Abstract
Purpose
To analyse characteristics from the SMR to explore the risk factors for visual acuity (VA) below ≤ 35 letters of the Early Treatment Diabetic Retinopathy Study (ETDRS) due to nAMD during a two‐year follow‐up.
Methods
This study evaluates 6142 treatment‐naïve eyes, with focus on a subgroup of 780 eyes with final VA outcome of ≤ 35 letters, regarding differences of baseline characteristics, change of VA, number of injections and choice of drug to predict visual outcome.
Results
Patients with final VA ≤ 35 letters were older; p < 0.0001, and received fewer injections, 6.2 ± 3.8 vs. 8.7 ± 5.4; p < 0.00001. Only 4% of all patients with ≥ 70 letters baseline VA decreased to a final VA of ≤ 35 letters. The two groups with a final VA of ≤ 35 letters and VA > 35 letters presented the following baseline lesion locations; p = 0.001; 61% vs. 57% subfoveal, 18% vs. 21% juxtafoveal and 4% vs. 6% extrafoveal. Lesion size, in the group with final VA ≤ 35 letters, was 2805 ± 2093 μm vs. 2440 ± 1637 μm in the group with a VA of > 35 letters; p = 0.005. A logistic regression analysis including baseline VA, best‐ or worse‐seeing eye, age, membrane size, membrane location, symptom duration showed VA; p = < 0.0001, best‐ or worse‐seeing eye; p = 0.026, age; p = < 0.0001, and membrane size; p = 0.002 to predict a decline of VA within 2 years.
Conclusions
In eyes treated for wet AMD and studied for 2 years, 12.7% of eyes declined to a final VA of ≤ 35 letters. Visual acuity, worse‐seeing eye treated, age and membrane size turned out as the baseline characteristics that had significantly influenced visual decline to ≤ 35 letters during the two‐year follow‐up.
Keywords: aflibercept, bevacizumab, neovascular age‐related macular degeneration, ranibizumab, Swedish Macula Registry
Introduction
The era of intravitreal treatment of neovascular age‐related macular degeneration (nAMD) has opened up to new medical and socioeconomic challenges. The difficulty is to balance the financial burden with patient benefits such as visual gain. The global prevalence of AMD is expected to rise to 288 million by 2040, which forces the question of when, who and how to treat this group of patients (Wong et al. 2014). In clinical practice, three different drugs are administered to treat nAMD. But only two, ranibizumab (Lucentis®; Genentech) and aflibercept (Eylea®; Bayer Pharma AG), are officially approved by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA). Bevacizumab (Avastin®; Genentech) is used worldwide as an off‐label drug. Monthly dosing as in the pivotal studies ANCHOR and MARINA with ranibizumab (Rosenfeld et al. 2006; Brown et al. 2009) is hardly manageable in a clinical setting. It has resulted in the development of different regimens such as pro re nata, treat and extend, observe and plan to conquer the amount of injections, and clinical visits. Many attempts have been made to define the predictors for the best visual treatment outcome and to identify patients in need of more or less frequent injection intervals. Several studies have identified baseline visual acuity (VA), lesion size and age as baseline predictors for the future visual outcome (Finger et al. 2014; Lövestam Adrian et al. 2019). Real‐world data have shown the importance of continuous treatment to achieve and maintain the visual gain (Holz et al. 2015). Despite all efforts, undertreatment is still a widespread problem.
However, even if treated on a regular basis, some patients lose vision for example due to fibrosis or macular atrophy. During a two‐year period of treatment, CATT study patients developed macular atrophy in 18.3% of patients (Grunwald et al. 2014).
In Sweden, we have a nationwide database of patients treated for nAMD, the Swedish Macula Registry (SMR) (Svenska Makula Registret 2018). Today, this Registry covers 84% of all patients treated for nAMD (Westborg et al. 2017).
The purpose of the present study was to analyse patient data from the registry to describe a subgroup of patients with vision loss during a two‐year follow‐up and possibly define characteristics to predict visual loss below the treatment criteria. We evaluated possible differences of baseline characteristics, number of injections and choice of drug to explore visual decline during a two‐year period with information collected from the SMR.
Methods
Population
Practicing ophthalmologists at 39 Swedish clinics enter the patient data into the Swedish Macula Registry when they decide to start intravitreal treatment for newly diagnosed neovascular AMD. Patient consent is obtained before entering data into the registry. Since 2008, the Swedish Macula Registry is a web‐based registry including patient data from 2007 onwards. It covers 84% of all patients in Sweden treated for nAMD. This study evaluates treatment‐naïve patients who were registered during the period from 1 January 2013, to 31 December 2014. The follow‐up period is 2 years. The study protocol was approved by the institutional review board in Lund and conducted in conformity with the Helsinki Declaration.
The baseline visit was the first visit at which wet AMD was diagnosed and treatment was prescribed. Data were obtained on patients’ sex, age, presenting symptoms (self‐reported symptoms connected to wet AMD that caused the patient to consult an ophthalmologist, that is metamorphopsia, reduced VA, problems with reading), macular lesion type and location, visual acuity (VA) of the treated eye measured with the Snellen and/or ETDRS charts, best‐ or worse‐seeing eye treated, total number of received injections. Where only VA had been measured using the Snellen chart, it was converted into ETDRS VA, according to Gregori et al. 2010. ETDRS VA was originally registered in around 80% of the visits at baseline and in 87% at 1‐year follow‐up. Visual acuity of hand movements and amaurosis were converted into ETDRS 0.001. The baseline characteristics described were included in a logistic regression analysis to predict the outcome of low VA within 2 years follow‐up.
Statistical analysis
Baseline characteristics and study outcomes were summarized for all eyes. Data were described using mean, median, 1st and 3rd quartile, standard deviation and range for continuous variables, and frequency counts and percentages for categorical variables. Comparisons between two groups for VA were based on Student's t‐test. Wilcoxon rank sum test was used when nonparametric values were analysed. Analysis of variance (ANOVA) was used to compare the number of injections between outcome groups’ gain in vision, and if statistically significant, pairwise comparisons between the groups were made using Student's t‐test. Associations between VA outcome and age, sex, membrane type and location, best‐ or worse‐seeing eye, symptom duration, baseline VA and number of injections were evaluated using logistic regression analysis. Statistical significance was assumed at the p‐value < 0.05 level.
Results
Baseline characteristics
We included a total of 6142 treatment‐naïve eyes at baseline, from the Swedish Macula Registry. The focus was on a subgroup of 780 eyes (12.7%) of 774 patients (in six patients both eyes were included) with a visual impairment to Snellen visual acuity (VA) ≤ 0.1 or ≤ 35 letters of the Early Treatment Diabetic Retinopathy Study (ETDRS) during the two‐year follow‐up. In the group with a retained VA of > 35 letters during the follow‐up period, the mean age was 78.7 ± 8.1 years, compared to 80.5 ± 6.8 years in the subgroup ≤ 35 letters; p < 0.0001. The sex distribution was similar, with 66% and 64% females, respectively; p = 0.8 (Table 1).
Table 1.
Baseline characteristics and choice of drug of the group with final visual acuity (VA) > 35 letters compared with the group with final VA ≤ 35 letters
| Baseline characteristics | Both groups | Final VA > 35 letters | Final VA ≤ 35 letters | p |
|---|---|---|---|---|
| No. of eyes | 6142 | 5362 | 780 | |
| Sex (%) | ||||
| Female | 65 | 66 | 64 | 0.8 |
| Mean age, years (SD) | 78.9 (7.9) | 78.7 (8.1) | 80.5 (6.8) | <0.0001 |
| Mean BCVA letters (SD) | 57.5 (15.3) | 58.1 (15.8) | 53.5 (10.6) | <0.0001 |
| Best‐/worse‐seeing eye, n (%) | ||||
| Worse‐seeing eye | 3194 (52) | 2735 (51) | 445 (57) | 0.007 |
| Lesion type, n (%) | ||||
| Minimally classic | 477 (8) | 420 (8) | 57 (7) | 0.24 |
| Predominantly classic | 1292 (21) | 1113 (21) | 179 (23) | |
| Occult | 2127 (35) | 1882 (35) | 245 (31) | |
| PCV | 178 (3) | 154 (3) | 24 (3) | |
| RAP | 849 (14) | 755 (14) | 94 (12) | |
| Unknown | 1219 (20) | 1038 (19) | 181 (24) | |
| Mean lesion size, n (% of total eyes) | 2245 (37) | 1964 (37) | 281 (36) | |
| Mean lesion size, μm (SD) | 2485.5 (1704.7) | 2439.7 (1637.3) | 2805.5 (2092.7) | 0.005 |
| Lesion location, n (%) | 0.001 | |||
| Subfoveal | 3525 (57) | 3050 (57) | 475 (61) | 0.034 |
| Juxtafoveal | 1289 (21) | 1150 (21) | 139 (18) | 0.02 |
| Extrafoveal | 372 (6) | 342 (6) | 30 (4) | 0.006 |
| Unknown | 956 (16) | 820 (16) | 136 (17) | 0.123 |
| Symptom duration, n (%) | ||||
| 0 – <2 months | 2551 (42) | 2237 (42) | 314 (40) | 0.84 |
| 2 – <4 months | 1488 (24) | 1290 (24) | 198 (25) | |
| 4–6 months | 906 (15) | 790 (15) | 116 (15) | |
| >6 months | 1197 (19) | 1045 (19) | 152 (19) | |
| Choice of drug, n (%) | ||||
| Bevacizumab | 1328 (22) | 1120 (21) | 208 (27) | 0.0002 |
| Bevacizumab/ Ranibizumab | 99 (2) | 93 (2) | 6 (1) | 0.046 |
| Aflibercept | 1911 (31) | 1690 (32) | 221 (28) | 0.073 |
| Aflibercept/ Bevacizumab | 367 (6) | 334 (6) | 33 (4) | 0.028 |
| Aflibercept/ Bevacizumab/Ranibizumab | 32 (1) | 31 (1) | 1 (0) | 0.103 |
| Aflibercept/ Ranibizumab | 949 (15) | 853 (16) | 96 (12) | 0.009 |
| Ranibizumab | 1456 (24) | 1241 (23) | 215 (28) | 0.007 |
PCV, polypoidal choroidal vasculopathy; RAP, retinal angiomatous proliferation.
Angiographic lesion
Lesion type
In 80% of all cases (n = 4923) eyes presented with an angiographic lesion classification in the registry at baseline; minimally classic in 8%, predominantly classic in 21%, 100% occult in 34%, polypoidal choroidal vasculopathy in 3% and retinal angiomatous proliferation in 14%.
In the group with a final VA of > 35 letters, 81% (n = 4324) of the eyes had a registered angiographic lesion type with a corresponding lesion percentage compared to all cases above.
In the group with a final VA of ≤ 35 letters, 76% (n = 599) had a known lesion; minimally classic in 7%, predominantly classic in 23%, 100% occult in 31%, polypoidal choroidal vasculopathy in 3% and retinal angiomatous proliferation in 12%. There was no difference regarding lesion types between the two visual outcome groups.
Lesion location
The Swedish Macula Registry complementary reported the location of lesions related to the fovea. The subgroup with a final VA of > 35 letters showed the following distribution; subfoveal in 57%, juxtafoveal in 21%, extrafoveal in 6%, unknown in 16% compared to the remaining group with worse VA outcome with a subfoveal lesion in 61%, juxtafoveal in 18%, extrafoveal in 4% and unknown in 17%.
The known lesion types location differed significantly; p = 0.001, between the two VA outcome subgroups.
Lesion size
In the group with a final VA of > 35 letters, the lesion size was 2439.7 ± 1637.3 μm in diameter, compared to 2805.5 ± 2092.7 μm in the group with a final VA of ≤ 35 letters; p = 0.005. For the two subgroups, information about lesion size was available in 37% (n = 1964), and 36% (n = 281) of eyes, respectively.
Symptom duration
The total group of 6142 eyes showed similar values of symptom duration before first contact with the healthcare system.
Eyes with a final VA of > 35 letters observed symptoms for 0‐ <2 months in 42% (n = 2237), 2‐ <4 months in 24% (n = 1290), 4–6 months in 15% (n = 790), > 6 months in 19% (n = 1045) prior to the clinical baseline visit. Eyes with final a VA of ≤ 35 letters experienced symptoms for 0‐ <2 months in 40% (n = 314), 2‐ <4 months in 25% (n = 198), 4–6 months in 15% (n = 116), and > 6 months in 19% (n = 152). The symptom duration between the two groups revealed no significant difference; p = 0.84.
Best‐ or worse‐seeing eye
Overall, in 52% the worse‐seeing eye was treated. It was more common that the worse‐seeing eye was treated in eyes that decreased to ≤ 35 letters (57%) than in eyes with final VA > 35 letters (51%); p = 0.007 (Table 1).
A logistic regression analysis including baseline VA, best‐ or worse‐seeing eye, age, membrane size, membrane location, symptom duration showed VA; p = < 0.0001, best‐ or worse‐seeing eye; p = 0.026, age; p = < 0.0001 and membrane size; p = 0.002 to predict a decline of VA within 2 years.
Mean change in visual acuity
Among the better‐seeing group, VA increased from 58.1 ± 15.8 letters at baseline to 60.9 ± 17.4 letters at 2 years. Whereas in the group with a worse final VA, vision decreased from 53.5 ± 10.6 to 25.4 ± 12.6 letters.
Of all 1469 eyes that presented with a VA of ≥ 70 letters at baseline, 4% (n = 61) decreased to ≤ 35 letters. Eyes with a baseline VA of 36–69 letters (n = 4076), 17% (n = 713) dropped to ≤ 35 letters. In the group with a baseline at VA ≤ 35 letters, (n = 597), 1% (n = 6) did not improve.
The mean time to a decrease of VA to ≤ 35 letters was 10.0 ± 6.8 months.
Injections
Overall, eyes were treated with a mean of 8.4 ± 5.3 injections over the 2 years. The mean number of injections in the group with a final VA of > 35 letters vision compared to the group with a final VA of ≤ 35 letters was 8.7 ± 5.4 vs. 6.2 ± 3.8 at 2 years; p < 0.00001.
By the end of year one, eyes with a final VA of > 35 letters had received 5.6 ± 2.6 vs 4.9 ± 2.1 injections in the group with a final VA of ≤ 35 letters; during the second year 3.1 ± 3.3 vs. 1.3 ± 2.2 injections. Eyes with a final VA of ≤ 35 letters were treated with a mean of 5.0 ± 3.0 injections until they dropped to a VA of ≤ 35 letters; followed by a mean of 1.2 ± 2.2 injections until the end of the two‐year follow‐up.
Choice of drug
In the total group of all eyes, 31% (n = 1911) received aflibercept, 24% (n = 1456) ranibizumab and 22% (n = 1328) bevacizumab; the remaining eyes were treated with a combination of two or three drugs named above. When we look at the total number of injections until a VA of ≤ 35 letters stratified by choice of anti‐VEGF, the mean number of injections is aflibercept 4.9 ± 3, ranibizumab 4.3 ± 2.5 and bevacizumab 4.8 ± 3 (Fig. 1).
Figure 1.
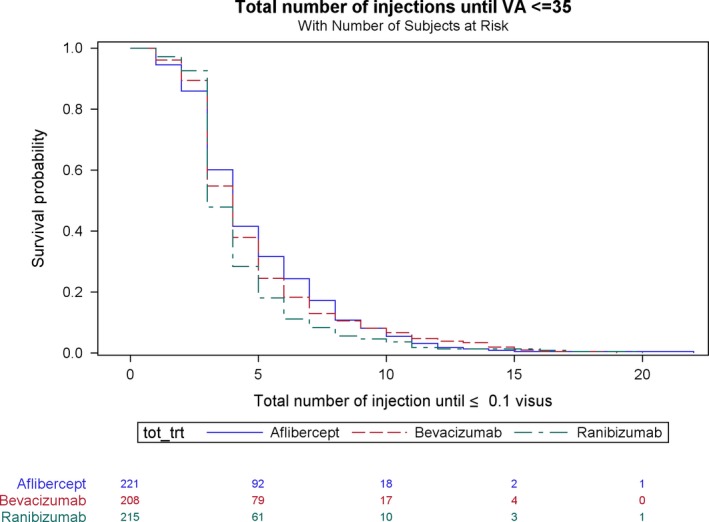
Total number of injections until VA ≤ 35 letters stratified by choice of anti‐VEGF.
In the group ending up with better final VA of > 35 letters, 32% (n = 1690) of eyes received aflibercept, 23% (n = 1241) ranibizumab, 21% (n = 1120) bevacizumab and the remaining 24% (n = 1311) of eyes were treated with two or three different drugs during follow‐up.
The subgroup with a final VA of ≤ 35 letters presented with 28% (n = 221) of eyes treated with aflibercept, 28% (n = 215) ranibizumab, 27% (n = 208) bevacizumab and the remaining 17% (n = 136) of eyes received injections with two or three different drugs. In this cohort, monotherapy from baseline with bevacizumab; p = 0.0002, and ranibizumab; p = 0.007, was significantly preferred compared to the group with final VA > 35 letters (Table 1).
Discussion
In the present study, we could show that almost 13% of the eyes followed for 2 years in the study declined to a VA of ≤ 35 letters, which is often regarded as the lower limit for continuing anti‐VEGF treatment. This figure is in agreement with (Kuroda et al. 2018), but lower than in the CATT study (Martin et al. 2011). The decline in VA was pronounced in the group with a final VA of ≤ 35 letters, with a drop of 28 letters, and we identified baseline VA, age, membrane size and worse‐seeing eye treated as baseline characteristics that significantly influenced visual decline to ≤ 35 letters during the two‐year follow‐up.
Further analysis of baseline VA showed that only 4% of patients with a baseline VA of ≥ 70 letters, compared to 17% with a baseline VA of 36–69 letters, ended up with a final VA of ≤ 35.
Treatment of the worse‐seeing eye was also predictive of a worse visual outcome. This is probably due to the fact that the patient is seeking with earlier symptoms when the better eye is affected.
The group with a VA outcome of > 35 letters increased from 58.1 ± 15.8 letters at baseline to 60.9 ± 17.4 letters at 2 years. These results are worse than in the controlled clinical trials (Brown et al. 2009; Schmidt‐Erfurth et al. 2014) and some real‐world studies (Barthelmes et al. 2018) but in agreement with others (Wolf & Kampik 2013; Holz et al. 2015).
Eyes with final VA of > 35 letters received more injections over the two‐year period compared to the group with a final VA of ≤ 35 letters, 8.7 ± 5.4 vs. 6.2 ± 3.8. Still, both groups are undertreated considering other real‐life studies (Gillies et al. 2015; Holz et al. 2015). In clinical practice, a level of 35 letters is often considered as the lower anti‐VEGF treatment limit. In spite of this, the present study showed that the group with a final VA of ≤ 35 letters received a mean of one more injection after the drop below this level. However, recent studies from the Swedish macular register, INSIGHT study, (Lövestam Adrian et al. 2019) have demonstrated that patients with baseline at VA ≤ 35 letters gained 13 letters after the two‐year follow‐up. Thus, it could make sense to try continuing treatment for a while even when vision is low, and in the present study, only a small group of 6 eyes with a baseline VA of ≤ 35 letters did not improve.
When analysing risk factors for a less favourable outcome we found, in agreement with previous studies, that eyes of patients with a final VA of ≤ 35 letters were older than patients with a final VA of > 35 letters (Nguyen et al. 2019). Despite our expectations, we did not see a difference in symptom duration before the first clinical visit between the group with a final VA of > 35 letters and the one with a final VA of ≤ 35 letters. Other authors pointed out the importance of the short time between diagnosis and treatment (Rasmussen et al. 2015).
When analysing the variable for different macular lesion types, we did not see a difference in lesion type between the group with a final VA outcome of > 35 letters and the group with an outcome of ≤ 35 letters. Previous studies reported better visual outcomes for patients with occult lesions and absence of retinal angiomatous proliferation lesions (Grunwald et al. 2014). The discrepancy to our study might be due to the fact that around 25% of the registered eyes did not have this parameter registered.
The lesion size was larger in the group that ended up with a final VA of ≤ 35 letters than the group with a final VA of > 35 letters. That is consistent with a former study (Brown et al. 2013). In the SMR, the diameter of the lesion size was measured in μm as in another database, the international Fight Retinal Blindness registry, (Nguyen et al. 2019). The overall median baseline lesion size of 2235 μm (1300–3340) (Q1‐Q3) in our study was almost identical compared to a study from the FRB with an overall median baseline lesion size of 2250 μm (1439–3200). Their group assessed the early response to intravitreal anti‐VEGF treatment and its association with a three‐year VA outcome in patients with treatment‐naïve nAMD. Other studies used disc areas or mm² to describe the lesion size (Busbee et al. 2013; Berg et al. 2016).
We could not identify any association between the choice of drug and visual outcome above or ≤ 35 letters. However, we discovered that eyes were preferably treated with bevacizumab and ranibizumab monotherapy in the group with final VA ≤ 35 letters.
As expected, there are limitations in our study. Unfortunately, optical coherence tomography (OCT) values such as central retinal thickness or morphology are not available parameters in the SMR. Thus, we have not been able to evaluate the influence of variables as intra‐ and subretinal fluid or atrophic changes on treatment outcome. However, the strength of our analysis is that the SMR covers 84% of all treated patients for nAMD in Sweden. This leads to a representative, large group of 6142 patients.
In conclusion: About 12.7% of all eyes presented with a final VA of ≤ 35 letters at two‐year follow‐up. These were older, more often treated in the worse‐seeing eye, had lower baseline VA, larger lesions and received fewer injections than eyes of patients with a final VA of > 35 letters.
This study was supported by the Skåne University Hospital (SUS) Research Grants, the Foundation for the Visually Impaired in the County of Malmöhus, and the Swedish Eye Foundation. The authors have full control of all primary data and agree to allow the journal to review the data on request. The authors have no conflicts of interest to disclose. The following clinics have contributed with data to the Swedish Macula Register: (1) Akademiska Uppsala, (2) Borås SÅS, (3) Capio Malmö, (4) Eksjö, (5) Eskilstuna, (6) Falu lasarett, (7) Gävle sjukhus, (8) Helsingborg, (9) Jönköping, (10) Kalmar, (11) Karlskrona, (12) Karlstad, (13) Kristianstad, (14) Landskrona, (15) Linköping, (16) Lund, Skånes Universitetssjukhus, (17) Lycksele, (18) Malmö, Skånes Universitetssjukhus, (19) Norrköping Vrinnevisjukhuset, (20) Nyköping, (21) Skellefteå, (22) Simrishamn, (23) Skövde, (24) Sollefteå, (25) St. Eriks Ögonsjukhus, (26) Stockholms Ögonklinik, (27) Sunderby, (28) Sundsvall– Härnösand, (29) Södersjukhuset, (30) Uddevalla, (31) Umeå NUS, (32) Värnamo, (33) Västervik, (34) Västerås, (35) Växjö, (36) Ängelholm, (37) Örebro, (38) Örnsköldsvik, (39) Östersund.
References
- Barthelmes D, Nguyen V, Daien V et al. (2018): Two‐year outcome of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age‐related macular degeneration. Retina 38: 20–28. [DOI] [PubMed] [Google Scholar]
- Berg K, Hadzalic E, Gjertsen I et al. (2016): Ranibizumab or bevacizumab for neovascular age‐related macular degeneration according to the lucentis compared to avastin study treat‐and‐extend protocol: two‐year results. Ophthalmology 123: 51–59. [DOI] [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP & Ianchulev T (2009): Ranibizumab versus verteporfin photodynamic therapy for neovascular age‐related macular degeneration: Two‐year results of the ANCHOR study. Ophthalmology 116: 57–65.e55. [DOI] [PubMed] [Google Scholar]
- Brown DM, Tuomi L & Shapiro H (2013): Anatomical measures as predictors of visual outcomes in ranibizumab‐treated eyes with neovascular age‐related macular degeneration. Retina 33: 23–34. [DOI] [PubMed] [Google Scholar]
- Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z, Rubio RG & Lai P (2013): Twelve‐month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age‐related macular degeneration. Ophthalmology 120: 1046–1056. [DOI] [PubMed] [Google Scholar]
- Finger RP, Wickremasinghe SS, Baird PN & Guymer RH (2014): Predictors of anti‐VEGF treatment response in neovascular age‐related macular degeneration. Surv Ophthalmol 59: 1–18. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Campain A, Barthelmes D et al. (2015): Long‐term outcomes of treatment of neovascular age‐related macular degeneration data from an observational study. Ophthalmology 122: 1837–1845. [DOI] [PubMed] [Google Scholar]
- Gregori NZ, Feuer W & Rosenfeld PJ (2010): Novel method for analyzing Snellen visual acuity measurements. Retina 30: 1046–1050. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Daniel E, Huang J et al. (2014): Risk of geographic atrophy in the comparison of age‐related macular degeneration treatments trials. Ophthalmology 121: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S et al. (2015): Multi‐country real‐life experience of anti‐vascular endothelial growth factor therapy for wet age‐related macular degeneration. Br J Ophthalmol 99: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Yamashiro K, Ooto S et al. (2018): Macular atrophy and macular morphology in aflibercept‐treated neovascular age.related macular degeneration. Retina 38: 1743–1750. [DOI] [PubMed] [Google Scholar]
- Lövestam Adrian M, Vassilev ZP & Westborg I (2019): Baseline visual acuity as a prognostic factor for visual outcomes in patients treated with aflibercept for wet age‐related macular degeneration: data from the INSIGHT study using the Swedish Macula Register. Acta Ophthalmol 97: 91–98. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maquire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ & CATT Research Group (2011): Ranibizumab and bevacizumab for neovascular age‐related macular degeneration. N Engl J Med 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, Gillies MC, Nguyen V, Daien V, Cohn A, Banerjee G, Arnold J & Fight Retinal Blindness Study Group (2019): Characterisation of poor visual outcomes of neovascular age‐related macular degeneration treated with anti‐vascular endothelial growth factor agents. Ophthalmology 126: 735–742. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Brandi S, Fuchs J, Hansen LH, Lund‐Andersen H, Sander B & Larsen M (2015): Visual outcomes in relation to time to treatment in neovascular age‐related macular degeneration. Acta Ophthalmol 93: 616–620. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS et al. (2006): Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik JF et al. (2014): Intravitreal aflibercept injection for neovascular age‐related macular degeneration: ninety‐six‐week results of the VIEW studies. Ophthalmology 121: 193–201. [DOI] [PubMed] [Google Scholar]
- Svenska Makula Registret (2018): Årsrapport 2017. Available at: http://rcsyd.se/makulareg/wp-content/uploads/sites/2/2018/11/%C3%85rsrapport-2017-Svenska-Makularegistret.pdf. (accessed on 9 June 2019)
- Westborg I, Granstam E, Rosso A, Albrecht S, Karlsson N & Lövestam‐Adrian M (2017): Treatment for neovascular age‐related macular degeneration in Sweden: outcomes at seven years in the Swedish Macula Register. Acta Ophthalmol 95: 787–795. [DOI] [PubMed] [Google Scholar]
- Wolf A & Kampik A (2013): Efficacy of treatment with ranibizumab in patients with wet age‐related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol 252: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WL, Su X1, Li X, Cheung CM, Klein R, Cheng CY & Wong TY (2014): Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta‐analysis. Lancet Glob Health 2: e106–e116. [DOI] [PubMed] [Google Scholar]


