Abstract
Maintenance of homeostasis at body barriers that are constantly challenged by microbes, toxins and potentially bioactive (macro)molecules requires complex, highly orchestrated mechanisms of protection. Recent discoveries in respiratory research have shed light on the unprecedented role of airway epithelial cells (AEC), which, besides immune cells homing to the lung, also significantly contribute to host defence by expressing membrane‐bound and soluble pattern recognition receptors (sPRR). Recent evidence suggests that distinct, evolutionary ancient, sPRR secreted by AEC might become activated by usually innocuous proteins, commonly referred to as allergens. We here provide a systematic overview on sPRR detectable in the mucus lining of AEC. Some of them become actively produced and secreted by AECs (like the pentraxins C‐reactive protein and pentraxin 3; the collectins mannose binding protein and surfactant proteins A and D; H‐ficolin; serum amyloid A; and the complement components C3 and C5). Others are elaborated by innate and adaptive immune cells such as monocytes/macrophages and T cells (like the pentraxins C‐reactive protein and pentraxin 3; L‐ficolin; serum amyloid A; and the complement components C3 and C5). Herein we discuss how sPRRs may contribute to homeostasis but sometimes also to overt disease (e.g. airway hyperreactivity and asthma) at the alveolar–air interface.
Keywords: Airway epithelial cells, Barrier tissues, Complement system, Innate defence, Soluble pattern recognition receptors
Airway epithelial cells actively contribute to protection of the respiratory tract, by locally secreting soluble pattern recognition receptors (sPRR). The sPRR play critical roles in the recognition of conserved motifs and are linked to complement activation. This article reviews the current knowledge on the involvement of sPRRs in the pathogenesis of allergic airway diseases.
Introduction
When resting, an average adult inhales about 11 000 L of ambient air per day. The inhaled air is neither sterile nor free of particles/macromolecules, and it potentially contains pathogens as well as allergens. Accordingly, the respiratory system has developed several safety mechanisms (e.g. mucus production by goblet cells, secretion of a plethora of antimicrobials, mucociliary transport by ciliated epithelial cells, coughing, etc.), which all together protect the body from harmful intruders at the alveolar–air interface [1]. Besides immune cells homing to this important organ, the cells lining the airways, that is airway epithelial cells, significantly contribute to host defence by expressing, amongst several other classes of antimicrobials, a multitude of pattern recognition receptors (PRR), which enables them to quickly respond to dangerous cues.
In the literature, PRRs are classically described as cytoplasmic (nucleotide‐binding oligomerization domain‐like receptors/retinoic acid‐inducible gene‐like receptors) or cell surface expressed (i.e. C‐type lectin‐like receptors and Toll‐like receptors) receptors. Less well known is the role of soluble pattern recognition receptors (sPRRs), which represent a group of several other evolutionarily ancient but secreted molecules. Soluble PRRs are key players of the humoral arm of innate immunity [2, 3]. Under physiological conditions, these molecules are expressed constitutively at low levels in the liver by hepatocytes [4] and by a variety of immune cells, but their production rapidly increases in response to pathogens, initiating innate immune responses that modulate mucosal immunity in extracellular compartments such as the bronchial and alveolar airspace [2, 5, 6]. Notably, in the respiratory tract of mice and men, airway epithelial cells (AECs) are a major source of sPRRs (Fig. 1) and protect this barrier tissue from potential injuries (Table 1). Moreover, cells of the macrophage lineage [7] but also T cells, monocytes, dendritic cells (DCs) and granulocytes contribute to protection of the respiratory tract, by locally secreting sPPRs [8, 9] (Table 1). Soluble PRRs share the capacity to bind various microbial and environmental proteins and eliminate them through common mechanisms including agglutination, neutralization, opsonization followed by phagocytosis, with some of them having the capacity to activate complement [10, 11, 12]. As such, sPRRs possess antibody‐like features and might, in fact, represent their functional ancestors [13]. In addition, they also interact with and regulate the function of membrane‐bound PRRs, which cooperate at the level of the recognition of environmental patterns and the regulation of inflammation [12]. Accordingly, innate humoral immune responses at the respiratory mucosa are shaped by the presence of (i) sPRR with the ability to activate complement, that is, the pentraxins PTX3, CRP; the collectin mannose‐binding lectin (MBL); the ficolins ficolin‐2 and ‐3; (ii) sPRR unable to activate complement (SAA) and (iii) complement components themselves (C3).
Figure 1.
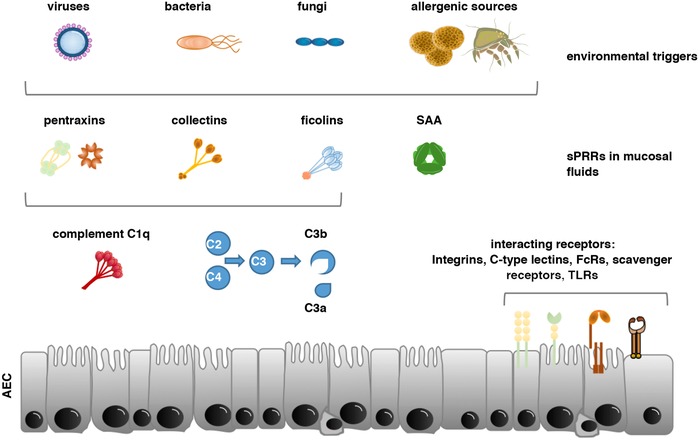
Humoral PRR within the mucosal lining of human airway epithelial cells. Shown are the different sPRRs within the mucus lining of AEC, their trigger factors and their primary mode of immune activation. The long pentraxin PTX3 has opsonic activity with a nonredundant protective role against selected pathogens, such as fungi, bacteria and viruses. Pentraxins are in addition complement activating proteins that lead to increased C3b levels and inflammatory signalling via C3a. The short pentraxins CRP and SAP are considered to bind to phosphorylcholine on pathogens and apoptotic cells, respectively. The collectin MBL is present primarily at mucosal surfaces including the airways and binds to a large array of pathogens, activating the lectin pathway of complement. Ficolins primarily function as opsonins, but can also activate the lectin pathway. SAA is a sPPR for Gram‐negative bacteria and blocks viral entry into cells. sPPRs also interact with and regulate the function of membrane bound PRRs such as Toll‐like receptors and C‐type lectin receptors but can also bind to integrins, Fcγ and scavenger receptors. Allergens might also have C3 convertase activity or might be recognized by C1q (in conjunction with specific antibodies). sPRRS might in addition function as an adjuvant and/or interact directly with environmental inhaled proteins to promote allergic sensitization.
Table 1.
Cellular source of soluble pattern recognition receptors in the lung
| sPRRs | Cellular sourcea | Evidence | Implication in allergic responses |
|---|---|---|---|
| Pentraxins | |||
| Pentraxin 3 (PTX3) |
Airway epithelial cells Airway smooth muscle cells Dendritic cells Macrophages Neutrophils |
Secreted protein and mRNA [41, 66] Secreted protein and mRNA [41, 66] Secreted protein and mRNA [64] Secreted protein and mRNA [64] Store and secrete protein but no mRNA expression [65] |
Increased BALF and serum levels in allergic asthma; levels correlate with disease severity [14, 21, 22, 41, 70]. |
| C‐reactive protein (CRP) |
Airway epithelial cells Alveolar macrophages |
Secreted protein from cell lines and primary cells, mRNA [105, 106, 107] Secreted protein and mRNA [108] |
Serum levels significantly elevated in asthmatics; correlate with reduced lung function and eosinophil numbers; associated with allergic sensitization and elevated risk of concomitant allergic airway inflammation [15, 34, 40, 98, 99, 103, 104]. |
| Serum amyloid P component (SAP) | Liver | Increased sputum levels in asthmatics [14] | |
| Collectins | |||
| Mannose Binding Lectin (MBL) | Liver, foetal lung | Secreted protein and mRNA [116] | Directly binds allergen extracts [131]; increased plasma levels in asthmatics that correlate with eosinophil numbers [132, 133] |
|
Surfactant protein A (SP‐A) |
Alveolar type II cells club cells |
Secreted protein and mRNA [136] Secreted protein and mRNA [136] |
Both SP‐A and SP‐D can bind allergenic components including pollen, house dust mite and A. fumigatus [150, 151, 152]; SP‐D serum and BALF increased in allergic asthmatics [160, 161]. |
|
Surfactant protein D (SP‐D) |
Alveolar type II cells club cells |
Secreted protein and mRNA [137] Secreted protein and mRNA [138] |
|
| Ficolins | |||
| L ficolins (ficolin‐2) | Liver | Deficiency is associated with allergic and infectious diseases [179] | Direct involvement in allergic inflammation currently unknown. |
| H ficolins (ficolin‐3) | Airway epithelial cells | Secreted protein and mRNA [167] | |
| Serum Amyloid A | |||
| Serum amyloid A (SAA) |
Alveolar epithelial lining Alveolar macrophages monocytes |
Secreted protein and mRNA [181] Secreted protein and mRNA [181] secreted protein and mRNA [182] |
Elevated sputum SAA levels correlate with CRS and asthma prevalence and disease severity [23, 24, 212] |
| Complement components | |||
| C3 |
Airway epithelial cells Dendritic cells Monocytes Neutrophils T cells |
Secreted protein from cell lines and primary cells [226, 227, 237] Secreted protein and mRNA [241] Secreted protein and mRNA [241] Secreted protein and mRNA [241] Secreted protein and mRNA [241] intracellularly stored protein [8] |
Allergens possess complement convertase activity and lead to C3a and C5a formation [243]; C3a levels are found upregulated in individuals with respiratory Allergies [250, 251]; positive correlation between C3 levels and asthma [252] |
| C5 |
Airway epithelial cells Dendritic cells T cells |
Secreted protein from cell lines [228, 237] Secreted protein and mRNA [241] Secreted protein and mRNA [241] intracellularly stored protein [8] |
|
Different cells are listed in alphabetical order.
Due to their dramatic systemic upregulation upon infection (i.e. by 500‐ to 1000‐fold), sPRRs have been termed ‘inflammation markers’, with some of them being extremely valuable in daily clinical practice (e.g. CRP, high‐sensitivity CRP and SAA) [14, 15, 16]. Airway mucosal inflammation in response to foreign antigens is an essential effector arm of innate host defence; however, the involvement of sPRRs in the regulation of the intensity and persistence of airway inflammation remains poorly understood. For instance, some of these sPRR, such as the collectin MBL [17] and the pentraxin PTX3, seem to inhibit, while others, like complement [18, 19], appear to aggravate infectious lung diseases triggered by deadly coronaviruses well known to induce severe acute respiratory syndrome and middle east respiratory syndrome [20].
Moreover, recent evidence suggests that sPRRs might also be causally involved in allergic airway inflammation [21, 22, 23, 24, 25]. In the following, we review the function of sPRRs present in the AEC mucus lining of mice and men and discuss their potential involvement in allergic inflammation in the lungs (Table 1).
Pentraxins
Pentraxins belong to a superfamily of cyclic multimeric sPRRs (Fig. 2) that can be divided into short and long pentraxins based on their primary structure [11, 12, 13, 26, 27]. Prototypes of short pentraxins are the serum amyloid P component (SAP) and C‐reactive protein (CRP) [26] that constitute the main acute phase proteins in mouse and human, respectively [27]. Short pentraxins show a pentameric radial symmetry consisting of five or ten identical subunits of 25 kDa in size [13, 28, 29, 30]. While CRP and SAP are mainly produced systemically in the liver in response to inflammatory stimuli such as IL‐6, pentraxin 3 (PTX3), the most commonly known long pentraxin, is induced locally at sites of inflammation or cell damage where it plays a similar role as CRP and SAP in the circulation [11, 12, 31]. In contrast to CRP, measurement of systemic levels of PTX3 seems to be a much faster (peak levels reached at 7.5 h versus 24 h after hospital admission, respectively) and more reliable (faster regression to normal values) marker for acute myocardial infarction [32, 33]. Increasing evidence now also suggests that the expression of pentraxins is increased in asthmatic patients, contributing to allergic airway inflammation [14, 21, 22, 28, 34, 35, 36, 37, 38, 39, 40, 41]. Accordingly, pentraxins can be regarded as markers for (allergic) inflammation in the airways.
Figure 2.
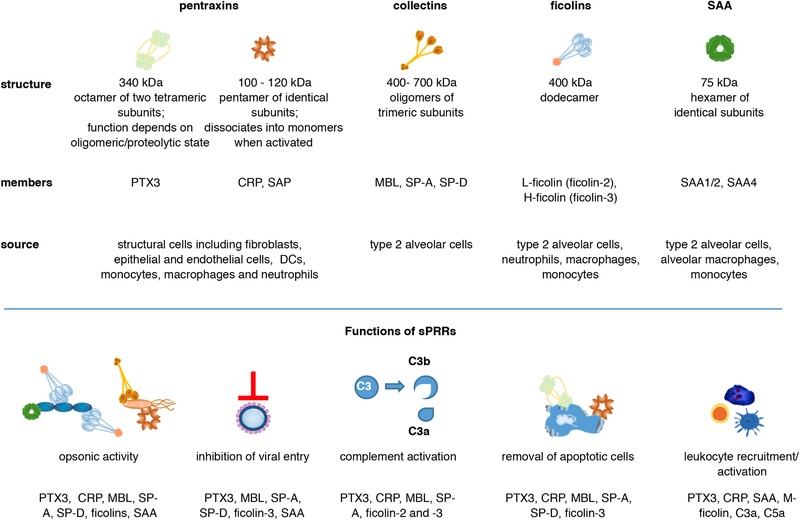
Protein structures, source and roles of sPRRs in the airways. Shown are the functional structures of pentraxins, collectins, ficolins and SAA proteins and their prominent family members. Local production of pentraxins in the lung could be detected in structural and myeloid cells as well as neutrophils. While the lung (type 2 alveolar cells) is the main synthesis site of the collectins SP‐A and SP‐D, MBL is primarily produced by the liver. Ficolins are produced in the liver by hepatocytes and in the lung by type 2 alveolar cells, as well as by neutrophils, macrophages and monocytes. SAA proteins, similar to pentraxins, were long thought to be liver‐derived serum factors but are also produced locally in the lung by type 2 alveolar cells, macrophages and monocytes. sPRRs share overlapping but also nonredundant roles in the first line of defence against microbial intruders through opsonic activity, inhibition of viral entry and the activation of the complement cascade. Some sPRRs play essential roles in the removal of apoptotic bodies, which is crucial for pulmonary immune tolerance and tissue homeostasis. In addition, several sPRRs are involved in leukocyte recruitment to the lung and local proinflammatory cytokine production.
Pentraxin 3 (PTX3)
PTX3: structure/function relationship
Thirty years ago, PTX3 was identified as a member of the pentraxin family and is now classified as the prototypic long pentraxin [42, 43]. PTX3 exists as a homo‐oligomer formed by eight identical, covalently linked subunits [44]. Protein oligomerization seems to be essential for PTX3 activity as other oligomeric forms of PTX3 have decreased functionality [45]. In addition, PTX3 function can be regulated by elastase and Aspergillus fumigatus proteases [46]. This indicates that PTX3 is capable to serve pleiotropic functions depending on its oligomeric and proteolytic state. As a sPRR, PTX3 has opsonic activity with a nonredundant protective role against selected pathogens, such as fungi, bacteria and viruses [2, 10, 13, 47]. PTX3 binds with high affinity to the outer membrane protein A from Klebsiella pneumoniae (KpOmpA) but does not interact with cellular receptors that bind this bacterial molecule. PTX3−/− mice show reduced local inflammation in response to KpOmpA, indicating that PTX3 might amplify KpOmpA‐induced responses [48].
PTX3: epidemiologic data‐lung immunity
Most recently, PTX3 was shown to be a critical positive regulator for inflammatory responses during pneumococcal pneumonia [49]. While PTX3 deficiency renders mice more susceptible to pulmonary aspergillosis due to a defective recognition of conidia by antigen presenting cells and an inappropriate induction of an adaptive type 2 response [50], administration of exogenous PTX3 is able to rescue antifungal resistance early in infection [51]. Similarly, transgenic overexpression of PTX3 is responsible for resistance to viruses (e.g. influenza virus, cytomegalovirus), pathogenic (e.g. Neisseria meningitidis) and opportunistic (e.g. Klebsiella pneumoniae and Pseudomonas aeruginosa) bacteria, including preclinical models of bacterial sepsis induced by cecal ligation puncture [52, 53, 54]. PTX3 also takes part in extracellular matrix formation [45, 47] and interacts with recognition molecules of the classical and lectin complement pathway, Fcγ receptor, fibroblast growth factor 2 and P‐selectin [10, 50, 55, 56]. A number of clinical studies have pointed to the important role of PTX3 in lung and bone marrow transplant recipients [57, 58, 59, 60, 61, 62]. In fact, patients with elevated pretransplant PTX3 plasma levels are more likely to suffer from ischemia reperfusion injury leading to primary graft dysfunction after lung transplantation [57, 58, 59]. In contrast, genetic deficiency of PTX3 predisposes to invasive lung aspergillosis in patients who underwent bone marrow transplantation [61]. Elevated bronchoalveolar lavage fluid (BALF) PTX3 levels might be indicative of invasive aspergillosis in lung transplant patients [62] and represent a more reliable biomarker than determination of galactomannan, which gives a high rate of false positive results [60]. Moreover, serum PTX3 levels were found to be higher in patients with lung cancer as compared to cancer‐free heavy smokers [63]. In the airways, several innate immune cells have been found to produce PTX3 locally including macrophages, DCs [64] and neutrophils that store and secrete PTX3 as part of neutrophil extracellular traps [65], highlighting its critical role in the humoral arm of innate immunity. Besides infiltrating inflammatory cells, also structural cells, particularly bronchial and alveolar epithelial cells [66, 67] and smooth muscle cells, are main sites of PTX3 expression, which is absent in resting and activated T or B lymphocytes and NK cells [41, 68, 69].
PTX: role in allergic diseases
In human patients, PTX3 levels in BALF and sputum significantly correlate with disease severity [21, 22] and have been associated with allergic asthma in adults [14, 21, 22, 41, 70] and children [14]. Moreover, patients with allergic asthma show significantly higher PTX3 expression in airway smooth muscle cells as compared to healthy donors [41]. PTX3 enhances smooth muscle cell eotaxin‐1 release, which contributes to eosinophilic airway inflammation and airway remodelling [41]. In children, a positive correlation was identified between high sputum PTX3 levels and decreased pulmonary function. Similarly, significant positive correlations were identified between PTX3 sputum concentrations and eosinophil counts in sputum and blood as well as total serum immunoglobulin E (IgE) levels [22]. These findings have been confirmed recently in an independent study in a group of over 100 children with allergic asthma [71]. Notably, there is also a role for PTX3 in children with allergic rhinitis [37]. Children monosensitized to seasonal or perennial allergens and those with allergic rhinitis presented higher PTX3 plasma levels which also correlated with symptom severity [37]. Interestingly, the human PTX3 gene is located on chromosome 3q24–28 [42], a region associated with sensitization to Der p 1, a major allergen from house dust mites, and total serum IgE levels in humans [72]. A connection between PTX3 and type 2 immune responses was also established in an experimental model of ovalbumin‐induced allergic asthma in mice [21]. PTX3‐deficient mice exhibited enhanced type 2 inflammation in the lungs after ovalbumin sensitization and challenge with increased airway hyperresponsiveness, mucus plugging of the airways as well as BALF and lung eosinophilia [21]. The allergic phenotype was associated with a Th17‐dominant inflammatory response with an enhanced recruitment of granulocytes, particularly eosinophils and neutrophils, and increased IgE/IgG2a secretion [21]. Mechanistically, the Th17‐dominant allergic response in these mice was supported by the Th17‐polarizing cytokines IL‐6 and IL‐23 from DCs [21]. In summary, serum PTX3 levels might be a valuable marker to demonstrate the presence of low‐grade inflammation in asthma [71]. However, serum PTX3 levels seem to be inadequate as marker for asthma management, and it remains a matter of debate whether PTX3 might be directly involved in the etiology of allergic airway inflammation.
C‐reactive protein
CRP has been named for its ability to bind and precipitate the ‘C’ polysaccharide contained in the pneumococcal cell wall in a Ca2+‐dependent manner [73]. Later on, this antigen has been identified as phosphocholine and related molecules on other microorganisms [74] but CRP also binds to phosphocholine exposed on the surface of damaged cells [75]. This involves CRP in the clearance of apoptotic cells [75]. Additional CRP ligands have been identified in recent years including nuclear antigens such as histones and chromatin as well as proteins from small nuclear RNP particles, fibronectin, laminin and polycations [76], which points to an important regulatory role of CRP in the development of autoimmunity [77].
CRP is an ideal marker for acute inflammation, showing a more than 1000‐fold increase in concentration under inflammatory conditions [75]. In recent years, more sensitive assays have been developed and high‐sensitivity CRP determination is considered as an indicator of low‐grade systemic inflammation that may be a useful predictor for the risk of atherosclerosis [78] and myocardial infarction [79]. It can also serve as an indicator of airway inflammation for many noncommunicable lung diseases including asthma [14, 34, 39, 40, 80].
CRP: structure/function relationship
The strongest activator for CRP transcription is interleukin‐6 [81], which requires the synergistic interaction of CCAAT/enhancer‐binding protein (C/EBP)‐beta and Rel p50 [82]. By contrast, interferon α significantly inhibits IL‐6‐induced CRP expression in hepatocytes [83], explaining why viral infections cause only modest elevations of CRP. Notably, the small molecule hypolipidemic drug Gemcabene not only lowers cholesterol but also CRP levels alone and synergistically with statins [84], and this is accomplished by interference with C/EBP‐delta and NF‐kB [85]. Once translated, monomeric CRP (mCRP; 21 kDa) assembles to pentamers in human plasma, which, in fact, might be in equilibrium with a decamer form under physiological conditions [86]. Mechanistically, CRP has homologous regions found in immunoglobins G (IgGs) and can therefore bind to FcγRI and FcγRIIa but also activate the complement cascade via C1q [87]. Moreover, an interesting dissociation mechanism has been described for pentameric CRP in the presence of bioactive lipids recently, suggesting that pentameric CRP which gives rise to mCRP [88, 89, 90, 91]. mCRP, formed on the surface of activated platelets, shows increased inflammatory properties and is rarely found in circulation, suggesting its predominant role in local inflammation that likely serves as a contributing factor atherosclerotic plaque formation [92]. However, mCRP also mitigates distinct immune reactions, for example, when bound to neutrophils via the FcγRIII, mCRP was shown to inhibit fMLP‐triggered chemotaxis [93], while aggregated CRP appeared to enhance reactive oxygen species production in response to aggregated IgG but not other stimuli, such as phorbol myristate acetate or opsonized zymosan [94]. Moreover, by recruiting factor H, mCRP also seems to be involved in blunting C3b activity, thereby contributing to the safe removal of opsonized bodily material [95].
CRP: epidemiologic data‐lung immunity
CRP is a well‐established biomarker of lung infection. In fact, CRP was first described in patients suffering from acute pneumonia due to Streptococcus pneumoniae infections [73]. Subsequently, increased CRP levels have been established as a systemic marker of reduced lung function also in chronic lung diseases such as cystic fibrosis [96] and chronic obstructive pulmonary disease [97]. The exact role of mCRP in the lung is still unknown.
CRP: role in allergic diseases
Serum CRP levels, as determined by high‐sensitivity CRP assays, have been found to be significantly elevated in asthma patients as compared to healthy controls [14, 34, 40, 98, 99], suggesting an association between plasma CRP levels and the severity of asthma [39, 100]. Indeed, elevated serum CRP levels have been correlated with decreased pulmonary function and increased sputum eosinophilic cationic protein levels and eosinophil numbers in steroid naïve asthma patients [34, 35, 39, 98, 99, 101, 102]. These studies were the first to indicate an association of systemic inflammation with airway inflammation. Similarly, studies in Danish school children as well as cohorts of U.S. children and adolescents reported that increased CRP levels were significantly associated with allergic sensitization and an elevated risk of concomitant allergic airway inflammation (rhinitis, asthma), however, independently of allergen type or clinical allergy symptoms [103, 104]. Yet, CRP cannot be used as a predictive biomarker in allergy as no associations were observed between CRP levels at 6 months of age with later development of allergic diseases [104]. Therefore, it was concluded that the low‐grade inflammation seen in children with allergic sensitization may be host intrinsic and part of a systemic inflammatory disorder rather than an isolated type 2 immune response or indirectly linked to sensitization through shared environmental risk factors (diet, microbiome) [104].
Apart from systemic alterations of serum CRP levels during lung diseases, there is clear evidence that CRP is also produced and secreted locally in the airways by epithelial cells such as nasal epithelial cells and the alveolar basal epithelial cell line A549 [105, 106, 107] and alveolar macrophages [108] Therefore, CRP may have direct and local effects on airway inflammation [109, 110] and contribute to bacterial clearance in the human respiratory tract [105].
With regard to the short pentraxin SAP, very little is known about its expression in allergic disease. Recently, Gao and coworkers found that sputum SAP levels in patients with eosinophilic asthma were significantly higher than in individuals suffering from noneosinophilic asthma or healthy controls [14].
Collectins
Collectins are collagen containing C‐type lectins comprising MBL, surfactant proteins A and D as well as organ‐specific collectins.
Mannose‐binding lectin
MBL: structure/function relationship
Human mannose (also mannan)‐binding lectin recognizes a large array of pathogens and activates the most ancient pathway of complement [111], very similar to C1q, which activates the classical pathway of complement [112]. MBL is composed of three identical 32 kDa polypeptide chains, which associate to a hexamer [113]. The individual polypeptide chains are composed of a carbohydrate binding and a neck domain, which is followed by a collagen domain. In circulation, MBL is complexed to the MBL‐associated serine proteases MASP‐1 and ‐2 [114, 115]. MBL is mainly expressed and produced in the liver [116].
MBL: epidemiologic data‐lung immunity
Studies in mouse and man have indicated that MBL is absent from the BALF of healthy individuals [117, 118], but can be clearly detected in the BALF of patients suffering from acute (pneumonia) [119] or chronic (protracted bacterial bronchitis) [120] respiratory diseases. Thus, detectable MBL levels in BALF can be regarded as a sign of inflammatory immune cell infiltration during infection [119].
With regard to its protective activity, MBL seems to be especially important during early childhood and in situations of immunosuppression. Some MBL variants fail to produce stable multimeric forms and are thus dysfunctional [116]. Indeed, MBL protects from infections and prolongs life expectancy in patients with congenital lung diseases such as cystic fibrosis, in which low MBL levels reduce life expectancy by 8 years [121]. The disease‐modifying role of MBL2 has been also correlated with the frequency of respiratory infection‐associated hospital admissions in chronic obstructive pulmonary disease patients [122], implicating a putative beneficial role for MBL replacement therapy in this disease entity [123]. Paradoxically, a recently performed long‐term follow‐up study showed that MBL‐deficient COPD patients had more polymorphic microbiota and less risk for infectious exacerbations when compared to patients with fully functional MBL. This was attributed to the fact that no oxidized and thus no macrophage‐inhibitory MBL could be formed in the collective of MBL‐deficient patients studied [124]. Hence, lung MBL can be regarded a double‐edged sword in situations of chronic inflammation, since oxidized forms of MBL might interfere with MBL‐oligomer formation and thus clearance of distinct microorganisms [125]
Apart from opsonizing pathogens and thus facilitating phagocytosis of microorganisms, MBL also largely contributes to the removal of apoptotic cells, especially within the lining of the lung alveoli, a process commonly referred to as efferocytosis [126]. Target cell bound MBL is recognized by calreticulin (the cC1qR), which is associated with CD91 (the α2 macroglobulin receptor), an endocytic receptor expressed on many cell types (hepatocytes, neurons, fibroblasts) including blood monocytes [127]. While the role of calreticulin for the uptake of MBL immune complexes is undisputed, some reports question a firm association of endocytosing calreticulin with CD91 [128]. This might indicate that other cell surface receptors are involved in calreticulin‐dependent endocytosis and thus require a redefinition of the clearance pathway for MBL bound. Intriguingly, the high frequency of MBL deficiency, amounting to 5% in the general population, represents a constant matter of debate, since only a selective advantage is able to establish such a high degree of ‘deficiency’ for a single protein playing otherwise an important role in humans. One could speculate that MBL deficiency potentially protects from autoimmunity, because in the absence of MBL the likelihood that the immune system becomes primed against constituents of apoptotic cell bodies or serum components is much lower [129]. Moreover, the absence of MBL might protect from intracellular parasitism/infections, since MBL might favour infection with mycobacteria (Mycobacterium tuberculosis and Mycobacterium leprae) [111]. However, also the exact opposite has been hypothesized, by showing that coincubation of T cells with MBL inhibits T cell activation, while the absence of functional MBL has been implicated to promote T cell driven autoimmune processes such as systemic lupus erythematosus and rheumatoid arthritis [130].
MBL: role in allergic diseases
MBL has been shown to bind to distinct allergen extracts and to activate complement via the lectin pathway, however, only in the presence of allergen‐specific IgG [131]. Moreover, significantly increased MBL plasma levels have been detected in children with asthma and in adults with asthma and associated allergic rhinitis [132], which correlated with peripheral blood eosinophil levels in children [133]. Similarly, patients suffering from Aspergillus fumigatus‐associated bronchopulmonary aspergillosis presented with elevated MBL serum levels [132], with the generated C3a and C5a fragments contributing to the bridging of innate and adaptive immune responses in asthma [134]. The possible involvement of MBL in the pathogenesis of airway hyperresponsiveness has been substantiated in preclinical studies in mMBL‐A knockout mice, which revealed a mitigated airway response upon challenge with Aspergillus fumigatus in the absence of mMBL‐A [135].
Surfactant proteins‐A and ‐D
Surfactant proteins‐A and‐D: structure/function relationship
In the lungs, surfactant protein‐A (SP‐A) and surfactant protein‐D (SP‐D) are expressed and produced by alveolar type II cells and club cells of the distal bronchioles [136, 137, 138]. Similar to C1q, SP‐A proteins form a bouquet‐like structure [139]. Within the alveolar compartment, SP‐A is tightly associated with phospholipids. SP‐A opsonizes Staphylococcus aureus but also Herpes simplex particles for uptake by alveolar macrophages [140, 141]. In contrast, SP‐D obtains a cruciform structure, which links its trimeric subunits [142]. In addition, SP‐D also exists as a nonlipid bound free form, especially in alveoli. Apart from saccharides, SP‐D also binds to phosphatidyl‐inositol and glucosylceramide [143, 144, 145]. SP‐D plays an important role in the microbicidal activity within the alveolar space, since it opsonizes a number of Gram‐negative bacteria including Escherichia coli, activates the respiratory burst in alveolar macrophages [146] and leads to uptake of E. coli particles by DCs followed by T cell activation [147].
Surfactant proteins A and D: epidemiologic data‐lung immunity
Lung diseases, which increase alveolar‐capillary leakage, such as lung fibrosis or interstitial pneumonia, are associated with increased serum levels of SP‐A and SP‐D, while the respective BALF levels are reduced [148], making SP‐A and SP‐D markers for alveolar integrity. In addition, increases in especially of SP‐D levels have been observed to correlate with the degree of lung pneumonitis upon irradiation therapy [149].
Surfactant proteins A and D: role in allergic diseases
SP‐A has been shown to bind to a number of grass pollen grains in a Ca2+‐ and carbohydrate‐specific manner [150]. Moreover, both SP‐A and SP‐D bind to house dust mite [151] and Aspergillus fumigatus [152] antigens, binding to the latter inhibits the allergen's recognition by specific IgE antibodies and consequently blunts IgE‐dependent effector cell [153] and T cell [154] activation. In line with these findings, intranasal application of SP‐D in mice inhibited airway hypersensitivity induced by Aspergillus fumigatus [155, 156] and Dermatophagoides pteronyssinus [157], while challenge with Aspergillus fumigatus of SP‐A or SP‐D knockout mice promoted type 2 immunity involving hypereosinophilia and a high IL‐13/IFN‐γ‐ratio, which, again, could be abrogated by SP‐A and/or SP‐D application [158]. Of relevance, once IL‐13 becomes produced, it reduces SP‐D production by alveolar type II cells [159]. Irrespective of this regulatory mechanism, SP‐D serum [160] and BALF [161] levels were found to be increased in patients with allergic asthma.
Ficolins
Ficolins: structure/function relationship
Ficolins represent a class of opsonins that are characterized by the presence of a C‐terminal fibrinogen‐like and an N‐terminal collagen‐like domain (fi‐col‐ins) [162] that have distinct ligand specificities (GlcNAc GalNAc, sialic acid, d‐fucose). The fibrinogen‐like domain specifically binds to pathogen‐associated carbohydrates, while the collagen‐like domain signals ligand binding to the MBL‐associated serine proteases leading to their activation, eventually leading to the cleavage of C2 and C4 and the formation of C3 convertase activity [116]. Ficolins are lectins, which assemble to form oligomeric structures that look like a ‘bunch of flowers’ and have high, calcium‐dependent binding specificity for N‐acetyl‐glucosamine (GlcNAc), which is an important component of the cell wall of Aspergillus fumigatus. Although primarily functioning as opsonins, orchestrating the phagocytosis of fungi, ficolins also contribute to the activation of the lectin pathway by interacting and activating the MBL‐associated serine proteases [163] and trigger the production of inflammatory cytokines and nitric oxide by macrophages [164].
Ficolins: Epidemiologic data‐lung immunity
Two serum soluble ficolins produced by hepatocytes exist, that is, L‐ficolin (ficolin‐2) [165] and H‐ficolin (ficolin‐3) [166]. Notably, the latter can be also produced and secreted by bronchial and alveolar type II epithelial cells [167]. Ficolin‐3 becomes increasingly produced by AEC upon systemic challenge with lipopolysaccharides (LPS) [168]. In addition, a membrane‐bound ficolin, M‐ficolin, for which binding to Escherichia coli has been described [169], is primarily expressed on monocytes [170]. While there is clear‐cut binding of ficolin‐3 to Aspergillus fumigatus conidia, its binding affinity is much lower than that of serum‐derived ficolin‐2. Moreover, low ficolin‐2 serum levels identify patients suffering from common variable immunodeficiency who are at risk to develop bronchiectases, a serious life‐shortening sequela of the disease [171]. Ficolin‐3 bound to sialylated glycans scavenges influenza A virus, which leads to the block of infectivity and hemagglutination activity of influenza A virus and its complement mediated destruction [172]. Ficolin‐3 deficiency due to mutations at position 1637 of the gene (1637delC) leads to immunodeficiency with recurrent lower respiratory infections, septicemia and warts [173], which can even lead to sudden death due to acute meningitis [174]. Interestingly, individuals heterozygous for the 1637delC mutation present with lower serum ficolin‐3 concentrations [175]. Moreover, ficolin‐3 has been shown to be responsible for the removal of late apoptotic cells [176]. This salient function might also be one of the reasons why ficolin‐3 is also a prominent target for autoantibodies (with DNA and other factors acting as adjuvant). Of note, anti‐ficolin‐3 antibodies are frequently (in one third) observed in systemic lupus erythematosus patients and currently have the strongest association of all autoantibodies with active lupus nephritis [177].
Ficolins: Role in allergic diseases
The direct involvement of ficolins in allergic inflammation is currently unknown. However, a retrospective study suggests that low ficolin‐2 serum levels are associated with atopic disorders [178], which subsequently was confirmed in a prospective study indicating that ficolin‐2 deficiency is associated with allergic and infectious respiratory diseases [179]. The authors speculated that low ficolin levels might lead to a lack of protection from microorganisms potentially complicating (driving) allergic diseases [178].
Serum amyloid A
Similar to the short pentraxin CRP, the serum amyloid A (SAA) proteins, have been classically viewed as highly inducible, liver‐derived factors in response to infection or trauma [180]. Today, baseline expression of SAA is well documented for many extrahepatic human tissues including the alveolar epithelial lining [181], alveolar macrophages [181] but also monocytes [182].
SAA: structure/function relationship
SAA is a sPRR for Gram‐negative bacteria [183, 184] and blocks entry of viruses (e.g. hepatitis C virus) into cells [185]. More recently, SAA1 was found to directly bind LPS to form a complex that promotes LPS clearance by macrophages, which offers partial protection against LPS‐induced inflammation and acute lung injury [186]. In addition, SAA proteins can bind and transport retinol during bacterial infection [187], thereby restricting their optimal growth and expansion. Moreover, mice lacking Saa3 express lower levels of retinoic acid receptors than their wild‐type littermates, which inhibits their ability to respond to retinoic acid and may lead to an incapacity to develop immune tolerance [188]. In humans, there are three SAA proteins that are encoded by the inducible genes, SAA1 and SAA2, and the SAA4 gene that is constitutively expressed [180, 189]. SAA3, which is a pseudogene in humans, encodes an additional inducible form of SAA in mice with high structural similarities to other SAA proteins [180, 189]. Locally produced, lipid‐free SAA1 can act through a number of potential SAA receptors [190] and has pleiotropic effects on the immune system including the recruitment of immune cells [191, 192, 193], epithelial wound repair [194] and proinflammatory cytokine production [185, 195, 196, 197, 198, 199, 200]. Considering the dramatic increase in SAA levels at sites of inflammation, the biological activities of SAA1 need to be tightly controlled, for example, by factors present in specific tissue microenvironments or by the ability of SAA1 to adopt different oligomeric states [187, 201, 202, 203, 204]. The structure of SAA, however, is different from chemokines and cytokines, and SAA seems to require ligand‐induced activation, which is not observed under physiological conditions [186, 205, 206]. For example, binding to plasma high‐density lipoprotein abrogates the cytokine‐like activities of SAA proteins [206].
SAA: epidemiologic data‐lung immunity
SAA3 knockout mice develop intrinsic airway hyperresponsiveness and show increased mortality to influenza A virus infection [188]. In mice, Saa3 may therefore have key homeostatic functions in lung development and inflammation [188]. The sequence similarity of mouse Saa3 and human SAA1/2 suggests that their functions during inflammatory processes might be similar.
In the lungs, SAA1 has been established as a mediator of local effector Th17 responses [197, 207, 208, 209]. This is consistent with the role for SAAs in retinol shuttling that is required to induce Th17 responses in the presence of infection and inflammation [187]. SAA‐induced Th17 responses are associated with robust airway neutrophilia [207] and more severe asthma phenotypes that are less responsive to corticosteroids [210].
SAA: role in allergic diseases
SAA is by far the most widely studied acute phase protein in the airways and has been repeatedly associated with chronic rhinosinusitis but also asthma symptoms [23, 24, 36, 186, 188, 207, 211, 212, 213, 214]. One such study analysed a fraction of the SARP‐3 study population (i.e. adult participants suffering from severe and nonsevere asthma), an NIH‐sponsored multisite cohort study conducted to investigate mechanisms of severe asthma. Notably, it was found that patients with severe asthma showed increased pruning of the pulmonary vasculature that was associated with decreased lung function, greater peripheral and sputum eosinophilia, and higher BAL SAA/lipoxin A4 ratio [87]. Moreover, there is a strong correlation between elevated blood as well as sputum SAA levels and asthma prevalence and/or allergic rhinitis and disease severity [23, 24, 212]. As described above, the ability of SAA1 to adopt different oligomeric states (monomers and oligomers) [202] or to transport retinols and related molecules [187, 188] might serve to scavenge and sequester essential vitamins (or even nutrients) to deter pathogen growth at mucosal surfaces [215] and may thus have important functional implications for promoting local inflammation. Considering that SAA is increased in allergic asthma [23, 24], it remains to be established whether SAA functions as an adjuvant and/or interacts directly with environmental airborne proteins to promote allergic sensitization. Preliminary evidence of the authors (Smole et al., in revision) suggests that a component within house dust mite extracts can lead to active forms of SAA which impact on AEC and the induction of type 2 immunity, thus contributing to the priming of exposed individuals for sensitization and allergic disease (Smole et al.). A better understanding of the diverse immune protective mechanisms of acute phase SAA at mucosal surfaces may help to develop novel therapies for infectious but also inflammatory diseases such as allergic asthma and rhinitis [186].
Complement components
The complement system represents a central and very effective innate protection mechanism, which received its name from Paul Ehrlich, who intended to indicate that it enhances (complements) the activity of antibodies and phagocytes. It can be regarded as one of the centrepieces of humoral innate immunity and critically contributes to defence mechanisms in the airways. An emerging theme is, however, the recently discovered noncanonical functions of complement [216, 217], which indicate that this system has several important functions beyond being ‘the guardian of the extracellular space’ and being an ‘important player within the innate sensor network’. These functions include but are not restricted to its important contributions to neuronal cell and organ development and brain function [218], tissue repair, regeneration [219] and metabolic programming (also referred to as complosome) [220, 221, 222, 223], including the modulation of immune cell effector functions.
Complement: structure/function relationship
The complement system encompasses more than 30 serum‐soluble and membrane‐expressed molecules [224, 225]. All three complement activation pathways (classic, nonclassic and lectin) eventually lead to C3‐ followed by C5‐convertase activity. Serine protease activity converts the evolutionary conserved C3 molecule, which is molecularly related to alpha‐2‐macroglobulin and highly abundant in human serum (1.2–1.3 g/L), into the small (10 kDa) and diffusible anaphylatoxin C3a and the larger opsonin fragment C3b [224, 225]. Similarly, C5 is converted into the small anaphylatoxin C5a (11 kDa) and the larger C5b fragment. The latter associates with C6 and C7, attaches to membranes (microbial or bodily) and completes the formation of the membrane attack complex by binding of C8 and C9 [223]. C3b‐dependent opsonization occurs upon conformational change of C3b and exposure of the hidden internal thioester bond which is highly reactive and covalently binds to nucleophils such as OH‐ or NH2‐groups in its immediate surroundings. The anaphylatoxins C3a and C5a are potent inflammatory mediators, which target a number of inflammatory and noninflammatory cell types.
Complement: epidemiologic data‐lung immunity
The central components of complement, that is, circulating C3 and C5, are largely produced in the liver. However, it has been clearly demonstrated that apart from other nonliver cells, such as endothelial cells and immune cells (granulocytes, monocytes, DCs, T cells), also airway epithelial cells, such as BEAS‐2B cells, constitutively synthesize and release C3 under serum‐free conditions which can be significantly upregulated by IL‐1 or TNF‐α but not IFN‐γ [226, 227]. In addition, lung cancer cells substantially generate and release C5a, which is believed to create a milieu favourable for tumour cells and thus contributing to lung cancer progression [228]. Intratracheal instillation of C3a or peptides thereof induces respiratory distress, which is accompanied by C3‐dependent bronchial smooth muscle contraction in bronchioles and hemodynamic effects due to C3a‐induced platelet aggregation in pulmonary arteries [229, 230]. AEC (and airway smooth muscle cells) can sense complement, since both human and murine AEC express C3a‐ and C5a‐receptors under steady‐state conditions, which become upregulated in situations of endotoxinemia and allergen‐induced asthma [231]. Targeting of these receptors on AEC by intratracheal instillation of antibodies has been shown to reduce pulmonary oedema and lung injury [232, 233]. Apart from soluble complement components, AEC under steady‐state conditions also express transmembrane complement regulatory proteins such as Membrane cofactor protein (CD46), Decay accelerating factor (CD55) and protectin (CD59), with IFN‐γ able to significantly upregulate DAF levels [226]. It is therefore not surprising that complement proteins can also be detected in the BALF of healthy individuals [234] and that complement levels are increased upon exposure to bacterial danger signals such as LPS [235]. Some of these complement regulatory proteins might also contribute to the overall reduction of inflammation, such as CD46, which was shown to downregulate IL‐1β levels induced by H2O2‐activated AEC due to induction of autophagy and associated downregulation of inflammasome components like pro‐IL‐1β and NLRP3 [236]. Moreover, AEC were shown to scavenge (actively take up) C3 from their surroundings, very similar to CD4 T cells. In its intracellularly stored form, C3 is supposed to play a cytoprotective role by potentially influencing oxidative stress of AEC [237]. Production but also storage of C3 by AEC might have important implications also for end‐stage lung diseases such as cystic fibrosis and chronic obstructive pulmonary disease in which C3 stores in AEC are found to be increased. C3 is stored within the endoplasmic reticulum and late endosomes in its mature form, ready to become activated by cleavage. Other cell types known to not only synthesize but also store large amounts of C3 are neutrophils [238] and monocytes [239]. Moreover, T cells are not only activated by complement split products but also contain C3 and C5 intracellularly [240]. Whether T cells express and are able to activation‐dependently upregulate C3 and C5, or rather accumulate preformed C3 and C5 proteins present in large amounts in bodily fluids is a matter of intense discussion and might critically depended on APC‐T cell interaction [8, 241]. However, it has been clearly shown that T cells have complement convertase activity and can activate intracellular C3 by virtue of T cell‐expressed cathepsin L [9]. Secretion of such activated C3 (C3a) contributes to T cell‐mediated inflammation in target tissues including the lung. This also fits to the recent finding that intracellular pools of C3 become reduced upon T cell activation [8].
Complement: role in allergic diseases
Early reports by Nagata and Glovsky have shown that different airborne allergens contain complement convertase activity and lead to C3a formation when incubated with serum [242]. Later on, these findings were corroborated by studies demonstrating that the serine protease from the house dust mite Dermatophagoides farinae generates the anaphylatoxins C3a and C5a [243], which already suggested a possible involvement of house dust proteases in the pathogenesis of allergic diseases [243]. Moreover, the mould component chitin, which is an important component of Aspergillus fumigatus, is a strong activator of the alternative complement pathway and thereby shapes the ensuing immune response upon inhalation [244].
Preclinical data confirmed the importance of anaphylatoxins in asthma pathogenesis. Along those lines, it was demonstrated that genetic deletion of C3aR on mouse AEC protects mice from lung dysfunction after allergen challenge [245]. Consistent with these findings, BALF of human patients with asthma [246, 247] but also respiratory distress syndrome [248] contain increased levels of C3a and C5a which might, at least in part, originate from AEC stores. Notably, the C3 pathway has been shown to be involved in the development of Th2 responses in allergic inflammation [245, 249], which is also reflected by the fact that C3a levels are found upregulated in individuals with respiratory allergies [250, 251] and asthma [252]. In addition, a positive correlation between C3 levels and asthma severity has been described [252]. Apart from lungs, also upper airway diseases, such as chronic rhinosinusitis, have been shown to be associated with increased local production of C3. Indeed, its neutralization has therapeutic effects in this difficult to treat condition [253].
In summary, the complement system and especially C3 is pivotal for the innate humoral protection of the airways. Humoral factors that are directly released by airway epithelial cells or immune cells associated with the epithelial cell lining diffuse into the mucus lining, and the majority of them eventually take advantage of the C3 convertase activity to signal the presence of danger.
Summary
Mucosal surfaces represent very critical barrier tissues of our body that ensure immune homeostasis in response to microorganism that ranges from host defence to active tolerance and symbiosis. Consequently, and among other protection mechanisms, sPRR play an important role in local immune surveillance of mucosal surfaces and can quickly neutralize, opsonize or induce phagocytosis of potentially harmful intruders. Ligand binding by sPRR very effectively activates and amplifies canonical pathways of humoral immunity such as the complement system. The central component of it, that is C3, is also found in the airway mucosal lining. As such, sPRRs within the airway epithelial lining represent a very effective form of ‘forward defence’, especially against highly virulent and/or invasive strains of microorganisms characterized by strain‐specific sugar or lipid moieties (e.g. Streptococcus pneumoniae, Aspergillus fumigatus, etc.), and thereby prevent early and devastating epithelial damage to occur. Although seemingly redundant, sPRRs have largely diverging ligand specificities, several of them converge by activating the complement cascade. A large body of evidence suggests that sPRR might also sense the presence of usually innocuous proteins (allergens), setting the stage for allergic sensitization and subsequent allergic disease. Future research will focus on dissecting the underlying cellular and molecular complexity of potential sPRR–allergen interactions and highlight approaches of how to interfere with such undesired, allergen‐driven activation mechanism.
Author contributions
U.S., B.K. and W.F.P. wrote the paper.
Conflict of interest
Winfried F. Pickl holds stocks of Biomay AG and receives honoraria from Novartis and Roche. All other authors have no additional commercial or financial conflict of interest.
Abbreviations
- AEC
airway epithelial cell
- BALF
bronchoalveolar lavage fluid
- CRP
C‐reactive protein
- DCs
dendritic cells
- MBL
mannose‐binding lectin
- mCRP
momomeric C‐reactive protein
- PTX
pentraxin
- SAA
serum amyloid A
- SAP
serum amyloid P
- SP‐A
surfactant protein‐A
- SP‐D
surfactant protein‐D
- sPRR
soluble pattern recognition receptors
Acknowledgements
This work was supported by grants DK‐W1248 and SFB F4609 of the Austrian Science Foundation (FWF).
References
- 1. Kay, A. B. , Allergy and hypersensitivity: history and concepts In Kay A. B., Kaplan A. P., Bousquet J. and Holt P. G. (Eds), Allergy and Allergic Diseases. Blackwell Publishing, Oxford, UK: 2008, pp 1–22. [Google Scholar]
- 2. Mantovani, A. , Valentino, S. , Gentile, S. , Inforzato, A. , Bottazzi, B. and Garlanda, C. , The long pentraxin PTX3: a paradigm for humoral pattern recognition molecules. Ann. N. Y. Acad. Sci. 2013. 1285: 1–14. [DOI] [PubMed] [Google Scholar]
- 3. Bottazzi, B. , Doni, A. , Garlanda, C. and Mantovani, A. , An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 2010. 28: 157–183. [DOI] [PubMed] [Google Scholar]
- 4. Tucci, A. , Goldberger, G. , Whitehead, A. S. , Kay, R. M. , Woods, D. E. and Colten, H. R. , Biosynthesis and postsynthetic processing of human C‐reactive protein. J. Immunol. 1983. 131: 2416–2419. [PubMed] [Google Scholar]
- 5. Litvack, M. L. and Palaniyar, N. , Soluble innate immune pattern‐recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation. Innate Immun. 2010. 16: 191–200. [DOI] [PubMed] [Google Scholar]
- 6. Martin, T. R. and Frevert, C. W. , Innate immunity in the lungs. Proc. Am. Thorac. Soc. 2005. 2: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu, Y. , Endo, Y. , Iwaki, D. , Nakata, M. , Matsushita, M. , Wada, I. , Inoue, K. et al., Human M‐ficolin is a secretory protein that activates the lectin complement pathway. J. Immunol. 2005. 175: 3150–3156. [DOI] [PubMed] [Google Scholar]
- 8. Hansen, C. B. , Willer, A. , Bayarri‐Olmos, R. , Kemper, C. and Garred, P. , Expression of complement C3, C5, C3aR and C5aR1 genes in resting and activated CD4(+) T cells. Immunobiology 2019. 224: 307–315. [DOI] [PubMed] [Google Scholar]
- 9. Liszewski, M. K. , Kolev, M. , Le Friec, G. , Leung, M. , Bertram, P. G. , Fara, A. F. , Subias, M. et al., Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation> Immunity 2013. 39: 1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inforzato, A. , Doni, A. , Barajon, I. , Leone, R. , Garlanda, C. , Bottazzi, B. and Mantovani, A. , PTX3 as a paradigm for the interaction of pentraxins with the complement system. Semin. Immunol. 2013. 25: 79–85. [DOI] [PubMed] [Google Scholar]
- 11. Mantovani, A. , Garlanda, C. , Doni, A. and Bottazzi, B. , Pentraxins in innate immunity: from C‐reactive protein to the long pentraxin PTX3. J. Clin. Immunol. 2008. 28: 1–13. [DOI] [PubMed] [Google Scholar]
- 12. Zhang, P. , Liu, X. and Cao, X. , Extracellular pattern recognition molecules in health and diseases. Cell. Mol. Immunol. 2015. 12: 255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deban, L. , Jaillon, S. , Garlanda, C. , Bottazzi, B. and Mantovani, A. , Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 2011. 343: 237–249. [DOI] [PubMed] [Google Scholar]
- 14. Gao, P. , Tang, K. , Wang, M. , Yang, Q. , Xu, Y. , Wang, J. , Zhao, J. et al., Pentraxin levels in non‐eosinophilic versus eosinophilic asthma. Clin. Exp. Allergy 2018. 48: 981–989. [DOI] [PubMed] [Google Scholar]
- 15. Olafsdottir, I. S. , Gislason, T. , Thjodleifsson, B. , Olafsson, I. , Gislason, D. , Jogi, R. and Janson, C. , C reactive protein levels are increased in non‐allergic but not allergic asthma: a multicentre epidemiological study. Thorax 2005. 60: 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malle, E. and De Beer, F. C. , Human serum amyloid A (SAA) protein: a prominent acute‐phase reactant for clinical practice. Eur. J. Clin. Invest. 1996. 26: 427–435. [DOI] [PubMed] [Google Scholar]
- 17. Zhou, Y. , Lu, K. , Pfefferle, S. , Bertram, S. , Glowacka, I. , Drosten, C. , Pohlmann, S. et al., A single asparagine‐linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose‐binding lectin through multiple mechanisms. J. Virol. 2010. 84: 8753–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gralinski, L. E. , Sheahan, T. P. , Morrison, T. E. , Menachery, V. D. , Jensen, K. , Leist, S. R. , Whitmore, A. et al., Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 2018. 9: e01753‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang, R. , Xiao, H. , Guo, R. , Li, Y. and Shen, B. , The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg. Microbes Infect. 2015. 4: e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park, M. , Thwaites, R. S. and Openshaw, P. J. M. , COVID‐19: lessons from SARS and MERS. Eur. J. Immunol. 2020. 50: 308–311. [Google Scholar]
- 21. Balhara, J. , Shan, L. , Zhang, J. , Muhuri, A. , Halayko, A. J. , Almiski, M. S. , Doeing, D. et al., Pentraxin 3 deletion aggravates allergic inflammation through a TH17‐dominant phenotype and enhanced CD4 T‐cell survival. J. Allergy Clin. Immunol. 2017. 139: 950–963 e959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim, M. J. , Lee, H. S. , Sol, I. S. , Kim, M. N. , Hong, J. Y. , Lee, K. E. , Kim, Y. H. et al., Sputum pentraxin 3 as a candidate to assess airway inflammation and remodeling in childhood asthma. Medicine (Baltimore) 2016. 95: e5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozseker, F. , Buyukozturk, S. , Depboylu, B. , Yilmazbayhan, D. , Karayigit, E. , Gelincik, A. , Genc, S. et al., Serum amyloid A (SAA) in induced sputum of asthmatics: a new look to an old marker. Int. Immunopharmacol. 2006. 6: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 24. Buyukozturk, S. , Gelincik, A. A. , Genc, S. , Kocak, H. , Oneriyidogan, Y. , Erden, S. , Dal, M. et al., Acute phase reactants in allergic airway disease. Tohoku J. Exp. Med. 2004. 204: 209–213. [DOI] [PubMed] [Google Scholar]
- 25. Haczku, A. , Cao, Y. , Vass, G. , Kierstein, S. , Nath, P. , Atochina‐Vasserman, E. N. , Scanlon, S. T. et al., IL‐4 and IL‐13 form a negative feedback circuit with surfactant protein‐D in the allergic airway response. J. Immunol. 2006. 176: 3557–3565. [DOI] [PubMed] [Google Scholar]
- 26. Bottazzi, B. , Inforzato, A. , Messa, M. , Barbagallo, M. , Magrini, E. , Garlanda, C. and Mantovani, A. , The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016. 64: 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pepys, M. B. , The pentraxins 1975–2018: serendipity, diagnostics and drugs. Front. Immunol. 2018. 9: 2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balhara, J. , Koussih, L. , Zhang, J. and Gounni, A. S. , Pentraxin 3: an immuno‐regulator in the lungs. Front. Immunol. 2013. 4: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emsley, J. , White, H. E. , O'Hara, B. P. , Oliva, G. , Srinivasan, N. , Tickle, I. J. , Blundell, T. L. et al., Structure of pentameric human serum amyloid P component. Nature 1994. 367: 338–345. [DOI] [PubMed] [Google Scholar]
- 30. Rubio, N. , Sharp, P. M. , Rits, M. , Zahedi, K. and Whitehead, A. S. , Structure, expression, and evolution of guinea pig serum amyloid P component and C‐reactive protein. J. Biochem. 1993. 113: 277–284. [DOI] [PubMed] [Google Scholar]
- 31. Clos Du, W. T., Pentraxins: structure, function, and role in inflammation. ISRN Inflamm. 2013. 2013: 379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peri, G. , Introna, M. , Corradi, D. , Iacuitti, G. , Signorini, S. , Avanzini, F. , Pizzetti, F. et al., PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 2000. 102: 636–641. [DOI] [PubMed] [Google Scholar]
- 33. Latini, R. , Maggioni, A. P. , Peri, G. , Gonzini, L. , Lucci, D. , Mocarelli, P. , Vago, L. et al., Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2004. 110: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 34. Allam, M. H. , Said, A. F. , El Samie Omran, A. A. , Abd El‐Reheim, D. M. and Kasem, A. H. , High sensitivity C‐reactive protein: its correlation with sputum cell counts in bronchial asthma. Respir. Med. 2009. 103: 1878–1884. [DOI] [PubMed] [Google Scholar]
- 35. Ceylan, E. , Bulut, S. , Yilmaz, M. , Orun, H. , Karadag, F. , Omurlu, I. K. , Kirdar, S. et al., The levels of serum biomarkers in stable asthma patients with comorbidities. Iran. J. Allergy Asthma Immunol. 2019. 18: 27–37. [PubMed] [Google Scholar]
- 36. Ho, J. , Hamizan, A. W. , Alvarado, R. , Rimmer, J. , Sewell, W. A. and Harvey, R. J. , Systemic predictors of eosinophilic chronic rhinosinusitis. Am J Rhinol Allergy 2018. 32: 252–257. [DOI] [PubMed] [Google Scholar]
- 37. Marseglia, G. L. , De Amici, M. , Leonardi, S. , Miraglia Del Giudice, M. , Salpietro, A. , La Rosa, M. , Caimmi, D. et al., Pentraxin 3 in children suffering from allergic rhinitis. J. Biol. Regul. Homeost. Agents 2012. 26: S109‐112. [PubMed] [Google Scholar]
- 38. Mauri, T. , Coppadoro, A. , Bellani, G. , Bombino, M. , Patroniti, N. , Peri, G. , Mantovani, A. et al., Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity. Crit. Care Med. 2008. 36: 2302–2308. [DOI] [PubMed] [Google Scholar]
- 39. Takemura, M. , Matsumoto, H. , Niimi, A. , Ueda, T. , Matsuoka, H. , Yamaguchi, M. , Jinnai, M. et al., High sensitivity C‐reactive protein in asthma. Eur. Respir. J. 2006. 27: 908–912. [DOI] [PubMed] [Google Scholar]
- 40. Yang, B. Y. , Markevych, I. , Harris, C. , Standl, M. , Schikowski, T. , Koletzko, S. , Herberth, G. et al., High‐sensitivity C‐reactive protein and allergic endpoints in German adolescents. Int. Arch. Allergy Immunol. 2019. 179: 152–157. [DOI] [PubMed] [Google Scholar]
- 41. Zhang, J. , Shan, L. , Koussih, L. , Redhu, N. S. , Halayko, A. J. , Chakir, J. and Gounni, A. S. , Pentraxin 3 (PTX3) expression in allergic asthmatic airways: role in airway smooth muscle migration and chemokine production. PLoS One 2012. 7: e34965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Breviario, F. , d'Aniello, E. M. , Golay, J. , Peri, G. , Bottazzi, B. , Bairoch, A. , Saccone, S. et al., Interleukin‐1‐inducible genes in endothelial cells. Cloning of a new gene related to C‐reactive protein and serum amyloid P component. J. Biol. Chem. 1992. 267: 22190–22197. [PubMed] [Google Scholar]
- 43. Lee, G. W. , Lee, T. H. and Vilcek, J. , TSG‐14, a tumor necrosis factor‐ and IL‐1‐inducible protein, is a novel member of the pentaxin family of acute phase proteins. J. Immunol. 1993. 150: 1804–1812. [PubMed] [Google Scholar]
- 44. Bottazzi, B. , Vouret‐Craviari, V. , Bastone, A. , De Gioia, L. , Matteucci, C. , Peri, G. , Spreafico, F. et al., Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C‐reactive protein and serum amyloid P component. J. Biol. Chem. 1997. 272: 32817–32823. [DOI] [PubMed] [Google Scholar]
- 45. Ievoli, E. , Lindstedt, R. , Inforzato, A. , Camaioni, A. , Palone, F. , Day, A. J. , Mantovani, A. et al., Implication of the oligomeric state of the N‐terminal PTX3 domain in cumulus matrix assembly. Matrix Biol. 2011. 30: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamon, Y. , Jaillon, S. , Person, C. , Ginies, J. L. , Garo, E. , Bottazzi, B. , Ghamrawi, S. et al., Proteolytic cleavage of the long pentraxin PTX3 in the airways of cystic fibrosis patients. Innate Immun. 2013. 19: 611–622. [DOI] [PubMed] [Google Scholar]
- 47. Garlanda, C. , Jaillon, S. , Doni, A. , Bottazzi, B. and Mantovani, A. , PTX3, a humoral pattern recognition molecule at the interface between microbe and matrix recognition. Curr. Opin. Immunol. 2016. 38: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeannin, P. , Bottazzi, B. , Sironi, M. , Doni, A. , Rusnati, M. , Presta, M. , Maina, V. et al., Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity 2005. 22: 551–560. [DOI] [PubMed] [Google Scholar]
- 49. Koh, S. H. , Shin, S. G. , Andrade, M. J. , Go, R. H. , Park, S. , Woo, C. H. and Lim, J. H. , Long pentraxin PTX3 mediates acute inflammatory responses against pneumococcal infection. Biochem. Biophys. Res. Commun. 2017. 493: 671–676. [DOI] [PubMed] [Google Scholar]
- 50. Moalli, F. , Doni, A. , Deban, L. , Zelante, T. , Zagarella, S. , Bottazzi, B. , Romani, L. et al., Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus . Blood 2010. 116: 5170–5180. [DOI] [PubMed] [Google Scholar]
- 51. D'Angelo, C. , De Luca, A. , Zelante, T. , Bonifazi, P. , Moretti, S. , Giovannini, G. , Iannitti, R. G. et al., Exogenous pentraxin 3 restores antifungal resistance and restrains inflammation in murine chronic granulomatous disease. J. Immunol. 2009. 183: 4609–4618. [DOI] [PubMed] [Google Scholar]
- 52. Diniz, S. N. , Nomizo, R. , Cisalpino, P. S. , Teixeira, M. M. , Brown, G. D. , Mantovani, A. , Gordon, S. et al., PTX3 function as an opsonin for the dectin‐1‐dependent internalization of zymosan by macrophages. J. Leukoc. Biol. 2004. 75: 649–656. [DOI] [PubMed] [Google Scholar]
- 53. Porte, R. , Davoudian, S. , Asgari, F. , Parente, R. , Mantovani, A. , Garlanda, C. and Bottazzi, B. , The long pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and sepsis. Front. Immunol. 2019. 10: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soares, A. C. , Souza, D. G. , Pinho, V. , Vieira, A. T. , Nicoli, J. R. , Cunha, F. Q. , Mantovani, A. et al., Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006. 8: 1321–1329. [DOI] [PubMed] [Google Scholar]
- 55. Deban, L. , Jarva, H. , Lehtinen, M. J. , Bottazzi, B. , Bastone, A. , Doni, A. , Jokiranta, T. S. et al., Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J. Immunol. 2008. 181: 8433–8440. [DOI] [PubMed] [Google Scholar]
- 56. Lu, J. , Mold, C. , Du Clos, T. W. and Sun, P. D. , Pentraxins and Fc receptor‐mediated immune responses. Front. Immunol. 2018. 9: 2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diamond, J. M. , Lederer, D. J. , Kawut, S. M. , Lee, J. , Ahya, V. N. , Bellamy, S. , Palmer, S. M. et al., Elevated plasma long pentraxin‐3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am. J. Transplant. 2011. 11: 2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Diamond, J. M. , Meyer, N. J. , Feng, R. , Rushefski, M. , Lederer, D. J. , Kawut, S. M. , Lee, J. C. et al., Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2012. 186: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diamond, J. M. and Wigfield, C. H. , Role of innate immunity in primary graft dysfunction after lung transplantation. Curr. Opin. Organ Transplant. 2013. 18: 518–523. [DOI] [PubMed] [Google Scholar]
- 60. Farmakiotis, D. , Le, A. , Weiss, Z. , Ismail, N. , Kubiak, D. W. and Koo, S. , False positive bronchoalveolar lavage galactomannan: effect of host and cut‐off value. Mycoses 2019. 62: 204–213. [DOI] [PubMed] [Google Scholar]
- 61. Cunha, C. , Aversa, F. , Lacerda, J. F. , Busca, A. , Kurzai, O. , Grube, M. , Loffler, J. et al., Genetic PTX3 deficiency and aspergillosis in stem‐cell transplantation. N. Engl. J. Med. 2014. 370: 421–432. [DOI] [PubMed] [Google Scholar]
- 62. Kabbani, D. , Bhaskaran, A. , Singer, L. G. , Bhimji, A. , Rotstein, C. , Keshavjee, S. , Liles, W. C. et al., Pentraxin 3 levels in bronchoalveolar lavage fluid of lung transplant recipients with invasive aspergillosis. J. Heart Lung Transplant. 2017. 36: 973–979. [DOI] [PubMed] [Google Scholar]
- 63. Infante, M. , Allavena, P. , Garlanda, C. , Nebuloni, M. , Morenghi, E. , Rahal, D. , Roncalli, M. et al., Prognostic and diagnostic potential of local and circulating levels of Pentraxin 3 in lung cancer patients. Int. J. Cancer 2016. 138: 983–991. [DOI] [PubMed] [Google Scholar]
- 64. Introna, M. , Alles, V. V. , Castellano, M. , Picardi, G. , De Gioia, L. , Bottazzai, B. , Peri, G. et al., Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood 1996. 87: 1862–1872. [PubMed] [Google Scholar]
- 65. Jaillon, S. , Peri, G. , Delneste, Y. , Fremaux, I. , Doni, A. , Moalli, F. , Garlanda, C. et al., The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 2007. 204: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Pottelberge, G. R. , Bracke, K. R. , Pauwels, N. S. , Vermassen, F. E. , Joos, G. F. and Brusselle, G. G. , COPD is associated with reduced pulmonary interstitial expression of pentraxin‐3. Eur. Respir. J. 2012. 39: 830–838. [DOI] [PubMed] [Google Scholar]
- 67. Mauri, T. , Coppadoro, A. , Bombino, M. , Bellani, G. , Zambelli, V. , Fornari, C. , Berra, L. et al., Alveolar pentraxin 3 as an early marker of microbiologically confirmed pneumonia: a threshold‐finding prospective observational study. Crit. Care 2014. 18: 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han, B. , Mura, M. , Andrade, C. F. , Okutani, D. , Lodyga, M. , dos Santos, C. C. , Keshavjee, S. et al., TNFα‐induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J. Immunol. 2005. 175: 8303–8311. [DOI] [PubMed] [Google Scholar]
- 69. Alles, V. V. , Bottazzi, B. , Peri, G. , Golay, J. , Introna, M. and Mantovani, A. , Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 1994. 84: 3483–3493. [PubMed] [Google Scholar]
- 70. Schwingel, F. L. , Pizzichini, E. , Kleveston, T. , Morato, E. F. , Pinheiro, J. T. , Steidle, L. J. , Dal‐Pizzol, F. et al., Pentraxin 3 sputum levels differ in patients with chronic obstructive pulmonary disease vs asthma. Ann. Allergy Asthma Immunol. 2015. 115: 485–489. [DOI] [PubMed] [Google Scholar]
- 71. Licari, A. , Marseglia, G. , De Amici, M. and Ciprandi, G. , Pentraxin 3 and D‐dimer in children with asthma: a real‐world study. Clin. Exp. Allergy 2019. 49: 550–551. [DOI] [PubMed] [Google Scholar]
- 72. Koppelman, G. H. , Stine, O. C. , Xu, J. , Howard, T. D. , Zheng, S. L. , Kauffman, H. F. , Bleecker, E. R. et al., Genome‐wide search for atopy susceptibility genes in Dutch families with asthma. J. Allergy Clin. Immunol. 2002. 109: 498–506. [DOI] [PubMed] [Google Scholar]
- 73. Tillett, W. S. and Francis, T. , Serological reactions in pneumonia with a non‐protein somatic fraction of pneumococcus. J. Exp. Med. 1930. 52: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mold, C. , Rodgers, C. P. , Kaplan, R. L. and Gewurz, H. , Binding of human C‐reactive protein to bacteria. Infect. Immun. 1982. 38: 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sproston, N. R. and Ashworth, J. J. , Role of C‐reactive protein at sites of inflammation and infection. Front. Immunol. 2018. 9: 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Black, S. , Kushner, I. and Samols, D. , C‐reactive protein. J. Biol. Chem. 2004. 279: 48487–48490. [DOI] [PubMed] [Google Scholar]
- 77. Clos Du, W. T., C‐Reactive protein as a regulator of autoimmunity and inflammation. Arthritis Rheum. 2003. 48: 1475–1477. [DOI] [PubMed] [Google Scholar]
- 78. Ridker, P. M. , Cushman, M. , Stampfer, M. J. , Tracy, R. P. and Hennekens, C. H. , Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997. 336: 973–979. [DOI] [PubMed] [Google Scholar]
- 79. Pai, J. K. , Pischon, T. , Ma, J. , Manson, J. E. , Hankinson, S. E. , Joshipura, K. , Curhan, G. C. et al., Inflammatory markers and the risk of coronary heart disease in men and women. N. Engl. J. Med. 2004. 351: 2599–2610. [DOI] [PubMed] [Google Scholar]
- 80. van Durme, Y. M. , Verhamme, K. M. , Aarnoudse, A. J. , Van Pottelberge, G. R. , Hofman, A. , Witteman, J. C. , Joos, G. F. et al., C‐reactive protein levels, haplotypes, and the risk of incident chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009. 179: 375–382. [DOI] [PubMed] [Google Scholar]
- 81. Castell, J. V. , Gomez‐Lechon, M. J. , David, M. , Fabra, R. , Trullenque, R. and Heinrich, P. C. , Acute‐phase response of human hepatocytes: regulation of acute‐phase protein synthesis by interleukin‐6. Hepatology 1990. 12: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 82. Agrawal, A. , Cha‐Molstad, H. , Samols, D. and Kushner, I. , Transactivation of C‐reactive protein by IL‐6 requires synergistic interaction of CCAAT/enhancer binding protein beta (C/EBP beta) and Rel p50. J. Immunol. 2001. 166: 2378–2384. [DOI] [PubMed] [Google Scholar]
- 83. Enocsson, H. , Sjowall, C. , Skogh, T. , Eloranta, M. L. , Ronnblom, L. and Wettero, J. , Interferon‐alpha mediates suppression of C‐reactive protein: explanation for muted C‐reactive protein response in lupus flares? Arthritis Rheum. 2009. 60: 3755–3760. [DOI] [PubMed] [Google Scholar]
- 84. Stein, E. , Bays, H. , Koren, M. , Bakker‐Arkema, R. and Bisgaier, C. , Efficacy and safety of gemcabene as add‐on to stable statin therapy in hypercholesterolemic patients. J. Clin. Lipidol. 2016. 10: 1212–1222. [DOI] [PubMed] [Google Scholar]
- 85. Srivastava, R. A. K. , Cornicelli, J. A. , Markham, B. and Bisgaier, C. L. , Gemcabene, a first‐in‐class lipid‐lowering agent in late‐stage development, down‐regulates acute‐phase C‐reactive protein via C/EBP‐delta‐mediated transcriptional mechanism. Mol. Cell. Biochem. 2018. 449: 167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Okemefuna, A. I. , Stach, L. , Rana, S. , Buetas, A. J. , Gor, J. and Perkins, S. J. , C‐reactive protein exists in an NaCl concentration‐dependent pentamer‐decamer equilibrium in physiological buffer. J. Biol. Chem. 2010. 285: 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ash, S. Y. , Rahaghi, F. N. , Come, C. E. , Ross, J. C. , Colon, A. G. , Cardet‐Guisasola, J. C. , Dunican, E. M. et al., Pruning of the pulmonary vasculature in asthma. The Severe Asthma Research Program (SARP) cohort. Am. J. Respir. Crit. Care Med. 2018. 198: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Braig, D. , Kaiser, B. , Thiele, J. R. , Bannasch, H. , Peter, K. , Stark, G. B. , Koch, H. G. et al., A conformational change of C‐reactive protein in burn wounds unmasks its proinflammatory properties. Int. Immunol. 2014. 26: 467–478. [DOI] [PubMed] [Google Scholar]
- 89. Eisenhardt, S. U. , Thiele, J. R. , Bannasch, H. , Stark, G. B. and Peter, K. , C‐reactive protein: how conformational changes influence inflammatory properties. Cell Cycle 2009. 8: 3885–3892. [DOI] [PubMed] [Google Scholar]
- 90. Salazar, J. , Martinez, M. S. , Chavez‐Castillo, M. , Nunez, V. , Anez, R. , Torres, Y. , Toledo, A. et al., C‐reactive protein: an in‐depth look into structure, function, and regulation. Int. Sch. Res. Notices 2014. 2014: 653045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thiele, J. R. , Habersberger, J. , Braig, D. , Schmidt, Y. , Goerendt, K. , Maurer, V. , Bannasch, H. et al., Dissociation of pentameric to monomeric C‐reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti‐inflammatory strategy. Circulation 2014. 130: 35–50. [DOI] [PubMed] [Google Scholar]
- 92. Eisenhardt, S. U. , Habersberger, J. , Murphy, A. , Chen, Y. C. , Woollard, K. J. , Bassler, N. , Qian, H. et al., Dissociation of pentameric to monomeric C‐reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ. Res. 2009. 105: 128–137. [DOI] [PubMed] [Google Scholar]
- 93. Heuertz, R. M. , Schneider, G. P. , Potempa, L. A. and Webster, R. O. , Native and modified C‐reactive protein bind different receptors on human neutrophils. Int. J. Biochem. Cell Biol. 2005. 37: 320–335. [DOI] [PubMed] [Google Scholar]
- 94. Zeller, J. M. and Sullivan, B. L. , C‐reactive protein selectively enhances the intracellular generation of reactive oxygen products by IgG‐stimulated monocytes and neutrophils. J. Leukoc. Biol. 1992. 52: 449–455. [DOI] [PubMed] [Google Scholar]
- 95. Mihlan, M. , Stippa, S. , Jozsi, M. and Zipfel, P. F. , Monomeric CRP contributes to complement control in fluid phase and on cellular surfaces and increases phagocytosis by recruiting factor H. Cell Death Differ. 2009. 16: 1630–1640. [DOI] [PubMed] [Google Scholar]
- 96. Levy, H. , Kalish, L. A. , Huntington, I. , Weller, N. , Gerard, C. , Silverman, E. K. , Celedon, J. C. et al., Inflammatory markers of lung disease in adult patients with cystic fibrosis. Pediatr. Pulmonol. 2007. 42: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Broekhuizen, R. , Wouters, E. F. , Creutzberg, E. C. and Schols, A. M. , Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 2006. 61: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fujita, M. , Ueki, S. , Ito, W. , Chiba, T. , Takeda, M. , Saito, N. , Kayaba, H. et al., C‐reactive protein levels in the serum of asthmatic patients. Ann. Allergy Asthma Immunol. 2007. 99: 48–53. [DOI] [PubMed] [Google Scholar]
- 99. Zietkowski, Z. , Tomasiak‐Lozowska, M. M. , Skiepko, R. , Mroczko, B. , Szmitkowski, M. and Bodzenta‐Lukaszyk, A. , High‐sensitivity C‐reactive protein in the exhaled breath condensate and serum in stable and unstable asthma. Respir. Med. 2009. 103: 379–385. [DOI] [PubMed] [Google Scholar]
- 100. Shimoda, T. , Obase, Y. , Kishikawa, R. and Iwanaga, T. , Serum high‐sensitivity C‐reactive protein can be an airway inflammation predictor in bronchial asthma. Allergy Asthma Proc. 2015. 36: e23‐28. [DOI] [PubMed] [Google Scholar]
- 101. Ko, A. R. , Kim, Y. H. , Sol, I. S. , Kim, M. J. , Yoon, S. H. , Kim, K. W. and Kim, K. E. , High‐sensitivity C‐reactive protein can reflect small airway obstruction in childhood asthma. Yonsei Med. J. 2016. 57: 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hoshino, M. , Ohtawa, J. and Akitsu, K. , Increased C‐reactive protein is associated with airway wall thickness in steroid‐naive asthma. Ann. Allergy Asthma Immunol. 2014. 113: 37–41. [DOI] [PubMed] [Google Scholar]
- 103. Visness, C. M. , London, S. J. , Daniels, J. L. , Kaufman, J. S. , Yeatts, K. B. , Siega‐Riz, A. M. , Liu, A. H. et al., Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 2009. 123: 1163–1169, 1169 e1161‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chawes, B. L. , Stokholm, J. , Schoos, A. M. , Fink, N. R. , Brix, S. and Bisgaard, H. , Allergic sensitization at school age is a systemic low‐grade inflammatory disorder. Allergy 2017. 72: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 105. Gould, J. M. and Weiser, J. N. , Expression of C‐reactive protein in the human respiratory tract. Infect. Immun. 2001. 69: 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ramage, L. and Guy, K. , Expression of C‐reactive protein and heat‐shock protein‐70 in the lung epithelial cell line A549, in response to PM10 exposure. Inhal. Toxicol. 2004. 16: 447–452. [DOI] [PubMed] [Google Scholar]
- 107. Ramage, L. , Proudfoot, L. and Guy, K. , Expression of C‐reactive protein in human lung epithelial cells and upregulation by cytokines and carbon particles. Inhal. Toxicol. 2004. 16: 607–613. [DOI] [PubMed] [Google Scholar]
- 108. Dong, Q. and Wright, J. R. , Expression of C‐reactive protein by alveolar macrophages. J. Immunol. 1996. 156: 4815–4820. [PubMed] [Google Scholar]
- 109. Paats, M. S. , Bergen, I. M. , Bakker, M. , Hoek, R. A. , Nietzman‐Lammering, K. J. , Hoogsteden, H. C. , Hendriks, R. W. et al., Cytokines in nasal lavages and plasma and their correlation with clinical parameters in cystic fibrosis. J. Cyst. Fibros. 2013. 12: 623–629. [DOI] [PubMed] [Google Scholar]
- 110. Schalek, P. , Hornackova, Z. and Hahn, A. , The relationship of C‐reactive protein levels and positive culture with quality of life in acute rhinosinusitis. Patient Prefer Adherence 2015. 9: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Casanova, J. L. and Abel, L. , Human Mannose‐binding lectin in immunity: friend, foe, or both? J. Exp. Med. 2004. 199: 1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lu, J. H. , Thiel, S. , Wiedemann, H. , Timpl, R. and Reid, K. B. , Binding of the pentamer/hexamer forms of mannan‐binding protein to zymosan activates the proenzyme C1r2C1s2 complex, of the classical pathway of complement, without involvement of C1q. J. Immunol. 1990. 144: 2287–2294. [PubMed] [Google Scholar]
- 113. Gadjeva, M. , Takahashi, K. and Thiel, S. , Mannan‐binding lectin—a soluble pattern recognition molecule. Mol. Immunol. 2004. 41: 113–121. [DOI] [PubMed] [Google Scholar]
- 114. Takahashi, M. , Endo, Y. , Fujita, T. and Matsushita, M. , A truncated form of mannose‐binding lectin‐associated serine protease (MASP)‐2 expressed by alternative polyadenylation is a component of the lectin complement pathway. Int. Immunol. 1999. 11: 859–863. [DOI] [PubMed] [Google Scholar]
- 115. Mayilyan, K. R. , Presanis, J. S. , Arnold, J. N. and Sim, R. B. , Discrete MBL‐MASP complexes show wide inter‐individual variability in concentration: data from UK vs Armenian populations. Int. J. Immunopathol. Pharmacol. 2006. 19: 567–580. [DOI] [PubMed] [Google Scholar]
- 116. Garred, P. , Honore, C. , Ma, Y. J. , Munthe‐Fog, L. and Hummelshoj, T. , MBL2, FCN1, FCN2 and FCN3—the genes behind the initiation of the lectin pathway of complement. Mol. Immunol. 2009. 46: 2737–2744. [DOI] [PubMed] [Google Scholar]
- 117. Reading, P. C. , Morey, L. S. , Crouch, E. C. and Anders, E. M. , Collectin‐mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 1997. 71: 8204–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gomi, K. , Tokue, Y. , Kobayashi, T. , Takahashi, H. , Watanabe, A. , Fujita, T. and Nukiwa, T. , Mannose‐binding lectin gene polymorphism is a modulating factor in repeated respiratory infections. Chest 2004. 126: 95–99. [DOI] [PubMed] [Google Scholar]
- 119. Fidler, K. J. , Hilliard, T. N. , Bush, A. , Johnson, M. , Geddes, D. M. , Turner, M. W. , Alton, E. W. et al., Mannose‐binding lectin is present in the infected airway: a possible pulmonary defence mechanism. Thorax 2009. 64: 150–155. [DOI] [PubMed] [Google Scholar]
- 120. Chang, A. B. , Yerkovich, S. T. , Gibson, P. G. , Anderson‐James, S. , Petsky, H. L. , Carroll, M. L. , Masters, I. B. et al., Pulmonary innate immunity in children with protracted bacterial bronchitis. J. Pediatr. 2012161: 621–625 e621. [DOI] [PubMed] [Google Scholar]
- 121. Garred, P. , Pressler, T. , Madsen, H. O. , Frederiksen, B. , Svejgaard, A. , Hoiby, N. , Schwartz, M. et al., Association of mannose‐binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J. Clin. Invest. 1999. 104: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yang, I. A. , Seeney, S. L. , Wolter, J. M. , Anders, E. M. , McCormack, J. G. , Tunnicliffe, A. M. , Rabnott, G. C. et al., Mannose‐binding lectin gene polymorphism predicts hospital admissions for COPD infections. Genes Immun. 2003. 4: 269–274. [DOI] [PubMed] [Google Scholar]
- 123. Bang, P. , Laursen, I. , Thornberg, K. , Schierbeck, J. , Nielsen, B. , Valdimarsson, H. , Koch, C. et al., The pharmacokinetic profile of plasma‐derived mannan‐binding lectin in healthy adult volunteers and patients with Staphylococcus aureus septicaemia. Scand. J. Infect. Dis. 2008. 40: 44–48. [DOI] [PubMed] [Google Scholar]
- 124. Dicker, A. J. , Crichton, M. L. , Cassidy, A. J. , Brady, G. , Hapca, A. , Tavendale, R. , Einarsson, G. G. et al., Genetic mannose binding lectin deficiency is associated with airway microbiota diversity and reduced exacerbation frequency in COPD. Thorax 2018. 73: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tran, H. B. , Ahern, J. , Hodge, G. , Holt, P. , Dean, M. M. , Reynolds, P. N. and Hodge, S. , Oxidative stress decreases functional airway mannose binding lectin in COPD. PLoS One 2014. 9: e98571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ogden, C. A. , deCathelineau, A. , Hoffmann, P. R. , Bratton, D. , Ghebrehiwet, B. , Fadok, V. A. and Henson, P. M. , C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001. 194: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hudig, D. , Hunter, K. W. , Diamond, W. J. and Redelman, D. , Properties of human blood monocytes. I. CD91 expression and log orthogonal light scatter provide a robust method to identify monocytes that is more accurate than CD14 expression. Cytometry B Clin. Cytom. 2014. 86: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Walters, J. J. and Berwin, B. , Differential CD91 dependence for calreticulin and Pseudomonas exotoxin‐A endocytosis. Traffic 2005. 6: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 129. Ulrich‐Merzenich, G. , Hausen, A. , Zeitler, H. , Goldmann, G. , Oldenburg, J. and Pavlova, A. , The role of variant alleles of the mannose‐binding lectin in the inhibitor development in severe hemophilia A. Thromb. Res. 2019. 179: 140–146. [DOI] [PubMed] [Google Scholar]
- 130. Zhao, N. , Wu, J. , Xiong, S. , Zhang, L. , Lu, X. , Chen, S. , Wu, Q. et al., Mannan‐binding lectin, a serum collectin, suppresses T‐cell proliferation via direct interaction with cell surface calreticulin and inhibition of proximal T‐cell receptor signaling. FASEB J. 2017. 31: 2405–2417. [DOI] [PubMed] [Google Scholar]
- 131. Varga, L. , Szilagyi, K. , Lorincz, Z. , Berrens, L. , Thiel, S. , Zavodszky, P. , Daha, M. R. et al., Studies on the mechanisms of allergen‐induced activation of the classical and lectin pathways of complement. Mol. Immunol. 2003. 39: 839–846. [DOI] [PubMed] [Google Scholar]
- 132. Kaur, S. , Gupta, V. K. , Shah, A. , Thiel, S. , Sarma, P. U. and Madan, T. , Plasma mannan‐binding lectin levels and activity are increased in allergic patients. J. Allergy Clin. Immunol. 2005. 116: 1381–1383. [DOI] [PubMed] [Google Scholar]
- 133. Uguz, A. , Berber, Z. , Coskun, M. , Halide Akbas, S. and Yegin, O. , Mannose‐binding lectin levels in children with asthma. Pediatr. Allergy Immunol. 2005. 16: 231–235. [DOI] [PubMed] [Google Scholar]
- 134. Hawlisch, H. , Wills‐Karp, M. , Karp, C. L. and Kohl, J. , The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol. Immunol. 2004. 41: 123–131. [DOI] [PubMed] [Google Scholar]
- 135. Hogaboam, C. M. , Takahashi, K. , Ezekowitz, R. A. , Kunkel, S. L. and Schuh, J. M. , Mannose‐binding lectin deficiency alters the development of fungal asthma: effects on airway response, inflammation, and cytokine profile. J. Leukoc. Biol. 2004. 75: 805–814. [DOI] [PubMed] [Google Scholar]
- 136. Phelps, D. S. and Floros, J. , Localization of pulmonary surfactant proteins using immunohistochemistry and tissue in situ hybridization. Exp. Lung Res. 1991. 17: 985–995. [DOI] [PubMed] [Google Scholar]
- 137. Voorhout, W. F. , Veenendaal, T. , Kuroki, Y. , Ogasawara, Y. , van Golde, L. M. and Geuze, H. J. , Immunocytochemical localization of surfactant protein D (SP‐D) in type II cells, Clara cells, and alveolar macrophages of rat lung. J. Histochem. Cytochem. 1992. 40: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 138. Crouch, E. , Parghi, D. , Kuan, S. F. and Persson, A. , Surfactant protein D: subcellular localization in nonciliated bronchiolar epithelial cells. Am. J. Physiol. 1992. 263: L60‐66. [DOI] [PubMed] [Google Scholar]
- 139. Voss, T. , Eistetter, H. , Schafer, K. P. and Engel, J. , Macromolecular organization of natural and recombinant lung surfactant protein SP 28–36. Structural homology with the complement factor C1q. J. Mol. Biol. 1988. 201: 219–227. [DOI] [PubMed] [Google Scholar]
- 140. van Iwaarden, F. , Welmers, B. , Verhoef, J. , Haagsman, H. P. and van Golde, L. M. , Pulmonary surfactant protein A enhances the host‐defense mechanism of rat alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1990. 2: 91–98. [DOI] [PubMed] [Google Scholar]
- 141. van Iwaarden, J. F. , van Strijp, J. A. , Ebskamp, M. J. , Welmers, A. C. , Verhoef, J. and van Golde, L. M. , Surfactant protein A is opsonin in phagocytosis of herpes simplex virus type 1 by rat alveolar macrophages. Am. J. Physiol. 1991. 261: L204‐209. [DOI] [PubMed] [Google Scholar]
- 142. Crouch, E. , Chang, D. , Rust, K. , Persson, A. and Heuser, J. , Recombinant pulmonary surfactant protein D. Post‐translational modification and molecular assembly. J. Biol. Chem. 1994. 269: 15808–15813. [PubMed] [Google Scholar]
- 143. Kuroki, Y. , Gasa, S. , Ogasawara, Y. , Shiratori, M. , Makita, A. and Akino, T. , Binding specificity of lung surfactant protein SP‐D for glucosylceramide. Biochem. Biophys. Res. Commun. 1992. 187: 963–969. [DOI] [PubMed] [Google Scholar]
- 144. Ogasawara, Y. , Kuroki, Y. and Akino, T. , Pulmonary surfactant protein D specifically binds to phosphatidylinositol. J. Biol. Chem. 1992. 267: 21244–21249. [PubMed] [Google Scholar]
- 145. Persson, A. V. , Gibbons, B. J. , Shoemaker, J. D. , Moxley, M. A. and Longmore, W. J. , The major glycolipid recognized by SP‐D in surfactant is phosphatidylinositol. Biochemistry 1992. 31: 12183–12189. [DOI] [PubMed] [Google Scholar]
- 146. Van Iwaarden, J. F. , Shimizu, H. , Van Golde, P. H. , Voelker, D. R. and Van Golde, L. M. , Rat surfactant protein D enhances the production of oxygen radicals by rat alveolar macrophages. Biochem. J. 1992. 286 (Pt 1): 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Brinker, K. G. , Martin, E. , Borron, P. , Mostaghel, E. , Doyle, C. , Harding, C. V. and Wright, J. R. , Surfactant protein D enhances bacterial antigen presentation by bone marrow‐derived dendritic cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2001. 281: L1453–1463. [DOI] [PubMed] [Google Scholar]
- 148. Kuroki, Y. , Tsutahara, S. , Shijubo, N. , Takahashi, H. , Shiratori, M. , Hattori, A. , Honda, Y. et al., Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis. Am. Rev. Respir. Dis. 1993. 147: 723–729. [DOI] [PubMed] [Google Scholar]
- 149. Sasaki, R. , Soejima, T. , Matsumoto, A. , Maruta, T. , Yamada, K. , Ota, Y. , Kawabe, T. et al., Clinical significance of serum pulmonary surfactant proteins A and D for the early detection of radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2001. 50: 301–307. [DOI] [PubMed] [Google Scholar]
- 150. Malhotra, R. , Haurum, J. , Thiel, S. , Jensenius, J. C. and Sim, R. B. , Pollen grains bind to lung alveolar type II cells (A549) via lung surfactant protein A (SP‐A). Biosci. Rep. 1993. 13: 79–90. [DOI] [PubMed] [Google Scholar]
- 151. Wang, J. Y. , Kishore, U. , Lim, B. L. , Strong, P. and Reid, K. B. , Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin. Exp. Immunol. 1996. 106: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Madan, T. , Eggleton, P. , Kishore, U. , Strong, P. , Aggrawal, S. S. , Sarma, P. U. and Reid, K. B. , Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect. Immun. 1997. 65: 3171–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Madan, T. , Kishore, U. , Shah, A. , Eggleton, P. , Strong, P. , Wang, J. Y. , Aggrawal, S. S. et al., Lung surfactant proteins A and D can inhibit specific IgE binding to the allergens of Aspergillus fumigatus and block allergen‐induced histamine release from human basophils. Clin. Exp. Immunol. 1997. 110: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wang, J. Y. , Shieh, C. C. , You, P. F. , Lei, H. Y. and Reid, K. B. , Inhibitory effect of pulmonary surfactant proteins A and D on allergen‐induced lymphocyte proliferation and histamine release in children with asthma. Am. J. Respir. Crit. Care Med. 1998. 158: 510–518. [DOI] [PubMed] [Google Scholar]
- 155. Madan, T. , Kishore, U. , Singh, M. , Strong, P. , Clark, H. , Hussain, E. M. , Reid, K. B. et al., Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J. Clin. Invest. 2001. 107: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Madan, T. , Kishore, U. , Singh, M. , Strong, P. , Hussain, E. M. , Reid, K. B. and Sarma, P. U. , Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect. Immun. 2001. 69: 2728–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Singh, M. , Madan, T. , Waters, P. , Parida, S. K. , Sarma, P. U. and Kishore, U. , Protective effects of a recombinant fragment of human surfactant protein D in a murine model of pulmonary hypersensitivity induced by dust mite allergens. Immunol. Lett. 2003. 86: 299–307. [DOI] [PubMed] [Google Scholar]
- 158. Madan, T. , Reid, K. B. , Singh, M. , Sarma, P. U. and Kishore, U. , Susceptibility of mice genetically deficient in the surfactant protein (SP)‐A or SP‐D gene to pulmonary hypersensitivity induced by antigens and allergens of Aspergillus fumigatus . J. Immunol. 2005. 174: 6943–6954. [DOI] [PubMed] [Google Scholar]
- 159. Ito, Y. and Mason, R. J. , The effect of interleukin‐13 (IL‐13) and interferon‐gamma (IFN‐gamma) on expression of surfactant proteins in adult human alveolar type II cells in vitro. Respir. Res. 2010. 11: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Koopmans, J. G. , van der Zee, J. S. , Krop, E. J. , Lopuhaa, C. E. , Jansen, H. M. and Batenburg, J. J. , Serum surfactant protein D is elevated in allergic patients. Clin. Exp. Allergy 2004. 34: 1827–1833. [DOI] [PubMed] [Google Scholar]
- 161. Erpenbeck, V. J. , Schmidt, R. , Gunther, A. , Krug, N. and Hohlfeld, J. M. , Surfactant protein levels in bronchoalveolar lavage after segmental allergen challenge in patients with asthma. Allergy 2006. 61: 598–604. [DOI] [PubMed] [Google Scholar]
- 162. Matsushita, M. , Endo, Y. , Taira, S. , Sato, Y. , Fujita, T. , Ichikawa, N. , Nakata, M. et al., A novel human serum lectin with collagen‐ and fibrinogen‐like domains that functions as an opsonin. J. Biol. Chem. 1996. 271: 2448–2454. [DOI] [PubMed] [Google Scholar]
- 163. Matsushita, M. , Endo, Y. and Fujita, T. , edge Cutting: complement‐activating complex of ficolin and mannose‐binding lectin‐associated serine protease. J. Immunol. 2000. 164: 2281–2284. [DOI] [PubMed] [Google Scholar]
- 164. Luo, F. , Sun, X. , Wang, Y. , Wang, Q. , Wu, Y. , Pan, Q. , Fang, C. et al., Ficolin‐2 defends against virulent Mycobacteria tuberculosis infection in vivo, and its insufficiency is associated with infection in humans. PLoS One 2013. 8: e73859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Endo, Y. , Sato, Y. , Matsushita, M. and Fujita, T. , Cloning and characterization of the human lectin P35 gene and its related gene. Genomics 1996. 36: 515–521. [DOI] [PubMed] [Google Scholar]
- 166. Sugimoto, R. , Yae, Y. , Akaiwa, M. , Kitajima, S. , Shibata, Y. , Sato, H. , Hirata, J. et al., Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J. Biol. Chem. 1998. 273: 20721–20727. [DOI] [PubMed] [Google Scholar]
- 167. Akaiwa, M. , Yae, Y. , Sugimoto, R. , Suzuki, S. O. , Iwaki, T. , Izuhara, K. and Hamasaki, N. , Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J. Histochem. Cytochem. 1999. 47: 777–786. [DOI] [PubMed] [Google Scholar]
- 168. Plovsing, R. R. , Berg, R. M. , Munthe‐Fog, L. , Konge, L. , Iversen, M. , Moller, K. and Garred, P. , Alveolar recruitment of ficolin‐3 in response to acute pulmonary inflammation in humans. Immunobiology 2016. 221: 690–697. [DOI] [PubMed] [Google Scholar]
- 169. Lu, J. , Le, Y. , Kon, O. L. , Chan, J. and Lee, S. H. , Biosynthesis of human ficolin, an Escherichia coli binding protein, by monocytes: comparison with the synthesis of two macrophage‐specific proteins, C1q and the mannose receptor. Immunology 1996. 89: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Teh, C. , Le, Y. , Lee, S. H. and Lu, J. , M‐ficolin is expressed on monocytes and is a lectin binding to N‐acetyl‐d‐glucosamine and mediates monocyte adhesion and phagocytosis of Escherichia coli . Immunology 2000. 101: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Metzger, M. L. , Michelfelder, I. , Goldacker, S. , Melkaoui, K. , Litzman, J. , Guzman, D. , Grimbacher, B. et al., Low ficolin‐2 levels in common variable immunodeficiency patients with bronchiectasis. Clin. Exp. Immunol. 2015. 179: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Verma, A. , White, M. , Vathipadiekal, V. , Tripathi, S. , Mbianda, J. , Ieong, M. , Qi, L. et al., Human H‐ficolin inhibits replication of seasonal and pandemic influenza A viruses. J. Immunol. 2012. 189: 2478–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Munthe‐Fog, L. , Hummelshoj, T. , Honore, C. , Madsen, H. O. , Permin, H. and Garred, P. , Immunodeficiency associated with FCN3 mutation and ficolin‐3 deficiency. N. Engl. J. Med. 2009. 360: 2637–2644. [DOI] [PubMed] [Google Scholar]
- 174. Dadfar, E. , Furuhjelm, C. , Nilsson, J. , Dahle, C. and Garred, P. , Fatal pneumococcus meningitis in a child with complement factor ficolin‐3 deficiency. J Allergy Clin Immunol Pract 2019. [DOI] [PubMed] [Google Scholar]
- 175. Munthe‐Fog, L. , Hummelshoj, T. , Ma, Y. J. , Hansen, B. E. , Koch, C. , Madsen, H. O. , Skjodt, K. et al., Characterization of a polymorphism in the coding sequence of FCN3 resulting in a Ficolin‐3 (Hakata antigen) deficiency state. Mol. Immunol. 2008. 45: 2660–2666. [DOI] [PubMed] [Google Scholar]
- 176. Honore, C. , Hummelshoj, T. , Hansen, B. E. , Madsen, H. O. , Eggleton, P. and Garred, P. , The innate immune component ficolin 3 (Hakata antigen) mediates the clearance of late apoptotic cells. Arthritis Rheum. 2007. 56: 1598–1607. [DOI] [PubMed] [Google Scholar]
- 177. Plawecki, M. , Lheritier, E. , Clavarino, G. , Jourde‐Chiche, N. , Ouili, S. , Paul, S. , Gout, E. et al., Association between the presence of autoantibodies targeting ficolin‐3 and active nephritis in patients with systemic lupus erythematosus. PLoS One 2016. 11: e0160879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Atkinson, A. P. , Cedzynski, M. , Szemraj, J. , St Swierzko, A. , Bak‐Romaniszyn, L. , Banasik, M. , Zeman, K. et al., L‐ficolin in children with recurrent respiratory infections. Clin. Exp. Immunol. 2004. 138: 517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Cedzynski, M. , Atkinson, A. P. , St Swierzko, A. , MacDonald, S. L. , Szala, A. , Zeman, K. , Buczylko, K. et al., L‐ficolin (ficolin‐2) insufficiency is associated with combined allergic and infectious respiratory disease in children. Mol. Immunol. 2009. 47: 415–419. [DOI] [PubMed] [Google Scholar]
- 180. Malle, E. , Sodin‐Semrl, S. and Kovacevic, A. , Serum amyloid A: an acute‐phase protein involved in tumour pathogenesis. Cell. Mol. Life Sci. 2009. 66: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Urieli‐Shoval, S. , Cohen, P. , Eisenberg, S. and Matzner, Y. , Widespread expression of serum amyloid A in histologically normal human tissues. Predominant localization to the epithelium. J. Histochem. Cytochem. 1998. 46: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 182. Jumeau, C. , Awad, F. , Assrawi, E. , Cobret, L. , Duquesnoy, P. , Giurgea, I. , Valeyre, D. et al., Expression of SAA1, SAA2 and SAA4 genes in human primary monocytes and monocyte‐derived macrophages. PLoS One 2019. 14: e0217005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hari‐Dass, R. , Shah, C. , Meyer, D. J. and Raynes, J. G. , Serum amyloid A protein binds to outer membrane protein A of Gram‐negative bacteria. J. Biol. Chem. 2005. 280: 18562–18567. [DOI] [PubMed] [Google Scholar]
- 184. Shah, C. , Hari‐Dass, R. and Raynes, J. G. , Serum amyloid A is an innate immune opsonin for Gram‐negative bacteria. Blood 2006. 108: 1751–1757. [DOI] [PubMed] [Google Scholar]
- 185. Cai, H. , Song, C. , Endoh, I. , Goyette, J. , Jessup, W. , Freedman, S. B. , McNeil, H. P. et al., Serum amyloid A induces monocyte tissue factor> J. Immunol. 2007. 178: 1852–1860. [DOI] [PubMed] [Google Scholar]
- 186. Cheng, N. , Liang, Y. , Du, X. and Ye, R. D. , Serum amyloid A promotes LPS clearance and suppresses LPS‐induced inflammation and tissue injury. EMBO Rep. 2018. 19: e45517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Derebe, M. G. , Zlatkov, C. M. , Gattu, S. , Ruhn, K. A. , Vaishnava, S. , Diehl, G. E. , MacMillan, J. B. et al., Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection. Elife 2014. 3: e03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ather, J. L. , Dienz, O. , Boyson, J. E. , Anathy, V. , Amiel, E. and Poynter, M. E. , Serum amyloid A3 is required for normal lung development and survival following influenza infection. Sci. Rep. 2018. 8: 16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sack, G. H., Jr. , Serum amyloid A—a review. Mol. Med. 2018. 24: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sun, L. and Ye, R. D. , Serum amyloid A1: structure, function and gene polymorphism. Gene 2016. 583: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Badolato, R. , Wang, J. M. , Murphy, W. J. , Lloyd, A. R. , Michiel, D. F. , Bausserman, L. L. , Kelvin, D. J. et al., Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J. Exp. Med. 1994. 180: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Su, S. B. , Gong, W. , Gao, J. L. , Shen, W. , Murphy, P. M. , Oppenheim, J. J. and Wang, J. M. , A seven‐transmembrane, G protein‐coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 1999. 189: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Gouwy, M. , De Buck, M. , Portner, N. , Opdenakker, G. , Proost, P. , Struyf, S. and Van Damme, J. , Serum amyloid A chemoattracts immature dendritic cells and indirectly provokes monocyte chemotaxis by induction of cooperating CC and CXC chemokines. Eur. J. Immunol. 2015. 45: 101–112. [DOI] [PubMed] [Google Scholar]
- 194. Hinrichs, B. H. , Matthews, J. D. , Siuda, D. , O'Leary, M. N. , Wolfarth, A. A. , Saeedi, B. J. , Nusrat, A. et al., Serum amyloid A1 is an epithelial prorestitutive factor. Am. J. Pathol. 2018. 188: 937–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Lee, K. , Choi, J. , Choi, B. K. , Gu, Y. M. , Ryu, H. W. , Oh, S. R. and Lee, H. J. , Picroside II isolated from Pseudolysimachion rotundum var. subintegrum inhibits glucocorticoid refractory serum amyloid A (SAA) expression and SAA‐induced IL‐33 secretion. Molecules 2019. 24: E2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Patel, H. , Fellowes, R. , Coade, S. and Woo, P. , Human serum amyloid A has cytokine‐like properties. Scand. J. Immunol. 1998. 48: 410–418. [DOI] [PubMed] [Google Scholar]
- 197. Poynter, M. E. , Airway epithelial regulation of allergic sensitization in asthma. Pulm. Pharmacol. Ther. 2012. 25: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sano, T. , Huang, W. , Hall, J. A. , Yang, Y. , Chen, A. , Gavzy, S. J. , Lee, J. Y. et al., An IL‐23R/IL‐22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses> Cell 2015. 163: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Migita, K. , Koga, T. , Torigoshi, T. , Maeda, Y. , Miyashita, T. , Izumi, Y. , Aiba, Y. et al., Serum amyloid A protein stimulates CCL20 production in rheumatoid synoviocytes. Rheumatology (Oxford) 2009. 48: 741–747. [DOI] [PubMed] [Google Scholar]
- 200. Migita, K. , Koga, T. , Torigoshi, T. , Motokawa, S. , Maeda, Y. , Jiuchi, Y. , Izumi, Y. et al., Induction of interleukin‐23 p19 by serum amyloid A (SAA) in rheumatoid synoviocytes. Clin. Exp. Immunol. 2010. 162: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Frame, N. M. , Jayaraman, S. , Gantz, D. L. and Gursky, O. , Serum amyloid A self‐assembles with phospholipids to form stable protein‐rich nanoparticles with a distinct structure: a hypothetical function of SAA as a “molecular mop” in immune response. J. Struct. Biol. 2017. 200: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lu, J. , Yu, Y. , Zhu, I. , Cheng, Y. and Sun, P. D. , Structural mechanism of serum amyloid A‐mediated inflammatory amyloidosis. Proc. Natl. Acad. Sci. U. S. A. 2014. 111: 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Wang, L. , Lashuel, H. A. , Walz, T. and Colon, W. , Murine apolipoprotein serum amyloid A in solution forms a hexamer containing a central channel. Proc. Natl. Acad. Sci. U. S. A. 2002. 99: 15947–15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Wang, Y. , Srinivasan, S. , Ye, Z. , Javier Aguilera, J. , Lopez, M. M. and Colon, W. , Serum amyloid A 2.2 refolds into a octameric oligomer that slowly converts to a more stable hexamer. Biochem. Biophys. Res. Commun. 2011. 407: 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kim, M. H. , de Beer, M. C. , Wroblewski, J. M. , Webb, N. R. and de Beer, F. C. , SAA does not induce cytokine production in physiological conditions. Cytokine 2013. 61: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Bjorkman, L. , Raynes, J. G. , Shah, C. , Karlsson, A. , Dahlgren, C. and Bylund, J. , The proinflammatory activity of recombinant serum amyloid A is not shared by the endogenous protein in the circulation. Arthritis Rheum. 2010. 62: 1660–1665. [DOI] [PubMed] [Google Scholar]
- 207. Ather, J. L. , Ckless, K. , Martin, R. , Foley, K. L. , Suratt, B. T. , Boyson, J. E. , Fitzgerald, K. A. et al., Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Immunol. 2011. 187: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ather, J. L. , Fortner, K. A. , Budd, R. C. , Anathy, V. and Poynter, M. E. , Serum amyloid A inhibits dendritic cell apoptosis to induce glucocorticoid resistance in CD4(+) T cells. Cell Death Dis. 2013. 4: e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Anthony, D. , Seow, H. J. , Uddin, M. , Thompson, M. , Dousha, L. , Vlahos, R. , Irving, L. B. et al., Serum amyloid A promotes lung neutrophilia by increasing IL‐17A levels in the mucosa and gammadelta T cells. Am. J. Respir. Crit. Care Med. 2013. 188: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Newcomb, D. C. and Peebles, R. S., Jr. , Th17‐mediated inflammation in asthma. Curr. Opin. Immunol. 2013. 25: 755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Bozinovski, S. , Uddin, M. , Vlahos, R. , Thompson, M. , McQualter, J. L. , Merritt, A. S. , Wark, P. A. et al., Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. U. S. A. 2012. 109: 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Jousilahti, P. , Salomaa, V. , Hakala, K. , Rasi, V. , Vahtera, E. and Palosuo, T. , The association of sensitive systemic inflammation markers with bronchial asthma. Ann. Allergy Asthma Immunol. 2002. 89: 381–385. [DOI] [PubMed] [Google Scholar]
- 213. Lu, H. , Lin, X. S. , Yao, D. M. , Zhuang, Y. Y. , Wen, G. F. , Shi, J. and Sun, Y. Q. , Increased serum amyloid A in nasal polyps is associated with systemic corticosteroid insensitivity in patients with chronic rhinosinusitis with nasal polyps: a pilot study. Eur. Arch. Otorhinolaryngol. 2018. 275: 401–408. [DOI] [PubMed] [Google Scholar]
- 214. Ricklefs, I. , Barkas, I. , Duvall, M. G. , Cernadas, M. , Grossman, N. L. , Israel, E. , Bleecker, E. R. et al., ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight 20183: 93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Eckhardt, E. R. , Witta, J. , Zhong, J. , Arsenescu, R. , Arsenescu, V. , Wang, Y. , Ghoshal, S. et al., Intestinal epithelial serum amyloid A modulates bacterial growth in vitro and pro‐inflammatory responses in mouse experimental colitis. BMC Gastroenterol. 2010. 10: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Kemper, C. and Kohl, J. , Back to the future—non‐canonical functions of complement. Semin. Immunol. 2018. 37: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Hajishengallis, G. , Reis, E. S. , Mastellos, D. C. , Ricklin, D. and Lambris, J. D. , Novel mechanisms and functions of complement. Nat. Immunol. 2017. 18: 1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Schafer, D. P. , Lehrman, E. K. , Kautzman, A. G. , Koyama, R. , Mardinly, A. R. , Yamasaki, R. , Ransohoff, R. M. et al., Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron 2012. 74: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Mastellos, D. , Papadimitriou, J. C. , Franchini, S. , Tsonis, P. A. and Lambris, J. D. , A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 2001. 166: 2479–2486. [DOI] [PubMed] [Google Scholar]
- 220. Elvington, M. , Liszewski, M. K. and Atkinson, J. P. , Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol. Rev. 2016. 274: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Arbore, G. , Kemper, C. and Kolev, M. , Intracellular complement—the complosome—in immune cell regulation. Mol. Immunol. 2017. 89: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Pandey, M. K. , Burrow, T. A. , Rani, R. , Martin, L. J. , Witte, D. , Setchell, K. D. , McKay, M. A. et al., Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature 2017. 543: 108–112. [DOI] [PubMed] [Google Scholar]
- 223. Ricklin, D. , Hajishengallis, G. , Yang, K. and Lambris, J. D. , Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010. 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Walport, M. J. , Complement. Second of two parts. N. Engl. J. Med. 2001. 344: 1140–1144. [DOI] [PubMed] [Google Scholar]
- 225. Walport, M. J. , Complement. First of two parts. N. Engl. J. Med. 2001. 344: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 226. Varsano, S. , Kaminsky, M. , Kaiser, M. and Rashkovsky, L. , Generation of complement C3 and expression of cell membrane complement inhibitory proteins by human bronchial epithelium cell line. Thorax 2000. 55: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ali, M. , Lillehoj, E. P. , Park, Y. , Kyo, Y. and Kim, K. C. , Analysis of the proteome of human airway epithelial secretions. Proteome Sci 2011. 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Corrales, L. , Ajona, D. , Rafail, S. , Lasarte, J. J. , Riezu‐Boj, J. I. , Lambris, J. D. , Rouzaut, A. et al., Anaphylatoxin C5a creates a colourable microenvironment for lung cancer progression. J. Immunol. 2012. 189: 4674–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Stimler, N. P. , Hugli, T. E. and Bloor, C. M. , Pulmonary injury induced by C3a and C5a anaphylatoxins. Am. J. Pathol. 1980. 100: 327–348. [PMC free article] [PubMed] [Google Scholar]
- 230. Huey, R. , Erickson, B. W. , Bloor, C. M. and Hugli, T. E. , Contraction of guinea pig lung by synthetic oligopeptides related to human C3a. Immunopharmacology 1984. 8: 37–45. [DOI] [PubMed] [Google Scholar]
- 231. Drouin, S. M. , Kildsgaard, J. , Haviland, J. , Zabner, J. , Jia, H. P. , McCray, P. B., Jr. , Tack, B. F. et al., Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J. Immunol. 2001. 166: 2025–2032. [DOI] [PubMed] [Google Scholar]
- 232. Stevens, J. H. , O'Hanley, P. , Shapiro, J. M. , Mihm, F. G. , Satoh, P. S. , Collins, J. A. and Raffin, T. A. , Effects of anti‐C5a antibodies on the adult respiratory distress syndrome in septic primates. J. Clin. Invest. 1986. 77: 1812–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Czermak, B. J. , Breckwoldt, M. , Ravage, Z. B. , Huber‐Lang, M. , Schmal, H. , Bless, N. M. , Friedl, H. P. et al., Mechanisms of enhanced lung injury during sepsis. Am. J. Pathol. 1999. 154: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Watford, W. T. , Ghio, A. J. and Wright, J. R. , Complement‐mediated host defense in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2000. 279: L790‐798. [DOI] [PubMed] [Google Scholar]
- 235. Bolger, M. S. , Ross, D. S. , Jiang, H. , Frank, M. M. , Ghio, A. J. , Schwartz, D. A. and Wright, J. R. , Complement levels and activity in the normal and LPS‐injured lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2007. 292: L748‐759. [DOI] [PubMed] [Google Scholar]
- 236. Tsai, Y. G. , Wen, Y. S. , Wang, J. Y. , Yang, K. D. , Sun, H. L. , Liou, J. H. and Lin, C. Y. , Complement regulatory protein CD46 induces autophagy against oxidative stress‐mediated apoptosis in normal and asthmatic airway epithelium. Sci. Rep. 2018. 8: 12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kulkarni, H. S. , Elvington, M. L. , Perng, Y. C. , Liszewski, M. K. , Byers, D. E. , Farkouh, C. , Yusen, R. D. et al., Intracellular C3 protects human airway epithelial cells from stress‐associated cell death. Am. J. Respir. Cell Mol. Biol. 2019. 60: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Botto, M. , Lissandrini, D. , Sorio, C. and Walport, M. J. , Biosynthesis and secretion of complement component (C3) by activated human polymorphonuclear leukocytes. J. Immunol. 1992. 149: 1348–1355. [PubMed] [Google Scholar]
- 239. Einstein, L. P. , Hansen, P. J. , Ballow, M. , Davis, A. E. , III, Davis , t. J. S., Alper, C. A. , Rosen, F. S. et al., Biosynthesis of the third component of complement (C3) in vitro by monocytes from both normal and homozygous C3‐deficient humans. J. Clin. Invest. 1977. 60: 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Kohl, J. , The role of complement in danger sensing and transmission. Immunol. Res. 2006. 34: 157–176. [DOI] [PubMed] [Google Scholar]
- 241. Strainic, M. G. , Liu, J. , Huang, D. , An, F. , Lalli, P. N. , Muqim, N. , Shapiro, V. S. et al., Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells> Immunity 2008. 28: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Nagata, S. and Glovsky, M. M. , Activation of human serum complement with allergens. I. Generation of C3a, C4a, and C5a and induction of human neutrophil aggregation. J. Allergy Clin. Immunol. 1987. 80: 24–32. [DOI] [PubMed] [Google Scholar]
- 243. Maruo, K. , Akaike, T. , Ono, T. , Okamoto, T. and Maeda, H. , Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J. Allergy Clin. Immunol. 1997. 100: 253–260. [DOI] [PubMed] [Google Scholar]
- 244. Roy, R. M. , Paes, H. C. , Nanjappa, S. G. , Sorkness, R. , Gasper, D. , Sterkel, A. , Wuthrich, M. et al., Complement component 3C3 and C3a receptor are required in chitin‐dependent allergic sensitization to Aspergillus fumigatus but dispensable in chitin‐induced innate allergic inflammation. mBio 20134: e00162‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Humbles, A. A. , Lu, B. , Nilsson, C. A. , Lilly, C. , Israel, E. , Fujiwara, Y. , Gerard, N. P. et al., A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature 2000. 406: 998–1001. [DOI] [PubMed] [Google Scholar]
- 246. van de Graaf, E. A. , Jansen, H. M. , Bakker, M. M. , Alberts, C. , Eeftinck Schattenkerk, J. K. and Out, T. A. , ELISA of complement C3a in bronchoalveolar lavage fluid. J. Immunol. Methods 1992. 147: 241–250. [DOI] [PubMed] [Google Scholar]
- 247. Teran, L. M. , Campos, M. G. , Begishvilli, B. T. , Schroder, J. M. , Djukanovic, R. , Shute, J. K. , Church, M. K. et al., Identification of neutrophil chemotactic factors in bronchoalveolar lavage fluid of asthmatic patients. Clin. Exp. Allergy 1997. 27: 396–405. [PubMed] [Google Scholar]
- 248. Zilow, G. , Joka, T. , Obertacke, U. , Rother, U. and Kirschfink, M. , Generation of anaphylatoxin C3a in plasma and bronchoalveolar lavage fluid in trauma patients at risk for the adult respiratory distress syndrome. Crit. Care Med. 1992. 20: 468–473. [DOI] [PubMed] [Google Scholar]
- 249. Drouin, S. M. , Corry, D. B. , Hollman, T. J. , Kildsgaard, J. and Wetsel, R. A. , Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J. Immunol. 2002. 169: 5926–5933. [DOI] [PubMed] [Google Scholar]
- 250. Jun, S. W. , Kim, T. H. , Lee, H. M. , Lee, S. H. , Kim, W. J. , Park, S. J. and Kim, Y. S. , Overexpression of the anaphylatoxin receptors, complement anaphylatoxin 3a receptor and complement anaphylatoxin 5a receptor, in the nasal mucosa of patients with mild and severe persistent allergic rhinitis. J. Allergy Clin. Immunol. 2008. 122: 119–125. [DOI] [PubMed] [Google Scholar]
- 251. Krug, N. , Tschernig, T. , Erpenbeck, V. J. , Hohlfeld, J. M. and Kohl, J. , Complement factors C3a and C5a are increased in bronchoalveolar lavage fluid after segmental allergen provocation in subjects with asthma. Am. J. Respir. Crit. Care Med. 2001. 164: 1841–1843. [DOI] [PubMed] [Google Scholar]
- 252. Nakano, Y. , Morita, S. , Kawamoto, A. , Suda, T. , Chida, K. and Nakamura, H. , Elevated complement C3a in plasma from patients with severe acute asthma. J. Allergy Clin. Immunol. 2003. 112: 525–530. [DOI] [PubMed] [Google Scholar]
- 253. Mulligan, J. K. , Patel, K. , Williamson, T. , Reaves, N. , Carroll, W. , Stephenson, S. E. , Gao, P. et al., C3a receptor antagonism as a novel therapeutic target for chronic rhinosinusitis. Mucosal Immunol. 2018. 11: 1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]


