Abstract
Edible insects are advocated as sustainable and healthy food and feed. However, commercially produced insects are often low in n‐3 fatty acids and have suboptimal n‐6/n‐3 ratios. A certain amount and proportion of these FAs is required to optimize human health. Flaxseed oil consists primarily (57%) out of alpha‐linolenic acid. An experiment was conducted to quantify the effect of flaxseed oil provision on fatty acid composition and to determine the quantity needed to attain a beneficial n‐6/n‐3 ratio. Three species were used in the experiment: house crickets (Acheta domesticus [L.]), lesser mealworms (Alphitobius diaperinus [Pfanzer]) and black soldier flies (Hermetia illucens [L.]). These were provided with either a control diet or a diet enriched with 1%, 2%, or 4% flaxseed oil during their larval/nymphal stage. Fatty acid profiles of diets and insects were determined via GC‐MS. The three species had distinct fatty acid profiles on all four diets, but responded similarly to flaxseed oil addition. For each percent added to the diet, the alpha‐linolenic acid content of the insects increased by 2.3%–2.7%. Four percent addition increased the n‐3 fatty acid content 10–20 fold in the three species and thereby strongly decreased n‐6/n‐3 ratios from 18–36 to 0.8–2.4. A ratio below 5 is considered optimal for human health and was achieved by 2% flaxseed oil inclusion for house crickets and lesser mealworms, and at 1% inclusion for black soldier flies. Adding a source of n‐3 fatty acids to insect diets can thus improve the nutritional quality of insects.
Keywords: Acheta domesticus, Alphitobius diaperinus, diet, fatty acids, Hermetia illucens
Introduction
As incomes rise and the world population grows, the demand for animal protein increases (Sans & Combris, 2015). The current production of animal protein is already associated with a large environmental impact (Herrero et al., 2015). Hence, more sustainable sources of animal protein are being investigated, including edible insects. These are considered a relatively sustainable source of animal protein and are perceived as highly nutritious (van Huis & Oonincx, 2017). Most insects are rich sources of protein, minerals, and certain vitamins, although great variation exists between species and life stages, and due to production conditions (Finke & Oonincx, 2017; Oonincx et al., 2018). Additionally, most insect species contain large amounts of fat. For instance on a dry matter (DM) basis the fat content of house crickets is between 17% and 28% (Finke, 2015; Oonincx et al., 2015b), for lesser mealworms this is between 21% and 31% (Despins & Axtell, 1995; Bjørge et al., 2018) and for larvae of the black soldier fly this is between 6.6% and 39% (Oonincx et al., 2015b; Barragan‐Fonseca et al., 2017). In certain species this fat is largely composed of long chained polyunsaturated fatty acids (PUFAs) (Finke & Oonincx, 2017). These PUFAs can be distinguished based on the position of their first double bond. The two most important types, omega 3 (n‐3) and omega 6 (n‐6) fatty acids, are required in mammalian diets because mammals cannot synthesize these de novo (Anderson & Ma, 2009). Both the ingested amounts and proportions of these fatty acids are important for human health. Western diets generally contain too little n‐3 and too much n‐6 PUFAs (Simopoulos, 2002). This imbalance is associated with health issues in humans, such as coronary heart disease, cancer and autoimmune and inflammatory diseases (Tokudome et al., 2000; Trautwein, 2001; Simopoulos, 2002; Bagga et al., 2003; Robinson & Stone, 2006). Early studies that demonstrated beneficial effects of n‐3 fatty acids on human health focused on marine sources rich in C20:5n3 (eicosapentaenoic acid; EPA) and C22:6n3 (docosahexaenoic acid; DHA) (Antruejo et al., 2011). Later studies included the plant based fatty acid C18:3n3 (alpha‐linolenic acid; ALA) and indicated that also ALA can be an effective way to achieve desired health effects in humans (Mantzioris et al., 2000; Bemelmans et al., 2002; Singh et al., 2002; Zhao et al., 2004; Campos et al., 2008). ALA can be converted to EPA and DHA via several enzymatic steps (Thais et al., 2013). The same enzymes can also elongate C18:2n6 (linoleic acid; LA). Therefore, due to substrate competition, both the levels of and the ratio between n‐3 and n‐6 PUFAs determine the quantity of the formed end products (Gerster, 1998). The human diet in our early evolutionary period had a n6/n3 ratio of approximately 1–2, whereas this has increased to ∼16 in current Western diets (Simopoulos, 1999; Simopoulos, 2002; Simopoulos, 2009; Melvin & Boyd, 2010). For optimal human health this ratio should be around 5 (Gerster, 1998; Kouba & Mourot, 2011). Therefore, if insects are to be part of a sustainable and nutritious diet it would be advantageous if they contain relatively large proportions of n‐3 PUFA's, which would result in more favorable n‐6/n‐3 ratios.
The n‐6/n‐3 ratios in terrestrial insects are on average three times higher than in aquatic insects (Fontaneto et al., 2011). This is because microalgae produce n‐3 PUFAs de novo and these fatty acids are selectively accumulated via the trophic chain (Gladyshev et al., 2013). However, most of the insect species which are produced for human or animal consumption are terrestrial. Furthermore, commercially produced insects generally contain higher levels of n‐6 than species collected in the wild, which leads to elevated n‐6/n‐3 ratios in these produced species (Finke & Oonincx, 2017). The fatty acid composition of insects is in part determined by the fatty acid composition of their diet (St‐Hilaire et al., 2007; Komprda et al., 2013; van Broekhoven et al., 2015; Oonincx et al., 2015b; Hussein et al., 2017; Starčević et al., 2017). Higher levels of n‐3 PUFAs in insect diets would therefore increase n‐3 PUFA concentrations and decrease n‐6/n‐3 ratios in the insects. However, differences between species in accumulation efficiency and in de novo synthesis of fatty acids are expected to lead to distinct fatty acid profiles in different species, even if they are provided with the same diet. Therefore, an experiment was conducted to quantify the effects of dietary n‐3 levels on the fatty acid profiles of three taxonomically distinct insect species commonly used as feed or food.
Materials and methods
Diets
Four diets differing in fatty acid composition (Table 1) were made by adding flaxseed oil (Lijnzaadolie koudgeperst—article #082167, Holland & Barrett B.V., Amsterdam, the Netherlands), a well‐known source of n‐3 fatty acids (Antruejo et al., 2011), to a basal diet.
Table 1.
Main fatty acid composition (as a percentage of total fatty acids†) of a control diet (0%) and diets enriched with 1%, 2%, or 4% of flax seed oil (Mean ± SD; n = 6). If superscripts in the same column have no letters in common, means differ significantly (Kruskal–Wallis test followed by Dunn–Bonferroni post hoc test; P < 0.05)
| Flaxseed oil | C12:0 | C14:0 | C16:0 | C18:0 | C18:1n9 | C18:1n7 | C18:2n6 | C18:3n3 | C20:5n3 |
|---|---|---|---|---|---|---|---|---|---|
| 0% | 1.5 ± 0.06a | 1.1 ± 0.03a | 22.2 ± 0.22a | 3.6 ± 0.05a | 30.2 ± 0.19a | 0.5 ± 0.07a | 34.7 ± 0.29a | 2.5 ± 0.05a | 0.5 ± 0.01a |
| 1% | 1.1 ± 0.09ab | 0.8 ± 0.04ab | 17.9 ± 0.15ab | 4.0 ± 0.03ab | 28.1 ± 0.16a | 0.4 ± 0.06ab | 30.8 ± 0.31ab | 13.8 ± 0.21ab | 0.3 ± 0.01ab |
| 2% | 0.9 ± 0.05bc | 0.7 ± 0.04bc | 16.0 ± 1.01bc | 4.4 ± 0.30bc | 27.6 ± 1.88ab | 0.4 ± 0.05ab | 25.3 ± 4.85bc | 21.9 ± 1.46bc | 0.3 ± 0.02bc |
| 4% | 0.6 ± 0.08c | 0.5 ± 0.04c | 12.6 ± 0.34c | 4.6 ± 0.10c | 25.1 ± 0.26b | 0.3 ± 0.09b | 23.9 ± 0.34c | 30.3 ± 0.86c | 0.2 ± 0.02c |
†Fatty acids <0.5% of total fatty acids are excluded.
This basal diet consisted of a complete chicken feed (Opfokmeel farmfood, Agruniek Rijnvallei Voer BV, Wageningen, the Netherlands) and contained approximately 4% fat per kg of which 2.5% was ALA and 0.5% was EPA (Table 1). One kilogram of diet containing 0, 10, 20 or 40 g of flaxseed oil (0%, 1%, 2%, or 4%) was made for each treatment. First tert‐butylhydroquinone (200 μg/g oil) was added to the flaxseed oil to prevent oxidation (Omar et al., 2010). Then 2% Tween‐20 (Sigma‐Aldrich) and 2 g of chicken feed were added to create a carrier. This carrier was then hand‐mixed thoroughly with a spoon for 10 min with the rest of the chicken feed. All four diets were stored in sealed plastic boxes at −20 °C until further use. Six samples were taken per diet to verify the homogeneity of fatty acid concentrations.
Animals and experimental setup
Three insect species were selected: house crickets (Acheta domesticus L.; Orthoptera: Gryllidae), lesser mealworms (Alphitobius diaperinus Panzer; Coleoptera: Tenebrionidae), and black soldier flies (Hermetia illucens L.; Diptera: Stratiomyidae). Eggs from the first two species were obtained from established colonies at the Laboratory of Entomology, Wageningen University, the Netherlands and first‐stage larvae of the lesser mealworm were provided by a commercial insect producer (Kreca V.O.F., Ermelo, the Netherlands).
House crickets: 100 nymphs, less than 24 h old, were placed in transparent plastic containers (356 mm × 234 mm × 228 mm; Faunarium type pt2665, Hagen, Holm, Germany). The tops of these enclosures were covered with a lid and a net (mesh width 1 mm) to prevent escape while providing ample ventilation. Fifteen hollow plastic tubes (200 mm long and 30 mm in diameter) were placed in each container to provide the nymphs with shelter. A water dispenser (Gebroeders de Boon, Gorinchem, the Netherlands) with tissue paper in its opening was placed in each container to provide water.
Lesser mealworms: 100 first instar larvae were placed in a plastic container (178 mm × 114 mm × 65 mm) of which the lid was perforated with 60 small holes to allow air exchange. Three times per week carrot pieces were provided as a source of moisture.
Black soldier flies: 100 larvae, less than 24 h old, were placed in a plastic container (178 mm × 114 mm × 65 mm) of which the lid was perforated with 60 small holes to allow air exchange.
The larvae or nymphs of all three species were randomly allocated to one of the four dietary treatments. Per species, six replicates were used for each treatment. Feed was provided ad libitum. House crickets and lesser mealworms were provided with the diet as is, whereas the feed for the black soldier flies was first mixed with tap water (2 mL per gram of diet). The experiments were carried out in a climate‐controlled room at 28 °C, 70% relative humidity, and a 12 h photoperiod. Boxes were randomly rotated on a weekly basis via the randomize function in Microsoft Office Excel 2013 (Microsoft, Redmond, WA, USA).
Sampling and calculations
When the first adult (house cricket), pupa (lesser mealworm), or prepupa (black soldier fly) was seen in a container all animals in that container were harvested. Development time was calculated as the number of days between the start of the experiment and the moment of harvesting. Prior to harvesting the container was placed at –20 °C for circa 15 min. All insects were taken from their containers and counted to determine their survival [(number of surviving insects/100) × 100%]. The house crickets and lesser mealworms were weighed directly per container. The BSF larvae were first put in a sieve, rinsed under running water and dried with tissue paper to remove feed adhering to their integument and then weighed per container.
Subsequently, all insect and diet samples were freeze‐dried using a Vaco5 Drytec (Zirbus technology, Bad Grund, Germany) until a stable weight was reached. The dry matter content of the insects was determined by dividing the dry weight by the fresh weight. Average weight was calculated by dividing the total weight by the number of surviving insects. The dried samples were then milled to a fine powder with an A11 Basic IKA mill (IKA®, Staufen, Germany).
Fat extraction and fatty acid profile analyses
Fats were extracted in accordance with the method of Folch et al. (1957) and crude fat content was determined in accordance with AOAC (1990); first the ground samples (1 g for house cricket and black soldier fly, 0.5 g for lesser mealworm, and 4 g of diet) were mixed with 0.2 g sodium sulphate. Then 15 mL of 2:1 (v/v) chloroform/methanol was added, the mixture was stirred for 20 min at room temperature, and then filtered (>10 μm; Whatman 595½ GE Health care, Kent, UK). Subsequently, 10 mL of chloroform/methanol (2:1, v/v) was added to the residue and the mixture was stirred again for 15 min and filtered. This filtrate was transferred to a tube and evaporated under nitrogen at 42 °C. The resulting extracts were automatically methylated with a Gerstel MPS injector (Da Vinci Laboratory Solutions BV, Rotterdam, the Netherlands). First, they were heated to 80 °C for 1 min and then 400 μL sodium methoxide solution (0.5 mol/L in methanol, Sigma Aldrich, Zwijndrecht, the Netherlands) was added and mixed for 10 min at 70 °C at 500 r/min. Then 400 μL boron trifluoride (20% in methanol, Sigma Aldrich, Zwijndrecht, the Netherlands) was added, mixed for 5 min at 80 °C at 500 r/min, after which 450 μL iso‐octane (99% HPLC quality, Biosolve, Valkenswaard, the Netherlands) was added and samples were again mixed at 80 °C at 500 r/min for 1 min. Subsequently, 400 μL saturated sodium chloride solution was added, and samples were mixed again for 1 min at 80 °C at 500 r/min.
All samples were kept at room temperature until two phases had separated after which a sample from the upper organic layer (2 μL for the insects and 1 μL for the feeds) was injected in the gas chromatograph (GC). Thirty fatty acid methyl esters of feed, house crickets and lesser mealworms were quantified on a Thermo Focus gas chromatograph equipped with a FAME Agilent CP‐7489 column (100 m × 0.25 mm) and a flame ionization detector. Helium was used as the carrier gas. The GC was set up with the following temperature program: 60 °C for 5 min, ramp at 15 °C/min, held at 165 °C for 1 min, followed by a ramp at 1 °C/min hold at 225 °C for 23 min. Data were integrated with Chromquest 5.0 version 3.2.1. Fatty acid methyl esters of black soldier flies were quantified on an Agilent 7890A gas chromatograph equipped with a FAME Agilent CP7419 column (50 m × 0.25 mm) and a flame ionization detector. Helium was used as the carrier gas. The GC was set up with the following temperature program: 100 °C for 1 min, ramp at 5 °C/min, hold at 230 °C for 9 min. Data was integrated with EZChrom Elite software and expressed as a percentage of total fatty acids.
Statistical analysis
Statistical analysis was performed with SPSS 23.0 (IBM Corporation, Armonk, NY, USA). A General Linear Model using treatment as a fixed factor was used to analyze fatty acid profile differences between species, and was followed by a Tukey's HSD. Animal performance data that were normally distributed and had homogeneous variances were analyzed for significant differences via an ANOVA followed by a Tukey HSD test. Data that were not normally distributed or had inhomogeneous variances were analyzed for significant differences (P < 0.05) via a Kruskal–Wallis test. Subsequent post hoc testing was conducted via a Dunn–Bonferroni post hoc test. Most of the fatty acid data did not meet ANOVA prerequisites and these were therefore also analyzed via the latter procedure. Linear regression was used to quantify the relationship between flaxseed oil inclusion and ALA content.
Results
The three species survived and developed well on the control diet (Table 2). Similar to the composition of the control diet, the three species had a high content of C18:1n9 (oleic acid; 12%–30% of TFA) and LA, whereas their ALA content was low (Table 3). Addition of flaxseed oil to the feed strongly increased the relative abundance of ALA (from 2.5% to 30.3%), slightly increased C18:0 (stearic acid; from 3.6% to 4.6% of total fatty acids; TFA) and decreased the relative abundance of the other fatty acids by dilution (Table 1). The four dietary treatments did not affect survival, development time, live weight, dry weight or dry matter content in any of the three species (Table 2). The crude fat content of both the house crickets (P = 0.023) and the lesser mealworms (P = 0.043) increased due to the addition of flaxseed oil. The crude fat content of the black soldier flies was not determined due to a human error.
Table 2.
Survival, development time, live and dry weight, dry matter (DM) content, and fat content of house crickets, lesser mealworms, and black soldier flies on a control diet (0%) and diets enriched with 1%, 2%, or 4% of flax seed oil (mean ± SD; n = 6). If for a species superscripts in the same column have no letters in common, means differ significantly (ANOVA followed by Tukey's HSD; P < 0.05)
| Treatment | Survival | Development time | Live weight | Dry weight | DM content | Fat content | |
|---|---|---|---|---|---|---|---|
| (flaxseed oil) | (%) | (days) | (mg) | (mg) | (% live weight) | (% DM) | |
| House cricket | 0% | 69 ± 16.6 | 44 ± 0.0 | 238 ± 24.1 | 71 ± 10.1 | 30 ± 1.2 | 29 ± 1.4a |
| 1% | 65 ± 5.9 | 44 ± 0.5 | 236 ± 15.6 | 72 ± 6.5 | 30 ± 0.9 | 32 ± 1.7b | |
| 2% | 64 ± 10.9 | 45 ± 0.6 | 253 ± 17.4 | 79 ± 7.3 | 31 ± 0.8 | 32 ± 1.4b | |
| 4% | 55 ± 10.7 | 46 ± 0.5 | 252 ± 23.2 | 78 ± 9.4 | 31 ± 0.9 | 32 ± 1.6b | |
| Lesser mealworm | 0% | 75 ± 10.1 | 53 ± 1.3 | 21.3 ± 3.57 | 7.7 ± 1.99 | 36 ± 3.5 | 31 ± 1.8a |
| 1% | 63 ± 20.4 | 52 ± 1.5 | 19.6 ± 2.42 | 6.7 ± 0.90 | 34 ± 1.2 | 33 ± 1.5ab | |
| 2% | 70 ± 9.9 | 51 ± 2.4 | 18.7 ± 1.61 | 6.1 ± 0.65 | 33 ± 0.9 | 31 ± 1.5ab | |
| 4% | 78 ± 3.0 | 53 ± 2.4 | 21.2 ± 2.34 | 7.4 ± 0.98 | 35 ± 1.5 | 34 ± 2.2b | |
| Black soldier flies | 0% | 83 ± 12.5 | 17 ± 0.5 | 135 ± 14.1 | 47 ± 3.7 | 35 ± 2.9 | N/A |
| 1% | 84 ± 20.0 | 17 ± 1.5 | 132 ± 9.4 | 45 ± 6.7 | 34 ± 5.4 | N/A | |
| 2% | 85 ± 11.2 | 17 ± 0.6 | 140 ± 17.7 | 50 ± 7.0 | 35 ± 1.1 | N/A | |
| 4% | 90 ± 7.7 | 17 ± 0.0 | 145 ± 11.4 | 50 ± 4.0 | 34 ± 0.7 | N/A |
Table 3.
Main fatty acid composition (as a percentage of total fatty acids†) of house crickets, lesser mealworms, and black soldier flies on a control diet (0%) and diets enriched with 1%, 2%, or 4% of flax seed oil (mean ± SD; n = 6). If for a species superscripts in the same column have no letters in common, means differ significantly (Kruskal–Wallis test followed by Dunn–Bonferroni post hoc test; P < 0.05)
| Flaxseed oil | C10:0 | C12:0 | C14:0 | C16:0 | C16:1n9 | C18:0 | C18:1n9 | C18:1n7 | C18:2n6 | C18:3n3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| House | 0% | 0.0 ± 0.00 | 0.1 ± 0.00a | 0.7 ± 0.03a | 27.8 ± 0.25a | 1.0 ± 0.05a | 8.2 ± 0.32 | 29.8 ± 1.21a | 0.3 ± 0.02a | 28.7 ± 0.96a | 0.8 ± 0.04a |
| cricket | 1% | 0.0 ± 0.00 | 0.1 ± 0.00a | 0.7 ± 0.01a | 27.7 ± 1.76a | 0.9 ± 0.04ab | 8.1 ± 0.23 | 29.1 ± 1.32ab | 0.3 ± 0.02ab | 26.7 ± 1.17ab | 4.1 ± 0.18ab |
| 2% | 0.0 ± 0.00 | 0.1 ± 0.01ab | 0.7 ± 0.02ab | 25.3 ± 0.63ab | 0.8 ± 0.03bc | 8.1 ± 0.19 | 28.9 ± 0.76ab | 0.4 ± 0.01bc | 26.3 ± 1.01ab | 7.2 ± 0.36bc | |
| 4% | 0.0 ± 0.00 | 0.1 ± 0.00b | 0.6 ± 0.02b | 23.1 ± 0.92b | 0.6 ± 0.04c | 7.6 ± 0.43 | 27.1 ± 1.13b | 0.4 ± 0.03c | 25.5 ± 1.27b | 12.7 ± 1.05c | |
| Lesser | 0% | 0.0 ± 0.00 | 0.1 ± 0.02a | 0.8 ± 0.02a | 23.7 ± 3.46 | 0.4 ± 0.04a | 8.8 ± 1.27 | 34.9 ± 2.40a | 0.4 ± 0.03a | 26.9 ± 2.22 | 1.2 ± 0.11a |
| mealworm | 1% | 0.0 ± 0.00 | 0.1 ± 0.01ab | 0.8 ± 0.02ab | 21.4 ± 1.66 | 0.4 ± 0.02a | 8.4 ± 0.69 | 34.5 ± 1.17ab | 0.4 ± 0.03ab | 27.1 ± 1.55 | 4.4 ± 0.23ab |
| 2% | 0.0 ± 0.00 | 0.1 ± 0.01ab | 0.7 ± 0.02bc | 20.8 ± 2.38 | 0.4 ± 0.03ab | 8.5 ± 0.87 | 33.4 ± 1.68ab | 0.4 ± 0.04b | 26.0 ± 1.59 | 7.2 ± 0.26bc | |
| 4% | 0.0 ± 0.01 | 0.1 ± 0.01b | 0.7 ± 0.03c | 21.0 ± 4.29 | 0.3 ± 0.02b | 8.7 ± 1.79 | 31.4 ± 1.94b | 0.4 ± 0.07b | 23.9 ± 1.42 | 10.9 ± 3.04c | |
| Black | 0% | 1.1 ± 0.04a | 47.8 ± 1.20a | 9.2 ± 0.23a | 13.7 ± 0.35a | 2.5 ± 0.12a | 2.3 ± 0.12a | 11.7 ± 0.61a | 0.5 ± 0.11 | 9.1 ± 0.84 | 0.5 ± 0.14a |
| soldier | 1% | 1.0 ± 0.05ab | 44.4 ± 1.98ab | 8.9 ± 0.40a | 13.4 ± 0.57ab | 2.2 ± 0.15ab | 2.6 ± 0.27ab | 12.2 ± 0.54ab | 0.5 ± 0.11 | 9.7 ± 0.71 | 3.3 ± 0.46ab |
| fly | 2% | 0.9 ± 0.05bc | 43.2 ± 3.26ab | 8.4 ± 0.49ab | 12.8 ± 0.48ab | 1.9 ± 0.13bc | 2.6 ± 0.30ab | 12.5 ± 0.86ab | 0.5 ± 0.19 | 10.0 ± 1.21 | 5.5 ± 0.59bc |
| 4% | 0.8 ± 0.07c | 38.9 ± 2.52b | 7.7 ± 0.34b | 12.7 ± 0.37b | 1.5 ± 0.07c | 2.8 ± 0.24b | 13.4 ± 1.05b | 0.4 ± 0.10 | 10.4 ± 0.83 | 9.7 ± 0.87c |
†Fatty acids < 0.5% of total fatty acids are excluded.
In general, the FA profiles of the three insect species followed the changes in the dietary fatty acid profiles; higher inclusion levels of flaxseed oil increased ALA levels (R 2 = 0.85–0.97) and decreased the relative abundance of the other FAs (Table 3, 4). Two noticeable exceptions were the increased level of C18:1n9 (oleic acid) and the numerically increased level of LA in black soldier flies provided with higher dietary levels of flaxseed oil. For each unit increase of flaxseed oil inclusion, the ALA concentration in the TFA rose between 2.3% and 2.7% for all three species. When the effect of treatment was excluded, species specific differences in fatty acid profile were apparent. Black soldier fly larvae had higher C12:0 concentrations (44 vs. < 0.1% of TFA; P < 0.001) and lower ALA concentrations (4.8% vs. 5.9% of TFA; P = 0.004) than both the lesser mealworms and the house crickets. The C18:1n9 concentration was highest (P < 0.001) in the lesser mealworms (34% of TFA), followed by house crickets (28% of TFA).
Table 4.
Proportions of saturated fatty acids (SFA), mono‐unsaturated fatty acids (MUFA), poly‐unsaturated fatty acids (PUFA), total omega 3 fatty acids, total omega 6 fatty acids, and their ratio in experimental diets, house crickets, lesser mealworms, and black soldier flies (mean ± SD; n = 6). Different letters in superscript in the same column indicate significant differences (Kruskal–Wallis test followed by Dunn–Bonferroni post hoc test; P < 0.05)
| Flaxseed oil | SFA | MUFA | PUFA | n‐3 | n‐6 | n‐6/n‐3 | |
|---|---|---|---|---|---|---|---|
| Diet | 0% | 29.1 ± 0.30a | 31.5 ± 0.12a | 38.3 ± 0.31a | 3.0 ± 0.05a | 35.0 ± 0.29a | 11.8 ± 0.22a |
| 1% | 24.4 ± 0.26ab | 29.2 ± 0.12a | 45.5 ± 0.24ab | 14.2 ± 0.21ab | 31.1 ± 0.30ab | 2.2 ± 0.05ab | |
| 2% | 22.6 ± 1.42bc | 28.5 ± 1.92ab | 48.0 ± 3.38bc | 22.2 ± 1.48bc | 25.6 ± 4.82bc | 1.2 ± 0.27bc | |
| 4% | 18.7 ± 0.39c | 25.9 ± 0.24b | 54.9 ± 0.56c | 30.5 ± 0.85c | 24.2 ± 0.34c | 0.8 ± 0.03c | |
| House cricket | 0% | 37.3 ± 0.35a | 31.5 ± 1.25a | 29.8 ± 0.97a | 0.8 ± 0.04a | 28.8 ± 0.96a | 36.2 ± 1.32a |
| 1% | 37.0 ± 1.96a | 30.6 ± 1.40ab | 31.0 ± 1.22ab | 4.1 ± 0.18ab | 26.8 ± 1.17ab | 6.6 ± 0.38ab | |
| 2% | 34.6 ± 0.66ab | 30.4 ± 0.76ab | 33.7 ± 1.31bc | 7.2 ± 0.36bc | 26.4 ± 1.01ab | 3.7 ± 0.12bc | |
| 4% | 31.9 ± 1.36b | 28.4 ± 1.17b | 38.4 ± 2.23c | 12.7 ± 1.05c | 25.6 ± 1.27b | 2.0 ± 0.09c | |
| Lesser mealworm | 0% | 34.0 ± 4.68 | 36.0 ± 2.48a | 28.6 ± 2.30a | 1.2 ± 0.11a | 27.0 ± 2.22 | 21.7 ± 0.44a |
| 1% | 31.2 ± 2.27 | 35.6 ± 1.19ab | 31.9 ± 1.35ab | 4.4 ± 0.23ab | 27.2 ± 1.54 | 6.3 ± 0.63ab | |
| 2% | 30.7 ± 3.24 | 34.5 ± 1.73ab | 33.6 ± 1.74b | 7.2 ± 0.26bc | 26.1 ± 1.59 | 3.6 ± 0.19bc | |
| 4% | 31.0 ± 6.15 | 32.5 ± 2.02b | 35.2 ± 4.25b | 10.9 ± 3.04c | 24.0 ± 1.42 | 2.4 ± 1.03c | |
| Black soldier fly | 0% | 74.4 ± 1.04a | 15.1 ± 0.47 | 10.1 ± 0.72a | 0.5 ± 0.14a | 9.1 ± 0.84 | 18.3 ± 5.59a |
| 1% | 70.8 ± 1.60ab | 15.3 ± 0.64 | 13.3 ± 1.27ab | 3.3 ± 0.46ab | 9.7 ± 0.71 | 3.0 ± 0.24ab | |
| 2% | 68.4 ± 2.91b | 15.3 ± 1.18 | 15.8 ± 1.84bc | 5.5 ± 0.59bc | 10.0 ± 1.21 | 1.8 ± 0.11bc | |
| 4% | 63.5 ± 2.76b | 15.6 ± 1.21 | 20.3 ± 1.63c | 9.7 ± 0.87c | 10.4 ± 0.83 | 1.1 ± 0.02c |
Furthermore, the house crickets contained small amounts of EPA (0.2% of TFA) at all four treatment levels, whereas this FA was not detected in the two other species. No DHA was detected in any of the three species.
The increased dietary ALA levels increased insect PUFA content and strongly decreased their n‐6/n‐3 ratios (Fig. 1). An inclusion of 2% of flaxseed oil decreased this ratio from 36 to 4 in house crickets, and from 22 to 4 in lesser mealworms. Addition of 1% of flaxseed oil sufficed to decrease the n‐6/n‐3 ratio from 18 to 3 in black soldier flies.
Figure 1.
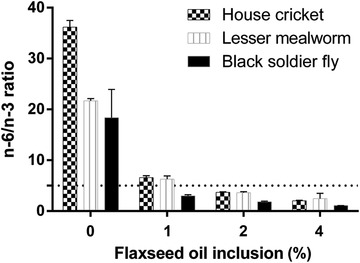
Omega 6/omega 3 (n‐6/n‐3) fatty acid ratio of three insect species provided with a control diet (0%) or a diet enriched with either 1%, 2%, or 4% of flaxseed oil (n = 6). The dotted line indicates the maximum ratio considered suitable for human health.
Discussion
The three insect species in this study had high n‐6/n‐3 ratios (18–36) when reared on their cereal‐based control diet, as is common in insect production systems (van Broekhoven et al., 2015; Oonincx et al., 2015b). These ratios are even higher than in current Western diets (∼15–17), which are already considered excessive and potentially detrimental (Simopoulos, 2002). Addition of 1% of flaxseed oil to the insect diets strongly lowered this ratio (house crickets: 6.6, lesser mealworms: 6.3, BSF larvae: 3.0) so that it approaches the ratio recommended for human health (∼5) (Gerster, 1998; Kouba & Mourot, 2011). Higher inclusion levels further lowered the n‐6/n‐3 ratios.
The n‐3 content of the diet, in the form of ALA, was increased via the addition of flaxseed oil. This did not affect survival, development, or weight in the three species in the current study. Contrarily, Starčević et al. (2017) reported reduced survival (from 37% to 24%) in Jamaican field crickets (Gryllus assimilis) when flaxseed oil inclusion was increased from 3% to 5% during a period of 50 d. Whether that cricket species responds differently to flaxseed oil than house crickets or that another factor caused low survival is unclear. Flaxseed contains antinutritional factors including linatin and phytic acid, which can inhibit growth in broilers (Lee et al., 1991; Bond et al., 1997; Treviño et al., 2000; Nguyen et al., 2003; Anjum et al., 2013) and pigs (Juárez et al., 2011). However, growth inhibition is not always apparent in these species (Matthews et al., 2000; Crespo & Esteve‐Garcia, 2002) and the responsible antinutritional factors seem to be retained in the seed cake after pressing (Lee et al., 1991).
Providing flaxseed (oil) to pigs (Matthews et al., 2000; Juárez et al., 2011; Turner et al., 2014) and broilers (Crespo & Esteve‐Garcia, 2002; Nguyen et al., 2003) increases their ALA content, as well as their EPA concentration. In the current, study trace amounts of EPA were found in the house crickets, whereas none was detected in the lesser mealworms and black soldier flies. The EPA traces (0.2% of TFA) found in the house crickets likely came from the feed which contained small amounts of EPA (0.2% – 0.5% of TFA; Table 1). Although previous studies suggest that house crickets can enzymatically synthesize EPA from ALA (Jurenka et al., 1988; Blomquist et al., 1991; Tzompa‐Sosa et al., 2014) no evidence for this was found in the current study. Perhaps house crickets only synthesize EPA de novo from ALA in specific tissues and in low amounts, thereby avoiding detection in the current study which determined the FA profile of the whole body. Whether such amounts are a nutritionally relevant contribution to TFA for people or animals consuming these crickets seems doubtful.
The data do indicate that the house crickets selectively retained EPA; dilution of the relative concentration of EPA in the feed resulted in constant relative concentrations in the house crickets. This concurs with published data on a large variety of insects, including Diptera, Orthoptera, Lepidoptera, and Coleoptera, which selectively retain EPA (St‐Hilaire et al., 2007; Finke, 2015; Spranghers et al., 2016; Hussein et al., 2017; Starčević et al., 2017). These studies also indicate that DHA is not retained in insects, which explains its absence in the insects in the current study.
When DHA is detected in insects, this is often due to feed residing in their gastrointestinal tract at the moment of sampling; their gutload. This gutload thus affects analyzed nutrient profiles. Finke (2003) reports a gutload content of approximately 5% of the total dry matter for adult house crickets and yellow mealworms. At the 4% inclusion level in the current study, this would result in an overestimation of approximately 1% of TFA.
In general, insects obtain fatty acids via absorption of dietary lipids through their midgut epithelium or generate them from sugars in their enterocytes (Chapman et al., 2013). The black soldier fly seems to synthesize C12:0 (Lauric acid) de novo from sugars as can be deduced from mass balance calculations from a previous dietary study (Oonincx et al., 2015b). Lauric acid is the most abundant fatty acid in most studies on black soldier flies (Finke & Oonincx, 2017) and the current study is no exception. This feature seems unique to the black soldier fly and accounts for the major difference in fatty acid profile compared to both the house cricket and the lesser mealworm. Contrary to the latter two species, higher inclusion levels of flaxseed oil increased stearic and oleic acid concentrations, and numerically increased LA concentrations in black soldier fly larvae. The increased abundance of these 18 carbon fatty acids at higher flax seed oil inclusion levels might be due to microbial biohydrogenation, as is known to occur in sheep and cattle (Jenkins et al., 2008).
Comparative data for the fatty acid profile of the lesser mealworm are limited (van Broekhoven et al., 2015), but follows the same general trend as found in the current study. The most abundant fatty acids in lesser mealworms are C16:0 (palmitic acid), stearic acid, oleic acid, and LA. The same fatty acids are reported to be the most abundant in commercially produced house crickets (Finke, 2002; Collavo et al., 2005; Oonincx et al., 2015a,b), which is in general agreement with the data in this study. In most of these studies the dietary n‐3 content is low, leading to a low n‐3 content and high n‐6/n‐3 ratio in these house crickets. Provision of ALA‐rich Chia seeds [Salvia hispanica (L.)] was tested during 10 d in Jamaican field crickets and giant mealworms [Zophobas atratus (Fabricius)] (Komprda et al., 2013). These seeds increased levels of ALA in both species, but the effect was more pronounced in the Jamaican field crickets. In the current study, ALA increased in both the lesser mealworms and the house crickets to a similar extent. Perhaps differences in fatty acid accumulation and synthesis between Jamaican and house crickets or between giant and lesser mealworms explain this difference. Alternatively, the difference in the duration of the experiment (10 d vs. the entire larval/nymphal stage) might be the reason for differences in the accumulation pattern. Future studies could determine to which extent shorter term provision of enriched diets is effective and whether this would be more cost‐effective than long‐term provision. This could lead to the incorporation of so‐called finishing diets, as commonly used to optimize the nutritional value of conventional production animals.
In conclusion, the n‐3 fatty acid content of insects produced on standard commercial diets is low and their n‐6/n‐3 ratio is excessively high. A desirable n‐6/n‐3 ratio can be obtained by enriching standard diets with 1%–2% of flaxseed oil in the three investigated species. If insects are to be consumed directly, the provision of an enriched diet is strongly recommended. Further studies should determine whether provision of n‐3 enrichment at a later stage of production is effective and potentially more efficient than long‐term provision.
Disclosure
The authors declare that they have no conflict of interest.
Acknowledgments
The authors kindly acknowledge Rita Boerrigter‐Eenling for conducting the fatty acid analysis and aiding with interpreting the data. Kreca VOF, now Proti‐Farm, is kindly acknowledged for providing first instar larvae of the lesser mealworm. Lastly, Guido Bosch is kindly acknowledged for his careful review of this manuscript. Funding for this study was provided by the Dutch ministry of Economic Affairs via the In2food Project.
References
- Anderson, B.M. and Ma, D.W. (2009) Are all n‐3 polyunsaturated fatty acids created equal? Lipids in Health and Disease, 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum, F.M. , Haider, M.F. , Khan, M.I. , Sohaib, M. and Arshad, M.S. (2013) Impact of extruded flaxseed meal supplemented diet on growth performance, oxidative stability and quality of broiler meat and meat products. Lipids in Health and Disease, 12, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists ., & Helrich, K. (1990) Official methods of analysis of the Association of Official Analytical Chemists. Arlington, VA: The Association. [Google Scholar]
- Antruejo, A. , Azcona, J. , Garcia, P. , Gallinger, C. , Rosmini, M. , Ayerza, R . et al (2011) Omega‐3 enriched egg production: the effect of α‐linolenic ω‐3 fatty acid sources on laying hen performance and yolk lipid content and fatty acid composition. British Poultry Science, 52, 750–760. [DOI] [PubMed] [Google Scholar]
- Bagga, D. , Wang, L. , Farias‐Eisner, R. , Glaspy, J.A. and Reddy, S.T. (2003) Differential effects of prostaglandin derived from ω‐6 and ω‐3 polyunsaturated fatty acids on COX‐2 expression and IL‐6 secretion. Proceedings of the National Academy of Sciences USA, 100, 1751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan‐Fonseca, K.B. , Dicke, M. and van Loon, J.J.A. (2017) Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—a review. Journal of Insects as Food and Feed, 3, 105–120. [Google Scholar]
- Bemelmans, W.J. , Broer, J. , Feskens, E.J. , Smit, A.J. , Muskiet, F.A. , Lefrandt, J.D . et al (2002) Effect of an increased intake of α‐linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean alpha‐linolenic enriched groningen dietary intervention (MARGARIN) study. The American Journal of Clinical Nutrition, 75, 221–227. [DOI] [PubMed] [Google Scholar]
- Bjørge, J.D. , Overgaard, J. , Malte, H. , Gianotten, N. and Heckmann, L.H. (2018) Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor . Journal of Insect Physiology, 107, 89–96. [DOI] [PubMed] [Google Scholar]
- Blomquist, G.J. , Borgeson, C.E. and Vundla, M. (1991) Polyunsaturated fatty acids and eicosanoids in insects. Insect Biochemistry, 21, 99–106. [Google Scholar]
- Bond, J. , Julian, R. and Squires, E. (1997) Effect of dietary flaxseed on broiler growth, erythrocyte deformability, and fatty acid composition of erythrocyte membranes. Canadian Journal of Animal Science, 77, 279–286. [Google Scholar]
- Campos, H. , Baylin, A. and Willett, W.C. (2008) α‐Linolenic acid and risk of nonfatal acute myocardial infarction. Circulation, 118, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, R.F. , Simpson, S.J. and Douglas, A.E. (2013) The Insects: Structure and Function. Cambridge: Cambridge University Press. [Google Scholar]
- Collavo, A. , Glew, R.H. , Huang, Y.S. , Chuang, L.T. , Bosse, R. and Paoletti, M.G. (2005) House cricket small‐scale farming Ecological Implications of Minilivestock: Potential of Insects, Rodents, Frogs and Snails (ed. Paoletti M. G.). Science Publishers Inc, Enfield, New Hampshire, USA. [Google Scholar]
- Crespo, N. and Esteve‐Garcia, E. (2002) Dietary linseed oil produces lower abdominal fat deposition but higher de novo fatty acid synthesis in broiler chickens. Poultry Science, 81, 1555–1562. [DOI] [PubMed] [Google Scholar]
- Despins, J.L. and Axtell, R.C. (1995) Feeding behavior and growth of broiler chicks fed larvae of the darkling beetle. Alphitobius diaperinus. Poultry Science, 74, 331–336. [DOI] [PubMed] [Google Scholar]
- Finke, M.D. (2002) Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biology, 21, 269–285. [Google Scholar]
- Finke, M.D. (2003) Gut loading to enhance the nutrient content of insects as food for reptiles: a mathematical approach. Zoo Biology, 22, 147–162. [Google Scholar]
- Finke, M.D. (2015) Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo Biology, 34, 554–564. [DOI] [PubMed] [Google Scholar]
- Finke, M.D. and Oonincx, D.G.A.B. (2017) Nutrient content of insects (eds. van Huis A. & Tomberlin J. K.). Insects as Food and Feed: From Production to Consumption. Wageningen Academic Publishers, Wageningen, the Netherlands. [Google Scholar]
- Folch, J. , Lees, M. and Sloane Stanley, G. (1957) A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509. [PubMed] [Google Scholar]
- Fontaneto, D. , Tommaseo‐Ponzetta, M. , Galli, C. , Risé, P. , Glew, R.H. and Paoletti, M.G. (2011) Differences in fatty acid composition between aquatic and terrestrial insects used as food in human nutrition. Ecology of Food and Nutrition, 50, 351–367. [DOI] [PubMed] [Google Scholar]
- Gerster, H. (1998) Can adults adequately convert α‐linolenic acid (18:3n‐3) to eicosapentaenoic acid (20:5n‐3) and docosahexaenoic acid (22:6n‐3)? International Journal for Vitamin and Nutrition Research, 68, 159–173. [PubMed] [Google Scholar]
- Gladyshev, M.I. , Sushchik, N.N. and Makhutova, O.N. (2013) Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins and other Lipid Mediators, 107, 117–126. [DOI] [PubMed] [Google Scholar]
- Herrero, M. , Wirsenius, S. , Henderson, B. , Rigolot, C. , Thornton, P. , Havlík, P . et al (2015) Livestock and the environment: what have we learned in the past decade? Annual Review of Environment and Resources, 40, 177–202. [Google Scholar]
- Hussein, M. , Pillai, V.V. , Goddard, J.M. , Park, H.G. , Kothapalli, K.S. , Ross, D.A . et al (2017) Sustainable production of housefly (Musca domestica) larvae as a protein‐rich feed ingredient by utilizing cattle manure. PLoS ONE, 12, e0171708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, T. , Wallace, R. , Moate, P. and Mosley, E. (2008) Board‐invited review: recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem 1. Journal of Animal Science, 86, 397–412. [DOI] [PubMed] [Google Scholar]
- Juárez, M. , Dugan, M. , Aldai, N. , Aalhus, J. , Patience, J. , Zijlstra, R . et al (2011) Increasing omega‐3 levels through dietary co‐extruded flaxseed supplementation negatively affects pork palatability. Food Chemistry, 126, 1716–1723. [DOI] [PubMed] [Google Scholar]
- Jurenka, R.A. , Stanley‐Samuelson, D.W. , Loher, W. and Blomquist, G.J. (1988) De novo biosynthesis of arachidonic acid and 5, 11, 14‐eicosatrienoic acid in the cricket Teleogryllus commodus . Biochimica et Biophysica Acta (BBA)—Lipids and Lipid Metabolism, 963, 21–27. [DOI] [PubMed] [Google Scholar]
- Komprda, T. , Zorníková, G. , Rozíková, V. , Borkovcová, M. and Przywarová, A. (2013) The effect of dietary Salvia hispanica seed on the content of n‐3 long‐chain polyunsaturated fatty acids in tissues of selected animal species, including edible insects. Journal of Food Composition and Analysis, 32, 36–43. [Google Scholar]
- Kouba, M. and Mourot, J. (2011) A review of nutritional effects on fat composition of animal products with special emphasis on n‐3 polyunsaturated fatty acids. Biochimie, 93, 13–17. [DOI] [PubMed] [Google Scholar]
- Lee, K.‐H. , Olomu, J. and Sim, J.S. (1991) Live performance, carcass yield, protein and energy retention of broiler chickens fed canola and flax full‐fat seeds and the restored mixtures of meal and oil. Canadian Journal of Animal Science, 71, 897–903. [Google Scholar]
- Mantzioris, E. , Cleland, L.G. , Gibson, R.A. , Neumann, M.A. , Demasi, M. and James, M.J. (2000) Biochemical effects of a diet containing foods enriched with n‐3 fatty acids. The American Journal of Clinical Nutrition, 72, 42–48. [DOI] [PubMed] [Google Scholar]
- Matthews, K.R. , Homer, D.B. , Thies, F. and Calder, P.C. (2000) Effect of whole linseed (Linum usitatissimum) in the diet of finishing pigs on growth performance and on the quality and fatty acid composition of various tissues. British Journal of Nutrition, 83, 637–643. [DOI] [PubMed] [Google Scholar]
- Melvin, K. and Boyd, E.S. (2010) Paleolithic nutrition. Nutrition in Clinical Practice, 25, 594–602. [DOI] [PubMed] [Google Scholar]
- Nguyen, C. , Smulikowska, S. and Mieczkowska, A. (2003) Effect of linseed and rapeseed or linseed and rapeseed oil on performance, slaughter yield and fatty acid deposition in edible parts of the carcass in broiler chickens. Journal of Animal and Feed Sciences, 12, 271–288. [Google Scholar]
- Omar, K.A. , Shan, L. , Wang, Y.L. and Wang, X. (2010) Stabilizing flaxseed oil with individual antioxidants and their mixtures. European Journal of Lipid Science and Technology, 112, 1003–1011. [Google Scholar]
- Oonincx, D. , van Leeuwen, J. , Hendriks, W. and van der Poel, A. (2015a) The diet of free‐roaming Australian central bearded dragons (Pogona vitticeps). Zoo Biology, 34, 271–277. [DOI] [PubMed] [Google Scholar]
- Oonincx, D.G.A.B. , van Broekhoven, S. , van Huis, A. and van Loon, J.J.A. (2015b) Feed conversion, survival and development, and composition of four insect species on diets composed of food by‐products. PLoS ONE, 10, e0144601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonincx, D.G.A.B. , van Keulen, P. , Finke, M.D. , Baines, F.M. , Vermeulen, M. and Bosch, G. (2018) Evidence of vitamin D synthesis in insects exposed to UVb light. Scientific Reports, 8, 10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J.G. and Stone, N.J. (2006) Antiatherosclerotic and antithrombotic effects of omega‐3 fatty acids. American Journal of Cardiology, 98, 39–49. [DOI] [PubMed] [Google Scholar]
- Sans, P. and Combris, P. (2015) World meat consumption patterns: An overview of the last fifty years (1961–2011). Meat Science, 109, 106–111. [DOI] [PubMed] [Google Scholar]
- Simopoulos, A.P. (1999) Evolutionary aspects of omega‐3 fatty acids in the food supply. Prostaglandins Leukotrienes and Essential Fatty Acids, 60, 421–429. [DOI] [PubMed] [Google Scholar]
- Simopoulos, A.P. (2002) The importance of the ratio of omega‐6/omega‐3 essential fatty acids. Biomedicine and Pharmacotherapy, 56, 365–379. [DOI] [PubMed] [Google Scholar]
- Simopoulos, A.P. (2009) Omega‐6/omega‐3 essential fatty acids: biological effects. World Review of Nutrition and Dietetics, 99, 1–16. [DOI] [PubMed] [Google Scholar]
- Singh, R.B. , Dubnov, G. , Niaz, M.A. , Ghosh, S. , Singh, R. , Rastogi, S.S . et al (2002) Effect of an Indo‐Mediterranean diet on progression of coronary artery disease in high risk patients (Indo‐Mediterranean diet heart study): a randomised single‐blind trial. The Lancet, 360, 1455–1461. [DOI] [PubMed] [Google Scholar]
- Spranghers, T. , Ottoboni, M. , Klootwijk, C. , Ovyn, A. , Deboosere, S. , DeMeulenaer, B . et al (2016) Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. Journal of the Science of Food and Agriculture, 97, 2594–2600. [DOI] [PubMed] [Google Scholar]
- St‐Hilaire, S. , Cranfill, K. , Mcguire, M.A. , Mosley, E.E. , Tomberlin, J.K. , Newton, L . et al (2007) Fish offal recycling by the black soldier fly produces a foodstuff high in omega‐3 fatty acids. Journal of the World Aquaculture Society, 38, 309–313. [Google Scholar]
- Starčević, K. , Gavrilović, A. , Gottstein, Ž. and Mašek, T. (2017) Influence of substitution of sunflower oil by different oils on the growth, survival rate and fatty acid composition of Jamaican field cricket (Gryllus assimilis). Animal Feed Science and Technology, 228, 66–71. [Google Scholar]
- Thais, S.R. , Sophie, V.D.S. , Catherine, D. , Evert, T. , Ronald, E.V.K. , Nele, J . et al (2013) Endogenous pathway for LC‐PUFA synthesis DHA, EPA and ARA by enzymatic desaturation and chain elongation steps, PloS one 8, no. 6 (2013): e68000.
- Tokudome, S. , Nagaya, T. , Okuyama, H. , Tokudome, Y. , Imaeda, N. , Kitagawa, I . et al (2000) Japanese versus Mediterranean diets and cancer. Asian Pacific Journal of Cancer Prevention, 1, 61–66. [PubMed] [Google Scholar]
- Trautwein, E.A. (2001) n‐3 Fatty acids—physiological and technical aspects for their use in food. European Journal of Lipid Science and Technology, 103, 45–55. [Google Scholar]
- Treviño, J. , Rodríguez, M.L. , Ortiz, L.T. , Rebolé, A. and Alzueta, C. (2000) Protein quality of linseed for growing broiler chicks. Animal Feed Science and Technology, 84, 155–166. [Google Scholar]
- Turner, T. , Mapiye, C. , Aalhus, J. , Beaulieu, A. , Patience, J. , Zijlstra, R . et al (2014) Flaxseed fed pork: n‐3 fatty acid enrichment and contribution to dietary recommendations. Meat Science, 96, 541–547. [DOI] [PubMed] [Google Scholar]
- Tzompa‐Sosa, D.A. , Yi, L. , van Valenberg, H.J. , van Boekel, M.A. and Lakemond, C.M. (2014) Insect lipid profile: aqueous versus organic solvent‐based extraction methods. Food Research International, 62, 1087–1094. [Google Scholar]
- van Broekhoven, S. , Oonincx, D.G.A.B. , van Huis, A. and van Loon, J.J.A. (2015) Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by‐products. Journal of Insect Physiology, 73, 1–10. [DOI] [PubMed] [Google Scholar]
- van Huis, A. and Oonincx, D.G.A.B. (2017) The environmental sustainability of insects as food and feed. A review. Agronomy for Sustainable Development, 37, 43. [Google Scholar]
- Zhao, G. , Etherton, T.D. , Martin, K.R. , West, S.G. , Gillies, P.J. and Kris‐Etherton, P.M. (2004) Dietary α‐linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. The Journal of Nutrition, 134, 2991–2997. [DOI] [PubMed] [Google Scholar]


