Summary
The aim of this study was to investigate whether there is an impact of donation rates on the quality of lungs used for transplantation and whether donor lung quality affects post‐transplant outcome in the current Lung Allocation Score era. All consecutive adult LTx performed in Eurotransplant (ET) between January 2012 and December 2016 were included (N = 3053). Donors used for LTx in countries with high donation rate were younger (42% vs. 33% ≤45 years, P < 0.0001), were less often smokers (35% vs. 46%, P < 0.0001), had more often clear chest X‐rays (82% vs. 72%, P < 0.0001), had better donor oxygenation ratios (20% vs. 26% with PaO2/FiO2 ≤ 300 mmHg, P < 0.0001), and had better lung donor score values (LDS; 28% vs. 17% with LDS = 6, P < 0.0001) compared with donors used for LTx in countries with low donation rate. Survival rates for the groups LDS = 6 and ≥7 at 5 years were 69.7% and 60.9% (P = 0.007). Lung donor quality significantly impacts on long‐term patient survival. Countries with a low donation rate are more oriented to using donor lungs with a lesser quality compared to countries with a high donation rate. Instead of further stretching donor eligibility criteria, the full potential of the donor pool should be realized.
Keywords: donation, donor, expanded donor pool, lung clinical, outcome
Introduction
Early and late survival have improved over the last decades leading to an extension of listing indications. As a result, referral for lung transplantation increased such that the number of patients on the lung transplant waiting list outpaced the availability of donor organs. In 2018, 1036 patients were on the lung transplant waiting list in Eurotransplant at year‐end, while 719 had received a lung transplant and 137 patients died awaiting an organ offer 1.
Worldwide, only 20–30% of organ donors become lung donors 2, 3. The dramatic organ shortage encourages centers to expand lung donor suitability criteria in order to maximize recovery and usage rate of every reported lung donor. Lung donor yield can be improved by increased utilization of extended‐criteria donors. This percentage of used extended‐criteria donors varies widely across centers ranging from 24% to 77% of the total transplant volume 2.
Out of the eight countries that collaborate within Eurotransplant, four had active lung transplant programs in the study period. These four countries have different donor legislative frameworks: Austria and Belgium use an opting‐out system, where every citizen is considered an organ donor unless an active registration against donation has taken place. Germany and the Netherlands apply an opting‐in system which requires an active registration in order to be considered as organ donor. As a consequence, the number of lung donors used for transplantation per million population was in 2018 for Austria: 9.8; for Belgium: 10.8; for Germany: 3.8; and for the Netherlands: 4.7 (Fig. 1). Furthermore, waiting list mortality rates in countries with low donation rates (Germany and the Netherlands) are higher compared to those in countries with high donation rates (Austria and Belgium): 12% vs. 7% at 1 year 1.
Figure 1.

Donation rates per million population of used diseased donor lungs, by year, by donor country.
Because of the large discrepancies among the Eurotransplant countries, Dutch members of parliament have asked for a change in legislation. The proposal for change toward an opting‐out scheme was successfully passed in 2018, and the new organ donor law will be implemented July 1, 2020 4. Parliamentary discussions in Germany have just started, and the authors hope that with additional insight gained by the current study, Germany will, as the last country in Eurotransplant, also adopt the opting‐out system.
In May 2005, the Lung Allocation Score (LAS) was implemented in the United States. This allocation system replaced a scheme based solely on waiting time. There were three objectives: reduce the number of deaths on the lung transplant waiting list; increase the survival benefit for lung recipients; and ensure the efficient and equitable allocation of lungs to transplant candidates 5. Germany was the first country to adopt the LAS as national allocation policy on December 10, 2011; the Netherlands followed on April 22, 2014 6.
The aim of this study was to investigate whether there is an impact of donation rates on the quality of lungs used for transplantation and whether donor lung quality affects post‐transplant outcome in the current LAS era.
Patients and methods
Definitions
The lung donor score (LDS) is a Eurotransplant adaptation of the Oto score 7, 8, where the ideal donor has a LDS value of 6. This LDS is an instrument to gauge donor quality based on six preprocurement variables: general and smoking history, age, arterial blood gases, chest X‐ray, and bronchoscopic findings (Table 1). The LDS of six points is equivalent to the ISHLT definition of standard donor lung with the exception that chest X‐ray images showing edema or atelectasis, bronchoscopy findings of nonpurulent secretions, and PaO2/FiO2 measurements between 300 and 350 mmHg do not increase the score 9. The lung donor score’s variables are registered electronically in Eurotransplant as of 2002.
Table 1.
The Eurotransplant lung donor score.
| Factor | Points |
|---|---|
| Donor age (year) | |
| <45 | 1 |
| 45–54 | 1 |
| 55–59 | 2 |
| 60+ | 3 |
| Donor history | |
| Compromised* | 4 |
| Uncompromised | 1 |
| Smoking history | |
| Yes | 2 |
| No | 1 |
| NA | 1 |
| Chest X‐ray | |
| Clear | 1 |
| Edema | 1 |
| Shadow | 2 |
| Atelectasis | 1 |
| Consolidation | 2 |
| NA | 1 |
| Bronchoscopy | |
| Clear | 1 |
| Non purulent | 1 |
| Purulent | 2 |
| Inflammation | 3 |
| Visualized tumor | 5 |
| NA | 1 |
| PO2/FiO2 (mmHg) | |
| >450 | 1 |
| 351–450 | 1 |
| 301–350 | 2 |
| ≤300 | 3 |
| NA | 2 |
The donor history is compromised in case of a malignancy, sepsis, drug abuse, meningitis, or a positive virology (HBsAg, HBcAb, and HCVAb) was registered.
Patients were classified into four groups depending on their underlying disease: Group A, obstructive airway diseases (e.g., chronic obstructive pulmonary disease [COPD]); Group B, diseases of the pulmonary circulation (e.g., idiopathic pulmonary arterial hypertension); Group C, suppurative lung diseases (e.g., cystic fibrosis [CF]); and Group D, restrictive lung diseases (e.g., pulmonary fibrosis).
Throughout the manuscript Austria and Belgium were labeled as “high donation rate” countries and Germany and the Netherlands as “low donation rate” countries.
Lung Allocation Score
The LAS is a numerical value used to assign relative priority in distributing donated lungs. The LAS evaluates several parameters of patient health to direct organ donation toward patients obtaining greatest benefit from lung transplantation. The score is calculated from objective clinical measures of the patient’s current health status to estimate survival probability and projected duration of 1‐year survival with or without a lung transplant. LAS values range from 0 to 100, with higher scores indicative of greater predicted survival benefit, directing priority toward these patients, and hence excluding wait list time 10. Although eight countries collaborate in Eurotransplant, in this study period only four countries (Austria, Belgium, Germany, and the Netherlands) had active lung transplant programs. Germany and the Netherlands use the LAS scoring system for their national allocation while all 4 countries use the LAS scoring system for international donor lung exchange since December 10, 2011.
Study design
Historical prospective study including all adult (≥16 years) consecutive lung‐only transplant recipients in the Eurotransplant area between January 1, 2012, and December 31, 2016.
Statistical analysis
Continuous variables were analyzed using the Wilcoxon–Mann–Whitney test, while chi‐square statistics were used to compare categorical variables. Survival rates were examined with time‐to‐event analysis in which the event was defined as patient death. Patients were followed up until December 31, 2018. Univariable survival analyses were performed by Kaplan–Meier method. Survival rates were compared using the log‐rank test. Multivariable analysis was performed with Cox’s proportional hazards model and included the following factors: recipient primary diagnosis, recipient age, LAS at transplant, lung donor score, DCD/DBD donor, and transplant country. Missing data were included in the LDS model as a “non available” class.
All analyses were performed using sas statistical program version 9.1 (SAS Institute, Indianapolis, IN, USA). A P‐value below 0.05 was considered statistically significant.
Results
Demographics
The study population included 3053 lung transplants, of which 1118 (37%) were performed in Austria and Belgium (A/B) and 1935 (63%) in Germany and the Netherlands (G/N; Table 2).
Table 2.
Demographic statistics.
| ALL | Transplant country | P‐value | ||
|---|---|---|---|---|
| Austria/Belgium | Germany/Netherlands | |||
| Total | 3053 | 1118 | 1935 | |
| Recipient | ||||
| Age (years) | ||||
| <45 | 764 (25%) | 300 (27%) | 464 (24%) | <0.0001 |
| 45–54 | 718 (24%) | 217 (20%) | 501 (26%) | |
| 55–59 | 713 (23%) | 261 (23%) | 452 (23%) | |
| ≥60 | 858 (28%) | 340 (30%) | 518 (27%) | |
| Diagnosis group | ||||
| Obstructive (Group A) | 1302 (43%) | 574 (51%) | 728 (38%) | <0.0001 |
| Vascular (Group B) | 136 (4%) | 74 (7%) | 62 (3%) | |
| Infectious (Group C) | 484 (16%) | 159 (14%) | 325 (17%) | |
| Restrictive (Group D) | 965 (32%) | 269 (24%) | 696 (36%) | |
| Other | 166 (5%) | 42 (4%) | 124 (6%) | |
| LAS | ||||
| 1–30 | 104 (4%) | 100 (13%) | 4 (0%) | <0.0001* |
| 30–34 | 865 (33%) | 313 (43%) | 552 (30%) | |
| 35–39 | 501 (19%) | 101 (14%) | 400 (21%) | |
| 40–49 | 535 (21%) | 90 (12%) | 445 (24%) | |
| 50+ | 593 (23%) | 128 (18%) | 465 (25%) | |
| Missing | 455 | 386 | 69 | |
| BMI (median IQR) | 22 (19–26) | 22 (19–25) | 23 (20–26) | <0.0001 |
| Donor | ||||
| Age (years) | ||||
| <45 | 1119 (37%) | 467 (42%) | 652 (33%) | <0.0001 |
| 45–54 | 887 (29%) | 315 (28%) | 572 (30%) | |
| 55–59 | 405 (13%) | 150 (13%) | 255 (13%) | |
| ≥60 | 642 (21%) | 186 (17%) | 456 (24%) | |
| Smoking history | ||||
| Yes | 1149 (42%) | 334 (35%) | 815 (46%) | <0.0001* |
| No | 1594 (56%) | 622 (65%) | 972 (54%) | |
| Missing | 310 | 162 | 148 | |
| Bronchoscopy | ||||
| Clear | 1707 (86%) | 304 (88%) | 1403 (85%) | 0.38* |
| Non purulent | 77 (3%) | 8 (2%) | 69 (4%) | |
| Purulent | 134 (7%) | 22 (6%) | 112 (7%) | |
| Inflammation | 70 (4%) | 11 (4%) | 59 (4%) | |
| NA | 1065 | 773 | 292 | |
| Chest X‐ray | ||||
| Clear | 2050 (75%) | 766 (82%) | 1284 (72%) | <0.0001* |
| Edema | 244 (9%) | 53 (6%) | 191 (11%) | |
| Shadow | 83 (3%) | 19 (2%) | 64 (4%) | |
| Atelectasis | 156 (6%) | 45 (5%) | 111 (6%) | |
| Consolidation | 179 (7%) | 53 (5%) | 126 (7%) | |
| NA | 341 | 182 | 159 | |
| Donor history | ||||
| Compromised | 134 (4%) | 35 (3%) | 99 (5%) | 0.010 |
| Uncompromised | 2919 (96%) | 1083 (97%) | 1836 (95%) | |
| PaO2/FiO2 (mmHg) | ||||
| ≤300 | 716 (24%) | 222 (20%) | 494 (26%) | <0.0001 |
| 301–350 | 369 (12%) | 114 (10%) | 255 (13%) | |
| 351–450 | 926 (30%) | 327 (29%) | 599 (31%) | |
| >450 | 942 (31%) | 419 (38%) | 523 (27%) | |
| NA | 100 (3%) | 36 (3%) | 64 (3%) | |
| LDS | ||||
| 6 | 638 (21%) | 317 (28%) | 321 (17%) | <0.0001 |
| ≥7 | 2415 (79%) | 801 (72%) | 1614 (83%) | |
| Donor type | ||||
| DCD† | 272 (9%) | 125 (11%) | 147 (8%) | 0.001 |
| DBD | 2781 (81%) | 993 (89%) | 1788 (92%) | |
| Transplantation | ||||
| Single lung | 287 | 38 (3%) | 249 (13%) | <0.0001 |
| Double lung | 2766 | 1080 (97%) | 1696 (87%) | |
| Cold ischemia time (h) | ||||
| Median (IQR) | 6 (5.5–6.4) | 6 (5.2–6.0) | 6 (5.7–7.1) | 0.37 |
P‐value without NA/missing class.
DCD donation and transplantation is legally not allowed in Germany.
Compared with Germany and the Netherlands, patients transplanted in Austria and Belgium were more often aged <45 years (27% vs. 24%) and more often aged ≥60 years (30% vs. 27%, P < 0.0001). Their primary diagnosis was more often Obstructive (51% vs. 38%) and less often Restrictive (24% vs. 36%, P < 0.0001). Patients transplanted in A/B were less often transplanted with a high LAS value (18% vs. 25% with LAS ≥50, P < 0.0001), compared with patients transplanted in G/N. Lung donors used for transplantation in A/B were younger (42% vs. 33% in age class ≤45 years, P < 0.0001), were less often smokers (35% vs. 46%, P < 0.0001), had more often a clear chest X‐ray (82% vs. 72%, P < 0.0001), had less often a compromised donor history (3% vs. 5%, P = 0.010), had better donor oxygenation ratio [partial arterial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2); 20% vs. 26% with PaO2/FiO2 ≤ 300 mmHg, P < 0.0001], and had better LDS values (28% vs. 17% with LDS = 6, P < 0.0001).
Six transplant centers were active in the high donation rate countries; the annual transplant volume was as follows: 1–4 LTx: 1 center; 10–19 LTx: 2 centers; 20–29 LTx: 1 center; and 50+ LTx: 2 centers. In the low donation rate countries, 18 centers had an active LTx program with the following annual transplant volume: 1–4 LTx: 2 centers; 5–9 LTx: 5 centers; 10–19 LTx: 7 centers; 30–39 LTx: 2 centers; and 50+ LTx: 2 centers.
Donor quality over time
The distribution of the LDS among patients transplanted in the period 2002–2018 is shown in Fig. 2. In the years 2003, 2008, 2012, and 2017, the proportion of patients transplanted with a lung with LDS of 6 decreased from 44% to 31% to 22% to 17%.
Figure 2.

Proportion of lung‐only transplants by lung donor score over time.
Donor age distribution since the start of the first lung transplant is shown in Fig. 3. All other components of the LDS are systematically recorded since 2002 and represented in Fig. 4a–d. In the most recent decades, the usage of older donors, donors with a smoking history, donors without a clear chest X‐ray, and donors with a low PaO2/FiO2 ratio has increased compared with the earlier transplants.
Figure 3.

Proportion of lung‐only transplants by donor age over time.
Figure 4.
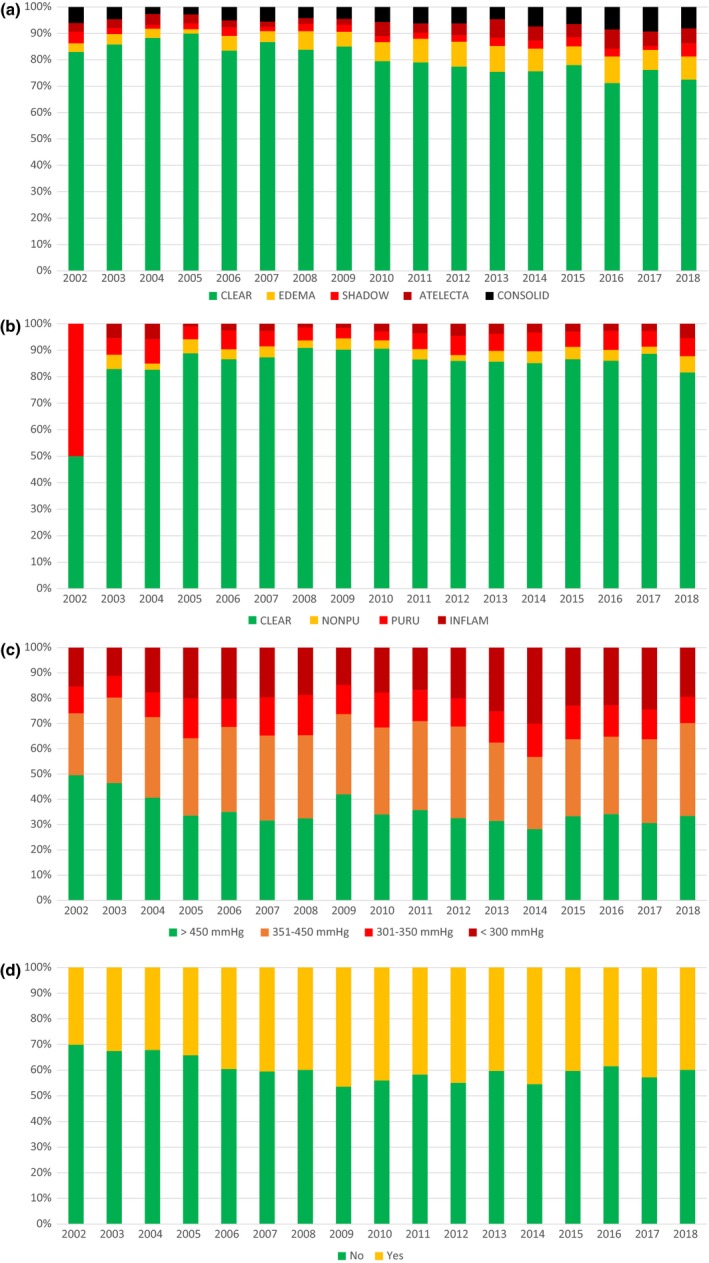
(a) Proportion of lung‐only transplants by donor X‐ray classes over time. (b) Proportion of lung‐only transplants by donor bronchoscopy classes over time. (c) Proportion of lung‐only transplants by donor PaO2/FiO2 classes over time. (d) Proportion of lung‐only transplants by donor smoking status over time.
Post‐transplant survival
The survival rates for the groups LDS = 6 and LDS ≥7 at 1, 2, and 5 years were 85.9%, 79.3%, and 69.7% and 82.9%, 76.1%, and 60.9%, respectively (P = 0.007; Fig. 5).
Figure 5.

Post‐transplant survival by lung donor score [LDS = 6 (N = 638) dark blue line and LDS ≥7 (N = 2415) light green line].
Factors associated with overall patient survival
Table 3 shows the unadjusted and adjusted hazard rates on post‐transplant survival.
Table 3.
Univariate and multivariate analysis of post‐transplant survival.
| Factor | N | Unadj HR (95% CI) | P‐value | Adj HR | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Recipient age (years) | ||||||
| <45 | 764 | 1 | <0.0001 | 1 | 0.12 | |
| 45–54 | 718 | 1.03 (0.84–1.26) | 0.96 | 0.75–1.23 | ||
| 55–59 | 713 | 1.25 (1.02–1.52) | 1.14 | 0.89–1.46 | ||
| ≥60 | 858 | 1.46 (1.22–1.76) | 1.20 | 0.94–1.53 | ||
| Diagnosis | ||||||
| Obstructive | 1302 | 1 | <0.0001 | 1 | 0.009 | |
| Vascular | 136 | 1.22 (0.88–1.69) | 1.53 | 1.08–2.17 | ||
| Infectious | 484 | 0.88 (0.71–1.09) | 1.01 | 0.77–1.34 | ||
| Restrictive | 965 | 1.40 (1.20–1.63) | 1.26 | 1.06–1.50 | ||
| Other | 166 | 1.29 (0.96–1.73) | 1.36 | 0.99–1.86 | ||
| LAS | ||||||
| <50 | 2005 | 1 | <0.0001 | 1 | <0.0001 | |
| ≥50 | 593 | 1.81 (1.56–2.11) | 1.60 | 1.35–1.88 | ||
| 0 | 455 | 1.05 (0.85–1.28) | 1.26 | 0.90–1.41 | ||
| BMI | 1.004 (0.99–1.01) | 0.24 | 1 | 0.99–1.01 | 0.97 | |
| LDS | ||||||
| 6 | 638 | 1 | 0.006 | 1 | 0.001 | |
| ≥7 | 2415 | 1.27 (1.07–1.52) | 1.35 | 1.13–1.61 | ||
| Donor type | ||||||
| DBD | 2781 | 1 | 0.032 | 1 | 0.10 | |
| DCD | 272 | 0.75 (0.57–0.98) | 0.79 | 0.59–1.05 | ||
| Transplant country | ||||||
| A/B | 1118 | 1 | 0.029 | 1 | 0.30 | |
| G/N | 1935 | 1.18 (1.02–1.35) | 0.90 | 0.74–1.10 | ||
| Transplant volume (number/year) | ||||||
| 50+ | 1661 | 1 | <0.0001 | 1 | <0.0001 | |
| 1–4 | 35 | 3.11 (1.94–5.00) | 4.40 | 2.71–7.12 | ||
| 5–9 | 173 | 2.34 (1.84–2.98) | 2.83 | 2.18–3.69 | ||
| 10–19 | 744 | 1.67 (1.42–1.95) | 1.69 | 1.42–2.01 | ||
| 20–29 | 135 | 2.19 (1.67–2.87) | 3.15 | 2.30–4.31 | ||
| 30–39 | 305 | 1.11 (0.86–1.43) | 1.09 | 0.82–1.45 | ||
| Type of LTx | ||||||
| Double | 2766 | 1 | <0.0001 | 1 | <0.0001 | |
| Single | 287 | 2.14 (1.78–2.57) | 1.90 | 1.57–2.31 | ||
| Cold ischemic | ||||||
| <6 | 896 | 1 | 0.001 | 1 | <0.0001 | |
| Time (h) | ||||||
| ≥6 | 2157 | 1.30 (1.12–1.51) | 1.76 | 1.48–2.08 | ||
The unadjusted HR for the factor that represents the transplant countries is 1.18 (95% CI 1.02–1.35) P = 0.029 and the 5‐year survival rates are 66.7% for the high donation rate countries (A/B) and 60.8% for the low donation rate countries (G/N; P = 0.001). In the multivariable model, the effect of the factor “Transplant Country” on survival is no longer observed [HR: 0.90 (95% CI 0.74–1.10) P = 0.30].
Unadjusted survival rates in low donation rate countries were lower compared to high donation rate countries with 5‐year survival rates at 60.8% and 66.7% for low and high donation rate countries, respectively (P = 0.001). However when corrected for confounding factors, like the LDS, this country effect disappeared. This observation implies that part of the country effect can be explained by the difference in the quality of organs used for transplantation.
The multivariable model showed that the factors transplant volume, type of lung transplant (double vs. single), duration of cold ischemia time, primary diagnosis, LAS value at transplantation, and the lung donor score [HR: 1.35 (95% CI: 1.13–1.61), P = 0.001] were found to be independent predictors of survival (Table 3).
Discussion
The Oto LDS was the first attempt at quantifying overall donor lung quality 7, 8, 11. Eurotransplant’s adaptation of this score has been shown to be associated with donor usage. Reported donor lungs which in reality were judged to be unsuitable for transplantation and hence discarded were those with a higher LDS at time of reporting. In addition, post‐transplant recipient outcome of donors with a higher LDS was found to be significantly worse compared to transplants performed with better quality lungs.
In the last 15 years, donor quality of the transplanted lungs, as measured by the LDS has decreased: in 2003, 44% of all donors were ideal donors with a LDS of 6, and in 2017 this proportion dropped to 17%. This reduction in quality of used donor lungs can be attributed to the increase in donor age, to the increase of the usage of donors with a smoking history, donors without a clear chest X‐ray, and donors with a lower PaO2/FiO2 ratio.
The observed decline in lung allograft quality raises the question of its impact on outcome. Our data show that lung recipients from donors with a LDS ≥7 had a significantly jeopardized long‐term outcome compared to those with an optimal lung quality: 69.7% and 60.9% for LDS = 6 and ≥7 at 5 years, respectively (P = 0.007). A considerable number of studies are published showing no disadvantage when extended‐criteria donor lungs were used 3, 12, 13, 14. Liberalization of donor criteria and retaining optimal patient outcome is also a result of increased experience and might explain these discrepancies.
Our study hypothesis was that there is an impact of donation rates on the quality of lungs used for transplantation; this hypothesis was confirmed by our data: lung donors used for transplantation in countries with a high donation rate were younger, were less often smokers, had more often a clear chest X‐ray, less often a compromised donor history, had a better donor oxygenation, and had a lower LDS compared with donors used for transplantation in countries with a low donation rate.
Doctors are trained to solve problems, not to create them. Hence, faced with an organ shortage lung donor suitability criteria become wider in order to maximize recovery and usage rate of every reported lung donor. But should these criteria be further stretched if the national lung donation rate is half that of other countries?
Optimally exploiting the potential of deceased organ donation could substantially increase the donor pool. Roels et al. showed that more than 57% of deceased potential donors were missed along the donation pathway because of nonidentification, no referral, no approach of relatives, or objections to donate. In countries with lower donation rates, expectedly more potential donors are missed proportionally 15. Efforts to increase the organ pool should therefore focus on optimizing clinical practices in deceased organ donation in addition to installing an opting‐out system.
Ex vivo lung perfusion has recently emerged as a new technology to safely prolong cross‐clamp time for standard‐criteria donor lungs 16, 17 and to re‐evaluate questionable lungs from extended‐criteria donors such as older donor lungs, DCD lungs, lungs with low oxygenation capacity, and lungs with expected long cold ischemic times 18. These strategies may help to increase the donor pool in the future in countries with lower organ donation rates. However, the concept of using DCD lungs is not legally allowed in Germany, which is the country in Eurotransplant with the largest number of potential donors, but with the lowest number of actual donors per inhabitants. Some of the lung transplant centers in Eurotransplant have now started using EVLP as a tool to reassess donor lungs of inferior quality. However, the impact on increasing the actual donor pool in the individual ET countries remains unknown and could not be examined in the present study.
There are various strategies to increase the donor pool; these include legislative action, public campaigns, in‐hospital training programs, extending the selection criteria for lung donors, and using from lungs from donors who died after circulatory arrest (DCD) 2. A study from the ISHLT DCD registry showed that outcomes of DCD were similar to DBD 19. This is confirmed in our cohort: [HR: 0.79 (95% CI: 0.59–1.05), P = 0.10]. In several Eurotransplant countries, there are still legal barriers that preclude DCD donation as is now the case in Germany. Removing these barriers could further expand the lung donor pool by 20%, which might lower the usage of lung donors with a high LDS 20, 21.
It has been advocated and shown that increasing organ donation rates can be achieved by introducing presumed consent legislation 22, 23. But presumed consent alone cannot explain all the variation in organ donation rates between different countries 24, and opting‐out systems have also been shown not to increase donation rates on its own 25, 26. Establishing an optimal legal framework should be aided by public support, public trust, and the role of the family in donation decisions 27.
This study has several limitations inherent to a multicenter registry. Although Eurotransplant collects a robust set of donor variables on a large number of records, data are missing, but these missing values are modeled and reported as such. No information on primary graft dysfunction nor on chronic rejection is available. As with any observational study, associations may not be causal. The center experience in assessing donor quality, reconditioning of donor lungs with EVLP, selecting suitable candidates, and excelling in the practice of using nonideal donors is not modeled and constitutes a serious bias in this analysis.
Our data show that donor lung quality impacts on long‐term patient survival and that higher quality donor lungs are more often used for transplantation in countries with a high donation rate compared to countries with a low donation rate. In the quest for finding more suitable organs, first the full potential of the donor pool should be realized. Professionals working in countries with a low donation rate should make every effort to convince policy makers to change their current national donor legislation by introducing opting‐out as well as DCD legislation.
Funding
The authors have declared no funding.
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1. Annual Report 2018. http://www.eurotransplant.org.
- 2. Van Raemdonck D, Neyrinck A, Verleden GM, et al Lung donor selection and management. Proc Am Thorac Soc 2009; 6: 28. [DOI] [PubMed] [Google Scholar]
- 3. Somers J, Ruttens D, Verleden SE, et al A decade of extended‐criteria lung donors in a single center: was it justified? Transplant Int 2015; 28: 170. [DOI] [PubMed] [Google Scholar]
- 4. https://www.government.nl/topics/organ-tissue-donation/new-donor-act-active-donor-registration (assessed May 19, 2019).
- 5. Egan TM, Murray S, Bustami RT, et al Development of the new lung allocation system in the United States. Am J Transplant 2006; 6: 1212. [DOI] [PubMed] [Google Scholar]
- 6. Gottlieb J, Greer M, Sommerwerck U, et al Introduction of the lung allocation score in Germany. Am J Transplant 2014; 14: 1318. [DOI] [PubMed] [Google Scholar]
- 7. Oto T, Levvey BJ, Whitford H, et al Feasibility and utility of a lung donor score: correlation with early post‐transplant outcomes. Ann Thorac Surg 2007; 83: 257. [DOI] [PubMed] [Google Scholar]
- 8. Smits JM, van der Bij W, Van Raemdonck D, et al Defining an extended criteria donor lung: an empirical approach based on the Eurotransplant experience. Transpl Int 2011; 24: 393. [DOI] [PubMed] [Google Scholar]
- 9. Orens JB, Boehler A, de Perrot M, et al Pulmonary Council, International Society for Heart and Lung Transplantation. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant 2003; 22: 1183. [DOI] [PubMed] [Google Scholar]
- 10. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf (assessed November 4, 2019).
- 11. Porro GA, Valenza F, Coppola S, et al Use of the Oto lung donor score to analyze the 2010 donor pool of the Nord Italia transplant program. Transpl Proc 2012; 44: 1830. [DOI] [PubMed] [Google Scholar]
- 12. Sommer W, Ius F, Salman J, et al Survival and spirometry outcomes after lung transplantation from donors aged 70 years and older. J Heart Lung Transplant 2015; 34: 1325. [DOI] [PubMed] [Google Scholar]
- 13. Shigemura N, Horai T, Bhama JK, et al Lung transplantation with lungs from older donors: recipient and surgical factors affect outcomes. Transplantation 2014; 98: 903. [DOI] [PubMed] [Google Scholar]
- 14. Zych B, García Sáez D, Sabashnikov A, et al Lung transplantation from donors outside standard acceptability criteria – are they really marginal? Transpl Int 2014; 27: 1183. [DOI] [PubMed] [Google Scholar]
- 15. Roels L, Smits J, Cohen B. Potential for deceased donation not optimally exploited: donor action data from six countries. Transplantation 2012; 94: 1167. [DOI] [PubMed] [Google Scholar]
- 16. Warnecke G, Van Raemdonck D, Ardehali A, et al Normothermic ex‐vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open‐label, non‐inferiority, phase 3 study. Lancet Respir Med 2018; 6: 357. [DOI] [PubMed] [Google Scholar]
- 17. Slama A, Schillab L, Barta M, et al Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. J Heart Lung Transplant 2017; 36: 744. [DOI] [PubMed] [Google Scholar]
- 18. Loor G, Warnecke G, Villavicencio MA, et al Portable normothermic ex‐vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended‐criteria donor (EXPAND): a single‐arm, pivotal trial. Lancet Respir Med 2019; 7: 975. [DOI] [PubMed] [Google Scholar]
- 19. Van Raemdonck DE, Keshavjee S, Levvey B, et al 5‐year results from the ISHLT DCD lung transplant registry confirm excellent recipient survival from donation after circulatory death donors. J Heart Lung Transplant 2019; 38(4S): S103. [Google Scholar]
- 20. Reeb J, Keshavjee S, Cypel M. Expanding the lung donor pool: advancements and emerging pathways. Curr Opin Organ Transplant 2015; 20: 498. [DOI] [PubMed] [Google Scholar]
- 21. Martens A, Van Raemdonck DE, Smits J, et al A retrospective database analysis to evaluate the potential of ex vivo lung perfusion to recruit declined lung donors. Transpl Int 2017; 30: 1002. [DOI] [PubMed] [Google Scholar]
- 22. Horvat LD, Cuerden MS, Kim SJ, Koval JJ, Young A, Garg AX. Informing the debate: rates of kidney transplantation in nations with presumed consent. Ann Intern Med. 2010; 153: 641. [DOI] [PubMed] [Google Scholar]
- 23. Saab S, Saggi SS, Akbar M, Choi G. Presumed consent: a potential tool for countries experiencing an organ donation crisis. Dig Dis Sci 2019; 64: 1346. [DOI] [PubMed] [Google Scholar]
- 24. Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A. A systematic review of presumed consent systems for deceased organ donation. Health Technol Assess 2009; 13: iii, ix–xi, 1–95. [DOI] [PubMed] [Google Scholar]
- 25. Domínguez J, Rojas JL. Presumed consent legislation failed to improve organ donation in Chile. Transplant Proc 2013; 45: 1316. [DOI] [PubMed] [Google Scholar]
- 26. Arshad A, Anderson B, Sharif A. Comparison of organ donation and transplantation rates between opt‐out and opt‐in systems. Kidney Int 2019; 95: 1453. [DOI] [PubMed] [Google Scholar]
- 27. Jensen AMB, Larsen JB. The public debate on organ donation and presumed consent in Denmark: are the right issues being addressed? Scand J Public Health 2019: 1403494819833797 10.1177/1403494819833797 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


