) 2 months after the open‐label session (i.e. treatment exit); for the crossover group, outcomes were assessed 2 months after the third open‐label session (i.e. treatment exit). The long‐term follow‐up outcome measures were administered 12 months after treatment concluded, at which point all participants had received at least one dose of active MDMA during the blinded or open‐label segment. In addition to the PTGI and CAPS‐IV presented in this paper, several other secondary and exploratory measures were administered in individual studies to gather preliminary data regarding efficacy and mechanisms of action of MDMA‐assisted psychotherapy.
Measures
Posttraumatic growth
The PTGI (Tedeschi & Calhoun, 1996) is a 21‐item, validated measure of perceived positive outcomes of a traumatic event. The measure includes five subscales: Personal Strength (e.g., “I have a greater feeling of self‐reliance”), Spiritual Change (e.g., “I have a better understanding of spiritual matters”), Relating to Others (e.g., “I have more compassion for others”), Appreciation of Life (e.g., “I can better appreciate each day”), and New Possibilities (e.g., “I established a new path for my life”). Respondents rate each item using a 6‐point Likert scale that ranges from 0 (no change) to 5 (great change). The original five‐factor model identified by Tedeschi and Calhoun (1996) was found to provide good fit (Taku et al., 2008). The total score, which can range from 0 to 105, is interpreted as a measure of magnitude in PTG. Participants were instructed to rate each item according to the degree to which this change occurred in their life as a result of the crisis or disaster. At baseline, participants were instructed to complete the PTGI using time since the trauma as the reference, but on subsequent administrations of the PTGI, participants were instructed to respond using the beginning of their participation in the study as a reference. Cronbach's alpha values for the PTGI total score at baseline, treatment exit, and 12‐month follow‐up were .827, .899, and .882, respectively. These values were derived from subscales, due to item‐level responses not being available.
PTSD
The CAPS‐IV is a semistructured interview regarded as the gold standard measure of PTSD symptomology (Bovin et al., 2016). The CAPS‐IV provides a total symptom severity rating; severity indices for each PTSD symptom cluster (reexperiencing symptoms, avoidance and numbing symptoms, and hyperarousal symptoms); and a diagnostic score (Blake et al., 1995). For the present study, blinded independent raters not present during therapy sessions administered the CAPS‐IV. Cronbach's alpha values for the CAPS‐IV total severity score at baseline, treatment exit, and 12‐month follow‐up were .794, .887, and .921, respectively.
Data Analysis
Analyses for this paper aggregated participants into either an active MDMA dose group (“MDMA group;” 75–125 mg of MDMA, n = 45) or a placebo/active control group (“control group;” 0–40 mg of MDMA, n = 15). Justification for aggregating data into two groups is based on the designation of these doses as control or active in individual study protocols and by inspection of posttreatment CAPS‐IV scores, which clearly indicated separation of individual doses into two groups (Mithoefer et al., 2019). The modified intent‐to‐treat set included pooled data of randomized participants who completed at least one blinded experimental session and a follow‐up assessment. Missing data were not imputed. The primary aim of this article was to evaluate differences in PTG between treatment groups at the primary endpoint. Independent samples t tests were used to analyze the change in PTGI and CAPS‐IV total scores from baseline to the primary endpoint. The alpha level indicating significance for analyses was .05 (two‐tailed). Between‐group effect sizes were calculated as Hedges’ g. Diagnostic criteria from CAPS‐IV for PTSD presence or absence was summarized descriptively.
Twelve‐month follow‐up outcomes were compared to scores at baseline and treatment exit (i.e., 2 months after the last active‐dose MDMA session or the last observation carried forward in cases where a participant had completed at least one active‐dose session). Because participants from both groups had received active doses of MDMA in the blinded or open‐label stages, outcome scores from all participants were aggregated at three time points and analyzed with a mixed‐effect repeated measure model (MMRM). The base model included time (baseline, treatment exit, 12‐month follow‐up), baseline CAPS‐IV score, and study as a fixed effect; participant was specified as a random effect. To assess the relation between age, PTSD duration, sex, race, and prestudy self‐reported “ecstasy” use (i.e., substances assumed to contain MDMA), these covariates were added to the base model one at a time.
A secondary study aim was to evaluate whether changes in PTSD symptoms correlated with PTG. For each treatment group in the blinded segment, Pearson correlations (two‐tailed) were used to evaluate scores at baseline as well as change in CAPS‐IV and PTGI scores from baseline to the primary endpoint. Correlation coefficients were compared between groups. To better understand the association between change in CAPS‐IV and PTGI scores, a regression model included a nonlinear term in the model. Analyses were completed using SPSS (Version 20; IBM Corp., 2011), and SAS (Version 9.3; SAS Institute Inc., 2003) was used for the MMRM.
Results
Sample Characteristics
The sample in the pooled dataset (Table 1) was nearly equally split by gender (29 women and 31 men), with a nearly homogenous racial sample (51 Caucasian, three Latino/Hispanic, two Native American, and four “other” participants) and a mean participant age of 40 years (range: 22–66). Qualifying traumatic events included war‐related trauma (n = 21), childhood sexual abuse (n = 7), childhood physical abuse (n = 7), adult sexual abuse (n = 8), adult physical abuse (n = 2), accident (n = 2), natural disaster (n = 2), and “other” (n = 9). At enrollment, the mean participant CAPS‐IV total and PTGI scores were 89.4 (SD = 16.71) and 37.8 (SD = 22.78), respectively. Due to the small sample size and normal distribution of these data, outlier labeling was conducted using a Hedges’ g value of 2.2, following Haoglin, Iglewicz, and Tukey (1986). No outliers were identified for baseline PTGI or CAPS‐IV total scores in either group (i.e., treatment or control). At baseline, there were no between‐group differences for CAPS‐IV total score, p = .721, or PTGI total score, p = .565.
Table 1.
Demographics and Baseline Characteristics
| Control | Active | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 15) | (n = 45) | |||||||
| Variable | M | SD | n | % | M | SD | n | % |
| Age (years) | 40.2 | 10.17 | 40.6 | 12.36 | ||||
| Sex | ||||||||
| Male | 7 | 46.7 | 24 | 53.3 | ||||
| Female | 8 | 53.3 | 21 | 46.7 | ||||
| Race/ethnicity | ||||||||
| White/Caucasian | 13 | 86.7 | 38 | 84.4 | ||||
| Latino/Hispanic | 1 | 6.7 | 2 | 4.4 | ||||
| Native American | 1 | 6.7 | 1 | 2.2 | ||||
| Other/Biracial | 0 | 0.0 | 4 | 8.9 | ||||
| Prestudy ecstasy use | ||||||||
| Yes | 3 | 20.0 | 16 | 35.6 | ||||
| No | 12 | 80.0 | 29 | 64.4 | ||||
| BMI, kg/m2 | 28.6 | 6.89 | 25.9 | 4.96 | ||||
| PTSD duration (months) | 145.9 | 139.42 | 234.5 | 229.72 | ||||
| Baseline CAPS‐IV score | 88.0 | 11.48 | 89.8 | 18.21 | ||||
| Baseline PTGI scorea | 35.0 | 20.00 | 38.8 | 23.76 | ||||
Note. BMI = body mass index; PTSD = posttraumatic stress disorder; CAPS = Clinician‐Administered PTSD Scale; PTGI = Posttraumatic Growth Inventory.
At baseline, 14 participants in the control group and 43 participants in the active group completed the PTGI.
Primary Outcome
Table 2 depicts blinded CAPS‐IV and PTGI outcomes at the primary endpoint. The change from baseline to the primary endpoint was significantly different between groups for both the CAPS‐IV, p < .001, and PTGI, p < .001, with the active MDMA group exhibiting the largest improvement. Hedges’ g between‐group effect sizes were large for both measures: CAPS‐IV, 0.88, 95% CI [−0.28, 1.5]; PTGI: 1.14, 95% CI [0.49, 1.78]. At the primary endpoint, more participants in the active group (52.3%) did not meet PTSD diagnostic criteria than in the control group (33.3%). Changes in PTG and PTSD symptom scores are shown in Figure 1.
Table 2.
Outcome Measures at Primary End Point After Two Blinded Sessions
| Control | Active | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 15) | (n = 45) | ||||||||||
| Variable | n | % | M | SD | n | % | M | SD | p | Hedges’ g | 95% CI |
| CAPS‐IV total | |||||||||||
| Baseline | 15 | 88.0 | 11.48 | 45 | 89.8 | 18.21 | |||||
| Primary endpoint | 15 | 75.2 | 21.53 | 44 | 53.8 | 32.52 | |||||
| Changea | 15 | −12.8 | 15.88 | 44 | −35.1 | 27.45 | < .001 | 0.88 | [− 0.28, 1.5] | ||
| PTGI totalb | |||||||||||
| Baseline | 14 | 35.0 | 20.00 | 43 | 38.8 | 23.76 | |||||
| Primary endpoint | 15 | 30.1 | 27.66 | 43 | 60.3 | 25.50 | |||||
| Changea | 14 | −3.2 | 18.70 | 41 | 23.0 | 24.35 | < .001 | 1.14 | [0.49, 1.78] | ||
| PTSD diagnostic met primary endpoint | |||||||||||
| Yes | 10 | 66.7 | 21 | 47.7 | — | — | — | ||||
| No | 5 | 33.3 | 23 | 52.3 | — | — | — | ||||
Note. CAPS = Clinician‐Administered PTSD Scale; PTGI = Posttraumatic Growth Inventory; PTSD = posttraumatic stress disorder.
Change from baseline to primary endpoint.
Different participants were missing data at baseline and primary endpoint. The change was calculated only for participants with scores from both visits.
Figure 1.
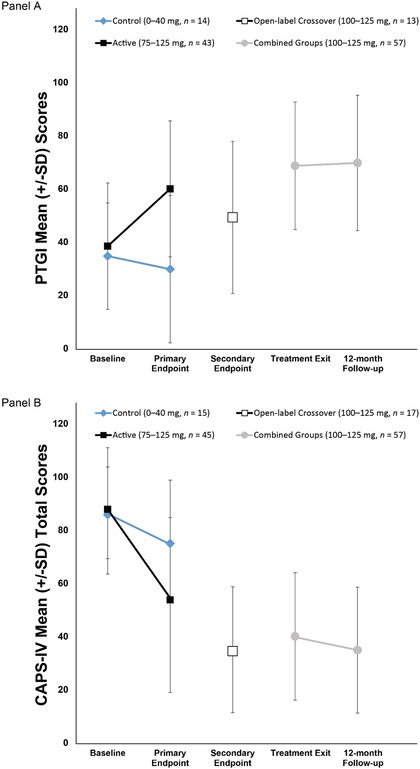
Change over time in (A) Posttraumatic Growth Inventory (PTGI) and (B) Clinician‐Administered PTSD Scale (CAPS‐IV) total scores. The primary endpoint occurred 1 month after the second blinded MDMA/placebo session. The blind was broken after the primary endpoint. The active‐dose groups (100–125 mg) had one additional open‐label MDMA session and completed an assessment 2 months after the third session (treatment exit). The comparator group (0–40 mg) and the 75 mg group crossed over to receive three open‐label (100–125 mg) sessions, with an assessment 1 month after the second open‐label MDMA session (secondary endpoint) and again 2 months after the third open‐label MDMA session (treatment exit). The 12‐month follow‐up visit occurred after the final open‐label MDMA session. Groups were pooled for treatment exit and 12‐month follow‐up endpoints, as all participants had received active doses of MDMA in either the blinded or open‐label crossover segments.
At baseline, neither group had significant correlations of PTGI and CAPS‐IV scores: placebo group (n = 14): r = −.09, p = .773; MDMA group (n = 43): r = −.01, p = .956. In the MDMA group (n = 41), the CAPS‐IV score change from baseline to primary endpoint was negatively correlated with the change in PTGI score, r = −.42, p = .006, 95% CI [−0.64, −0.13], showing that PTSD symptoms decreased as PTG increased. There was no significant correlation for the control group (n = 14), r = −.04, p = .895. However, a comparison of correlation coefficients between groups did not reach statistical significance, p = .234. A correlation plot of change scores for the PTGI and CAPS‐IV from baseline to primary endpoint is shown in Figure 2. Results from a regression model with a nonlinear term indicated that there was no evidence of nonlinearity between change in PTGI and CAPS‐IV scores.
Figure 2.
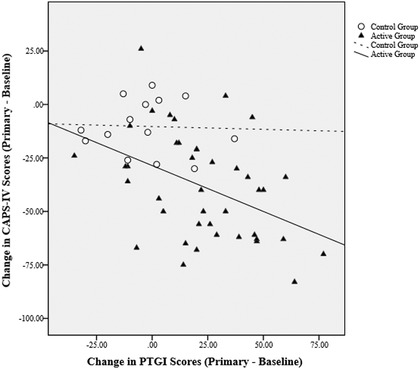
Correlation plot of change from baseline to primary endpoint on the Posttraumatic Growth Inventory (PTGI) and Clinician‐Administered PTSD Scale for DSM‐IV (CAPS‐IV). For the active MDMA group, change scores on the PTGI and CAPS‐IV were negatively correlated, indicating that as posttraumatic stress disorder (PTSD) symptoms decreased, posttraumatic growth increased. Scores were not correlated for the control group.
Treatment Exit and 12‐Month Follow‐up
To examine the change in outcomes from baseline to treatment exit to long‐term follow‐up (i.e., 12 months after the last active‐dose MDMA session), both groups were combined as described earlier. The results of the MMRM (Table 3) demonstrated that PTSD symptom severity and PTG significantly improved at treatment exit compared to baseline (n = 54) for the CAPS‐IV, p < .001, and PTGI, p < .001, with positive gains sustained at the 12‐month follow‐up. Compared to baseline, scores on both measures were significantly improved at the 12‐month endpoint: CAPS‐IV, p < .001; PTGI, p < .001. Duration of PTSD, sex, race, and prestudy self‐reported ecstasy use were not significant covariates in the model.
Table 3.
Within‐Subject Outcome Measure Scores at Baseline, Treatment Exit, and 12‐Month Follow‐Upa
| Total | |||
|---|---|---|---|
| (N = 57) | |||
| Variable | LS | SE | p |
| CAPS‐IV total score | |||
| Baseline | 89.9 | 2.25 | |
| Treatment exit | 39.9 | 3.60 | |
| Change: Baseline to treatment exit | −49.9 | 3.45 | < .001 |
| 12‐month follow‐up | 35.6 | 3.33 | |
| Change: Treatment exit to 12‐month follow‐up | −4.3 | 2.77 | .122 |
| Change: Baseline to 12‐month follow‐up | −54.3 | 3.50 | < .001 |
| PTGI total score | |||
| Baseline | 37.9 | 3.02 | |
| Treatment exit | 69.0 | 3.24 | |
| Change: Baseline to treatment exit | 31.2 | 4.20 | < .001 |
| 12‐month follow‐up | 70.1 | 3.43 | |
| Change: Treatment exit to 12‐month follow‐up | 1.0 | 2.45 | .680 |
| Change: Baseline to 12‐month follow‐up | 32.2 | 4.02 | < .001 |
Note. LS = least squares; CAPS = Clinician‐Administered PTSD Scale; PTGI = Posttraumatic Growth Inventory; PTSD = posttraumatic stress disorder.
At 12‐month follow‐up, 18 participants (32.7%) met diagnostic criteria for PTSD; 37 participants (67.3%) did not meet criteria.
Discussion
The current study was the first to examine PTG as a novel mechanism of action in MDMA‐assisted psychotherapy for PTSD. Data were pooled from three clinical studies that employed triple‐blind crossover designs for the treatment of PTSD. The results from the current analyses are important for three reasons. First, they confirm previous findings of clinically significant reductions in symptom severity following MDMA‐assisted psychotherapy for individuals diagnosed with PTSD. Second, participants who received the MDMA treatment showed significantly more PTG and improvement in PTSD symptoms than those in the control group, and this effect was of a large magnitude. Finally, symptom improvement correlated with PTG only for the active‐dose group. These are important findings that indicate MDMA‐assisted psychotherapy facilitates self‐reported improvements in interpersonal relationships, spirituality, sense of possibility, assessment of personal strengths, and appreciation of life. After two sessions, active doses of MDMA combined with psychotherapy resulted in decreased PTSD symptom severity and increased PTG with large between‐group effect sizes: g = 0.88 for the CAPS‐IV and g = 1.14 for the PTGI. Furthermore, substantial positive gains were observed at long‐term follow‐up as compared to baseline. The results at long‐term follow‐up suggest these clinical improvements are durable, lasting at least 1 year. Importantly, safety outcomes of MDMA in a PTSD population demonstrated that limited doses of MDMA were safe to use in a controlled clinical setting. There have been no reported unexpected serious adverse events and no related adverse events for problematic substance use or compulsive drug seeking for ecstasy during the treatment period in these trials (Feduccia et al., 2017; Mithoefer et al., 2019).
There have been numerous studies published regarding the association between PTSD symptom severity and PTG (Grubaugh & Resick, 2007; Rabe, Zöllner, Maercker, & Karl, 2006; Roepke, 2015; Zoellner & Maercker, 2006; Zoellner, Rabe, Karl, & Maercker, 2008). Contrary to expectations, some literature has found high levels of PTG to be correlated with high levels of posttraumatic stress symptoms several years after the traumatic incident (Blix et al., 2016; Engelhard et al., 2014; Frazier et al., 2009). The results of the current analyses showed that reductions in PTSD symptom severity were correlated with improvements in PTG 1 month after treatment. However, from the data available, it remains unknown whether MDMA treatment itself facilitates PTG, or if the decrease is a consequence of PTSD symptom decline (Richardson, 2011). It is possible that PTG may serve as an independent psychological mechanism of action in PTSD remission. When studying PTSD, a sole focus on clinical symptomatology may limit recovery or mask other growth processes (Roepke, 2015).
The use of MDMA‐assisted psychotherapy may be particularly suited for promoting PTG in people living with PTSD. The compound MDMA is associated with the release of serotonin, dopamine, and norepinephrine, which further stimulates release of adrenaline, oxytocin, vasopressin, and cortisol (de la Torre et al., 2004). The subjective effects commonly induced by MDMA include a sense of well‐being, elevated mood, euphoria, a feeling of closeness with others, and increased sociability (Stevens, Smith, & Reiner, 2009). In addition, MDMA has been reported to facilitate introspection, interest and capability for intimacy, temporary freedom from anxiety, and emotional openness (Baggott et al., 2016) while allowing for a clear‐headed and alert state of consciousness (Greer & Tolbert, 1998; Mithoefer et al., 2011). The pharmacological effects of MDMA in combination with the psychotherapeutic processing of trauma appear to have a synergistic effect to reduce PTSD symptoms.
The impact of MDMA‐assisted psychotherapy on PTG is not entirely unsurprising considering some of the theorized mechanisms of action in the psychotherapy and pharmacological action of MDMA. Increases in personal strength, as measured by the PTGI, may reflect the largely nondirective approach, which is aimed at empowering the participant to find their own resolutions and understandings of difficulties in their lives. Other PTGI factors, such as “appreciation of life” and “new possibilities,” may be connected to evidence of long‐term changes in the personality domains of openness and neuroticism following MDMA‐assisted psychotherapy (Mithoefer et al., 2018; Wagner et al., 2017). Improvement in the factor “relating to others” may be linked to the release of oxytocin and serotonin, which may acutely enhance closeness in the therapeutic relationship and may spur global improvements in interpersonal functioning. Finally, MDMA may have an effect on spirituality as measured by the “spiritual change” and other factors of the PTGI.
These novel findings should be qualified by some limitations inherent to the current data presentation. First, the pooled data originated from three different trials that had variations in study design, tested doses, and sample sizes. Many participants and therapists were able to correctly guess treatment conditions (Mithoefer et al., 2018; Ot'alora et al., 2018), which can influence report on outcome measures. However, masked, independent, blinded raters administered the CAPS‐IV to mitigate bias. Additionally, the authors of one of the studies (Mithoefer et al., 2018) looked at different doses to address this interpretation and determined that incorrect guesses among cotherapists and participants were more common between active doses (75 mg and 125 mg) rather than between active doses and a low dose (30 mg). Despite this limitation, within‐group effects were significant at the 12‐month follow‐up visit, which would not be expected if positive outcomes were attributed to a placebo response from surmising an MDMA group assignment. A second limitation of this study was the use of self‐report measures, particularly regarding the PTGI, which does not include implicit or behavioral validation of the changes reported by the participant. Third, the sample size was relatively small and uneven between groups; however, significant group differences were detected due to large treatment effects. The sample disproportionately comprised White/Caucasian participants, which is consistent with other samples used in studies of psychedelic‐assisted psychotherapy (Michaels, Purdon, Collins, & Williams, 2018), and results may not generalize to other racial, ethnic, and cultural groups.
The current study was the first to evaluate PTG after MDMA‐assisted psychotherapy for PTSD. At posttreatment assessment, the MDMA group experienced higher levels of PTG and larger reductions in PTSD symptom severity compared to the placebo group. Overall, these improvements were enduring at the 12‐month follow‐up. What is remarkable about these results is the large magnitude of treatment effects at both posttreatment and at the 12‐month follow‐up. Although PTG has not yet been rigorously studied in a drug‐assisted psychotherapy trial, the current results suggest that it is correlated with PTSD symptom severity. Future research of MDMA‐assisted psychotherapy should include robust measurement and analysis of PTG as secondary outcomes for treating PTSD.
Supporting information
Supplementary Table 1 Summary of Individual Study Designs
Supplementary Table 2 Summary of PTGI Subscales at Baseline and Endpoints
Supplementary Table 3 Summary of CAPS‐IV Subscales at Baseline and Endpoints
This work was supported in part by The Multidisciplinary Association for Psychedelic Studies (MAPS), a nonprofit organization. The authors wish to express their gratitude to the individuals who participated in these three studies for committing to the deep and difficult journey of healing; the therapists, Michael Mithoefer, Ann Mithoefer, Marcela Ot'alora, Bruce Poulter, Will Van Derveer, Jim Grigsby, Saj Razvi, Sandra Van Der Veer, Sara Gael Giron, Alison McQueen, Ingrid Pacey, and Hayden Rubensohn, who supported participants throughout their time in the studies; the study coordinators, Sarah Sadler, Peggy Ivers, and Katrina Blommaert, who provided organization and support; Michael Mithoefer, Annie Mithoefer, and Marcela Ot'alora for training therapists; the independent raters, Joy Wymer, Mark Wagner, Carla Clements, Kathryn Kaye, and Zach Walsh; the independent rater intern, Matthew Campeau; the study pharmacists, Kimm Singer, Mel Rauton and Colin Holyk; the clinical research associates, Rebecca Matthews, Charlotte Harrison, and Elizabeth Heimler, who monitored data collection; the adherence raters, who assessed adherence to the manualized therapy; the night attendants, who cared for participants during their overnight stays; and all of the other volunteers, who tirelessly supported the study. The sponsor played a role in the study design, data analysis, and writing of the report (the authors performed all data analyses). Two authors, Ingmar Gorman and Alexander B. Belser, receive consultation fees from Akeso Therapeutics as Coclinical Investigator and Subinvestigator, respectively, for a clinical trial site for the open label multisite study of safety and effects of MDMA‐assisted psychotherapy for treatment of PTSD (ClinicalTrials.gov identifier NCT03282123).
References
- American Psychiatric Association . (1994). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author; 10.1176/appi.books.9780890423349.7336 [DOI] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.).Washington, DC: Author; 10.1176/appi.books.9780890425596.529303 [DOI] [Google Scholar]
- Antoni, M. H. , Lehman, J. M. , Klibourn, K. M. , Boyers, A. E. , Culver, J. L. , Alferi, S. M. , … Harris, S. D. (2001). Cognitive‐behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early‐stage breast cancer. Health Psychology, 20, 20–32. 10.1037/0278-6133.20.1.20 [DOI] [PubMed] [Google Scholar]
- Baggott, M. J. , Coyle, J. R. , Siegrist, J. D. , Garrison, K. J. , Galloway, G. P. , & Mendelson, J. E. (2016). Effects of 3,4‐methylenedioxymethamphetamine on socioemotional feelings, authenticity, and autobiographical disclosure in healthy volunteers in a controlled setting. Journal of Psychopharmacology, 30, 378–387. 10.1177/0269881115626348 [DOI] [PubMed] [Google Scholar]
- Bedi, G. , Van Dam, N. T. , & Redman, J. (2010). Ecstasy (MDMA) and high prevalence psychiatric symptomatology: Somatic anxiety symptoms are associated with polydrug, not ecstasy, use. Journal of Psychopharmacology, 24, 233–240. 10.1177/0269881108097631 [DOI] [PubMed] [Google Scholar]
- Bershad, A. K. , Miller, M. A. , Baggott, M. J. , & de Wit, H. (2016). The effects of MDMA on socio‐emotional processing: Does MDMA differ from other stimulants?. Journal of Psychopharmacology, 30, 1248–1258. 10.1177/0269881116663120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a clinician‐administered PTSD scale. Journal of Traumatic Stress, 8, 75–90. 10.1037/e572192010-002 [DOI] [PubMed] [Google Scholar]
- Blix, I. , Birkeland, M. S. , Hansen, M. B. , & Heir, T. (2016). Posttraumatic growth—An antecedent and outcome of posttraumatic stress: Cross‐lagged associations among individuals exposed to terrorism. Clinical Psychological Science, 4, 620–628. 10.1177/2167702615615866 [DOI] [Google Scholar]
- Bouso, J. C. , Doblin, R. , Farré, M. , Alcázar, M. Á. , & Gómez‐Jarabo, G. (2008). MDMA‐assisted psychotherapy using low doses in a small sample of women with chronic posttraumatic stress disorder. Journal of Psychoactive Drugs, 40, 225–236. 10.1080/02791072.2008.10400637 [DOI] [PubMed] [Google Scholar]
- Bovin, M. J. , Marx, B. P. , Weathers, F. W. , Gallagher, M. W. , Rodriguez, P. , Schnurr, P. P. , & Keane, T. M. (2016). Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders–fifth edition (PCL‐5) in veterans. Psychological Assessment, 28, 1379 10.1037/pas0000504 [DOI] [PubMed] [Google Scholar]
- Calhoun, L. G. , & Tedeschi, R. G. (1998). Beyond recovery from trauma: Implications for clinical practice and research. Journal of Social Issues, 54, 357–371. 10.1111/j.1540-4560.1998.tb01223.x [DOI] [Google Scholar]
- Calhoun, L. G. , & Tedeschi, R. G. (1999). Facilitating posttraumatic growth: A clinician's guide. New York, NY: Routledge; 10.4324/9781410602268 [DOI] [Google Scholar]
- De la Torre, R. , Farré, M. , Roset, P. N. , Pizarro, N. , Abanades, S. , Segura, M. , … Camí, J. (2004). Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Therapeutic Drug Monitoring, 26, 137–144. 10.1097/00007691-200404000-00009 [DOI] [PubMed] [Google Scholar]
- Engelhard, I. M. , Lommen, M. J. , & Sijbrandij, M. (2015). Changing for better or worse? Posttraumatic growth reported by soldiers deployed to Iraq. Clinical Psychological Science, 3, 789–796. 10.1177/2167702614549800 [DOI] [Google Scholar]
- Feduccia, A. A. , Holland, J. , & Mithoefer, M. C. (2017). Progress and promise for the MDMA drug development program. Psychopharmacology, 235, 561–571. 10.1007/s00213-017-4779-2 [DOI] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. W. (1997) Structured Clinical Interview for DSM‐IV Axis I Disorders—patient edition (Version 2.0, 4/97 revision). New York, NY: New York State Psychiatric Institute. [Google Scholar]
- Frazier, P. , Tennen, H. , Gavian, M. , Park, C. , Tomich, P. , & Tashiro, T. (2009). Does self‐reported posttraumatic growth reflect genuine positive change? Psychological Science, 20, 912–919. 10.1111/j.1467-9280.2009.02381.x [DOI] [PubMed] [Google Scholar]
- Greer, G. , & Tolbert, R. (1986). Subjective reports of the effects of MDMA in a clinical setting. Journal of Psychoactive Drugs, 18, 319–327. 10.1080/02791072.1986.10472364 [DOI] [PubMed] [Google Scholar]
- Greer, G. R. , & Tolbert, R. (1998). A method of conducting therapeutic sessions with MDMA. Journal of Psychoactive Drugs, 30, 371–379. 10.1080/02791072.1998.10399713 [DOI] [PubMed] [Google Scholar]
- Grubaugh, A. L. , & Resick, P. A. (2007). Posttraumatic growth in treatment‐seeking female assault victims. Psychiatric Quarterly, 78, 145–155. 10.1007/s11126-006-9034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaglin, D. C. , Iglewicz, B. , & Tukey, J. W. (1986). Performance of some resistant rules for outlier labeling. Journal of the American Statistical Association, 81, 991–999. 10.1080/01621459.1986.10478363 [DOI] [Google Scholar]
- Hysek, C. M. , Simmler, L. D. , Nicola, V. G. , Vischer, N. , Donzelli, M. , Krähenbühl, S. , … Liechti, M. E. (2012). Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo‐controlled laboratory study. PloS one, 7(5), e36476 10.1371/journal.pone.0036476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp . (2011). IBM SPSS Statistics for Windows (Version 20.0). Armonk, NY: IBM Corp. [Google Scholar]
- Kamilar‐Britt, P. , & Bedi, G. (2015). The prosocial effects of 3, 4‐methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals. Neuroscience & Biobehavioral Reviews, 57, 433–446. 10.1016/j.neubiorev.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , Berglund, P. , Demler, O. , Jin, R. , Merikangas, K. R. , & Walters, E. E. (2005). Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Knaevelsrud, C. , Liedl, A. , & Maercker, A. (2010). Posttraumatic growth, optimism and openness as outcomes of a cognitive‐behavioural intervention for posttraumatic stress reactions. Journal of Health Psychology, 15, 1030–1038. 10.1177/1359105309360073 [DOI] [PubMed] [Google Scholar]
- McFarland, C. , & Alvaro, C. (2000). The impact of motivation on temporal comparisons: Coping with traumatic events by perceiving personal growth. Journal of Personality and Social Psychology, 79, 327–343. 10.1037/0022-3514.79.3.327 [DOI] [PubMed] [Google Scholar]
- Michaels, T. I. , Purdon, J. , Collins, A. , & Williams, M. T. (2018). Inclusion of people of color in psychedelic‐assisted psychotherapy: a review of the literature. BMC psychiatry, 18(1), 245. 10.2139/ssrn.3311241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer, M. (2017). A manual for MDMA‐assisted psychotherapy in the treatment of posttraumatic stress disorder (Version 8). http://www.maps.org/research/mdma/mdma-research-timeline/4887-a-manual-for-mdma-assisted-psychotherapy-in-the-treatment-of-ptsd [DOI] [PMC free article] [PubMed]
- Mithoefer, M. C. , Feduccia, A. A. , Jerome, L. , Mithoefer, A. , Wagner, M. , Walsh, Z. , … Doblin, R. (2019). MDMA‐assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology, 236, 2735–2745. 10.1007/s00213-019-05249-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mithoefer, M. C. , Mithoefer, A. T. , Feduccia, A. A. , Jerome, L. , Wagner, M. , Wymer, J. , … Doblin, R. (2018). 3, 4‐methylenedioxymethamphetamine (MDMA)‐assisted psychotherapy for post‐traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double‐blind, dose‐response, phase 2 clinical trial. The Lancet Psychiatry, 5, 486–497. 10.1016/s2215-0366(18)30135-4 [DOI] [PubMed] [Google Scholar]
- Mithoefer, M. C. , Wagner, M. T. , Mithoefer, A. T. , Jerome, L. , & Doblin, R. (2011). The safety and efficacy of 3, 4‐methylenedioxymethamphetamine‐assisted psychotherapy in subjects with chronic, treatment‐resistant posttraumatic stress disorder: The first randomized controlled pilot study. Journal of Psychopharmacology, 25, 439–452. 10.1177/0269881110378371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer, M. C. , Wagner, M. T. , Mithoefer, A. T. , Jerome, L. , Martin, S. F. , Yazar‐Klosinski, B. , … Doblin, R. (2013). Durability of improvement in post‐traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3, 4‐methylenedioxymethamphetamine‐assisted psychotherapy: A prospective long‐term follow‐up study. Journal of Psychopharmacology, 27, 28–9. 10.1177/0269881112456611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen, P. , Traber, R. , Widmer, V. , & Schnyder, U. (2013). A randomized, controlled pilot study of MDMA (±3, 4‐methylenedioxymethamphetamine)‐assisted psychotherapy for treatment of resistant, chronic post‐traumatic stress disorder (PTSD). Journal of Psychopharmacology, 27, 40–2. 10.1177/0269881112464827 [DOI] [PubMed] [Google Scholar]
- Ot'alora G., M., Grigsby , J., Poulter , B., Van Derveer , III, J. W., Giron, S. G. , Jerome, L. , … Mithoefer, M. C. (2018). Journal of Psychopharmacology, 32, 1295–1307. 10.1177/0269881118806297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penedo, F. J. , Molton, I. , Dahn, J. R. , Shen, B. J. , Kinsinger, D. , Traeger, L. , … Antoni, M. (2006). A randomized clinical trial of group‐based cognitive‐behavioral stress management in localized prostate cancer: Development of stress management skills improves quality of life and benefit finding. Annals of Behavioral Medicine, 31, 261–270. 10.1207/s15324796abm3103_8 [DOI] [PubMed] [Google Scholar]
- Rabe, S. , Zöllner, T. , Maercker, A. , & Karl, A. (2006). Neural correlates of posttraumatic growth after severe motor vehicle accidents. Journal of Consulting and Clinical Psychology, 74, 880–886. 10.1037/0022-006X.74.5.880 [DOI] [PubMed] [Google Scholar]
- Richardson, J. T. E. (2011). Eta squared and partial eta squared as measurements of effect size in educational research. Educational Research Review, 6, 135–147. 10.1016/j.edurev.2010.12.001 [DOI] [Google Scholar]
- Rodriguez, P. , Holowka, D. W. , & Marx, B. P. (2012). Assessment of posttraumatic stress disorder‐related functional impairment: A review. Journal of Rehabilitation Research & Development, 49, 649 10.1682/jrrd.2011.09.0162 [DOI] [PubMed] [Google Scholar]
- Roepke, A. M. (2015). Psychosocial interventions and posttraumatic growth: A meta‐analysis. Journal of Consulting and Clinical Psychology, 83, 129–142. 10.1037/a0036872 [DOI] [PubMed] [Google Scholar]
- SAS Institute . (2013). SAS/ACCESS® 9.3 interface to ADABAS: Reference. Cary, NC: SAS Institute. [Google Scholar]
- Shakespeare‐Finch, J. , & Lurie‐Beck, J. (2014). A meta‐analytic clarification of the relationship between posttraumatic growth and symptoms of posttraumatic distress disorder. Journal of Anxiety Disorders, 28, 223–229. 10.1016/j.janxdis.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Simmler, L. D. , Buser, T. A. , Donzelli, M. , Schramm, Y. , Dieu, L. H. , Huwyler, J. , … Liechti, M. E. (2013). Pharmacological characterization of designer cathinones in vitro. British Journal of Pharmacology, 168, 458–470. 10.1016/j.toxlet.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin‐Spenny, O. M. , Cohen, J. L. , Oberleitner, L. M. , & Lumley, M. A. (2011). The effects of different methods of emotional disclosure: Differentiating post‐traumatic growth from stress symptoms. Journal of Clinical Psychology, 67, 993–1007. 10.1002/jclp.20750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, P. , Smith, R. L. , & Reiner, S. M. (2009). Substance abuse counseling: Theory and practice (4th ed.). Upper Saddle River, NJ: Merrill/Pearson. [Google Scholar]
- Taku, K. , Cann, A. , Calhoun, L. G. , & Tedeschi, R. G. (2008). The factor structure of the posttraumatic growth inventory: A comparison of five models using confirmatory factor analysis. Journal of Traumatic Stress, 21, 158–164. 10.1002/jts.20305 [DOI] [PubMed] [Google Scholar]
- Tedeschi, R. G. , & Calhoun, L. G. (1996). The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress, 9, 455–471. 10.1007/bf02103658 [DOI] [PubMed] [Google Scholar]
- Tedeschi, R. G. , & Calhoun, L. G. (2004). Posttraumatic growth: Conceptual foundations and empirical evidence. Psychological Inquiry, 15, 1–18. 10.1207/s15327965pli1501_02 [DOI] [Google Scholar]
- Wagner, B. , Knaevelsrud, C. , & Maercker, A. (2007). Post‐traumatic growth and optimism as outcomes of an internet‐based intervention for complicated grief. Cognitive Behaviour Therapy, 36, 156–161. 10.1080/16506070701339713 [DOI] [PubMed] [Google Scholar]
- Wagner, M. T. , Mithoefer, M. C. , Mithoefer, A. T. , MacAulay, R. K. , Jerome, L. , Yazar‐Klosinski, B. , & Doblin, R. (2017). Therapeutic effect of increased openness: Investigating mechanism of action in MDMA‐assisted psychotherapy. Journal of Psychopharmacology, 31, 967–974. 10.1177/0269881117711712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoellner, T. , & Maercker, A. (2006). Posttraumatic growth in clinical psychology: A critical review and introduction of a two component model. Clinical Psychology Review, 26, 626–653. 10.1016/j.cpr.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Zoellner, T. , Rabe, S. , Karl, A. , & Maercker, A. (2008). Posttraumatic growth in accident survivors: Openness and optimism as predictors of its constructive or illusory sides. Journal of Clinical Psychology, 64, 245–263. 10.1002/jclp.2044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Summary of Individual Study Designs
Supplementary Table 2 Summary of PTGI Subscales at Baseline and Endpoints
Supplementary Table 3 Summary of CAPS‐IV Subscales at Baseline and Endpoints


