Abstract
In adults with end‐stage liver disease concurrent changes in pro‐ and antihemostatic pathways result in a rebalanced hemostasis. Children though, have a developing hemostatic system, different disease etiologies, and increased risk of thrombosis. This study aimed to assess the hemostatic state of children during and after liver transplantation. Serial blood samples were obtained from 20 children (≤16 years) undergoing primary liver transplantation (September 2017‐October 2018). Routine hemostasis tests, thrombomodulin‐modified thrombin generation, clot lysis times, and hemostatic proteins were measured. Reference values were established using an age‐matched control group of 30 children. Thrombocytopenia was present in study patients. Von Willebrand factors were doubled and ADAMTS13 levels decreased during and after transplantation up until day 30, when platelet count had normalized. Whereas prothrombin time and activated partial thromboplastin time were prolonged during transplantation, thrombin generation was within normal ranges, except during perioperative heparin administration. Fibrinogen, factor VIII levels, and clot lysis time were elevated up until day 30. In conclusion, children with end‐stage liver disease are in tight hemostatic balance. During transplantation a temporary heparin‐dependent hypocoagulable state is present, which rapidly converts to a hemostatic balance with distinct hypercoagulable features that persist until at least day 30. This hypercoagulable state may contribute to the risk of posttransplant thrombosis.
Keywords: clinical research/practice, liver allograft function/dysfunction, liver transplantation/hepatology, pediatrics, thrombosis and thromboembolism
Short abstract
This prospective exploratory study shows that children with end‐stage liver disease undergoing liver transplantation are in a fragile hemostatic balance, with a temporary heparin‐induced hypocoagulable state during transplantation, which rapidly converts to a rebalanced hemostatic state with distinct hypercoagulable features posttransplantation.
Abbreviations
- ADAMTS13
a disintegrin and metalloproteinase with thrombospondin motifs type 13
- APTT
activated partial thromboplastin time
- CLT
clot lysis time
- ETP
endogenous thrombin potential
- IQR
interquartile ranges
- MELD score
model for end‐stage liver disease score
- PAI‐1
plasminogen activator inhibitor type 1
- PELD score
pediatric end‐stage liver disease score
- PT
prothrombin time
- VWF
von Willebrand factor
1. INTRODUCTION
Bleeding and thrombosis are serious causes of morbidity and mortality in pediatric liver transplantation.1, 2, 3, 4 Incidences of 5%‐18% for hepatic artery thrombosis and 5%‐10% for portal vein thrombosis have been reported.1, 2, 3, 5 Bleeding complications occur in 5%‐20% and may complicate surgery, warranting proactive hemostatic management.4, 6 It remains unpredictable which patients are at risk of thrombotic complications and which patients are at risk of bleeding complications.
In contrast to the historical view that patients with end‐stage liver disease have an increased bleeding tendency, over the past decade it has been demonstrated that adult patients are in a state of “rebalanced hemostasis.”7, 8 Conventional coagulation tests such as prothrombin time (PT) and activated partial thromboplastin time (APTT) suggest a hypocoagulable state in these patients, but these tests only indicate decrease in procoagulant proteins and are unable to measure the concurrent reduction of anticoagulant proteins.9 More advanced hemostatic tests such as thrombomodulin‐modified thrombin generation assays have demonstrated intact or enhanced thrombin generation, indicating a normal to hypercoagulable hemostatic state in these patients.10, 11, 12, 13
This rebalanced hemostatic state is the result of a commensurate decrease in pro‐ and antihemostatic factors occurring in adults with end‐stage liver disease, affecting primary hemostasis, coagulation, and fibrinolysis (Table S1). Regarding primary hemostasis, thrombocytopenia is present in most patients. Meanwhile, increased levels of platelet adhesive protein von Willebrand factor (VWF), combined with decreased levels of VWF‐cleaving protease, a disintegrin and metalloproteinase with thrombospondin motifs type 13 (ADAMTS13), provide adequate platelet adhesion and aggregation.14, 15 Similarly, although plasma levels of fibrinogen and procoagulant proteins are decreased, these defects are balanced by a simultaneous decline in plasma levels of the natural anticoagulants and with this adequate thrombin generation is still achieved.7, 8 Fibrinolysis is also reequilibrated, as low plasminogen levels are compensated by decreased levels of fibrinolytic inhibitors, and increased plasminogen activator levels by increased plasminogen activator inhibitor type 1 (PAI‐1).16, 17 Notably though, little is needed to disturb this delicate balance, which may degenerate into a hypo‐ or hypercoagulable state, especially during invasive procedures such as liver transplantation when pro‐ and antihemostatic factors decrease even further.18, 19, 20, 21
Pediatric patients have different disease etiologies, higher incidences of posttransplant thrombosis and a still developing hemostatic system.22, 23 Hemostasis is an evolving age‐dependent process that begins in utero and continues throughout life. Because most maturation of the hemostatic system occurs in childhood, hemostasis in children is characterized by age‐related changes in the coagulation system, with most hemostatic proteins present in lower levels in children compared to adults.
Up to now, there is a lack of data on the hemostatic state in pediatric patients with liver disease. Thrombin generation capacity in pediatric patients with liver diseases has previously been studied by Magnusson et al,24 who reported comparable thrombin generation capacity in patients and age‐matched healthy controls. Notably, PT and APTT levels were comparable for their study patients and controls, suggesting these patients were in a relatively early disease stage. More knowledge of the hemostatic state in pediatric patients is required to safely and efficiently prevent and treat both thrombotic and bleeding complications during liver transplantation.
The aim of this study was to assess hemostatic balance in pediatric patients undergoing liver transplantation. We hypothesized that whereas routine laboratory tests demonstrate a hypocoagulable state, more advanced hemostatic tests would indicate intact or hypercoagulable hemostasis, as we previously have demonstrated in adults.25, 26
2. METHODS
2.1. Study design
This is a prospective exploratory cohort study comparing 20 pediatric patients who underwent a liver transplantation with age‐matched controls in the University Medical Center Groningen between September 2017 and October 2018. The study protocol was a priori approved by the Medical Ethical Committee (NL61164.042.17). Informed consent was obtained from all patients (if ≥12 years) and/or parents/guardians prior to inclusion.
2.2. Study participants
All pediatric patients (≤16 years) listed for primary liver transplantation were screened for eligibility for this study. Exclusion criteria were acute liver failure, retransplantation, or combined organ transplantation. Additionally, an age‐matched control group was included, consisting of 30 healthy individuals who underwent minor surgery, specifically inguinal surgery (n = 20), and resection of thyroglossal duct cysts (n = 3) or benign soft tissue tumors (n = 7) in our center. Exclusion criteria were preterm birth, comorbidities known to affect the hemostatic system, a medical history of bleeding or thrombosis, and use of medication affecting hemostasis (Figure 1).
Figure 1.
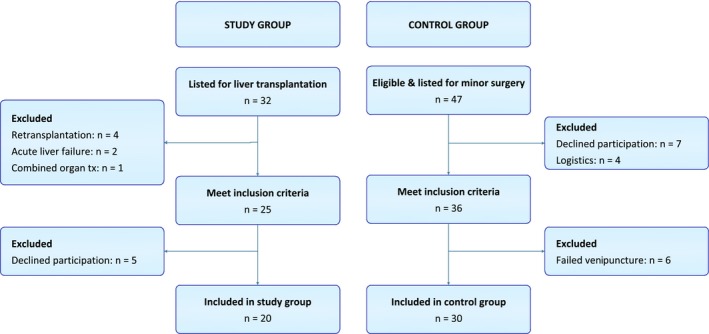
Flowchart of patients included in the study group and control group [Color figure can be viewed at http://www.wileyonlinelibrary.com]
2.3. Setting
Study patients were treated according to standard clinical practice. Liver grafts were classically (n = 1) or piggyback (n = 19) implanted. Vascular reconstructions were performed using end‐to‐end anastomoses, biliary reconstructions with a Roux‐and‐Y hepaticojejunostomy (n = 19) or duct‐to‐duct anastomosis (n = 1). Immunosuppressive therapy consisted of triple therapy including tacrolimus (Prograft®), with basiliximab (Simulect®) and prednisone as induction. To prevent thrombotic complications, all recipients received continuous intravenous unfractionated heparin posttransplantation for 1 week, followed by 3 months oral acetylsalicylic acid, according to our antithrombotic therapy protocol (Figure 2). Continuous infusion of unfractionated heparin was started at the end of transplantation or shortly afterwards, if PT values were <20 seconds, APTT < 50 seconds, and platelet counts >30 × 109/L. In some cases, heparin infusion was already started after graft reperfusion, based on the clinical judgment of the transplant surgeon and anesthesiologist.
Figure 2.
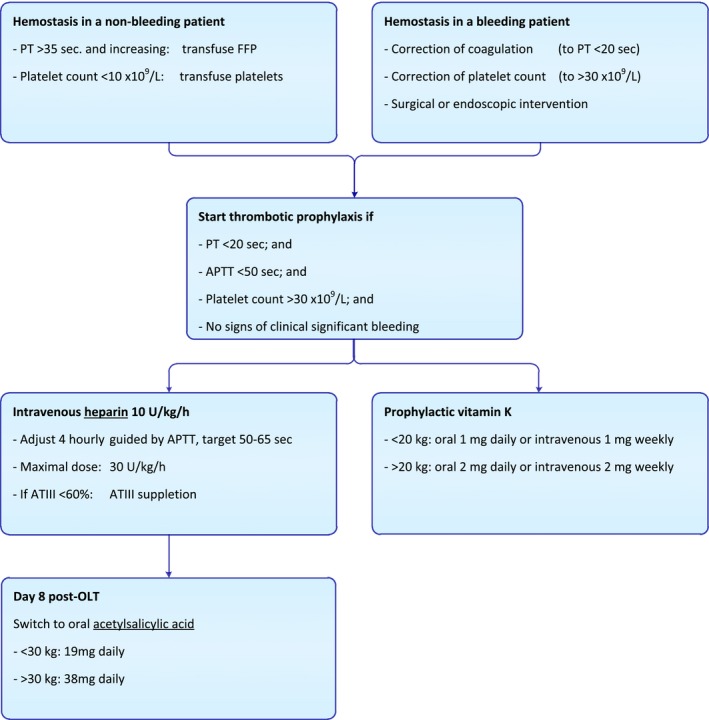
Clinical practice protocol regarding hemostasis and antithrombotic therapy in pediatric liver transplantation. FFP, fresh frozen plasma; PT, prothrombin time; APTT, activated partial thromboplastin time; ATIII, antithrombin III [Color figure can be viewed at http://www.wileyonlinelibrary.com]
2.4. Data collection
Patient and donor characteristics were documented, including transplant‐specific details and perioperative administered blood and coagulation products. To assess hemostasis, blood samples were obtained from study patients at the following time points: 30 minutes after induction of anesthesia, 30 minutes after start of anhepatic phase, 30 minutes after reperfusion, the end of transplantation, days 1, 3, 6, and 30 after transplantation. Blood samples in the control group were drawn after induction of anesthesia for minor surgery. Samples were drawn by venipuncture or derived from central venous lines in 3.2% sodium citrate tubes. After collection samples were double centrifuged at 18°C for 10 minutes at 2000 and 10 000 g, respectively, and subsequently stored at −80°C until use.
2.5. Hemostatic assays
Primary hemostasis was assessed by measuring platelet count (in our routine diagnostic laboratory), levels of VWF, and ADAMTS13. VWF levels were determined with an enzyme‐linked immunosorbent assay (ELISA) using commercially available polyclonal antibodies against VWF (DAKO, Santa Clara, CA). Plasma activity of ADAMTS13 was measured using the FRETS‐VWF73 assay (Peptanova, Sandhausen, Germany) as previously described by Kokame et al,27, 28 with the addition of bilirubin oxidase.
Coagulation was examined with routine tests including PT and APTT and by measuring plasma levels of more specific markers including fibrinogen, factor II, VIII, and antithrombin by using an automated coagulation analyzer (ACL 300 TOP) with reagents (Recombiplastin 2G for PT and factor II; Hemosil SynthaSil for APTT, fibrinogen and factor VIII; Liquid Antithrombin reagent for antithrombin), and protocols from the manufacturer (Instrumentation Laboratory, Bedford, MA). Factor levels are given as percentages relative to pooled normal plasma. Thrombin generation assays were performed in platelet‐poor plasma samples by using the fluorimetric method as previously described by Hemker et al.29 Thrombin generation capacity was measured with calibrated automated thrombography, in clotting plasma in the presence of thrombomodulin using a micro titer plate reading fluorometer (Fluoroskan Ascent) with reagents and protocols from Thrombinoscope (Maastricht, the Netherlands). Results were showed as the area under the thrombin generation curve, or so called endogenous thrombin potential (ETP; nM IIa*min).
Fibrinolysis was assessed by clot lysis time (CLT) and PAI‐1 levels. CLT was determined by monitoring changes in turbidity during clot formation and subsequent lysis by exogenous tissue plasminogen activator as described previously.30 PAI‐1 levels were determined with an ELISA kit (R&D Systems, Minneapolis, MN).
2.6. Statistical analysis
Data are presented as mean (standard deviation), median (interquartile range; IQR), or number (percentage) as appropriate. To test for differences between study and control group, Mann‐Whitney U test and Pearson chi‐square tests were used for continuous and categorical variables, respectively. Potential differences in the thrombin generation parameters at different time points in study group were compared to the values in the reference group with the Kruskal Wallis analysis using the Dunn's‐post tests. All reported P values are 2‐tailed and considered statistically significant if <.05. Statistical analyses were performed using IBM Statistics SPSS, version 23 (IBM Inc) and GraphPad Prism 7.02.
3. RESULTS
3.1. Baseline characteristics
Twenty pediatric liver transplant recipients were included in this study with a median age of 2.3 (IQR 0.6‐6.0) years, 55% was female (Table 1). Indications for liver transplantation were biliary atresia (45%), congenital cholestasis (30%), metabolic diseases (20%), and hepatoblastoma (5%). Sixteen partial and four full‐size grafts were derived from 13 living and 7 postmortal donors (Table 2). Baseline characteristics were comparable for study and control group.
Table 1.
Basic characteristics of study group (pediatric patients undergoing liver transplantation) and control group (pediatric patients undergoing minor surgery)
| Variable | Study group (n = 20) | Control group (n = 30) | P value |
|---|---|---|---|
| Gender, female | 11 (55%) | 11 (37%) | .20 |
| Age, y | 2.3 (0.6‐6.0) | 3.3 (1.2‐5.7) | .62 |
| Weight, kg | 14 (8‐21) | 16 (11‐22) | .31 |
| Length, cm | 90 (68‐114) | 108 (83‐124) | .09 |
Data presented as median (interquartile range) or number (%) where appropriate. P value using two sample Mann‐Whitney U test and Pearson chi‐square tests.
Table 2.
Basic characteristics of pediatric patients undergoing liver transplantation
| (n = 20) | |
|---|---|
| Pretransplant characteristics | |
| Indication for transplantation | |
| Biliary atresia | 9 (45%) |
| Congenital cholestasis | 6 (30%) |
| Metabolic | 4 (20%) |
| Hepatoblastoma | 1 (5%) |
| PELD score | 28 (28‐30) |
| Lab MELD score | 15 (6‐22) |
| High urgency | 3 (15%) |
| Previous portal vein thrombosis | 2 (10%) |
| Hemoglobin, mmol/L | 5.8 (4.7‐6.4) |
| Platelet count, 109/L | 145 (98‐195) |
| Serum creatinine, µmol/L | 21 (15‐37) |
| Serum bilirubin, µmol/L | 213 (36‐355) |
| Aspartate aminotransferase, U/L | 114 (75‐215) |
| Alanine transaminase, U/L | 76 (48‐106) |
| International normalized ratio | 1.4 (1.1‐1.8) |
| Donor characteristics | |
| Gender, female | 8 (40%) |
| Age, y | 33 (23‐43) |
| Weight, kg | 76 (65‐83) |
| Length, cm | 174 (165‐182) |
| Donor type | |
| Living donor | 13 (65%) |
| Brain death | 5 (25%) |
| Circulatory death | 2 (10%) |
| Graft type | |
| Left lateral segments | 15 (75%) |
| Left lobe | 1 (5%) |
| Full size | 4 (20%) |
| Graft weight, g | 316 (254‐413) |
| Graft/recipient weight ratio | 2.3 (1.7‐3.4) |
| Surgical characteristics | |
| Cold ischemia time, min | 61 (47‐404) |
| Warm ischemia time, min | 34 (29‐39) |
| Operation time, min | 472 (426‐526) |
| Blood loss, mL/kg | 67 (35‐116) |
| Blood transfusion, mL/kg | 32 (13‐68) |
| Platelet transfusion, mL/kg | 0 (0‐18)a |
| FFP transfusion, mL/kg | 0 (0‐52)a |
| Fibrinogen, mg/kg | 0 (0‐15)a |
Data presented as median (interquartile range) or number (%) where appropriate. P value using two sample Mann‐Whitney U test and Pearson chi‐square tests.
Abbreviations: FFP, fresh frozen plasma; MELD score, model for end‐stage liver disease score; PELD score, pediatric end‐stage liver disease score; U/L, units per liter.
Ranges instead of interquartile range values are given.
3.2. Primary hemostasis
Before, during, and after transplantation thrombocytopenia was present in most study patients, which normalized 30 days after transplantation (Figure 3A). Conversely, elevated VWF levels were present in study patients from start to end of transplantation and further increased in the week after transplantation. An opposite trend was seen for ADAMTS13, which was reduced in study patients at start of transplantation and further decreased during and after transplantation. A substantial number of patients had undetectable ADAMTS13 levels at certain time points. Both VWF and ADAMTS13 were outside our reference ranges at 30 days after transplantation (Figure 3B, C).
Figure 3.
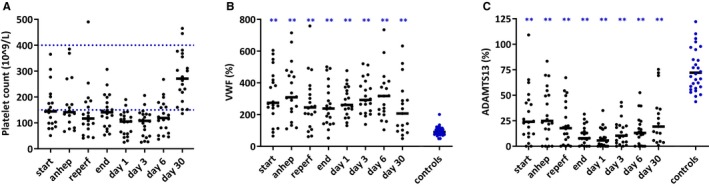
Platelet count (A), VWF (B), and ADAMTS13 (C) levels at various time points in 20 pediatric patients during and after liver transplantation and in 30 healthy controls. Small horizontal lines indicate medians. Blue horizontal dotted lines indicate reference values (A). Anhep, anhepatic phase; Reperf, reperfusion phase; VWF, von Willebrand factor; ADAMTS13, a disintegrin and metalloproteinase with thrombospondin 13.*P < .05, **P < .01 compared to controls [Color figure can be viewed at http://www.wileyonlinelibrary.com]
3.3. Coagulation
Routine laboratory tests PT and APTT were substantially prolonged in study patients at start of transplantation (Figure 4A, B) and further prolonged during transplantation. In half of study patients no clot formation was measured after reperfusion and/or at the end of transplantation. Posttransplantation PT and APTT gradually shortened and normalized on day 6. From days 1 to 6, APTT levels were influenced by continuous administration of intravenous heparin, the dose of which was guided by APTT levels, targeting 50‐65 seconds (Figure 4B).
Figure 4.
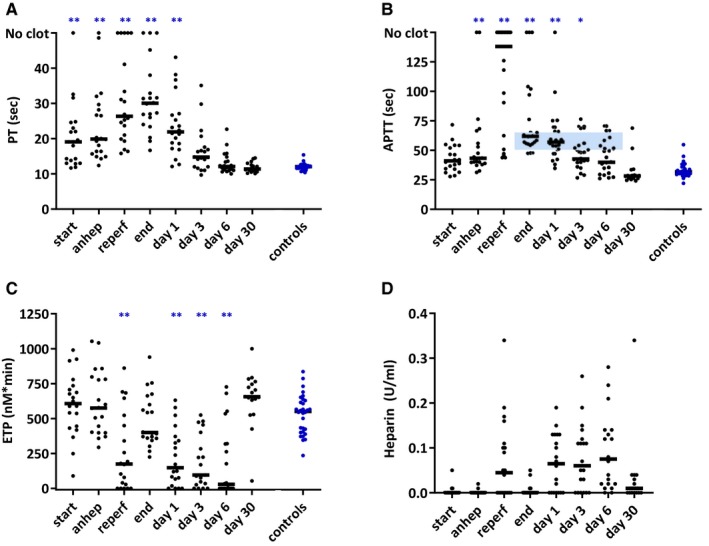
PT (A), APTT (B), thrombin generation capacity (C), and heparin concentrations (D) at various time points in 20 pediatric patients during and after liver transplantation and in 30 healthy controls. The small horizontal lines indicate medians. The horizontal blue highlighted area at 50 and 65 s. indicate target levels for heparin dosage (B). Thrombin generation capacity estimated with endogenous thrombin potential (C). Anhep, anhepatic phase; Reperf, reperfusion phase; PT, prothrombin time; APTT, activated partial thromboplastin time; ETP, endogenous thrombin potential. *P < .05, **P < .01 compared to controls [Color figure can be viewed at http://www.wileyonlinelibrary.com]
In contrast to the PT and APTT, the ETP was comparable for study patients and controls at baseline. After reperfusion ETP dropped significantly below the level of controls, which could be the result of locally supplied heparin during generation of the vascular anastomosis. Indeed heparin was detected in peripheral blood samples taken after reperfusion (Figure 4C, D). The ETP normalized at the end of transplantation and was decreased from days 1 to 6 posttransplantation under continuous heparin administration. ETPs levels were significantly lower in patients with detectable heparin concentrations than in patients without detectable heparin concentrations (44 vs 535 nmol/L IIa *min; P < .001). Thirty days after transplantation, both PT and APTT as well as ETPs were comparable in study patients and controls.
Preoperatively, prothrombin and antithrombin levels were decreased in study patients, further decreased during surgery, and normalized on day 30. Fibrinogen levels were also low at the start of surgery but recovered to supranormal levels on day 30. Factor VIII levels were high at the start of surgery, low during surgery, and recovered to supranormal levels at day 30 (Figure 5).
Figure 5.
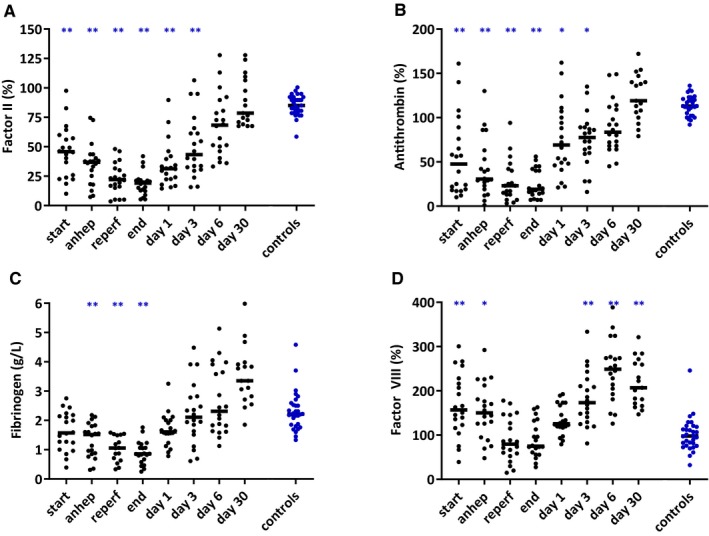
Factor II (A), antithrombin (B), fibrinogen (C), and factor VIII (D) levels at various time points in 20 pediatric patients during and after liver transplantation and 30 healthy controls. The small horizontal lines indicate medians. Factor levels are given as percentages relative to pooled normal plasma. Anhep, anhepatic phase; Reperf, reperfusion phase. *P < .05, **P < .01 compared to controls [Color figure can be viewed at http://www.wileyonlinelibrary.com]
3.4. Fibrinolysis
CLT was comparable for study and control group at start of transplantation, although the variation in CLT was much more profound in study patients. CLT was low after reperfusion, and at end of transplantation CLT was significantly prolonged in study patients, with no detectable fibrinolysis in the majority of patients. In the early postoperative period, CLT was variable but comparable to controls, but was prolonged at 30 days after transplantation. PAI‐1 was significantly higher than controls during the perioperative period in study patients (Figure 6).
Figure 6.
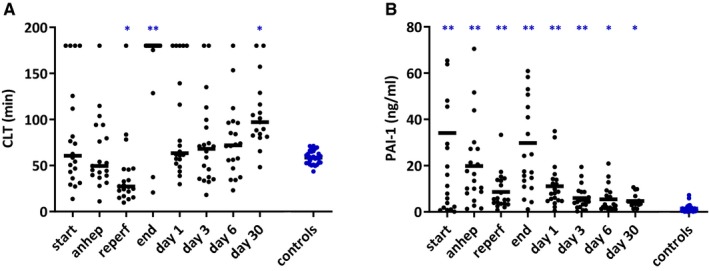
Clot lysis time (A) and PAI‐1 levels (B) at various time points in 20 pediatric patients during and after liver transplantation and in 30 healthy controls. The horizontal lines indicate medians. Anhep, anhepatic phase; Reperf, reperfusion phase; CLT, clot lysis time; PAI‐1, Plasminogen activator inhibitor 1. *P < .05, **P < .01 compared to controls
4. DISCUSSION
This study shows that pediatric patients with end‐stage liver disease are in a normal to hypercoagulable hemostatic state. During and shortly after liver transplantation, a temporary heparin‐dependent hypocoagulable state develops, which reverses to hemostatic balance with distinct hypercoagulable features on day 30. These observations extend our previous findings on hemostatic balance in adults with end‐stage liver disease.13, 25 The results of this study suggest that restrictive transfusion policy and proactive postoperative antithrombotic treatment, as is increasingly used in the adult population, is justified.7, 31
Notably, whereas conventional laboratory tests (platelet count, PT, APTT) are suggestive of a hypocoagulable state prior to, during, and shortly after pediatric liver transplantation, analyses using state‐of‐the‐art hemostasis tests are indicative of rebalanced hemostasis. Specifically, we found a profound VWF/ADAMTS13 unbalance that likely counteracts thrombocytopenia, intact secondary hemostasis, and normal to defective fibrinolytic capacity.
If we compare our results in pediatric patients to those of adult patients with end‐stage liver disease, both PT and APTT showed comparable changes over time during liver transplantation, with maximal values during reperfusion and at end of transplantation respectively. For ETP values, at the start of transplantation ETP values in adults are significantly elevated compared to controls, whereas in pediatric patients levels were comparable with those in age‐matched controls.25 During transplantation a comparable evolution of parameters over time was present in pediatrics compared to controls with one notable exception. In the first week posttransplantation, ETPs in pediatric patients were very low, whereas in adults ETP values were higher in patients compared to controls. This is likely the result of the intravenous administration of unfractionated heparin to pediatric patients, which is not performed in adults.
In aggregate, our data suggest that prior to transplantation, pediatric patients with end‐stage liver disease do not have a tendency to hemostasis‐related bleeding, although they may have a bleeding tendency related to portal hypertension. Intraoperatively, the use of local supplied heparin results in systemic heparinization, and combined with the temporary hyperfibrinolytic state after reperfusion, may contribute to perioperative bleeding.32 In the early postoperative period continuous heparinization led to a clear anticoagulant effect in most, but not all patients, whereas at day 30 after cessation of heparin therapy, there was a hemostatic balance with distinct hypercoagulable features. These hypercoagulable features, notably a persisting VWF/ADAMTS13 unbalance with normal platelet count, hyperfibrinogenemia, and a hypofibrinolytic state may contribute to thrombotic risk.
It has previously been demonstrated that a VWF/ADAMSTS13 unbalance might contribute to the risk of perioperative thrombotic complications.26, 33, 34 In addition, in living donor liver transplantation perioperative ADAMTS13 deficiency has been associated with early graft dysfunction, which might be related to intrahepatic microthrombosis.35 As isolated ADAMTS13 deficiency could result in (episodic) thrombotic microangiopathy, which is successfully prevented by plasma exchange, and possibly by administration of ADAMTS13 concentrate (ClinicalTrials.gov Identifier: NCT03393975). It may be that patients undergoing liver transplantation that have low or undetectable ADAMTS13 plasma levels can benefit from ADAMTS13 concentrate.
Although the PT and APTT suggest profound perioperative coagulation failure, thrombomodulin‐modified thrombin generation testing shows normal thrombin generating capacity in those samples not containing heparin. These results reconfirm that the PT and APTT should not be used alone to assess hemostatic status in patients with complex alterations in the coagulation system and indicate that prophylactic administration of prohemostatic therapy, with the aim to avoid perioperative bleeding, is not justified.9, 21, 36
The fibrinolytic status varied during liver transplantation, with individual patients above or below the reference range at all time points measured. On a group level, a clear hyperfibrinolytic state occurred after reperfusion, which is in line with results in adult transplantation.20, 37 The subsequent profound hypofibrinolytic state at the end of transplantation is also in line with previous observations in adult and pediatric transplantation.25, 38, 39 Although PAI‐1 levels were elevated in patients at all time points, PAI‐1 levels did not fully explain the hypofibrinolytic status, and other factors, notably low plasminogen levels likely contribute.20, 38, 40 The persistent high levels of PAI‐1 and postoperative hypofibrinolytic state may be clinically relevant because increased PAI‐1 levels and prolonged CLT are associated with an increased risk of venous and arterial thrombosis.41 In a retrospective study from our center, we demonstrated that postoperative thrombosis in pediatric liver transplant recipients mostly emerged at postoperative days 1 to 4.42
Because postoperative thrombosis is one of the main challenges in contemporary pediatric liver transplantation, routine antithrombotic therapy is increasingly used. Most centers have included a form of antithrombotic therapy in their clinical practice, mostly using heparin and/or acetylsalicylic acid.43 Our clinical antithrombotic protocol includes continuous intravenous heparin posttransplantation, targeting an APTT of 50‐65 seconds, replaced by acetylsalicylic acid (19 mg, once daily) after 1 week. In our study, targeted APTT levels were not reached in more than half of study patients (Figure 4B). This finding might be related to the increased use of thromboelastography to guide heparin levels, and by clinical aspects, notably perceived risk of ongoing bleeding.
Internationally there is a wide variation in antithrombotic therapy strategies, and there is no consensus about the best therapeutic agent and the optimal dose yet. The role of anticoagulant therapy in pediatric liver transplantation needs further investigation and clinical guidelines should be developed. The results of this study contribute to understanding the hemostatic state in these patients, and perhaps thrombin generation tests can play an important role in more rational anticoagulant management strategies. Note that point‐of‐care thrombin generation tests are in development. Whether current development of point‐of‐care thrombin generation tests will provide a solution needs to be determined with further research.
The hypercoagulable features present on day 30 after liver transplantation are in line with those noted in previous studies in adult transplantation, showing hypercoagulable features up to 1 year after transplantation.20, 44 A previous study in pediatric transplantation found complete normalization of hemostasis at 30 days,39 but a very different experimental approach was taken in this study. This hypercoagulable state posttransplantation is probably the result of low‐grade endothelial activation due to immunosuppressive therapy, combined with the hyperreactive hemostatic state in these patients. The elevated levels of VWF, fibrinogen, and FVIII support this theory. It might be that prolonged antithrombotic therapy is indicated after pediatric liver transplantation. Indeed, in adult liver transplantation prolonged administration of acetylsalicylic acid was shown to substantially reduce the incidence of late hepatic artery thrombosis.45 Clinical studies will be required to assess safety and efficacy of prolonged antithrombotic treatment in the pediatric population.
Although this is a relatively small study, including 20 patients with end‐stage liver disease, we were able to present a first detailed analysis of the hemostatic balance in pediatric patients undergoing liver transplantation and make a comparison of their hemostatic values to that of healthy controls. These results provide novel information but need to be confirmed and extended in further studies. It would be interesting to explore differences in hemostasis between etiologies of liver disease and between deceased vs living donor grafts. Furthermore, the hemostatic balance in pediatric patients undergoing acute liver transplantation or retransplantation needs further exploration, because these patients were excluded from the current study. Notably, study patients had relatively short cold ischemia times, and it needs to be further investigated if the hemostatic balance changes in patients receiving grafts with longer cold ischemia times.
In conclusion, pediatric patients with end‐stage liver disease are in a fragile hemostatic balance. During transplantation a temporary hypocoagulable state is present under heparin administration, which rapidly converts to a rebalanced hemostatic state. Distinct hypercoagulable features persist until at least day 30, which contribute to the risk of posttransplant thrombosis.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
Research funding was obtained by the University Medical Center Groningen Transplantation Research Foundation, Groningen, the Netherlands.
Werner MJM, de Meijer VE, Adelmeijer J, et al. Evidence for a rebalanced hemostatic system in pediatric liver transplantation: A prospective cohort study. Am J Transplant. 2020;20:1384–1392. 10.1111/ajt.15748
DATA AVAILABILITY STATEMENT
Deidentified individual participant data and hemostatic assay results are available on request through contact with the corresponding author.
REFERENCES
- 1. Kamran Hejazi Kenari S, Mirzakhani H, Eslami M, Saidi RF. Current state of the art in management of vascular complications after pediatric liver transplantation. Pediatr Transplant. 2015;19(1):18‐26. [DOI] [PubMed] [Google Scholar]
- 2. Nacoti M, Corbella D, Fazzi F, Rapido F, Bonanomi E. Coagulopathy and transfusion therapy in pediatric liver transplantation. World J Gastroenterol. 2016;22(6):2005‐2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziaziaris WA, Darani A, Holland AJA, et al. Reducing the incidence of hepatic artery thrombosis in pediatric liver transplantation: Effect of microvascular techniques and a customized anticoagulation protocol. Pediatr Transplant. 2017;21(4):e12917. [DOI] [PubMed] [Google Scholar]
- 4. Borst AJ, Sudan DL, Wang LA, Neuss MJ, Rothman JA, Ortel TL. Bleeding and thrombotic complications of pediatric liver transplant. Pediatr Blood Cancer. 2018;65(5):e26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: A systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9(4):746‐757. [DOI] [PubMed] [Google Scholar]
- 6. Kaneko J, Sugawara Y, Tamura S, et al. Coagulation and fibrinolytic profiles and appropriate use of heparin after living‐donor liver transplantation. Clin Transplant. 2005;19(6):804‐809. [DOI] [PubMed] [Google Scholar]
- 7. Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: Evidence and clinical consequences. Blood. 2010;116(6):878‐885. [DOI] [PubMed] [Google Scholar]
- 8. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147‐156. [DOI] [PubMed] [Google Scholar]
- 9. Lisman T, Porte RJ. Value of preoperative hemostasis testing in patients with liver disease for perioperative hemostatic management. Anesthesiology. 2017;126(2):338‐344. [DOI] [PubMed] [Google Scholar]
- 10. Tripodi A, Salerno F, Chantarangkul V, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41(3):553‐558. [DOI] [PubMed] [Google Scholar]
- 11. Gatt A, Riddell A, Calvaruso V, Tuddenham EG, Makris M, Burroughs AK. Enhanced thrombin generation in patients with cirrhosis‐induced coagulopathy. J Thromb Haemost. 2010;8(9):1994‐2000. [DOI] [PubMed] [Google Scholar]
- 12. Lebreton A, Sinegre T, Pereira B, Lamblin G, Duron C, Abergel A. Plasma hypercoagulability in the presence of thrombomodulin but not of activated protein C in patients with cirrhosis. J Gastroenterol Hepatol. 2017;32(4):916‐924. [DOI] [PubMed] [Google Scholar]
- 13. Fisher C, Patel VC, Stoy SH, et al. Balanced haemostasis with both hypo‐ and hyper‐coagulable features in critically ill patients with acute‐on‐chronic‐liver failure. J Crit Care. 2018;43:54‐60. [DOI] [PubMed] [Google Scholar]
- 14. Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of von Willebrand factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 15. Uemura M, Fujimura Y, Matsumoto M, et al. Comprehensive analysis of ADAMTS13 in patients with liver cirrhosis. Thromb Haemost. 2008;99(6):1019‐1029. [DOI] [PubMed] [Google Scholar]
- 16. Leebeek FW, Kluft C, Knot EA, de Maat MP, Wilson JH. A shift in balance between profibrinolytic and antifibrinolytic factors causes enhanced fibrinolysis in cirrhosis. Gastroenterology. 1991;101(5):1382‐1390. [DOI] [PubMed] [Google Scholar]
- 17. Lisman T, Leebeek FWG, Mosnier LO, et al. Thrombin‐activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology. 2001;121(1):131‐139. [DOI] [PubMed] [Google Scholar]
- 18. de Boer MT, Molenaar IQ, Hendriks HG, Slooff MJ, Porte RJ. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22(4):265‐275. [DOI] [PubMed] [Google Scholar]
- 19. Lisman T, Caldwell SH, Burroughs AK, et al. Hemostasis and thrombosis in patients with liver disease: the ups and downs. J Hepatol. 2010;53(2):362‐371. [DOI] [PubMed] [Google Scholar]
- 20. Arshad F, Lisman T, Porte RJ. Hypercoagulability as a contributor to thrombotic complications in the liver transplant recipient. Liver Int. 2013;33(6):820‐827. [DOI] [PubMed] [Google Scholar]
- 21. Weeder PD, Porte RJ, Lisman T. Hemostasis in liver disease: implications of new concepts for perioperative management. Transfus Med Rev. 2014;28(3):107‐113. [DOI] [PubMed] [Google Scholar]
- 22. Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80(8):1998‐2005. [PubMed] [Google Scholar]
- 23. Monagle P, Ignjatovic V, Savoia H. Hemostasis in neonates and children: pitfalls and dilemmas. Blood Rev. 2010;24(2):63‐68. [DOI] [PubMed] [Google Scholar]
- 24. Magnusson M, Berndtsson M, Fischler B, et al. Thrombin generation test in children and adolescents with chronic liver disease. Thromb Res. 2015;135(2):382‐387. [DOI] [PubMed] [Google Scholar]
- 25. Lisman T, Bakhtiari K, Pereboom IT, Hendriks HG, Meijers JC, Porte RJ. Normal to increased thrombin generation in patients undergoing liver transplantation despite prolonged conventional coagulation tests. J Hepatol. 2010;52(3):355‐361. [DOI] [PubMed] [Google Scholar]
- 26. Pereboom IT, Adelmeijer J, van Leeuwen Y, Hendriks HG, Porte RJ, Lisman T. Development of a severe von willebrand factor/ADAMTS13 dysbalance during orthotopic liver transplantation. Am J Transplant. 2009;9(5):1189‐1196. [DOI] [PubMed] [Google Scholar]
- 27. Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS‐VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93‐100. [DOI] [PubMed] [Google Scholar]
- 28. Eckmann CM, De Laaf RT, Van Keulen JM, Van Mourik JA, De Laat B. Bilirubin oxidase as a solution for the interference of hyperbilirubinemia with ADAMTS‐13 activity measurement by FRETS‐VWF73 assay. J Thromb Haemost. 2007;5(6):1330‐1331. [DOI] [PubMed] [Google Scholar]
- 29. Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4‐15. [DOI] [PubMed] [Google Scholar]
- 30. Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105(3):1102‐1105. [DOI] [PubMed] [Google Scholar]
- 31. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost. 2017;1(2):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tran LT, Mazariegos GV, Damian D, Davis PJ. Red blood cell transfusion in pediatric orthotopic liver transplantation: what a difference a few decades make. Anesth Analg. 2019;129(4):1087-1092. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi S‐I, Yokoyama Y, Matsushita T, et al. Increased von willebrand factor to ADAMTS13 ratio as a predictor of thrombotic complications following a major hepatectomy. Arch Surg. 2012;147(10):909‐917. [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi T, Wada H, Nishioka N, et al. ADAMTS13 related markers and von willebrand factor in plasma from patients with thrombotic microangiopathy (TMA). Thromb Res. 2008;121(6):849‐854. [DOI] [PubMed] [Google Scholar]
- 35. Ko S, Okano E, Kanehiro H, et al. Plasma ADAMTS13 activity may predict early adverse events in living donor liver transplantation: observations in 3 cases. Liver Transpl. 2006;12(5):859‐869. [DOI] [PubMed] [Google Scholar]
- 36. Lisman T, Caldwell SH, Porte RJ, Leebeek FW. Consequences of abnormal hemostasis tests for clinical practice. J Thromb Haemost. 2006;4(9):2062‐2063. [DOI] [PubMed] [Google Scholar]
- 37. Lisman T, Leebeek FW, Meijer K, Van Der Meer J, Nieuwenhuis HK, De Groot PG. Recombinant factor VIIa improves clot formation but not fibrolytic potential in patients with cirrhosis and during liver transplantation. Hepatology. 2002;35(3):616‐621. [DOI] [PubMed] [Google Scholar]
- 38. Leaker MT, Brooker LA, Mitchell LG, Weitz JI, Superina R, Andrew ME. Fibrin clot lysis by tissue plasminogen activator (tPA) is impaired in plasma from pediatric patients undergoing orthotopic liver transplantation. Transplantation. 1995;60(2):144‐147. [PubMed] [Google Scholar]
- 39. Mimuro J, Mizuta K, Kawano Y, et al. Impact of acute cellular rejection on coagulation and fibrinolysis biomarkers within the immediate post‐operative period in pediatric liver transplantation. Pediatr Transplant. 2010;14(3):369‐376. [DOI] [PubMed] [Google Scholar]
- 40. Praetner M, Zuchtriegel G, Holzer M, et al. Plasminogen activator inhibitor‐1 promotes neutrophil infiltration and tissue injury on ischemia‐reperfusion. Arterioscler Thromb Vasc Biol. 2018;38(4):829‐842. [DOI] [PubMed] [Google Scholar]
- 41. Meltzer ME, Lisman T, de Groot PG, et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI‐1. Blood. 2010;116(1):113‐121. [DOI] [PubMed] [Google Scholar]
- 42. Werner MJM, de Kleine RHJ, de Boer MT, et al. Anticoagulation in pediatric liver transplantation; the pros and cons. Am J Transplant. 2018;18:384. [Google Scholar]
- 43. Calinescu AM, Karam O, Wilde JCH, Ansari M, McLin VA, Wildhaber BE. International survey on anticoagulation and antiplatelet strategies after pediatric liver transplantation. Pediatr Transplant. 2018:e13317. [DOI] [PubMed] [Google Scholar]
- 44. Lisman T, Platto M, Meijers JC, Haagsma EB, Colledan M, Porte RJ. The hemostatic status of pediatric recipients of adult liver grafts suggests that plasma levels of hemostatic proteins are not regulated by the liver. Blood. 2011;117(6):2070‐2072. [DOI] [PubMed] [Google Scholar]
- 45. Vivarelli M, La Barba G, Cucchetti A, et al. Can antiplatelet prophylaxis reduce the incidence of hepatic artery thrombosis after liver transplantation? Liver Transpl. 2007;13(5):651‐654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified individual participant data and hemostatic assay results are available on request through contact with the corresponding author.


