Abstract
Purpose
To describe predisposing risk factors, causative microorganisms and their antibiotic susceptibility patterns in infectious keratitis during an 11‐year period in Region Örebro County, Sweden.
Methods
This is a descriptive study conducted as a retrospective audit of clinical records. Patients who received treatment for infectious keratitis at any of the three ophthalmological departments within Region Örebro County, Sweden, between 2004 and 2014 were included if they fulfilled the predefined criteria for infectious keratitis. Data regarding culture results, antibiotic susceptibility pattern and risk factors for infectious keratitis were obtained from medical records and microbiological reports.
Results
In total, 398 episodes of infectious keratitis in 392 patients were included, and 285 were culture positive. The most common predisposing risk factor was contact lens wear (45%). Coagulase‐negative staphylococci (39.6%) was the most commonly isolated type of organism. Staphylococcus aureus (15.1%) followed by Moraxella spp. (7.4%) and Pseudomonas aeruginosa (6.7%) were among the most common isolated bacteria not considered to be commensal. Reduced susceptibility to fluoroquinolones was observed in five of 43 S. aureus isolates and in four of nine Streptococcus pneumoniae isolates.
Conclusion
The most common predisposing risk factor for keratitis was contact lens wear. Among the most common microbes, not considered to be exclusively commensals, isolated from the cornea in microbial keratitis were S. aureus, Moraxella spp. and P. aeruginosa. The antibiotic susceptibility patterns showed low proportion of resistance. Empiric treatment of suspected infectious keratitis with topical fluoroquinolones and chloramphenicol might be considered in a setting like ours pending culture results.
Keywords: antibiotic susceptibility, keratitis, microbes
Introduction
Infectious keratitis is an acute and potentially sight‐threatening infection of the cornea that can lead to permanent, severe sight loss. A common predisposing risk factor in individuals with otherwise healthy eyes is contact lens wear (CLW) (Dart et al. 1991; Erie et al. 1993; Ladage et al. 2001; Bourcier et al. 2003; Keay et al. 2006; Stapleton & Carnt 2012). It has been suggested that the posterior contact lens surface enables biofilm formation that makes known corneal pathogens such as Pseudomonas aeruginosa more resistant to the antibacterial effects of the tear film and the defence mechanisms of the immune system (Tam et al. 2010).
The surface of the eye consists of the cornea and the conjunctiva. The conjunctiva communicates with the mucous membrane of the nose via the lacrimal sac. Under normal circumstances, the surface of the eye is colonized with commensals such as coagulase‐negative staphylococci (CoNS), Corynebacterium spp. and Propionibacterium spp. (Fleiszig & Efron 1992; Willcox 2013). The microbiology of infectious keratitis is well studied but there are conflicting data in the literature regarding the rate of positive corneal cultures and the most commonly isolated microbes (Bourcier et al. 2003; Yeh et al. 2006; Green et al. 2008; Lichtinger et al. 2012; Hoddenbach et al. 2014).
In a previous study of all hospitalized cases of contact lens induced keratitis in Sweden during 1989–1991(Nilsson & Montan 1994), bacterial growth was found in 54% of the patients. Among the most commonly isolated bacteria reported were P. aeruginosa (24%), Staphylococcus epidermidis (10%) and Staphylococcus aureus (6%). Acanthamoeba was isolated in 6% of the patients. There is another Swedish study from the same time period, prospectively including all cases, both contact lens induced and not, of infectious keratitis presenting in the greater Gothenburg region(Neumann & Sjostrand 1993). In this study, corneal cultures were positive in 46% (22/48) of the patients. Pseudomonas was isolated in 6% (3/48), S. epidermidis in 8% (4/48) and S. aureus in 13% (6/48) of the corneal cultures.
There are no recent studies from Sweden describing the causative microorganisms and their antibiotic susceptibility pattern in infectious keratitis. Since antimicrobial therapy needs to be initiated before the results of the corneal cultures are available, knowledge of the spectrum of causative micro‐organisms and their antibiotic susceptibility pattern is vital when choosing adequate empiric treatment for patients with infectious keratitis.
The aim of the present study was to describe predisposing risk factors, isolated micro‐organism and their antibiotic susceptibility pattern, in patients with infectious keratitis treated in a region in the central part of Sweden.
Materials and Methods
Study population
This is a descriptive study conducted as a retrospective audit of clinical records and microbiological reports. The study was approved by the Regional Ethical Review Board of Uppsala (No 2015/335). Patients eligible for inclusion were identified through two pathways; a database search for keratitis (diagnosis code H16.9 in the International Classification of Disease, version 10) was performed at the three ophthalmology departments in Region Örebro County: at Örebro University Hospital, Karlskoga County Hospital, and Lindesberg County Hospital between 1 January 2004 and 31 December 2014, and a search of the database at the Department of Laboratory Medicine, Clinical Microbiology, Örebro University Hospital, was performed to identify all patients who had undergone corneal culture during the study period (Fig. 1). Patients undergoing corneal culture due to presumed infectious keratitis were included if they met at least one of two inclusion criteria (Stapleton et al. 2012); (1) positive corneal culture (all growth was considered positive); (2) and/or stromal infiltration with overlying epithelial defect in combination with at least one of the following; lesion within or overlapping the central 4 mm of the cornea and/or uveitis and/or pain.
Figure 1.
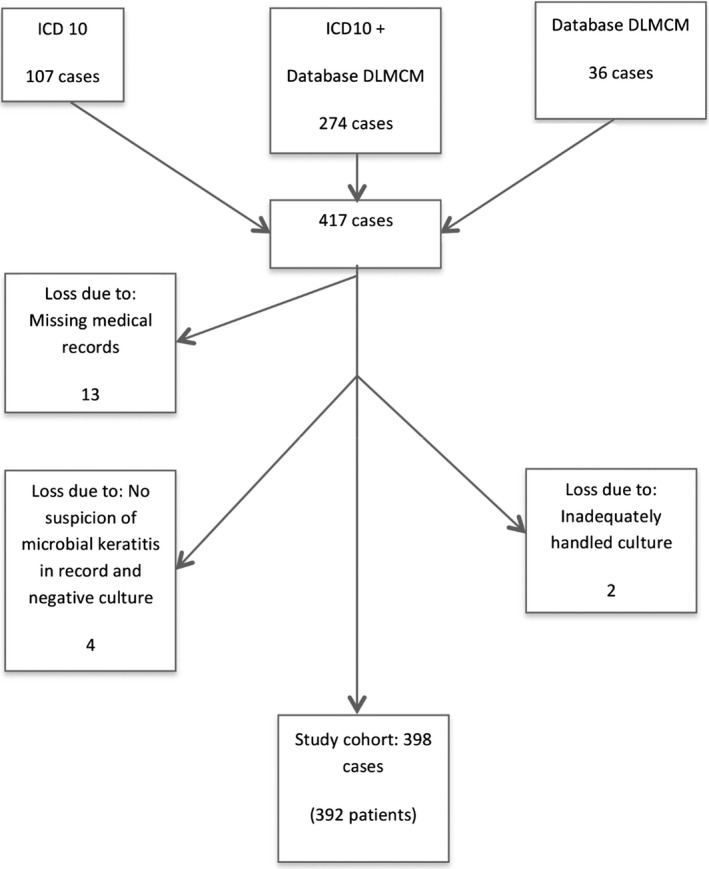
Study population: Flow chart showing how corneal cultured patients with infectious keratitis were identified during the 11‐year study period (2004–2014). Database search for diagnosis code H16.9 in the International Classification of Disease version 10 (ICD10) at the three ophthalmology departments in Region Örebro County, Sweden; database search at the Department of Laboratory Medicine, Clinical Microbiology (DLMCM), Örebro University Hospital, Sweden; or both.
Microbiological cultures
Corneal cultures were performed by either an ophthalmologist or a resident within ophthalmology according to written instructions available at the relevant time period. These written instructions were in accordance with the Swedish State of the Art Document on Infectious Keratitis Caused by Bacteria, Yeast, and Protozoa (2002) and can be considered to describe a routine procedure.
Prior to sampling, topical preservative‐free anaesthesia was applied. Microbial/bacterial samples were collected according to the following standard procedures. Samples from the infiltrates were obtained with both sterile cotton tipped applicators and a sterile number 15 knife blade. The corneal samples were inoculated on gonococcal agar, blood agar and Sabouraud agar and in fastidious anaerobic broth. If any clinical suspicion of Acanthamoeba, a final scraping in Page's saline solution or sodium chloride solution 0.9% was performed and sent to the national reference laboratory at the Public Health Agency of Sweden for further culture.
Typing of isolated microbes was carried out with Analytical Profile Index kits or with matrix‐assisted laser desorption/‐ionization time‐of‐flight mass spectrometry. Antibiotic susceptibility patterns were determined by the disc diffusion method or a gradient test such as the E‐test, according to the guidelines of the Swedish Reference Group of Antibiotic Issues (RAF; www.sls.se/raf) and later of the European Committee on Antimicrobial Susceptibility Testing (EUCAST; www.eucast.org). In this study, both I and R according to Susceptible Intermediate Resistant (SIR) system (EUCAST) are reported as reduced susceptibility, and not reported separately. Since the Department of Ophthalmology at the University Hospital of Örebro is a tertiary ophthalmology referral centre, some of the patients included in this study (n = 16) were cultured at ophthalmological departments other than the three in Region Örebro County. These cultures may not have been performed according to the method described above.
Statistical analyses were calculated in version 22 of IBM (Armonk, NY, USA) SPSS, using Pearson's chi‐square test or Fisher's exact test as appropriate. A significance level of p < 0.05 was chosen.
Results
Study population
During the 11 year study period, 392 patients with 398 episodes of infectious keratitis were included. Three women and three men had two separate episodes each.
Almost 10% of the study population (41 patients with 42 episodes) was referred from ophthalmology departments outside Region Örebro County. The remaining 351 patients, comprising 356 episodes, were inhabitants of Örebro County; six of these were initially diagnosed and treated elsewhere.
The median age was 49.5 years, ranging from 5 to 98 years. The study population consisted of 57% women, and 51.5% of episodes were in right eye. Additional surgical intervention and/or corneal cross‐linking (CXL) was performed in 27% (104/391) of the included patients. For one patient, it is unknown whether additional treatment was performed or not and another six patients are excluded from these calculations since they were involved in a prospective study of CXL as primary intervention (Makdoumi et al. 2012) (1).
Table 1.
Patients with infectious keratitis requiring additional intervention in relation to corneal culture outcome
| Total | AMT only | CXL only | CT only | Evisc/Enuc only | AMT + CXL | AMT + CT | CT + CXL | CXL + Evisc/Enuc | AMT + CXL + Evisc/Enuc | AMT + CT + CXL | CT + CXL + Evisc/Enuc | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Culture positive (n = 278) | 84 | 22 | 20 | 5 | 1 | 20 | 4 | 3 | 4 | 2 | 2 | 1 |
| Culture negative (n = 113) | 20 | 9 | 2 | 0 | 2 | 5 | 0 | 0 | 1 | 0 | 1 | 0 |
| Total* (n = 391) | 104 | 31 | 22 | 5 | 3 | 25 | 4 | 3 | 5 | 2 | 3 | 1 |
AMT = amniotic membrane transplant; CT = corneal transplant; CXL = corneal cross‐linking; Evisc./Enuc. = Evisceration/Enucleation.
* For one patient with positive corneal culture, it is unknown whether an additional intervention was performed or not. Six patients that at the study period was involved in another study (Makdoumi et al. 2012) regarding CXL as primary treatment of bacterial keratitis are excluded from these calculations.
Culture outcome
Of the 398 episodes, 285 (71.6%) were culture positive, of these 98% (279/285) had pure bacterial growth, and 2% (6/285) growth of fungi of which three had pure growth of fungi, and three in combination with bacterial growth of anaerobe microbes: Cutibacterium acnes (formerly Propionibacterium acnes) and anaerobe Gram‐positive cocci. Despite 124 corneal cultures for Acanthamoeba during the study period of 11 years, there were no findings of the protozoa in the study group.
However, in the retrospective audit of medical journals, one case with a documented persisting clinical suspicion of Acanthamoeba, despite negative culture was identified. Polymerase Chain Reaction and confocal microscopy were not performed. The patient, a contact lens wearer, was in severe pain and did not respond to treatment with topical fluoroquinolones, or fortified drops of vancomycin and ceftazidime. Improvement was noted after a couple of days treatment with chlorhexidine in combination with tobramycin.
The most common risk factor for infectious keratitis, regardless of whether the corneal cultures were positive or not, was contact lens wear which was seen in 44.5% (127/285) of those with positive cultures and in 47.8% (54/113) of those with negative cultures (Table 2).
Table 2.
Predisposing risk factors, prevalence of previous antibiotic treatment prior to culture and corneal culture outcome in patients with infectious keratitis
| Total | CLW | No known | OSD | OT | Corneal transplant | Eyelid disorder | Blind/severely diseased eye | Other ocular surgery | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 398 | 181 | 76 | 43 | 32 | 28 | 22 | 9 | 7 |
|
Culture positive (% of total in group) (No prior antibiotic/prior antibiotic) |
285* (72%) (236/48) |
127* (70%) (113/13) |
54 (71%) (46/8) |
29 (67%) (24/5) |
22 (69%) (13/9) |
25 (89%) (21/4) |
17 (77%) (12/5) |
7 (78%) (5/2) |
4 (57%) (2/2) |
| Polymicrobial growth [% of culture positive in group] | 77* [27%] | 26* [20%] | 21 [39%] | 6 [21%] | 6 [27%] | 10 [40%] | 7 [41%] | 1 [14%] | 0 [0%] |
|
Culture negative (% of total in group) (No prior antibiotic/prior antibiotic) |
113 (28%) (86/27) |
54 (30%) (49/5) |
22 (29%) (19/3) |
14 (33%) (9/5) |
10 (31%) (6/4) |
3 (11%) (1/2) |
5 (23%) (1/4) |
2 (22%) (0/2) |
3 (43%) (1/2) |
Corneal transplant: Penetrating keratoplasty, DSAEK, Superficial lamellar keratoplasty. Eyelid disorder: closure defect, trachoma, entropion, ectropion. Percentages within parentheses () refer to the proportions of culture positive and culture negative cases, respectively, in the respective risk factor group. Percentages within square brackets [] refer to the proportion of polymicrobial growth, in the respective risk factor groups of positive corneal culture cases.
CLW = contact lens wear; OSD = ocular surface disease; OT = ocular trauma.
* For one patient, it was unknown whether antibiotic treatment had been initiated prior to sampling.
The most commonly isolated microorganisms among the 285 positive corneal cultures were CoNS (39.6%, n = 113) and anaerobic diphtheroid rods (25.6%, n = 73), predominantly C. acnes (Table 3). S. aureus (15.1%, n = 43) was the most frequently isolated microbe among the Gram‐positives not considered a commensal. Moraxella spp. (7.4%, n = 21) and P. aeruginosa (6.7%, n = 19) were among the most commonly isolated Gram‐negative bacteria.
Table 3.
Corneal culture findings in relation to predisposing risk factor in patients with infectious keratitis
| Total | CLW | NK | OSD | OT | CT | ELD | SI/B | OOS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| All microbes isolated | 378 | 156 | 83 | 37 | 30 | 36 | 24 | 8 | 4 | |
| Gram‐positive | Staphylococcus aureus | 43 | 10 | 11 | 8 | 6 | 5 | 2 | – | 1 |
| Coagulase‐negative staphylococci | 113 | 49 | 26 | 7 | 10 | 10 | 9 | 1 | 1 | |
| Streptococcus pneumoniae | 9 | 1 | 1 | 4 | 1 | 1 | – | 1 | – | |
| Alpha‐haemolytic streptococci | 24 | 6 | 4 | 4 | 6 | 3 | 1 | – | – | |
| Beta‐haemolytic streptococci | 4 | 1 | 2 | 1 | – | – | – | – | – | |
| Corynebacterium spp. | 34 | 13 | 8 | 5 | – | 5 | 2 | 1 | – | |
| Micrococcus spp. | 5 | 1 | 1 | 1 | – | – | – | – | 2 | |
| Miscellaneous* | 3 | 1 | 2 | – | – | – | – | – | – | |
| Total Gram‐positive | 235 | 82 | 55 | 30 | 23 | 24 | 14 | 3 | 4 | |
| Gram‐negative | Haemophilus influenzae | 4 | 1 | – | – | – | 1 | 1 | 1 | – |
| Moraxella spp. | 21 | 2 | 10 | 2 | 1 | 2 | 2 | 2 | – | |
| Neisseria meningitidis | 3 | 3 | – | – | – | – | – | – | – | |
| Pseudomonas aeruginosa | 19 | 16 | – | – | – | 1 | 2 | – | – | |
| Enterobacteriaceae† | 9 | 5 | – | – | – | 1 | 2 | 1 | – | |
| Miscellaneous‡ | 6 | 2 | 1 | 2 | 1 | – | – | – | – | |
| Total Gram‐negative | 62 | 29 | 11 | 4 | 2 | 5 | 7 | 4 | – | |
| Anaerobes | Anaerobic diphteroid rods§ | 73 | 41 | 16 | 3 | 4 | 6 | 3 | – | – |
| Anaerobic Gram‐positive cocci | 2 | 1 | 1 | – | – | – | – | – | – | |
| Total anaerobes | 75 | 42 | 17 | 3 | 4 | 6 | 3 | – | – | |
| Fungi | Candida albicans | 2 | 1 | – | – | – | 1 | – | – | – |
| Miscellaneous¶ | 4 | 2 | – | – | 1 | – | – | 1 | – | |
| Total fungi | 6 | 3 | – | – | 1 | 1 | – | 1 | – | |
CLW = contact lens wear; CT = corneal transplant; ELD = eye lid disorder; NK = no known; OOS = other ocular surgery; OSD = ocular surface disease; OT = ocular trauma; SI/B = severely ill/blind eye.
* Miscellaneous Bacillus spp., Clostridium spp.
† Enterobacteriaceae Serratia spp., Escherichia coli, Enterobacteriaceae spp. Klebsiella oxytoca, Proteus mirabilis.
‡ Miscellaneous Aggregatibacter segnis, Stenotrophomonas maltophilia, Chrysoebacterium indologenes, Pasteurella multocida. Pseudomonas spp., Gram‐negative rod not specified.
§ Anaerobic diphteroid rods: Cutibacterium acnes, anaerobic diphteroid rods.
¶ Miscellaneous: Aspergillus fumigatus, Alternaria spp., Fusarium spp., mould non‐specified.
There was no statistically significant difference (p = 0.11, chi‐square test) in corneal culture outcome between the group that was cultured prior to antibiotic treatment compared to the group that was cultured after initial treatment. However, a statistically significantly higher number of cultures with polymicrobial growth were detected in the group not treated with antibiotics prior to culture (30.1%, 71/236) in comparison with those treated with antibiotics prior to culture (10.4%, 5/48; p = 0.005, chi‐square test). For one patient, there was no information on whether culture had been taken prior to antibiotic treatment; this patient had a history of contact lens wear and had polymicrobial growth with two different microbes.
In 75 patients, the corneal culture was preceded by antibiotic treatment. Fluoroquinolones in 21 cases of which 6 were culture negative, fusidic acid in 18 cases of which 7 were culture negative, chloramphenicol in 16 cases of which six were culture negative and a combination of fluoroquinolones and chloramphenicol in 10 cases of which four were culture negative. Remaining 10 patients were treated with polymyxin B, bibrocathol, tobramycin, or a combination of fusidic acid and chloramphenicol, fluoroquinolones and cefuroxime, or fluoroquinolones and fusidic acid.
The mean duration of combination treatment with topical chloramphenicol and fluoroquinolones prior to culture in the six patients with positive corneal culture was 3.5 days (range: 1–8 days). All six patients with a positive corneal culture after initial treatment with a combination of chloramphenicol and fluoroquinolones had pure growth of one microbe each; three with C. acnes, of which one had no reduced susceptibility and in the other two antibiotic susceptibility testing, was not performed. The forth patient, a contact lens wearer, had growth of P. aeruginosa – susceptible to ciprofloxacin and levofloxacin, this patient needed an amniotic membrane transplant. The fifth patient had growth with Proteus mirabilis – susceptible to ciprofloxacin. This was a previously blind eye due to secondary glaucoma and the eye was eviscerated. The sixth patient had growth of CoNS, susceptible to levofloxacin, ciprofloxacin and chloramphenicol. This patient had ocular surface disease and needed an amniotic membrane transplant and tarsorrhaphy.
Antibiotic susceptibility pattern
Reduced susceptibility to fluoroquinolones was seen in 5/43 (12%) of S. aureus isolates and in 4/9 (44%) of Streptococcus pneumoniae isolates. One of the S. aureus isolates with reduced susceptibility was treated with clindamycin prior to culture. Determination of Minimum Inhibitory Concentration (MIC) level was carried out in one of the isolated S. pneumonia and was determined to 0.5 mg/L. The remaining three isolates were I according to the SIR system (EUCAST.org).
Reduced susceptibility to ceftazidime was seen in 1/43 (2%) of S. aureus isolates.
One Pseudomonas sp. isolate (5%) showed reduced susceptibility to gentamicin which was from a patient who had not been treated with antibiotics prior to culture. Reduced susceptibility to tobramycin was seen in 1/4 (25%) isolates of beta‐haemolytic streptococci; this patient was not treated with antibiotics prior to culture.
Among the yeasts, one of two Candida albicans isolates showed reduced susceptibility to fluconazole (MIC 4 mg/L). This patient had received levofloxacin prior to corneal culture.
Discussion
During 2004–2014, we identified and included 392 patients with 398 episodes of infectious keratitis. Coagulase‐negative staphylococci (CoNS), S. aureus and P. aeruginosa were among the most frequently isolated microbes which was also the case in the previous two Swedish studies from the 90s (Neumann & Sjostrand 1993; Nilsson & Montan 1994).
Our results regarding the predominantly isolated bacteria and frequency of polymicrobial growth are also similar to those reported by Yeh et al. (2006) from North Carolina, USA with a similar study design as ours. Bourcier et al. (2003) in Paris, France, also reported similar results, with a frequency of CoNS in 48% (100/207) and P. aeruginosa in 10% (21/207) of those with positive cultures.
Comparisons between studies are difficult since frequencies of isolated microbes are reported in different ways. We have chosen to report the frequency of isolated microbes in relation to all culture positive cases. Prevalence of risk factors, climate and study design may also influence the results.
During 11 years not a single isolate of Acanthamoeba was detected despite 124 cultures was performed, and a high prevalence of contact lens wearers were present in the study group. Our lack of positive cultures for Acanthamoeba, and only one documented case with a persisting clinical suspicion of Acanthamoeba despite negative culture, are in contrast to previously findings from the early 90s by Skarin et al. They reported eight cases of verified Acanthamoeba during 2 years in the south of Sweden (Skarin et al. 1996). We have no explanation for this, but the study periods are more than 10 years apart. Differences in contact lens hygiene routines and contact lens wear schedule may among other factors, such as the method of corneal sampling, be different between the present study population and the one reported by Skarin et al.
The low rate of fungi in our study 2% (6/285) is similar to the study by Hoddenbach et al. (2014) who found 2% (1/59) of fungi in corneal cultures from patients admitted to hospital for treatment of contact lens related keratitis. In Denmark, Nielsen et al. (2015) reported 25 cases of fungal keratitis during 14 years giving an incidence of 0.6 per million per year. They found a significant delay of 24 days before correct diagnosis (Nielsen et al. 2015). In our study, a correct diagnosis was delayed in one‐third of the patients with growth of fungi since two of the patients with growth of fungi were cultured after an initial treatment with antibiotics.
The frequency of positive corneal cultures in our study (72%) was at the higher end of the frequencies previously reported in the literature. This might be due to our sampling method but could also be due to our definition of positive culture. In terms of positive or negative culture outcome, there was no statistically significant difference between patients treated with antibiotics prior to culture and those who were not. This might be due to patient compliance with local eye drops/ointment, or to selection bias. The finding of three positive cultures with aerobic microbes sensitive to fluoroquinolones, chloramphenicol or both despite that treatment with these two antibiotics preceded the corneal culture indicate that compliance and other mechanism, not fully understood may be involved. The lower rate of polymicrobial growth among the patients cultured after prior antibiotic treatment compared to those cultured prior to treatment was however statistically significant. This might be due to the effect of antibiotic treatment on some of the susceptible microorganisms, but might also be due to the fact that antibiotic treatment alters the bacterial load on the conjunctiva and eyelids, resulting in less contamination from commensal bacteria.
Our results revealed a low frequency (5%) of reduced susceptibility to gentamicin among isolated P. aeruginosa. This is consistent with the findings by Yeh et al. (2006) Lichtinger et al. (2012) and Sand et al. (2015). None of the S. aureus isolates in our study were methicillin resistant (MRSA). This finding is similar to the reported frequency of 1.3% MRSA in Toronto (Lichtinger et al. 2012), but in contrast to a reported frequency of 44% in Los Angeles (Sand et al. 2015).
The absence of MRSA in our study may reflect its low prevalence in the Nordic countries. In Sweden for instance, every case of MRSA is retraced in order to prevent further spread, antibiotic prescription is restricted, and strict hygiene measures are in place at all healthcare facilities.
We chose to apply the criteria of Stapleton et al. (2012) with both clinical and laboratory criterion to define an episode of infectious keratitis. Clinical course and risk factors for infectious keratitis are not included in this definition. The clinical relevance of corneal culture findings in patients with infectious keratitis is difficult to interpret. Small sample size may result in false‐negative cultures. Since the surface of the eye under normal circumstances is not sterile, there is always a risk of false‐positive cultures. In a clinical setting, the clinical presentation, disease course, local and systemic risk factors guide us in the interpretation of a corneal culture result in a patient with suspected infectious keratitis.
The strengths of this study are the size of the study group, with a relatively high number of positive corneal cultures and the likelihood of only few missing cases, since we identified cases both by diagnosis code and via the database at the Department of Laboratory Medicine.
The major limitation of this study is its retrospective design. Information in the medical records and microbiology reports are not always complete. Furthermore, since the culture findings had to be interpreted retrospectively the relation to the clinical course and local and systemic risk factors was challenging to take account for.
In conclusion, among the most common microbes, not considered commensals, isolated from the cornea in microbial keratitis were S. aureus, Moraxella spp. and P. aeruginosa. Contact lens wear was the most common predisposing risk factor. The antibiotic susceptibility patterns showed low frequency of resistance. Consequently, due to the spectrum of isolated microbes and their antibiotic susceptibility pattern, empiric treatment in a setting like ours, a combination of topical fluoroquinolones and chloramphenicol could be considered as an appropriate treatment for bacterial keratitis pending corneal culture results.
This study has in part been presented as an abstract at the annual ARVO meeting 29th April–3rd May 2018. The study was supported by Örebro County Council research committee. The funding organization had no role in the design or conduct of this research.
The authors wish to thank Bengt Hellmark, PhD, biomedical analyst, for explaining the handling of the corneal samples at the Laboratory of the Department of Microbiology and Anna Fält, MSc, for statistical advice.
References
- Bourcier T, Thomas F, Borderie V, Chaumeil C & Laroche L (2003): Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol 87: 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart JK, Stapleton F & Minassian D (1991): Contact lenses and other risk factors in microbial keratitis. Lancet 338: 650–653. [DOI] [PubMed] [Google Scholar]
- Erie JC, Nevitt MP, Hodge DO & Ballard DJ (1993): Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol 111: 1665–1671. [DOI] [PubMed] [Google Scholar]
- Fleiszig SM & Efron N (1992): Microbial flora in eyes of current and former contact lens wearers. J Clin Microbiol 30: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Apel A & Stapleton F (2008): Risk factors and causative organisms in microbial keratitis. Cornea 27: 22–27. [DOI] [PubMed] [Google Scholar]
- Hoddenbach JG, Boekhoorn SS, Wubbels R, Vreugdenhil W, Van Rooij J & Geerards AJ (2014): Clinical presentation and morbidity of contact lens‐associated microbial keratitis: a retrospective study. Graefes Arch Clin Exp Ophthalmol 252: 299–306. [DOI] [PubMed] [Google Scholar]
- Infektiös keratit orsakad av bakterier, svamp eller protozoer ‐ State of the Art. [Infectious keratitis caused by bacterial fungi or protozoa ‐ State of the Art] [homepage on the Internet] (2002). Stockholm: Socialstyrelsen; [The National Board of Health and Welfare]. Available at: https://cdn-swedeye.pressidium.com/wp-content/uploads/2010/02/2001-123-70.pdf. (Accessed on 5 November 2018). [Google Scholar]
- Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, Forde K & Stapleton F (2006): Microbial keratitis predisposing factors and morbidity. Ophthalmology 113: 109–116. [DOI] [PubMed] [Google Scholar]
- Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Petroll WM & Cavanagh HD (2001): Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology 108: 1279–1288. [DOI] [PubMed] [Google Scholar]
- Lichtinger A, Yeung SN, Kim P et al. (2012): Shifting trends in bacterial keratitis in Toronto: an 11‐year review. Ophthalmology 119: 1785–1790. [DOI] [PubMed] [Google Scholar]
- Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE & Crafoord S (2012): UVA‐riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefes Arch Clin Exp Ophthalmol 250: 95–102. [DOI] [PubMed] [Google Scholar]
- Neumann M & Sjostrand J (1993): Central microbial keratitis in a Swedish city population. A three‐year prospective study in Gothenburg. Acta Ophthalmol (Copenh) 71: 160–164. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Nielsen E, Julian HO, Lindegaard J, Hojgaard K, Ivarsen A, Hjortdal J & Heegaard S (2015): Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol 93: 54–58. [DOI] [PubMed] [Google Scholar]
- Nilsson SE & Montan PG (1994): The hospitalized cases of contact lens induced keratitis in Sweden and their relation to lens type and wear schedule: results of a three‐year retrospective study. CLAO J 20: 97–101. [PubMed] [Google Scholar]
- Sand D, She R, Shulman IA, Chen DS, Schur M & Hsu HY (2015): Microbial keratitis in los angeles: the doheny eye institute and the los angeles county hospital experience. Ophthalmology 122: 918–924. [DOI] [PubMed] [Google Scholar]
- Skarin A, Floren I, Kiss K, Miorner H & Stenevi U (1996): Acanthamoeba keratitis in the south of Sweden. Acta Ophthalmol Scand 74: 593–597. [DOI] [PubMed] [Google Scholar]
- Stapleton F & Carnt N (2012): Contact lens‐related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 26: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Edwards K, Keay L, Naduvilath T, Dart JK, Brian G & Holden B (2012): Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology 119: 1516–1521. [DOI] [PubMed] [Google Scholar]
- Tam C, Mun JJ, Evans DJ & Fleiszig SM (2010): The impact of inoculation parameters on the pathogenesis of contact lens‐related infectious keratitis. Invest Ophthalmol Vis Sci 51: 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox MD (2013): Characterization of the normal microbiota of the ocular surface. Exp Eye Res 117: 99–105. [DOI] [PubMed] [Google Scholar]
- Yeh DL, Stinnett SS & Afshari NA (2006): Analysis of bacterial cultures in infectious keratitis, 1997 to 2004. Am J Ophthalmol 142: 1066–1068. [DOI] [PubMed] [Google Scholar]


