Abstract
Objective
Primary renal lymphoma (PRL) is defined as a non‐Hodgkin lymphoma (NHL) restricted to kidneys without extensive nodal disease. The literature on epidemiology and outcome in PRL is limited to case reports and small case series.
Methods
We utilized Surveillance, Epidemiology, and End Result database (1984‐2015) to study the demographic, clinical, and pathological characteristics of PRL. We conducted analysis to assess factors associated with overall survival (OS) and cause‐specific survival (CSS).
Results
A total of 599 (0.17% of all NHL) patients were eligible for the study. The age‐adjusted incidence was 0.035/100,000 population and is increasing. The median age was 72 years, and most of the patients were Caucasians and were males. Most of the patients had unilateral tumors, and diffuse large B‐cell lymphoma (DLBCL) was the most common histologic type. The median OS was 112 months, while median CSS was not reached. Age ≥ 60 years was the strongest independent risk factor for worse OS and CSS, while non‐DLBCL histology was associated with better OS and CSS.
Discussion
Primary renal lymphoma is a rare lymphoma with increasing incidence in more recent years. In this study, we describe demographic, clinical, and pathological characteristics of PRL and factors affecting survival among these patients.
Keywords: non‐hodgkin lymphoma, primary lymphoma, primary renal lymphoma, rare lymphomas, renal lymphoma
1. INTRODUCTION
The presence of renal involvement is common in disseminated nodal and extranodal lymphomas. These are called secondary renal lymphomas (SRL). Primary renal lymphoma (PRL) is defined as a non‐Hodgkin lymphoma (NHL) involving the kidney primarily, with the absence of known extrarenal primary lymphatic disease. Since the kidney is not a lymphatic organ, the origin of renal lymphoma has been debatable.1 The evidence for existence of the entity stems from histologic examination and the observations that nephrectomy improved survival.2, 3 While PRL was first described by Gibson in 1948,4 the first case confirming PRL was reported by Coggins et al in 1980.5Even though secondary renal involvement by disseminated NHL is seen in 30%‐60% cases of NHL, PRL is an extremely rare disease accounting for less than 0.7% of NHL in North America.6, 7
The etiology of PRL is not well understood. Patients with immunosuppression in the form of human immunodeficiency virus (HIV) have a higher incidence of lymphoproliferative disorder; however, only isolated cases of PRL in HIV positive patients have been reported in literature.8, 9 Among the various theories being suggested for its etiopathogenesis, a popular one is the inflammatory nidus theory also implicated in cases of lymphoma arising out of chronic thyroiditis and H. pylori gastritis.10, 11 Chronic inflammation invades lymphoid cells which undergo oncogenic transformation in situ.3, 12 Reports of PRL arising in patients with chronic pyelonephritis support this theory 3; however, PRL is known to occur in patients without any primary kidney disease.7 Association of PRL with other chronic inflammatory and infectious diseases such as Sjogren's disease, systemic lupus erythematosus, and Epstein‐Barr virus infections has also been reported.3 Another theory states that PRL originates in the lymphatics surrounding the renal capsule and progress to infiltrate the kidney manifesting as solitary or multiple focal masses, large infiltrations in unilateral kidney or diffuse bilateral infiltrations.13
Renal lymphoma can mimic renal cell carcinoma (RCC).14 In patients with lymphoma, there is increased propensity for development of RCC due to several genetic and environmental factors.15, 16 However, it is important to differentiate between the two as the treatment is very different. While we use chemotherapy as primary treatment for PRL, the treatment for RCC is primarily surgical resection. Surgical resection is difficult for PRL because of the pattern of lymphomatous involvement and vascular arrangement. As per a study done by Memorial Sloan Kettering, 18F‐FDG PET/CT can be used to differentiate between RCC and PRL as the latter shows higher SUVs than the former.17
As per current literature, PRL has been reported mostly in middle aged adults and affects males more often than females. The most common symptom is flank pain, followed by fever and hematuria. The presentation can be age specific as younger patients (Ages 18‐50) present frequently with abdominal and flank pain whereas older patients (Age > 50) present with weight loss and hematuria.18 The disease is rapidly progressive and can present as renal failure.1 The prognosis has been reported very poor with median survival less than a year.17
As per a recent study, no more than 150 cases of PRL are reported in literature, with most cases being described only in recent years.18 Here, we present a series of 599 patients diagnosed with PRL obtained from the Surveillance, Epidemiology, and End Results (SEER) database to investigate the epidemiology, tumor characteristics, and survival of these patients.
2. MATERIALS AND METHODS
2.1. Data sources and study subjects
The population in SEER represents 28% of US population based on 2010 US census and includes incident malignancies from 19 cancer registries across the United States which includes Alaska, Georgia, California, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Rural Georgia, California, San Francisco‐Oakland, San Jose‐Monterey, Seattle, Utah, Kentucky, Los Angeles, Louisiana (including areas impacted by Hurricane Katrina), New Jersey, and Greater Georgia. The SEER database is considered to be a standard for population study in the United States with a case ascertainment rate of 98%.
2.2. Patient selection
In this retrospective population‐based study, we identified patients with a diagnosis of NHL with origin in the kidneys or renal pelvis using SEER database. We restricted year of diagnosis from 1984 onwards as Ann Arbor stages were consistently recorded in SEER database from 1984 onwards. We selected patients with stages I and II extranodal NHL as by definition PRL is restricted to the kidneys (stage I) or surrounding tissues (stage II). Widely spread lymphoma (stage III and IV) is often believed to arise from lymphoreticular system rather a primary extranodal organ. Patients were excluded, if they were diagnosed after death on autopsy.
The SEER registries record data on patient characteristics including gender, age at diagnosis, ethnicity, geographic regions, stage on diagnosis, primary tumor site, month and year of diagnosis, type of treatment (radiation/surgery/chemotherapy), vital status, cause of death, the month, and year of last follow‐up.
Associations between the demographic, clinical, and pathologic characteristics of patients and survival were assessed. Demographic variables included gender, age at diagnosis, year of diagnosis, race/ethnicity, and SEER registries. In our analysis of association between age and survival, we analyzed age as a categorical variable, with the following age intervals: ages 0‐59 years and 60 years. Initially, we grouped ages into 10‐year age‐groups and conducted a univariate analysis to estimate the hazard ratios (HRs). We grouped ages with similar hazard ratios into two groups, therefore, categorizing into 0‐59 years and 60 years age‐groups. Age 60 years is also considered an adverse prognostic factor for patients with aggressive lymphoma as per international prognostic index. Year of diagnosis was also studied as categorical variable from period prior to and after 1997 (calendar year corresponding to the approval of rituximab for treatment of lymphoma in the United States). Pathological information included stage at diagnosis and type of lymphoma (DLBCL vs non‐DLBCL). The patients were censored at the date of last follow‐up in SEER, death or on December 31, 2015, whichever came first.
2.3. Statistical analysis
Descriptive statistics were used to describe patient baseline characteristics. Incidence rates were calculated for each year using SEER*Stat software (version 8.0.5; Surveillance Research Program of the National Cancer Institute). All rates were age adjusted to the year 2000 standard population. The trend in the incidence rates was analyzed, and average annual percent change (AAPC) was calculated using NCI's Joinpoint regression program version 4.6.0. Survival curves for overall survival (OS) and cause‐specific survival (CSS) were plotted using the Kaplan‐Meier method. Life tables were constructed to analyze the year‐wise OS and CSS and were shown as percentage with 95% confidence interval (95% CI). To study the predictors of outcome, covariates were first analyzed in a univariate model and those with a “P” value of <.2 were fitted into a multivariate model to analyze the effect of each covariate independent of others using STATA 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). The strength of association between each predictor and survival was expressed as a hazard ratio (HR) along with a 95% CI. All tests were 2‐sided, and a “P” value of <.05 was considered statistically significant.
3. RESULTS
Of 345 624 patients who were diagnosed with NHL in the SEER database during 1984‐2015, 599 (0.17% or 17.33/10 000 NHL cases) were eligible for this study. The patient selection is shown in Figure 1.
Figure 1.
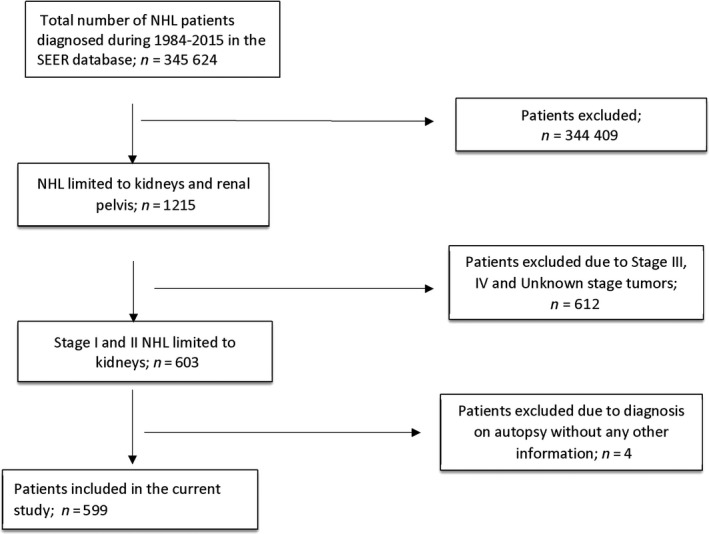
Flowchart to show the selection of eligible subjects
The age‐adjusted incidence rate (AAIR) of primary renal lymphoma was 0.035/100 000 population, and an increasing trend was observed during the study period with an AAPC of 3.3% (95%CI: 1.4‐5.3, P < .001) from 1984 to 2015. Of note, the trend for NHL also showed a rising trend during this period with an AAPC of 0.8 (95%CI: 0.5‐1, P < .001) (Figure 2). Over 87.8% of the patients were diagnosed between 1998 and 2015 compared with 12.2% of the patients diagnosed between 1984 and 1997.
Figure 2.
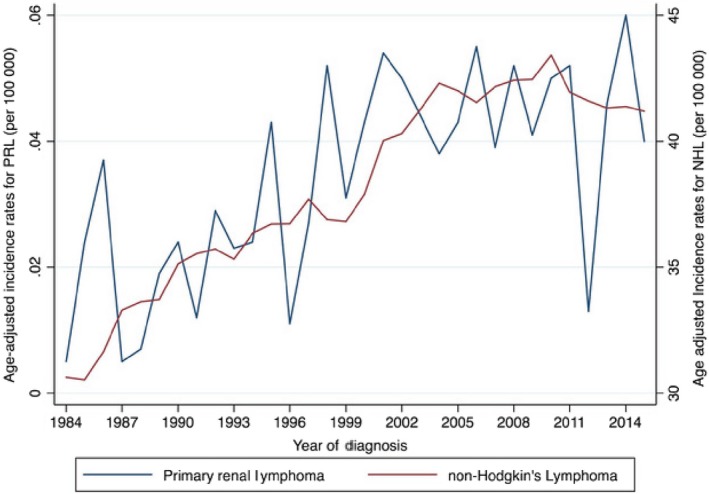
Age‐adjusted incidence rates for PRL and NHL
We have summarized the patient demographics in Table 1. The median age of the study population was 72 years (IQR: 62‐77.5). The vast majority of the patients were Caucasians (86%) and were males (63%). Eleven patients (1.8%) belonged to the 15 years or younger age‐group. Almost half (49.9%) of the patients were diagnosed in the Western region of the USA, whereas 17.5%, 13.2%, and 19.4% of the patients belonged to the northeast, Midwest, and southern regions, respectively. Most patients in our study had unilateral PRL—right and left sided PRL comprised of 42.1% and 52.1% cases, respectively. Bilateral PRL was seen in 2.7% of patients, and the laterality was unknown in another 3.2%.
Table 1.
Demographic and Clinical characteristics of the study population
| Characteristics | n = 599 (100) |
|---|---|
| Age (y) | |
| 1‐59 | 134 (22.4) |
| 60‐69 | 133 (22.2) |
| 70‐79 | 207 (34.6) |
| 80+ | 125 (20.9) |
| Year of diagnosis | |
| 1984‐1997 | 73 (12.2) |
| 1998‐2015 | 526 (87.8) |
| Gender | |
| Female | 221 (36.9) |
| Male | 378 (63.1) |
| Ethnicity | |
| White | 514 (85.8) |
| African‐American | 40 (6.7) |
| Others (American Indian, Asian/Pacific Islander/unknown) | 45 (7.5) |
| Geographic distribution | |
| Northeast | 105 (17.5) |
| Midwest | 79 (13.2) |
| South | 116 (19.4) |
| West | 299 (49.9) |
| Staging (Ann Arbor) | |
| 1 | 342 (57.1) |
| 2 | 257 (42.9) |
| Laterality | |
| Right | 252 (42.1) |
| Left | 312 (52.1) |
| Bilateral | 16 (2.7) |
| Unknown | 19 (3.2) |
| Sequence | |
| Only 1 primary tumor | 372 (62.1) |
| More than 1 primary tumors | 227 (37.9) |
| Treatment | |
| Chemotherapy only | 224 (37.4) |
| Surgery only | 129 (21.5) |
| Surgery plus chemotherapy | 100 (16.7) |
| No or unknown status | 146 (24.4) |
| Radiation | |
| Radiation (with or without chemo/surgery) | 46 (7.7) |
| Surgery plus chemotherapy plus radiation | 9 (1.5) |
Histologic classification of the lymphoma is summarized in Table 2. Majority (89.6%) of PRL was of B‐cell type NHL. The most common was diffuse large B‐cell lymphomas (DLBCL) followed by extranodal marginal zone lymphomas (MZL) and follicular lymphomas (FL). Only, 1.2% of the PRL were of the T‐cell type (Table 2).
Table 2.
Histologic subtype of primary renal lymphoma
| Non‐Hodgkin lymphoma | 599 (100) |
|---|---|
| Non‐Hodgkin lymphoma, B‐cell | 537 (89.6) |
| Diffuse large B‐cell lymphoma (DLBCL) | 274 (45.7) |
| Extranodal marginal zone lymphoma (MZL), MALT type | 86 (14.4) |
| Follicular lymphoma | 68 (11.4) |
| Chronic/Sm/Prolymphocytic/Mantle B‐cell NHL | 23 (3.8) |
| Lymphoplasmacytic lymphoma | 13 (2.2) |
| Burkitt lymphoma/leukemia | 9 (1.5) |
| Other B‐cell lymphoma | 64 (10.7) |
| Non‐Hodgkin lymphoma, T cell | 7 (1.2) |
| Non‐Hodgkin lymphoma, unclear lineage | 55 (9.2) |
Bold values denote categories based upon cell types (B‐cell and T‐cell).
About 37.4% of the patients received only chemotherapy for treatment, while 21.5% underwent only surgery, whereas 16.7% were treated with both chemotherapy and surgery. Radiation was used in only 7.7% of the patients. Combined treatment with surgery, chemotherapy, and radiation together was used in 1.5% of the patients (Table 1).
For cause‐specific survival, only those patients were included in whom PRL was the only or the first cancer. About 440 patients met the criteria. The median OS was 112 months while median cause‐specific survival was not reached (Figures 3 and 4). The 1‐ and 5‐year OS were 81.7% (95% CI: 77.4‐85.4) and 61.6% (95% CI: 55.9‐66.7) while 1‐ and 5‐ year cause‐specific survival were 86.2% (95% CI: 82.1‐89.3) and 72.9% (95% CI: 67.4‐77.5) months, respectively.
Figure 3.
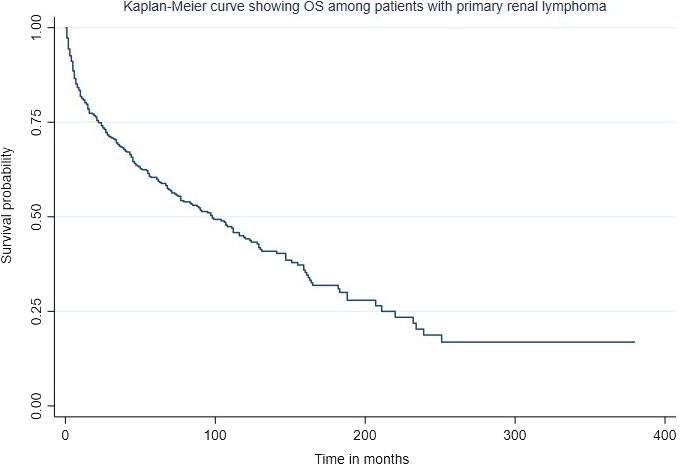
Kaplan‐Meier estimate of overall survival of patients with PRL
Figure 4.
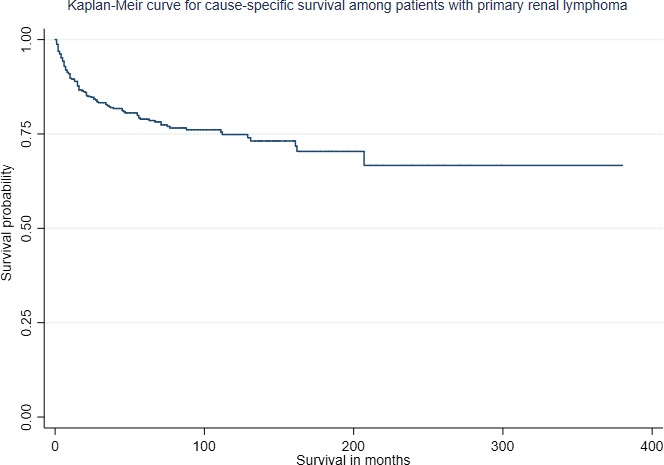
Kaplan‐Meier survival estimate of cause‐specific survival of patients with PRL
On univariate analysis, age, year of diagnosis, male gender, lymphoma histology, and stage were significantly associated with OS and cause‐specific survival. On fitting into the multivariate model, age ≥ 60 years was the strongest independent risk factor for both worse OS (HR 2.8; 95%CI: 2‐3.9, P < .01) and worse cause‐specific survival (HR 1.79; 95%CI: 1.11‐2.89, P = .02) (Figures 5A and 6A). The non‐DLBCL histology was associated with better OS (HR 0.7; 95%CI: 0.6‐0.9, P = .01) and better CSS (HR 0.64; 95%CI: 0.44‐0.94, P = .02) (Figures 5B and 6B). Other factors like stage II lymphoma (HR 1.56; 95%CI: 1.07‐2.3, P = .02) and more recent period of diagnosis (HR 0.54; 95%CI: 0.34‐0.86, P = .01) were significantly associated with cause‐specific survival but not with OS. We have summarized the results of the multivariate analysis in Table 3.
Figure 5.
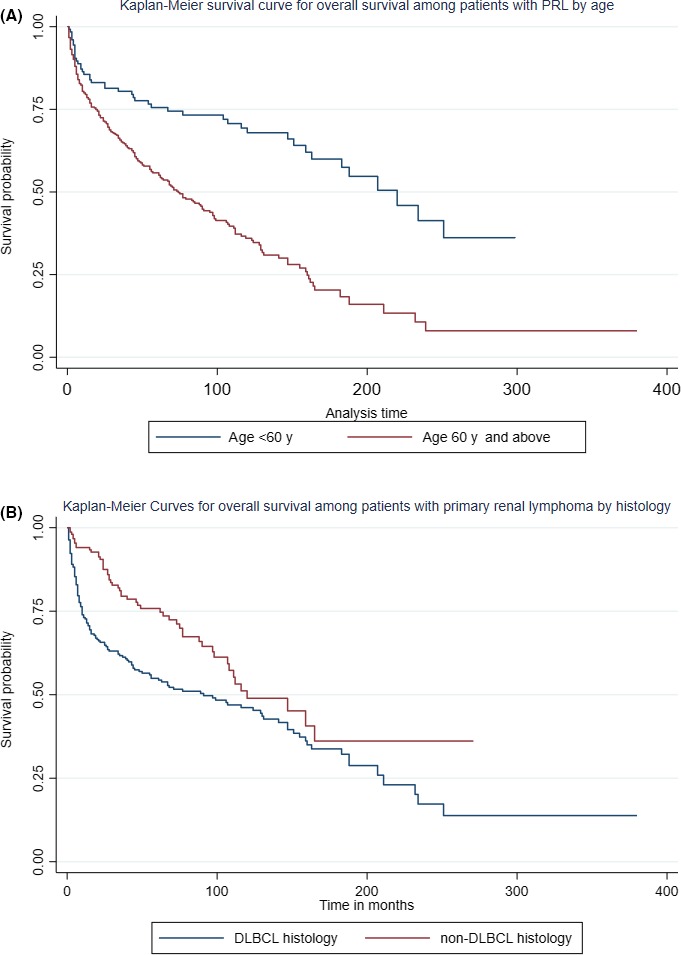
Kaplan‐Meier survival estimates for overall survival of PRL patients by (A) age and (B) histology
Figure 6.
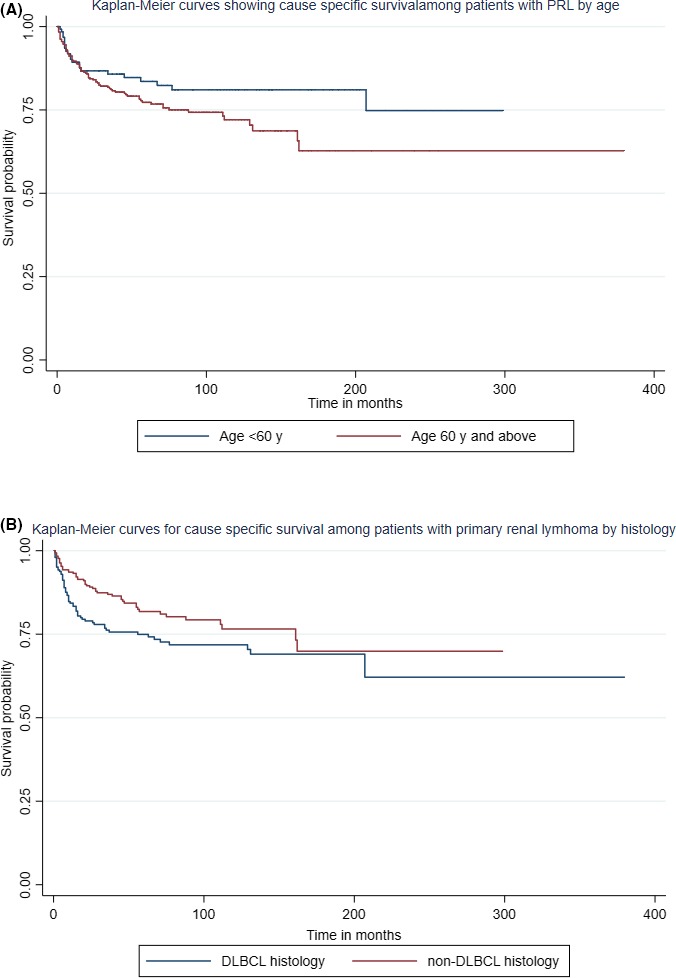
Kaplan‐Meier survival estimates for cause‐specific survival of PRL patients by (A) age and (B) histology
Table 3.
Multivariate analysis for cause‐specific survival and overall survival
| Covariates | Overall survival | Cause‐specific survival | ||
|---|---|---|---|---|
| Haz. ratio (95% CI) | “P” value | Haz. ratio (95% CI) | “P” value | |
| Age | ||||
| Age < 60 y | ref | ref | ||
| Age ≥ 60 y | 2.79 (2‐3.9) | <.001 | 1.79 (1.11‐2.89) | .02 |
| Gender | ||||
| Male | ref | ref | ||
| Female | 0.78 (0.61‐1) | .06 | 0.7 (0.47‐1.05) | .09 |
| Period of diagnosis | ||||
| Prior to 1997 | ref | ref | ||
| After 1997 | 0.81 (0.59‐1.12) | .20 | 0.54 (0.34‐0.86) | .01 |
| Histology | ||||
| DLBCL | ref | ref | ||
| Non‐DLBCL | 0.71 (0.56‐0.9) | .01 | 0.64 (0.44‐0.94) | .02 |
| Stage | ||||
| I | ref | ref | ||
| II | 1.06 (0.84‐1.35) | .61 | 1.56 (1.07‐2.27) | .02 |
Bold values denote P < .05.
4. DISCUSSION
Primary renal lymphoma is a rare entity and comprises less than 1% of all renal masses.3 So far, most of the literature is based upon isolated case reports and case series. To our knowledge, this report presents the largest series of PRL published so far.
It has been reported as of 2001 that the incidence of NHL had been rising by about 4% a year with plateauing over the last few years.19 We report a rising trend for PRL (AAPC of 3.3%) which mirrors the trends in NHL (AAPC of 0.8%) during 1984‐2015. In this study, 87.8% of the patients with PRL were diagnosed after 1998. The reason behind the surge is still unknown. However, it is plausible that due to recent increase in the understanding of the disease and improvement in diagnostic modalities, we are diagnosing PRL more now than before.19
PRL is mostly a disease of elderly although it can affect any age‐group, and 1.8% patients in the current study were in the <15 years age‐group. As per a study in 2011, the median age of DLBCL diagnosis was 70 years.20 We affirm this finding as median age in our study was 72 years, and more than three‐fourth patients were above 62.5 years. Most patients reported in literature are males with a skewed male to female ratio, with some studies reporting a ratio of 1.6:1.17 We confirm that there does seem to be a male predominance, and the ratio of male to female cases is 1.7:1 in our study as well. Most patients with PRL were white as is seen in most studies of NHL. We noted that a majority of patients diagnosed were from the Western region (49.9%) as compared with the rest of the United States. There have been studies in the past highlighting the geographic differences between different NHL subtypes around the world.21 Similarly, studies using the SEER database have shown significant variation in the geographic distribution of Hodgkin Lymphoma as well.22 Other studies using the SEER database have shown a higher incidence of other types of NHL in the Western region of USA.23 Whether there is an overall increased incidence of NHL in the Western region of USA, or the higher representation of western region in the SEER database, or even a combination of both is yet to be evaluated.
As per the available studies, bilateral PRL was seen in up to 20% of patients diagnosed with PRL. However, in our cohort of patients, about 95% of the patients had unilateral PRL, while only 2.5% had bilateral PRL. Both right and left sided PRL were common, with their prevalence being 41% and 53%, respectively.
Most PRLs described in literature are histologically DLBCLs followed by extranodal MZL.17, 24, 25 This is similar to our study in which DLBCL was the most common histologic subtype followed by extranodal MZL and FL. Other B‐cell lymphomas including small lymphocytic lymphoma, chronic lymphocytic leukemia, and Burkitt lymphoma each comprised less than 5% of PRLs.
Chemotherapy, radiation, and surgery are treatment modalities used by the patients with chemotherapy alone were used by majority of the patients (37.4) and combined with surgery in 16.7% of the patients. Surgery alone was the modality of treatment in 21.5% of the patients. Unfortunately, in the SEER database, we did not have information on the type of chemotherapy used. We also did not have any information on the adherence with chemotherapy, and therefore we did not include it in the survival analysis.
It has been known that PRL is a very aggressive tumor with median survival of less than one year.6 In a study of 49 patients, the mean survival time of 21 patients treated with chemotherapy was 15.8 months whereas 15 patients treated with combination chemotherapy and surgery was 49.4 months (P = .255).17 However, in our study, the median OS was 112 months (Figure 6). The OS was 81.7% at 1 year and 61.6% at 5 years while 1‐ and 5‐year CSS were 86.2% and 72.9%, respectively. The reason for this discrepancy is unclear. The previous reports are single centered with small sample size unlike present study which is population based. In the clinical practice, only patients with more aggressive disease are referred to a more advanced center.
In this study, we also analyzed factors associated with OS and CSS among patients with PRL. In our analysis, advanced age was the single most important risk factor for worse OS and CSS. Patients 60 years and above were ~3 times higher risk of dying as compared with the younger patients. Advanced age is also a risk factor for worse outcome among patients with aggressive NHL and has been included in international prognostic index (IPI).26 On contrary, Chen et al reported younger age (<18) as a poor prognostic factor.17 Non‐DLBCL was associated with better OS and CSS. DLBCL is an aggressive type of lymphoma with the worst outcome.27 More recent period of diagnosis which corresponds to the use of rituximab‐based regimens affected only CSS but did not have any effect on OS. Rituximab has considerably changed the outcome of patients with aggressive lymphoma and has improved both OS and progression free survival.28 However, its role on OS for patients with indolent lymphoma is still open to debate. In this study, we did not have any information on patients who received rituximab. Therefore, it warrants further testing. There are currently no clinical trials for treatment of PRL. Isolated case reports and case series have demonstrated the use of CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone) for PRL, albeit with a median survival of 6 months.3, 29, 30 However, survival could be improved by adding Rituximab to CHOP.30
Stages III and IV are considered as the marker of poor outcome in NHL.26 Due to design of our study, we excluded patients with Stage III and Stage IV disease due to concern of mislabeling patients with diffuse lymphoma with secondary renal involvement. In the present study, stage II was associated with worse CSS among patients with PRL but did not have any effect on OS. Extensive disease or heavier tumor burden as evidenced by higher stage (III and IV), more than 1 extranodal site involvement, serum LDH level (which is a marker of tumor burden in itself) are the prognostic factors which are included in IPI.26 In the present study, we could not include any of these due to unavailability of this data.
There are several limitations of the present study. Since we used the SEER database, we do not know the nature of chemotherapy used for the patients and cannot study the correlation of different agents with survival. Further, surgery is usually not the first‐line treatment for PRL, and in some cases, the diagnosis of PRL is made after nephrectomy.31 The SEER database does not give us detailed information about the indications for surgery or its timing. It also does not tell us about the patients’ comorbidities. Therefore, it is difficult to comment on the type of treatment most useful for PRL. There was no information on LDH, lymphoma biology, and genomic profile which are important in classifying lymphoma and predicting its outcome.
In conclusion, PRL is a rare form of extranodal NHL. It is most commonly seen in elderly white males. Bilateral PRL is a rare entity. Tumor subtype such as elderly patients (age > 60), DLBCL, male patients, and patients diagnosed before 1999 were associated with shorter OS. Further studies are warranted to confirm the findings from this study.
Taneja A, Kumar V, Chandra AB. Primary renal lymphoma: A population‐based analysis using the SEER program (1973‐2015). Eur J Haematol. 2020;104:390–399. 10.1111/ejh.13360
REFERENCES
- 1. Malbrain M.L., Lambrecht G.L., Daelemans R., Lins R.L., Hermans P., Zachée P.. Acute renal failure due to bilateral lymphomatous infiltrates. Primary extranodal non‐Hodgkin’s lymphoma (p‐EN‐NHL) of the kidneys: does it really exist? Clin Nephrol [Internet]. 1994;42(3):163‐169. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7994934. [PubMed] [Google Scholar]
- 2. Okuno S.H., Hoyer J.D., Ristow K., Witzig T.E.. Primary renal non‐Hodgkin's lymphoma. An unusual extranodal site. Cancer. 1995;75(9):2258‐2261. [DOI] [PubMed] [Google Scholar]
- 3. Stallone G., Infante B., Manno C., Campobasso N., Pannarale G., Schena F.P.. Primary renal lymphoma does exist: case report and review of the literature. J Nephrol. 2000;13(5):367–372. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11063141. [PubMed] [Google Scholar]
- 4. Gibson T.E.. Lymphosarcoma of the Kidney. J Urol. 1948;60(6):838‐854. [DOI] [PubMed] [Google Scholar]
- 5. Coggins C.H.. Renal failure in lymphoma. Kidney Int. 1980;17(6):847‐855. [DOI] [PubMed] [Google Scholar]
- 6. Ladha A., Haider G.. Primary renal lymphoma. J Coll Physicians Surg Pak. 2008;18(9):584‐585. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18803901. [PubMed] [Google Scholar]
- 7. Rissman C.M., Dagrosa L.M., Pettus J.R., Dillon J.L., Sverrisson E.F.. Primary renal lymphoma: an unusual finding following radical nephrectomy. Clin Nephrol Case Stud. 2017;5:1‐4. Available from: https://www.dustri.com/index.php?xml:id=8%26artId=15179%26doi=10.5414/CNCS108955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parsonnet J., Hansen S., Rodriguez L., et al. Helicobacter pylori Infection and Gastric Lymphoma. N Engl J Med. 1994;330(18):1267‐1271. http://www.nejm.org/doi/abs/10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 9. Busi Rizzi E., Schininà V., Cristofaro M., Bellussi A., Alba L., Bibbolino C.. Primary renal non‐Hodgkin’s lymphoma with inferior vena cava involvement: report of one case in HIV‐infected patient. Radiol Med. 2002;103(3):279‐282. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11976627. [PubMed] [Google Scholar]
- 10. Aozasa K., Inoue A., Tajima K., Miyauchi A., Matsuzuka F., Kuma K.. Malignant lymphomas of the thyroid gland: Analysis of 79 patients with emphasis on histologic prognostic factors. Cancer. 1986;58(1):100‐104. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3708539. [DOI] [PubMed] [Google Scholar]
- 11. Kandel L.B., McCullough D.L., Harrison L.H., Woodruff R.D., Ahl E.T., Munitz H.A.. Primary renal lymphoma. Does it exist? Cancer. 1987;60(3):386‐391. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3594375. [DOI] [PubMed] [Google Scholar]
- 12. Ganeshan D., Iyer R., Devine C., Bhosale P., Paulson E.. Imaging of Primary and Secondary Renal Lymphoma. Am J Roentgenol. 2013;201(5):W712‐W719. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24147501. [DOI] [PubMed] [Google Scholar]
- 13. Cyriac S., Rejiv R., Shirley S., Sagar G.T.. Primary renal lymphoma mimicking renal cell carcinoma. Indian J Urol. 2010;26(3):441 http://www.indianjurol.com/text.asp?2010/26/3/441/70591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kunthur A., Wiernik P.H., Dutcher J.P.. Renal parenchymal tumors and lymphoma in the same patient: Case series and review of the literature. Am J Hematol. 2006;81(4):271‐280. [DOI] [PubMed] [Google Scholar]
- 15. Lossos C., Ferrell A., Duncan R., Lossos I.S.. Association between non‐Hodgkin lymphoma and renal cell carcinoma. Leuk Lymphoma. 2011;52(12):2254‐2261. [DOI] [PubMed] [Google Scholar]
- 16. Nicolau C., Sala E., Kumar A., et al. Renal masses detected on FDG PET/CT in patients with lymphoma: imaging features differentiating primary renal cell carcinomas from renal lymphomatous involvement. Am J Roentgenol. 2017;208(4):849‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X., Hu D., Fang L., et al. Primary renal lymphoma: A case report and literature review. Oncol Lett. 2016;12(5):4001‐4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belbaraka R., Elyoubi M.B., Boutayeb S., Errihani H.. Primary renal non‐Hodgkin lymphoma: An unusual diagnosis for a renal mass. Indian J Cancer. 2011;48(2):255‐256. [DOI] [PubMed] [Google Scholar]
- 19. Garber K.. Lymphoma Rate Rise Continues to Baffle Researchers. JNCI. 2001;93(7):494‐496. [DOI] [PubMed] [Google Scholar]
- 20. Smith A., Howell D., Patmore R., Jack A., Roman E.. Incidence of haematological malignancy by sub‐type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson J.R., Armitage J.O., Weisenburger D.D.. Epidemiology of the non‐Hodgkin’s lymphomas: Distributions of the major subtypes differ by geographic locations. Ann Oncol. 1998;9(7):717‐720. [DOI] [PubMed] [Google Scholar]
- 22. Glaser S.L.. Regional variation in Hodgkin’s disease incidence by histologic subtype in the US. Cancer. 1987;60(11):2841‐2847. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs A.J., Michels R., Stein J., Levin A.S.. Socioeconomic and demographic factors contributing to outcomes in patients with primary lymphoma of bone. J Bone Oncol. 2015;4(1):32‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu L., Shi H.Z., Xiao Z.J., Wang D., Li C.L.. Primary renal lymphoma: a clinical analysis of 5 cases. Zhonghua Yi Xue Za Zhi. 2018;98(18):1443‐1445. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29804410. [DOI] [PubMed] [Google Scholar]
- 25. Hagihara M., Hua J., Iwaki Y., Inoue M., Sato T.. Primary Renal Lymphoma: A Case Report and Literature Review. Intern Med. 2015;54(20):2655‐2659. [DOI] [PubMed] [Google Scholar]
- 26. Shipp M.A. A predictive model for aggressive non‐Hodgkin's lymphoma. The international non‐Hodgkin's lymphoma prognostic factors project. N Engl J Med. 1993;329:987‐994. [DOI] [PubMed] [Google Scholar]
- 27. Non‐Hodgkin lymphoma ‐ The Lancet [Internet]. Accessed November 18, 2019. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60605-9/fulltext.
- 28. Dotan E., Aggarwal C., Smith M.R.. Impact of rituximab (Rituxan) on the treatment of B‐cell non‐Hodgkin’s lymphoma. P and T. 2010. [PMC free article] [PubMed]
- 29. Porcaro A.B., D’Amico A., Novella G., et al. Primary lymphoma of the kidney. Report of a case and update of the literature. Arch Ital di Urol Androl organo Uff [di] Soc Ital di Ecogr Urol e Nefrol. 2002;74(1):44–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12053451. [PubMed] [Google Scholar]
- 30. Vázquez‐Alonso F., Puche‐Sanz I., Sánchez‐Ramos C., Flores‐Martín J., Vicente‐Prados J., Cózar‐Olmo J.M.. Primary Renal Lymphoma: Long‐Term Results of Two Patients Treated with a Chemotherapy + Rituximab Protocol. Case Rep Oncol Med. 2012;2012:1–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22997596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vázquez Alonso F., Sánchez Ramos C., Vicente Prados F.J., et al. Primary renal lymphoma: report of three new cases and literature review. Arch Esp Urol. 2009;62(6):461‐465. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19736375. [PubMed] [Google Scholar]


