Abstract
Aim
India contributes towards a large part of the worldwide epidemic of diabetes and its associated complications. However, there are limited longitudinal studies available in India to understand the occurrence of diabetes complications over time. This pan‐India longitudinal study was initiated to assess the real‐world outcomes of diabetes across the country.
Methods
The LANDMARC study is the first prospective, multicentre, longitudinal, observational study investigating a large cohort of people with type 2 diabetes mellitus across India over a period of 3 years. The primary objective of this ongoing study is to determine the proportion of people developing macrovascular diabetes complications over the duration of the study (36 months ± 45 days) distributed over seven visits; the secondary objective is to evaluate microvascular diabetes complications, glycaemic control and time‐to‐treatment adaptation or intensification. Overall, 6300 participants (aged 25–60 years) diagnosed with type 2 diabetes for at least 2 years will be included from 450 centres across India. Data will be recorded for baseline demographics, comorbidities, glycaemic measurements, use of anti‐hyperglycaemic medications and any cardiovascular or other diabetes‐related events occurring during the observational study period.
Conclusions
The LANDMARC study is expected to reveal the trends in complications associated with diabetes, treatment strategies used by physicians, and correlation among treatment, control and complications of diabetes within the Indian context. The findings of this study will help to identify the disease burden, emergence of early‐onset complications and dose titration patterns, and eventually develop person‐centred care and facilitate public health agencies to invest appropriate resources in the management of diabetes. (Trial Registration No: CTRI/2017/05/008452).
Introduction
Worldwide, there is an increase in the prevalence of type 2 diabetes mellitus, probably due to an ageing population, economic development and increased urbanization, all of which lead to more sedentary lifestyles 1. Diabetes increases overall morbidity and mortality due to several complications that develop during the course of the disease 2. Diabetic nephropathy, neuropathy and retinopathy are the main microvascular complications induced by chronic hyperglycaemia 3. In 2017, ~ 425 million people aged between 20 and 79 years had diabetes, which contributed to ~ 11% of global all‐cause mortality among this group. If these trends continue, 629 million people in this age group will be diagnosed with diabetes by 2045 1.
India ranked second with ~ 72.9 million people with diabetes in 2017, and it is estimated that by 2045, ~ 134.3 million people in India will be diagnosed with diabetes 1. The A1chieve study, which included 20 554 persons with type 2 diabetes mellitus from India, reported that the mean HbA1c was 77 mmol/mol (9.2%), indicating poor glycaemic control 4. Mortality due to diabetes in India was ~ 1 million in 2017; the highest in Southeast Asia 1. These data clearly show a marked increase in the prevalence of diabetes in India, and the significant overall morbidity and mortality associated with it.
Several population‐based studies have been conducted in different regions of India to assess the prevalence and identify the complications of diabetes 5, 6. The Indian Council of Medical Research–India Diabetes (ICMR–INDIAB), an ongoing nationwide cross‐sectional study, aims to assess the prevalence of diabetes in urban and rural settings in 28 states of India. In the 15 sampled states, prevalence of diabetes ranged from 4.3% to 10% 7. Data from the A1chieve study indicated that in India, neuropathy (with a prevalence of nearly 25%) was the most common complication, followed by cardiovascular (~ 24%), renal (~ 21%) and eye (~ 17%) complications 4. In a cross‐sectional study performed in northwest India, among 11 157 persons with diabetes, retinopathy was diagnosed in ~ 33%, nephropathy in 30.2%, peripheral neuropathy in 26.8%, coronary heart disease in 25.8% and peripheral vascular disease in 28% 8.
Although several cross‐sectional studies have established the prevalence of diabetes in India, longitudinal studies to understand the development of diabetes complications over time and to explore their regional occurrence are limited. The LongitudinAl Nationwide stuDy on Management And Real‐world outComes of diabetes in India (LANDMARC) is the first prospective, observational study to investigate macrovascular and microvascular complications, glycaemic control and time‐to‐treatment adaptation in people with type 2 diabetes mellitus over a period of 3 years across India. This article presents the objectives, design, methodologies and expected benefits of the ongoing LANDMARC study.
Methods
Study design
The LANDMARC study (Trial Registration No: CTRI/2017/05/008452) is a multicentre, observational, prospective, longitudinal study investigating a large cohort of participants with type 2 diabetes mellitus across India. The primary objective of the LANDMARC study is to determine the development of macrovascular diabetes complications, including cardiovascular disease and peripheral vascular disease over a period of 3 years. The main secondary objective is to evaluate the development of microvascular complications (a composite of renal and retinal) and neuropathy. Other secondary objectives include assessment of glycaemic control, use of oral anti‐hyperglycaemic drugs (OAD) and insulin, dose titration, intensification and time‐to‐treatment adaptation over a period of 3 years. The primary and secondary objectives are described in Table 1 and the criteria/definition of macrovascular and microvascular diabetic complications are presented in Table 2 9, 10, 11, 12.
Table 1.
Study objectives
| Primary |
|---|
|
|
|
| Secondary |
|
|
|
|
|
|
|
Table 2.
Criteria/definition of macrovascular and microvascular diabetic complications
| Events | Criteria/definition |
|---|---|
| Myocardial infarction 9 |
Myocardial infarction is defined as an event that requires hospitalization and that fulfils two of the three below‐mentioned criteria:
|
| Stroke 10 |
Stroke is defined as one of the following:
Note: Retinal artery ischaemia or haemorrhage is included in the definition of stroke. |
| Cardiovascular death 11 | Cardiovascular death will include coronary death (fatal MI, sudden death, unwitnessed death in the absence of other likely noncoronary causes and death related to a coronary artery procedure) and cerebrovascular death (fatal stroke) |
| Nephropathy 12 | Progression to macroalbuminuria (urinary albumin‐to‐creatinine ratio, > 300 mg of albumin/g of creatinine); a doubling of the serum creatinine level, accompanied by an eGFR of ≤ 45 ml min 1.73 m−2, as calculated by the MDRD formula; the initiation of renal‐replacement therapy; or death from renal disease |
| Retinopathy | Initiation of retinal photocoagulation, vitreous haemorrhage and diabetes‐related blindness |
CK‐MB, creatine kinase‐muscle/brain; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; MI, myocardial infarction.
This study was approved by the ethics committee and is being conducted as per the guiding principles detailed in the 18th World Assembly (Declaration of Helsinki, 1964) and its subsequent amendments, and Good Epidemiology Practices 13, 14.
Sample size calculation
At least 4387 people with diabetes were required with a two‐sided 99% confidence interval (CI), assuming that after 3 years, ~ 3% of participants will have a composite incidence of non‐fatal myocardial infarction (MI), stroke and cardiovascular death 15. Inclusion of 6300 people with diabetes allows us to estimate this percentage with a precision of at least 1%, considering that 30% of participants would drop out from the study before the end of the third year.
Sampling design
The study was planned to be conducted at 450 representative centres across India. These centres represent the diversity of India with respect to geography, urban/rural practice and healthcare centres (clinics, government and corporate hospitals, and nursing homes). The pan‐India, urban and peri‐urban representation of study sites comprised clinics (67%), private hospitals (32%) and government hospitals (1%).
Investigator selection
Investigators were selected based on two modalities: (1) therapeutic specialty (general physicians, endocrinologists, diabetologists) and practice (government hospital, clinic, private hospital); and (2) investigator's experience and site facility (investigators who were interested in participating in the study, were assessed based on the requisite qualification, amenities and resources to conduct the study).
Participant selection
Men and women aged between 25 and 60 years diagnosed with type 2 diabetes mellitus for at least 2 years were enrolled in the study. Participants were required to be on two or more anti‐hyperglycaemic therapies with or without glycaemic control and had to sign the informed consent prior to data collection and documentation.
People with known type 1 diabetes mellitus and secondary diabetes, such as gestational diabetes and fibrocalculus pancreatic diabetes, were excluded from the study. Other exclusion criteria were limited life expectancy due to terminal diseases, such as (but not limited to) end‐stage renal disease, liver cirrhosis, congestive heart failure and active malignancy. Persons who were on an investigational drug or had participated in a clinical trial in the previous 3 months were not included in the study.
Persons fulfilling the eligibility criteria were enrolled in a consecutive manner by the investigators in both outpatient and inpatient departments; this recruitment method was preferred to limit bias in participant selection. Previous clinical notes and history were reviewed by the investigators and details were verified.
Study duration and endpoints
The estimated duration of the study is 4 years. The first participant first visit was performed on 20 April 2017 and the last participant first visit on 16 May 2018. The last participant last visit is expected to take place on 16 May 2021.
The primary endpoint of the study is to evaluate the proportion of participants with macrovascular disease (cardiovascular, including non‐fatal MI, non‐fatal stroke and cardiovascular death, and peripheral vascular disease, defined using the Edinburgh Claudication Questionnaire) over 36 months. The Edinburgh Claudication Questionnaire is a screening tool for diagnosing intermittent claudication in symptomatic persons. The questionnaire constitutes a series of six questions and a pain diagram that is completed by the participants 16. The diabetic neuropathy symptom (DNS) score will be used to assess neuropathy. DNS is a four‐item validated symptom score, with high predictive value to screen for neuropathy in diabetes 17. Symptoms of unsteadiness in walking, neuropathic pain, paraesthesia and numbness are elicited. The presence of one symptom is scored as 1 point, with the maximum score being 4 points. A score of 1 or higher is defined as positive for neuropathy 18. The secondary endpoints are summarized in Table 3.
Table 3.
Secondary endpoints and the assessment time points
| Secondary endpoint | 6 months | 12 months | 24 months | 36 months |
|---|---|---|---|---|
| First occurrence of composite renal and retinal microvascular outcomes, i.e. incident or worsening nephropathy, vitreous haemorrhage, retinal photocoagulation or diabetic retinopathy | ||||
| Neuropathy (DNS), nephropathy and retinopathy | × | × | × | |
| Controlled/uncontrolled [HbA1c ≥ 53 mmol/mol (7.0%)] | × | × | × | |
| Occurrence of microvascular and macrovascular complications in controlled/uncontrolled [HbA1c ≥ 53 mmol/mol (7.0%)] | × | |||
| Controlled [HbA1c < 53 mmol/mol (7.0%) and < 48 mmol/mol (6.5%)] | × | × | × | × |
| Time‐to‐treatment adaptation from baseline to end of study | × | × | × | |
| Urgent revascularization procedures, hospitalization due to acute coronary syndrome, heart failure or unstable angina | × | × | × | × |
| Compare glycaemic control and outcomes in participants with and without complications with respect to baseline | × | |||
| Composite of non‐fatal myocardial infarction, non‐fatal stroke, hospitalization for unstable angina and cardiovascular death | × |
DNS, diabetic neuropathy symptom.
Data collection
A tracking log form is used for recording data (such as participant number, whether included in the study or not, and the reason for non‐enrolment in the study) of enrolled participants with complete anonymity. The tracking log form will help ensure that consecutive recruitment was used, which will limit participant selection bias. Participants who are not be able to complete the study and for whom no endpoint data will be available are considered lost to follow‐up.
The duration of the study for a participant is 36 months (± 45 days). Data were recorded at visit 1 (baseline) and will be recorded prospectively during follow‐up at visit 2 [end of 6 months (±14 days)], visit 3 [end of 12 months (±25 days)], visit 4 [end of 18 months (±30 days)], visit 5 [end of 24 months (±30 days)], visit 6 [end of 30 months (±40 days)] and visit 7 [end of 36 months (±45 days)]. At each visit, data will be collected in the electronic case report form (eCRF). The study design and the schedule of participants’ visit to the clinic are shown in Fig. 1.
Figure 1.
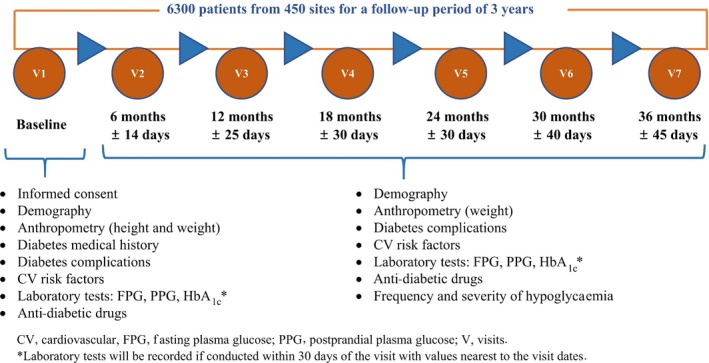
Study design including participants’ clinic visits.
Visit 1
At visit 1, the informed consent form was completed, the inclusion and exclusion criteria were reviewed and the following data were collected: demographics; anthropometrics (height and weight); medical history of diabetes (duration of diabetes mellitus); and diabetes‐associated complications at baseline, which included incidences of MI, stroke, peripheral vascular disease, neuropathy, nephropathy, retinopathy, acute coronary syndrome, heart failure and unstable angina. Cardiovascular risk factors were also collected at baseline, and included known incidences of hypertension, dyslipidaemia, albuminuria and family history of premature coronary disease. Laboratory tests, including fasting plasma glucose, postprandial plasma glucose and HbA1c, were performed. Class of OAD and injectable glucose‐lowering drug used was recorded.
Visits 2–7
From visit 2 to visit 7, the following data will be collected: weight; occurrence of diabetic complications, which includes MI, stroke, peripheral vascular disease, neuropathy, nephropathy and retinopathy (initiation of retinal photocoagulation, vitreous haemorrhage and diabetes‐related blindness); incident or worsening nephropathy; neuropathy as assessed by DNS score; frequency and severity of episodes of hypoglycaemia; hospitalization due to acute coronary syndrome; heart failure; unstable angina; urgent revascularization procedures; and cardiovascular risk factors including hypertension, dyslipidaemia and albuminuria. Laboratory tests for assessing glycaemic control, including fasting plasma glucose, postprandial plasma glucose and HbA1c, will be performed. Class of OADs (with change in dose) and injectable glucose‐lowering drugs that are used will be recorded. Visit 7 will be the end of the study.
Cardiovascular deaths including coronary death (fatal MI, sudden death, unwitnessed death in the absence of other likely non‐coronary causes and death related to a coronary artery procedure) and cerebrovascular death (fatal stroke) occurring during the study will be captured in the eCRF.
Because this is an observational study, it will mirror the real‐life management of participants, and none of the assessments, including study visits, is mandatory. Data will be collected whenever investigations are done as per routine clinical practice. As this is an observational study collecting real‐world data, no standardization of laboratory parameter was followed across sites.
Data quality control
Data quality control (site monitoring and/or phone quality control) will be performed randomly in 10% of the active sites (which have enrolled at least one participant).
Safety monitoring
The investigators are required to take necessary steps to ensure the safety of the enrolled participants, particularly for any adverse drug reactions associated with any Sanofi product. In the case of an adverse drug reaction related to a Sanofi product, participants will be followed up until complete recovery and normalization of the laboratory values or until the participant's condition stabilizes. The Sponsor will be responsible for reporting all the adverse drug reactions that meet the expedited reporting criteria to the Indian health authorities and institutional ethics committees/institutional review boards.
Statistical consideration
Continuous data will be summarized using the number of observations available (n), mean, standard deviation, median, minimum and maximum. Categorical data will be summarized using counts and percentages. In this study, analysis will be done on per‐protocol (evaluable) group for the primary endpoints and statistical testing will be conducted at a 5% significance level. Kaplan–Meier analysis will be done and time to event analysis will be performed. Time taken for the first occurrence of the diabetic complications will be analysed using Kaplan–Meier curves. A log rank test will be performed to examine whether there are any differences between the curves across subgroups (Table S1). Censoring will be done on participants who were lost to follow‐up on the last date of contact and those for whom events did not occur will be censored at the end of the study.
The association between primary endpoints (macrovascular diabetic complications, stroke, cardiovascular deaths and peripheral vascular disease) and baseline subgroups (Table S1) will be investigated using a stepwise logistic regression model. Binary response (occurrence of event) will be the dependent variable and baseline subgroups will be the independent variables. Significant subgroups will be maintained in the logistic model [meeting the entry criterion (value of 0.001 to 0.01 as entry criterion based on Akaike and Schwarz information criteria) for stepwise logistic regression model] and odds ratio along with 95% CI will be reported.
Missing data
Partial dates will be imputed using the following algorithm:
if month and year are present, impute the first day of the month;
if only year is present then, impute 01‐Jan;
if the complete date is not present, then leave it as missing.
Imputed dates will be used only for analyses and actual partial dates recorded in database will be listed.
Other missing data will first be analysed for trends. Missed visits and visit window deviations will be summarized and visualized by subgroups to identify whether they are missing completely at random (MCAR), missing at random (MAR) or missing not at random (MNAR). A multiple imputation technique may be adopted to impute missing data and analyses will be performed with and without this imputation.
Interim analyses
Interim analyses will be conducted to evaluate macrovascular events and glycaemic control among 2000 participants who would have completed 6 months of the study, and when the total population would have completed 1 and 2 years of follow‐up. Interim analyses are planned at the following milestones during the study: (1) analysis of 6300 participants based on data at V1; (2) analysis of 2000 participants based on data at V2; (3) analysis of 6300 participants based on data at V2; and (4) analysis of 6300 participants based on data at V3.
Current status
As of 29 October 2018, the study has completed recruitment by enrolling 6332 participants. In total, 388 sites pan‐India contributed towards enrolment of participants for the study.
Discussion
The increasing trend in the prevalence of diabetes and its associated complications contributing significantly to overall morbidity and mortality has been a constant challenge in India. Most studies that have been performed to date in India are population‐based cross‐sectional studies. LANDMARC is a first‐of‐its‐kind prospective, long‐term, longitudinal study conducted in India in a large cohort of persons with type 2 diabetes mellitus. The LANDMARC study aims not only to precisely determine the disease burden of diabetes in India, but also to evaluate the development of macrovascular and microvascular complications in these individuals over time and explore their regional occurrence. In this study, the role of effective treatment strategy in preventing diabetic complications and the course of the disease will be assessed.
In previous population‐based studies in India, the prevalence of diabetes and macrovascular (cardiovascular risk factors) complications were investigated at a smaller scale 19, 20. The pan‐India, urban and peri‐urban representation of participants in the LANDMARC study can provide valuable insights into the relationship between diabetes‐related complications and glycaemic control over time. This study is expected to help in understanding the emergence of complications at a relatively early age. It is known that diagnosis of diabetes takes place in India quite late in the disease cycle and that the complications of diabetes in Indians appear a decade earlier than in people with diabetes in the West 21, 22, 23, 24. The literature from population‐based studies in India indicate that disease diagnosis remains undetected in about half of the population with diabetes and may support the observation of a large majority of people having complications at the time of diagnosis itself 23, 24. Hence, we expect that the rate of complications will be truly representative of the real‐world situation in India. In addition, this study will also provide critical information on following common trends observed in India: delayed insulinization; increased use of OAD combinations; dose titration patterns of OADs, insulin and other injectable anti‐hyperglycaemic agents; patient and physician behaviours, etc. The study may help in identifying the role of optimal therapy (such as early insulinization and dose titration) in preventing complications and reducing the overall cost of diabetes management. Through this study, a person's journey, his/her response to various therapies over a period and the possible unmet needs can be identified. The study may provide an insight into changes in physician preferences and treatment patterns with the introduction of new therapies.
Because this is a disease observational study, as part of the study protocol, the process of spontaneous reporting is followed and no information about the individual drugs (INNs), including Sanofi products used to treat people with diabetes will be collected. Hence, only the name of the drug class and not individual drug names is recorded. The investigators are adequately instructed that based on their unbiased discretion, they should report any product related adverse effect to the respective drug manufacturers.
The strength of this study is the pan‐India representation of investigators and enrolment of a large cohort that will provide an in‐depth understanding of the disease landscape and real‐world management of type 2 diabetes mellitus. Because the study design mirrors real‐life normal routine practice of management of type 2 diabetes mellitus, the outcomes are expected to be a true reflection of the real world. Like many other longitudinal studies, the major limitation of this study is its prolonged duration, which may result in incomplete and interrupted follow‐up of individuals, and attrition with loss to follow‐up over time. This problem will be mitigated using survival analyses. In this study, data on lifestyle behavioural information, education level, minimal amount of diabetes education, smoking/alcohol intake status and physical activity or diet will not be collected as they are not part of the study protocol and not specifically collected during the study conduct; hence, this is a limitation.
Conclusion
The LANDMARC study intends to assess the trends of diabetes progression, its associated complications and treatment strategies within the Indian context. With the collective understanding of all the objectives of this study, public health agencies can be propelled to invest in relevant resources for the management of diabetes. Through this approach, person‐centred care can be developed and both treatment targets and treatment strategies can be tailored. The study can therefore be an impetus for redefining the approach towards diabetes management and control in India.
Funding sources
This study is funded by Sanofi, India.
Competing interests
AM, AGU, AKD and SJ received honoraria from Sanofi and other pharmaceutical companies. KMPK is on the advisory board of Sanofi and received honorarium for his talks. SK received honoraria/speaker fees from Eli Lilly, Novo Nordisk and Sanofi. HT received honoraria from MSD, Novartis, Sanofi and other companies for advice and lectures. BS received honorarium from Aventis, Novo Nordisk, Lilly, Boehringer Ingelheim and MSD. SC received honoraria/grants from Biocon, Boehringer Ingelheim, Intas, Novartis, Sanofi and Serdia. AHZ received honoraria from Novo Nordisk, Lilly, J & J, AstraZeneca, Boehringer Ingelheim and Sanofi. SJ received honoraria from Sanofi and other pharmaceutical companies. NR and SKW have nothing to declare. RG, AM, DC, SM, SKM, CT, MN, VK, VS and GC are the employees of Sanofi.
Data Sharing Statement
Qualified researchers may request access to person‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Person‐level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
Author contributions
AKD, MN, SM and CT were involved in the study concept and design. DC performed the statistical analyses of the data. All the authors participated in the interpretation of data and the writing, reviewing and editing of the manuscript and had final responsibility for approving the published version of the manuscript. AKD is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Table S1. Subgroups for analyses.
Acknowledgements
This study is funded by Sanofi, India. Part of the data presented in this paper (geographical representation and selection criteria for investigators) was presented at 45th Annual Meeting of The Research Society For The Study of Diabetes in India (Abstract Number: ABS‐177). The authors would like to thank the study participants, their families and caregivers who were involved in this study. Editorial support in preparation of this publication was provided by Archana Pandita of APCER Life Sciences India Ltd. and paid for by Sanofi. Editorial support was also provided by Anahita Gouri and Rohan Mitra of Sanofi, India. The authors are responsible individually and collectively for all content and editorial decisions, and did not receive any payment from Sanofi directly or indirectly (through a third party) related to the development or presentation of this publication. The authors acknowledge the role of DignoSearch for site management and monitoring activities, JSS Medical Research India Pvt. Ltd and Tech Observer Pvt. Ltd for site management and coordination support and Zifo R&D Solutions for data management services.
Diabet. Med. 37, 885–892(2020)
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 8th edition Brussels: International Diabetes Federation; Available at http://diabetesatlas.org/resources/2017-atlas.html Last accessed 10 May 2019. [Google Scholar]
- 2. World Health Organization . Global Report on Diabetes. Geneva: World Health Organization; Available at http://www.who.int/iris/handle/10665/204871 Last accessed 10 May 2019. [Google Scholar]
- 3. Papatheodorou K, Papanas N, Banach M, Papazoglou D, Edmonds M. Complications of diabetes 2016. J Diabetes Res 2016; 2016: 6989453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohan V, Shah S, Saboo B. Current glycemic status and diabetes related complications among type 2 diabetes patients in India: data from the A1chieve study. J Assoc Physicians India 2013; 61(Suppl 1): 12–15. [PubMed] [Google Scholar]
- 5. Moira A, D'Souza P, Kundapur R, Kiran NU. A cross sectional study to determine the prevalence of diabetes mellitus and its household awareness in the rural field practice areas of a medical college in Mangalore – a pilot study. NUJHS 2015; 5: 43–46. [Google Scholar]
- 6. Rathod HK, Darade SS, Chitnis UB, Bhawalkar JS, Jadhav SL, Banerjee A. Rural prevalence of type 2 diabetes mellitus: a cross‐sectional study. JOSH‐Diabetes 2014; 2: 82–86. [Google Scholar]
- 7. Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK et al Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population‐based cross‐sectional study. Lancet Diabetes Endocrinol 2017; 5: 585–596. [DOI] [PubMed] [Google Scholar]
- 8. Agrawal RP, Ola V, Bishnoi P, Gothwal S, Sirohi P, Agrawal R. Prevalence of micro and macrovascular complications and their risk factors in type‐2 diabetes mellitus. J Assoc Physicians India 2014; 62: 504–508. [PubMed] [Google Scholar]
- 9. O'Connor CM, Gottlieb S, Bourque JM, Krause‐Steinrauf H, Anand I, Anderson JL et al Impact of nonfatal myocardial infarction on outcomes in patients with advanced heart failure and the effect of bucindolol therapy. Am J Cardiol 2005; 95: 558–564. [DOI] [PubMed] [Google Scholar]
- 10. Bentley‐Lewis R, Aguilar D, Riddle MC, Claggett B, Diaz R, Dickstein K et al; ELIXA Investigators . Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long‐term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015; 169: 631–638.e7. [DOI] [PubMed] [Google Scholar]
- 11. Barrett‐Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K et al; MORE Investigators (Multiple Outcomes of Raloxifene Evaluation) . Raloxifene and cardiovascular events in osteoporotic postmenopausal women four‐year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002; 287: 847–857. [DOI] [PubMed] [Google Scholar]
- 12. Wanner Ch, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 13. International Society for Pharmacoepidemiology . Guidelines for Good Pharmacoepidemiology Practices (GPP). Bethesda, MD: International Society for Pharmacoepidemiology; Available at https://www.pharmacoepi.org/resources/policies/guidelines-08027/ Last accessed 10 May 2019. [Google Scholar]
- 14. International Epidemiological Association (IEA) European Federation . Good Epidemiological Practice (GEP) – IEA Guidelines for Proper Conduct of Epidemiological Research. Dundee: International Epidemiological Association, 2007. [Google Scholar]
- 15. Geiger MJ, Mehta C, Turner JR, Arbet‐Engels C, Hantel S, Hirshberg B et al Clinical development approaches and statistical methodologies to prospectively assess the cardiovascular risk of new antidiabetic therapies for type 2 diabetes. Ther Innov Regul Sci 2015; 49: 50–64. [DOI] [PubMed] [Google Scholar]
- 16. Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol 1992; 45: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 17. Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med 2002; 19: 962–965. [DOI] [PubMed] [Google Scholar]
- 18. Meijer JW, Bosma E, Lefrandt JD, Links TP, Smit AJ, Stewart RE et al Clinical diagnosis of diabetic polyneuropathy with the diabetic neuropathy symptom and diabetic neuropathy examination scores. Diabetes Care 2003; 26: 697–701. [DOI] [PubMed] [Google Scholar]
- 19. Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care 2008; 31: 893–898. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, Gupta R, Sharma KK, Lodha S, Achari V, Asirvatham AJ et al Prevalence of diabetes and cardiovascular risk factors in middle‐class urban participants in India. BMJ Open Diabetes Res Care 2014; 2: e000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viswanathan V, Rao VN. Problems associated with diabetes care in India. Diabetes Manage 2013; 3: 31–40. [Google Scholar]
- 22. Yajnik CS. The insulin resistance epidemic in India: fetal origins, later lifestyle, or both? Nutr Rev 2001; 59: 1–9. [DOI] [PubMed] [Google Scholar]
- 23. Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res 2007; 125: 217–230. [PubMed] [Google Scholar]
- 24. Joshi SR. Diabetes care in India. Ann Global Health 2015; 81: 830–838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subgroups for analyses.


