Abstract
Oxytocin neurones in the hypothalamus are activated by stressful stimuli and food intake. The oxytocin receptor is located in various brain regions, including the sensory information‐processing cerebral cortex; the cognitive information‐processing prefrontal cortex; reward‐related regions such as the ventral tegmental areas, nucleus accumbens and raphe nucleus; stress‐related areas such as the amygdala, hippocampus, ventrolateral part of the ventromedial hypothalamus and ventrolateral periaqueductal gray; homeostasis‐controlling hypothalamus; and the dorsal motor complex controlling intestinal functions. Oxytocin affects behavioural and neuroendocrine stress responses and terminates food intake by acting on the metabolic or nutritional homeostasis system, modulating emotional processing, reducing reward values of food intake, and facilitating sensory and cognitive processing via multiple brain regions. Oxytocin also plays a role in interactive actions between stress and food intake and contributes to adaptive active coping behaviours.
Keywords: active stress coping, food intake, oxytocin, stress
1. INTRODUCTION
Oxytocin, a nonapeptide, is mainly synthesised in magnocellular paraventricular and supraoptic neurones of the hypothalamus, in parvocellular paraventricular neurones and, in part, in neurones of the bed nucleus of the stria terminalis in mammals.1, 2 Hypothalamic parvocellular oxytocin‐synthesising neurones project their axons to various brain regions, including the spinal cord, dorsal vagal complex, nucleus tractus solitarius, rostral ventral medulla, ventral tegmental area and hypothalamus. Magnocellular oxytocin neurones, which mainly project their axon terminals to the neurohypophysis, also extend their axon collaterals to some brain regions, including the amygdala, lateral septum and nucleus of the horizontal limb of the diagonal band.3 Reproduction‐related stimuli, including mating, parturition and lactation, have been shown to activate magnocellular oxytocin neurones and facilitate oxytocin release from axon terminals of the neurohypophysis into the peripheral circulation. Oxytocin is released not only from neurohypophyseal axon terminals, but also from dendrites or somas of hypothalamic magnocellular oxytocin neurones within the hypothalamus4 or the medial amygdala.5 Oxytocin is also released from axon terminals in intracerebral target regions. Oxytocin has been shown to have multiple functions6, 7 by acting mainly on the oxytocin receptor and possibly on the vasopressin receptor.8
In this review, we focus on the role of oxytocin in the control of stress responses and food intake. In the last part, we briefly review several hypotheses for explaining multiple oxytocin functions to control social behaviour, as well as stress‐coping behaviours.
2. OXYTOCIN AND STRESS
2.1. Activation of oxytocin neurones after stressful stimuli
Stressful stimuli induce stereotypical alarm responses, especially in the neuroendocrine system, autonomic nervous system and immune system and in behaviours, and influence health conditions in humans.9 Some stressful stimuli, including noxious stimuli,10 conditioned fear stimuli,11 social defeat,12, 13, 14, 15, 16 immobilisation stress,17 shaker stress18 and forced swimming19, 20, as well as administration of lipopolysaccharide21 and interleukin‐1,22 activate oxytocin‐synthesising neurones in the hypothalamus and facilitate oxytocin release into the plasma or within the brain of mice or rats.23, 24 Exercise has also been shown to facilitate oxytocin release in horses.25 Oxytocin is released into peripheral circulation and within the brain. Parallel oxytocin release within the hypothalamus and into the peripheral circulation has been shown during forced swimming stress20 and shaker stress in rats.18 However, dissociations between peripheral and central release have also been reported in rats during social defeat stress,15 after adrenalectomy20 and after administration of α‐melanocyte‐stimulating hormone (α‐MSH).26 In humans, stressful stimuli such as physical running,27, 28, 29, 30 exposure to psychological stress induced by the Trier social stress test, which consists of public speaking and mental arithmetic in front of unknown jury members,28, 31 and interpersonal distress32 increase oxytocin in plasma or saliva, although no significant increase after psychological stress in urine of children33 or in plasma of female participants34 has also been reported.
Activation of oxytocin neurones in response to conditioned fear stimuli is mediated, at least in part, by A2 noradrenergic/prolactin‐releasing peptide (PrRP) neurones in the nucleus tractus solitarius of the medulla oblongata35 and by neurones of the medial amygdala,36 whereas activation of oxytocin neurones after noxious stimuli has been suggested to be mediated by A1 noradrenergic neurones in the medulla oblongata.10 The hypothalamic paraventricular and supraoptic nuclei have also been shown to receive inputs not only from noradrenergic neurones in the medulla oblongata, but also from neurones in other stress‐related areas, including the amygdala, septum, bed nucleus of the stria terminalis, dorsomedial hypothalamus, parabrachial nucleus and raphe nuclei.37 Further studies are needed to clarify the roles of these regions in activation of oxytocin neurones in response to stressful stimuli.
2.2. Roles of oxytocin in stress responses: attenuation or facilitation of stress responses
Oxytocin has been shown to modulate stress responses in the neuroendocrine system, autonomic nervous system and immune system and in behaviours. In laboratory animals and humans, oxytocin has been reported to reduce activity of the hypothalamic‐pituitary adrenal axis,38, 39, 40, 41, 42 attenuate inflammation43, 44 and reduce anxiety‐related behaviours.7, 39, 45, 46, 47, 48 Oxytocin‐deficient female mice have been shown to exhibit enhanced corticosterone release in response to shaker stress, enhanced novel environment‐induced hyperthermia and increased anxiety‐related behaviours in an elevated plus maze test.49, 50 These findings suggest that endogenous oxytocin has inhibitory actions on activity of the hypothalamic‐pituitary‐adrenal axis, sympathetic activity and anxiety‐related behaviour during exposure to stressful stimuli.51
Social relationships such as bonds between couples, mother‐offspring relationships and same‐sex platonic adult relationships have protective actions against adverse environments and evoke beneficial effects on health status.52, 53 This social interaction‐induced attenuation of stress responses is called social buffering.54 Social interactions ameliorate stress responses via sensation of visceral, tactile, olfactory, auditory and visual cues. Oxytocin has been suggested to be involved in social buffering in the neuroendocrine system and in behaviours of laboratory animals and humans.55
During sexual interactions, hypothalamic oxytocin neurones are activated in male rats.56 Oxytocin neurones are also activated in female rats under a paced mating condition in which females have the initiative to control sexual interactions.57 Experiments with oxytocin receptor antagonists have shown that endogenous oxytocin attenuates anxiety‐related behaviour in these mating conditions.
Oxytocin has also been suggested to reduce anxiety‐related behaviour or neuroendocrine stress responses during non‐mating social interactions in humans,58, 59 chimpanzees60 and prairie voles.61 In human children, comfort interactions with their mothers have been shown to increase urinary oxytocin and reduce an increase in cortisol after psychological stress.33 In chimpanzees, post‐conflict affiliations have been shown to increase urinary oxytocin.60 These findings suggest that activation of oxytocin neurones by social support reduces stress responses. In male prairie voles, it has been shown that oxytocin induces consolation behaviour towards their distressed female partners, resulting in induction of anxiolytic actions in both the giver and receiver of consolation.62 Oxytocin receptor‐dependent consolation behaviour toward distressed partners has also been reported in mandarin voles.63
Oxytocin has also been shown to have analgesic effects.64 Oxytocin neurones are activated after noxious stimuli10 and oxytocin acts on multiple sites, including the spinal cord and dorsal root ganglia, to induce analgesia.65, 66, 67, 68
As stated above, oxytocin has anti‐stress actions in many cases. However, oxytocin has also been shown to have anxiogenic effects in some conditions.69, 70 For example, it has been shown in humans that administration of oxytocin increases perceived social stress,71 facilitates fear conditioning,72 enhances startle reflex to unpredictable shocks73 and potentiates acoustic startle responses after exposure to negative emotional pictures.74
In a mouse model of social defeat stress, oxytocin has also been shown to facilitate stress‐related behaviours. Social defeat stress activated oxytocin‐synthesising neurones in the hypothalamus and bed nucleus of the stria terminalis, and oxytocin receptor‐deficient male mice showed a deficit in facilitation of social defeat posture observed during repeated social defeat, suggesting that the oxytocin receptor facilitates expression of social defeat posture.13 A local oxytocin receptor antagonist has also been shown to reduce social avoidance after social defeat stress in female mice, suggesting involvement of the oxytocin receptor in social avoidance.12
Oxytocin may also induce negative emotion via facilitation of negative emotional contagion. Emotional states, especially negative emotions, can be transmitted among in‐group familiar members. This is called emotional contagion. Intranasal oxytocin application and chemogenic activation of hypothalamic oxytocin neurones have been shown to enhance socially transmitted fear, whereas an oxytocin antagonist impairs observational fear in male mice, suggesting that oxytocin facilitates fear contagion.75
In summary, oxytocin has been shown to attenuate stress responses in the hypothalamo‐pituitary‐adrenal axis and the autonomic nervous system and in behaviour, especially in non‐competitive comfort situations. When facing stressful or threatening situations, seeking available social support by increasing social salience as a result of activation of the oxytocin system is an adaptive strategy when social support is expected. Indeed, oxytocin antagonist administration to couples of marmosets has been shown to decrease the time spent together in a novel‐housing stress condition.42 It is possible that oxytocin attenuates stress responses, at least in part, by inducing social support‐seeking behaviour and, as a result, reducing the risk of stressful stimuli.76 On the other hand, oxytocin appears to augment stress responses in socially aversive situations. When environments are severe with no hope of social support, augmentation of stress responses in the autonomic and behavioural systems may be more adaptive for coping with the situations.
2.3. Sites of action of oxytocin with respect to modulating stress responses
Oxytocin acts mainly on the oxytocin receptor. The oxytocin receptor is located in multiple brain areas that modulate stress responses, including the prefrontal cortex, limbic area, hypothalamus and medulla oblongata. Detailed downstream mechanisms of oxytocin remain to be clarified. However, stressful stimuli have been shown to activate oxytocin receptor‐expressing neurones in several distinct brains regions. For example, social defeat stress activated oxytocin receptor‐expressing neurones in the insula cortex, amygdala, paraventricular thalamic nucleus, posterior intralaminar thalamic nucleus, ventrolateral part of the ventromedial hypothalamus and ventrolateral periaqueductal gray,13 which have been shown to modulate autonomic or behavioural stress responses.
2.3.1. Medial prefrontal cortex
The medial prefrontal cortex of rodents consists of three parts, consisting of the anterior cingulate, prelimbic prefrontal cortex and infralimbic prefrontal cortex, and coordinates integrative responses in autonomic and behavioural systems to adapt to stressful environments.77
Activation of the oxytocin receptor in the medial prefrontal cortex has been shown to have anxiolytic actions (Table 1). Bilateral oxytocin administration into the prelimbic medial prefrontal cortex of rats has been reported to reduce anxiety‐related behaviour in an elevated plus maze test and an open field test and facilitate social interaction behaviours toward unfamiliar conspecifics.78 Oxytocin administration into the infralimbic region of the medial prefrontal cortex has also been shown to facilitate extinction of contextual conditioned fear and, as a result, induce attenuated freezing behaviour in response to conditioned fear stimuli in rats.79 Oxytocin has been shown to activate oxytocin receptor‐expressing GABAergic/corticotrophin‐releasing factor binding protein (CRFBP) interneurones in the medial prefrontal cortex, to induce anxiolytic actions in male mice via release of CRFBP, which suppresses activation of CRF receptor 1 (CRFR1)‐expressing neurones in layer 2/3,80 and to induce social approach toward male mice in female mice.81 Oxytocin in the infralimbic prefrontal cortex has also been shown to mediate social interaction‐induced facilitation of extinction of conditioned fear in rats.82
Table 1.
Roles of the oxytocin receptor in limbic areas in the control of anxiety‐related behaviours or conditioned fear responses
| Anxiety | Acquisition (treatments before fear conditioning) | Recall (treatments before recall test) | Extinction | ||
|---|---|---|---|---|---|
| Acquisition (treatments before recall test) | Consolidation (treatments after recall test | ||||
| Medial prefrontal cortex | |||||
| Prelimbic | ↓1 male rats, EPM, OT78 | ||||
| ↓1 male mice, EPM OF, optogenetic activation of OTR neurones, conditional OTR deletion80 | |||||
| Infralimbic | ±2 male rats, EPM, OT78 | ↓1 male rats, contextual CF, OTa82 | ↓1 male rats, contextual CF, OTa82 |
↓1 male rats, contextual CF, OT OTa79 |
|
| ±2juvenile male rats, contextual CF, OTa105 | |||||
| Basolateral amygdala | ↓1 male rats, contextual CF, OT100 | ↓1 male rats, contextual CF, OT100 | ↓1 male rats, contextual CF, OT100 | ±2 male rats, contextual CF, OT100 | |
| ↑1 male rats, contextual CF, OT OTa79 | ↑3 juvenile male rats, contextual CF, OTa105 | ||||
| ±2 juvenile male rats, contextual CF, OTa105 | ↓1 male rats, contextual CF, OTa79 | ||||
| ±2 male rats, contextual CF, OTa105 | |||||
| Central amygdala | ↓1 male mice, EZM, OTA102 | ↓1 male rats, contextual CF, OT OTa79, 100, 105 |
↓1 female rats, contextual CF, optogenetic activation of OT afferents3 |
↑3 male rats, contextual CF, OT100 | ↑3 male rats, contextual CF, OT100 |
| ↓1 female mandarin voles, EPM OF, OT OTA285 | ↓1 male rats, contextual CF, OT100 | ↓1 male rats, contextual CF, OTa104 | ±2 male rats, contextual CF, OT OTa79 | ||
| ↓1 male rats, contextual CF, OTa105 | ↑3 male rats, contextual CF, OT OTa OTA100 | ±2 juvenile male rats, contextual CF, OTa105 | |||
| ↑3 juvenile male rats, Contextual CF, OTa105 | |||||
| Bed nucleus of stria terminalis | ↑4 female California mice, social defeat‐induced social avoidance, OTA12 | ↑3 male rats, cued fear‐potentiated acoustic startle, OTA111 | |||
| Lateral septum | ±2 male mice, EPM, OT95 | ±2 lactating mice, social fear conditioning, OTA96 |
↓5 male mice, social fear conditioning, OT95 |
↓5 male mice, social fear conditioning, OT95 | |
| ↓5 lactating or virgin mice, social fear conditioning, OT OTA OTR overexpression chemogenetic silencing of OT afferents96 | |||||
| ±2 female mice, cued CF, OTR overexpression96 | |||||
±2, no significant change in anxiety‐related behaviours, freezing behaviour, or social investigation; ↑3, increased anxiety‐related behaviours or freezing behaviour (oxytocin having anxiogenic actions); ↑4, facilitation of social defeat‐induced social withdrawal (oxytocin inducing social avoidance); ↓1, reduced anxiety‐related behaviours or freezing behaviour (oxytocin having anxiolytic actions); ↓5, blockade of conditioned fear‐induced suppression of social investigation behaviour (oxytocin having anxiolytic actions); CF, conditioned fear; EPM, elevated plus maze test; EZM, elevated zero maze test; OF, open field test; OT, oxytocin; OTa, oxytocin receptor agonist; OTA, oxytocin receptor antagonist; OTR, oxytocin receptor.
In the experiments with conditioned fear, microinjections of oxytocin, oxytocin receptor agonists or oxytocin receptor antagonists or optogenetic manipulations were performed before fear conditioning training (pairings of conditioned stimuli and electric foot shocks), after fear conditioning training, before recall of conditioned responses (application of conditioned stimuli), and after recall of conditioned responses.
Reported functions of activation of the oxytocin receptor, sex and species, tests and local manipulations of oxytocin systems (optogenetic activation or microinjections of oxytocin, oxytocin receptor agonists or oxytocin receptor antagonists) are shown. To examine the roles of oxytocin in acquisition of fear conditioning, oxytocin manipulations were performed before fear conditioning and responses to conditioned stimuli were investigated. To examine the roles of oxytocin in recall of fear conditioning, oxytocin manipulations were performed before the recall test (application of conditioned fear). To examine the roles of oxytocin in acquisition of extinction learning, oxytocin manipulations were performed before the recall test. To examine the roles of oxytocin in consolidation of extinction learning, oxytocin manipulations were performed after the recall test.
In humans, intranasal oxytocin administration has been shown to increase activity of the prefrontal cortex and functional connectivity of the prefrontal cortex with the posterior cingulate cortex and precuneus, to decrease amygdala activity, and to facilitate extinction of fear conditioning.83
Oxytocin may also induce anxiolytic actions by evoking consolation behaviour via the oxytocin receptor in the anterior cingulate. Consolation behaviour of male prairie voles and mandarin voles toward distressed female partners has been shown to be mediated by the oxytocin receptor in the anterior cingulate.62, 63 Consolation reduces anxiety‐related behaviour and activity of the hypothalamo‐pituitary adrenal axis in both givers and receivers of consolation.
2.3.2. Nucleus accumbens
Male prairie voles show depression‐like behaviour (floating in a forced swimming test and tail suspension test) after separation from their female partners. Reduction of oxytocin transmission in the nucleus accumbens has been shown to be involved in this depression‐like behaviour in prairie voles.84 The oxytocin receptor in the nucleus accumbens has been shown to be up‐regulated via an epigenetic regulation, involving an increase of histone acetylation at a promoter region of the oxytocin receptor gene, after mating. This up‐regulation has been shown to facilitate partner preference formation in female85 and male86 prairie voles. Separation from partners reduces oxytocin synthesis in the hypothalamus and expression of the oxytocin receptor in the nucleus accumbens. Activation of the CRF receptor 2 in the nucleus accumbens suppresses local oxytocin release. Oxytocin administration into the nucleus accumbens shell has been shown to reverse separation‐induced passive coping behaviour, and local knockdown of the oxytocin receptor induces depression‐like passive coping behaviour, suggesting that separation increases CRFR2 signalling, which lowers oxytocin transmission within the nucleus accumbens, resulting in depression‐like behaviour. Consistent with the view of attenuating action of the oxytocin receptor on behavioural stress responses, oxytocin receptor binding in the nucleus accumbens in prairie voles has been shown to be reduced by immobilisation stress, and the presence of their partners dampens both reduction of the oxytocin receptor and anxiety‐related behaviour.87
2.3.3. Hypothalamic paraventricular nucleus
Bilateral infusions of oxytocin into the hypothalamic paraventricular nucleus of adult male rats88 and of female prairie voles89 have been shown to reduce anxiety‐related behaviour in an elevated plus maze test. The paraventricular oxytocin receptor is also involved in social buffering actions. Microinjection of oxytocin into the hypothalamic paraventricular nucleus has been reported to reduce stress responses of anxiety‐related behaviours and the hypothalamic‐adrenal axis, whereas that of an oxytocin antagonist impairs the social buffering actions on behavioural and neuroendocrine responses in female prairie voles.61
Activation of GABAergic neurones has been shown to be involved in the inhibitory actions of oxytocin on anxiety‐related behaviour and the hypothalamic‐pituitary‐adrenal axis. Pharmacological blockade of the GABAA receptor has been reported to block the actions of oxytocin in prairie voles.89 On the other hand, it has been reported that there is no mRNA expression of the oxytocin receptor in parvocellular hypothalamic CRH‐positive neurones of rats90, 91 and in hypothalamic CRH neurones of mice,92 and oxytocin has been shown to reduce the frequency of the spontaneous excitatory postsynaptic current in some CRH neurones of mice.93 These findings suggest that oxytocin suppresses the activity of hypothalamic CRH neurones also by suppressing excitatory synaptic transmission onto CRH neurones presynaptically.
2.3.4. Lateral septum
Deficiency of the oxytocin receptor in the lateral septum has been shown to reduce both social defeat‐induced facilitation of freezing behaviour in response to contextual fear and social buffering‐induced reduction of behavioural fear responses, suggesting that activation of the septal oxytocin receptor enhances social memory of negative or positive social interactions and, as a result, facilitates or reduces behavioural fear responses in mice.94
On the other hand, the oxytocin receptor in the lateral septum has been shown in mice to have behaviourally anxiolytic actions in certain conditions that cannot be explained by the facilitative action of oxytocin on social memory. Oxytocin injected into the dorsolateral septum has been shown to reduce social avoidance behaviour after social fear conditioning in which electric foot‐shocks were applied during investigation of a conspecific with social contact.95 Interestingly, lactating mice, whose oxytocin systems are activated, do not show this social avoidance. This avoidance has been shown to be recovered by local administration of an oxytocin receptor antagonist into the lateral septum, by region‐specific conditional deficiency of the oxytocin receptor in the lateral septum or by inactivation of oxytocin neurones projecting to the lateral septum, suggesting that oxytocin neurones projecting to the lateral septum are critical for suppression of social fear conditioning in lactating mice.96
2.3.5. Basolateral amygdala
Various studies have shown that oxytocin administration reduces amygdala activity in response to threatening social stimuli and, as a result, reduces anxiety in humans,97 although inconsistent reports98 and sexual difference99 have also been reported.
Administration of oxytocin into the basolateral amygdala before context‐shock pairings has been shown in rats to impair acquisition of context‐conditioned fear freezing and administration before re‐exposure to the context has been shown to suppress expression of conditioned freezing and facilitate its extinction in rodents.100 On the other hand, post‐session infusion of oxytocin into the basolateral amygdala has been shown to have no significant effects on consolidation of extinction learning.100 These findings suggest that the oxytocin receptor in the basolateral amygdala inhibits acquisition, suppresses recall and facilitates extinction of freezing behaviour in response to contextual fear conditioning, although contradictory results showing that oxytocin infusion into the basolateral amygdala enhances acquisition of contextual fear conditioning have also been reported.79
Oxytocin in the basolateral amygdala has also been shown to be involved not only in fear conditioning, but also in general discrimination learning. Microinjection of an oxytocin receptor agonist facilitates discrimination between conditioned signals for shocks and signals for the absence of shocks, being consistent with the view that oxytocin generally facilitates information processing of salient signals.101
2.3.6. Central amygdala
Activation of the oxytocin receptor in the central amygdala has been reported to reverse isolation stress‐induced anxiety‐related behaviour in mice.102 Administration of oxytocin into the central amygdala has also been shown to reduce anxiety‐related behaviour in female rats.103 Oxytocin has also been suggested to reduce conditioned fear‐induced freezing behaviour in rats. It has been shown in rats that local administration of an oxytocin agonist activates oxytocin receptor‐expressing GABAergic inhibitory neurones in the lateral part of the central amygdala and, as a result, inhibits neurones in the medial part of the central amygdala projecting to the ventrolateral part of the periaqueductal gray, leading to reduction of freezing behaviour in response to contextual fear stimuli without affecting the cardiovascular fear response.104 Furthermore, activation of oxytocin fibres projecting to the central amygdala has been shown to suppress the expression of freezing behaviour in response to contextual fear stimuli.3 All of these findings suggest that oxytocin in the central amygdala reduces the expression of conditioned fear‐induced freezing behaviour.
Oxytocin in the central amygdala is involved in not only the expression of fear responses, but also the acquisition of fear learning. Administration of oxytocin agonists into the central amygdala before context‐shock pairing of fear conditioning training has been reported to impair the expression of freezing behaviour during re‐exposure to the context, suggesting that activation of the oxytocin receptor in the central amygdala impairs acquisition of contextual conditioned fear in rats.79
On the other hand, contradictory findings have also been reported. Pharmacological studies in rats have shown that administration of oxytocin into the central amygdala before recall of conditioned fear (context re‐exposure) enhances freezing behaviour, whereas administration of an oxytocin receptor antagonist suppresses freezing behaviour in rats, suggesting that the oxytocin receptor in the central amygdala facilitates the expression of conditioned freezing behaviour.100 Infusion of oxytocin after a recall session (extinction session) has also been reported to enhance freezing behaviour during the next recall session, suggesting that oxytocin impairs extinction of conditioned freezing.100 Consistent with the facilitative role of oxytocin in fear conditioning, administration of an oxytocin agonist into the amygdala has been reported to enhance fear acquisition in juvenile rats.105
The discrepant findings regarding the role of central amygdala oxytocin in the control of fear responses remain to be clarified. However, the role of oxytocin may not be as simple as oxytocin directly facilitating or inhibiting fear responses. It has been proposed that oxytocin contributes to the selection of active coping behaviour rather than passive defensive behaviour toward an imminent threat. Oxytocin receptor‐expressing GABAergic neurones in the lateral part of the central amygdala innervating neurones of the medial division of the central amygdala play an important role in switching between active and passive responses to an imminent threat. Blockade of the oxytocin receptor in the central amygdala has been shown to reduce active escape behaviour and increase passive freezing behaviour, whereas activation of the oxytocin receptor has been shown to increase active escape performance and reduce freezing behaviour in rats.106
2.3.7. Bed nucleus of the stria terminalis
The bed nucleus of the stria terminalis plays an important role in neuroendocrine, autonomic and behavioural responses to an ambiguous threat or anxiety107, 108, 109 or those to discrete fear stimuli.110 Administration of an oxytocin receptor antagonist into the bed nucleus of the stria terminalis has been reported to impair acquisition of cued fear conditioning in a fear‐potentiated startle paradigm in rats, suggesting a facilitative role of the oxytocin receptor in the bed nucleus of the stria terminalis in acquisition of fear conditioning.111 On the other hand, oxytocin has been shown to reduce background anxiety in a fear‐potentiated startle paradigm in rats.112 Thus, the oxytocin receptor in the rat dorsolateral bed nucleus of the stria terminalis has been proposed to facilitate responses toward predictable, signalled fear but reduce responses to unsignalled threats or background anxiety via facilitating accurate discrimination between signals for threat and safety.113
The oxytocin receptor has also been shown to be involved in social avoidance after social defeat in female mice. Local administration of an oxytocin receptor antagonist into the anteromedial bed nucleus of the stria terminalis has been reported to increase the time spent for social interaction in socially defeated female California mice,12 suggesting that the oxytocin receptor in the bed nucleus of the stria terminalis facilitates social avoidance in socially defeated female mice.
2.3.8. Dorsal or median raphe serotoninergic nucleus
Serotoninergic neurones in the raphe nucleus express the oxytocin receptor. Activation of the oxytocin receptor in the median raphe nucleus has been shown to facilitate serotonin release, and oxytocin‐induced anxiolytic action is impaired by a serotonin 2A/2C antagonist, suggesting that oxytocin reduces anxiety‐related behaviour via facilitation of serotonin release,114 although it has been shown that oxytocin receptor deficiency in serotoninergic neurones has no significant effect on anxiety‐related behaviour but reduces aggressive behaviour in male mice.115
In summary, oxytocin in rodents has been shown to reduce anxiety‐related behaviours via acting on the medial prefrontal cortex, hypothalamic paraventricular nucleus, central amygdala and raphe nucleus; to attenuate depression‐like behaviour by acting on the nucleus accumbens; and to reduce neuroendocrine stress responses by acting on the hypothalamic paraventricular nucleus. Oxytocin has also been shown to reduce conditioned fear‐induced freezing behaviour by acting on the medial prefrontal cortex, basolateral amygdala and central amygdala. On the other hand, oxytocin has been shown to augment conditioned fear‐induced freezing behaviour, conditioned fear‐induced potentiation of startle responses or social avoidance by acting on the central amygdala or bed nucleus of the stria terminalis. It is likely that oxytocin suppresses or augments stress responses depending on the situation by acting on different brain regions.
2.4. Influence of early‐life experiences on stress responses
Oxytocin has been shown in laboratory animals to be involved in effects of childhood experiences on stress responses in adulthood. Expression of the oxytocin receptor is influenced by environments. An enriched environment has been shown to increase expression of the oxytocin receptor in the prefrontal cortex, anterior insula and basolateral amygdala in prairie voles.116 On the other hand, deprivation of early maternal care in rhesus macaques has been reported to suppress expression of the oxytocin receptor in the hippocampus via epigenetic regulation and induce higher separation anxiety, which was rescued by a certain oxytocin receptor gene single nucleotide polymorphism (SNP).117 Neonatal maternal separation has also been shown to induce larger responses to noxious stimuli in an oxytocin‐ and histone deacetylase‐dependent manner in rats.118 All of these reported findings suggest that expression of the oxytocin receptor is influenced by early experiences and modulates stress responsiveness in adulthood.
Oxytocin has been reported to play an important role in early experience‐dependent development of the sensory cortices in mice.119 Activation of the oxytocin receptor during a developmental period has also been shown to have epigenetic actions to modulate social behaviour and sensory functions.120 Prenatal activation of the oxytocin receptor has been shown to reduce the aggressiveness of male mice in adulthood.121 In female prairie voles, early adversity induced by repeated daily isolation over the first 2 weeks of life has been reported to induce deficits in partner preference in animals with lower oxytocin receptor density of the nucleus accumbens, and neonatal stimulation of oxytocin release has been shown to buffer against the effects of early social isolation.122 All of these findings suggest that activation of the oxytocin receptor in early life has plastic and organisation actions.
In humans, oxytocin‐oxytocin receptor systems have been suggested to be involved in developmental plasticity, especially as a result of early experiences. Childhood abuse has been reported to induce lower plasma oxytocin concentrations in males123 and lower CSF oxytocin in females.124 Carriers of the G‐allele of the oxytocin receptor gene SNP rs53576, a SNP in the third of four introns, have been reported to show higher emotional empathy,125 while carriers of the A allele show lower empathy and higher vulnerability to childhood maltreatment,126, 127, 128 possibly as a result of difficulties in taking appropriate stress‐coping behaviour. However, opposite results showing that G‐allele carriers have higher scores of depression and are more sensitive to childhood trauma have been obtained in other studies.129
3. OXYTOCIN AND FOOD INTAKE
3.1. Activation of oxytocin neurones by food intake
Food intake or gastric distension has been shown to activate oxytocin neurones in the hypothalamus and facilitate oxytocin release,130, 131, 132, 133 whereas fasting reduces oxytocin mRNA.134 Ingredients of food appear to be important for activation of oxytocin neurones. Oral injection of sucrose135 and intragastric ingestion of sweetened condensed milk, although not that of high‐fat cream,136 have been reported to activate oxytocin neurones in the hypothalamus. Although the detailed mechanisms for this macronutrient‐selective activation remain unclear, fibroblast growth factor 21 (FGF21) has been suggested to be involved. Sucrose ingestion has been shown to release FGF21 from the liver in rodents and humans, resulting in suppression of sucrose preference.137, 138, 139 β‐Klotho, which is the FGF21 co‐receptor, was expressed in hypothalamic oxytocin neurones, and peripheral FGF21 administration activated oxytocin neurones. Thus, FGF21 may contribute to activation of oxytocin neurones after sucrose ingestion.140
Activation of oxytocin neurones after food intake is mediated, at least in part, by noradrenergic projections to the hypothalamus from A2 noradrenergic neurones, especially a subpopulation of A2 neurones expressing PrRP in the nucleus tractus solitarius.23, 131 The PrRP/noradrenergic neurones are stimulated by activation of the cholecystokinin octapeptide (CCK)1 receptor on afferent neurones of the gastric vagus nerves. PrRP deficiency or destruction of noradrenergic inputs to the hypothalamus impairs the activation of oxytocin neurones. CCK1 receptor deficiency, PrRP deficiency and oxytocin receptor blockade have been shown to increase meal size, suggesting that the CCK‐PrRP‐oxytocin pathway plays an important role for termination of each meal.131 The roles of other pathways activated or factors released after food intake in activation of oxytocin neurones remain to be clarified.
In fact, not only CCK, but also other anorexigenic factors assumed to be released after food intake have been shown to activate oxytocin neurones. For example, oxytocin neurones of the supraoptic nucleus express glucokinase and the insulin receptor. Oxytocin neurones of rat hypothalamic explants have been reported to be activated in response to glucose and insulin.141 Oxytocin neurones have also been shown to be activated by leptin in rats,142 and reduction of leptin after fasting reduces hypothalamic oxytocin mRNA.143 Peripheral or central administration of glucagon‐like peptide 1 has been reported in rats to activate supraoptic nucleus neurones144 or hypothalamic paraventricular oxytocin neurones.145 α‐MSH has also been shown to facilitate dendritic oxytocin release but to decrease electrical activity of oxytocin neurones in rats.26, 146 Oestrogen treatment has also been reported to increase oxytocin mRNA and induce anorexia in rats.147 Among these anorexigenic substances, oxytocin has been suggested to be downstream of some anorexigenic substances to induce anorexia, including CCK,148, 149, 150 leptin,151, 152 α‐nesfatin‐1,153 α‐MSH154 and oestrogen.147
Consistent with the view that oxytocin neurones are activated after food intake, ghrelin, an orexigenic hormone that is released during fasting, has been reported to hyperpolarise the majority of oxytocin neurones in the hypothalamus,155 although i.c.v. administration of ghrelin has been reported to induce Fos protein expression in some oxytocin neurones.156 Relaxin‐3, an orexigenic peptide that has been suggested to induce stress‐induced hyperphagia,157 has also been shown to inhibit oxytocin neurones in the hypothalamus in rats.158
The sensitivity of oxytocin neurones in response to food intake may be different in light and dark periods. Percentages of oxytocin neurones activated by re‐feeding are similar during the night and daytime, although the amount of food intake during the night is larger than that during the daytime,131 being consistent with the view that oxytocin neurones are sensitive to food intake in the daytime, when food intake is low.
In summary, hypothalamic oxytocin neurones have been shown to be activated by food intake via activation of the vagal afferents‐PrRP/noradrenergic pathway and release of anorexic factors including gastrointestinal factors.
3.2. Roles of oxytocin in the control of food intake
Oxytocin has anorexigenic actions.130, 146, 159, 160 Oxytocin administration has been shown to reduce food intake in laboratory animals and humans.161, 162, 163 The anorexigenic effect of oxytocin has been shown to be stronger in diet‐induced obese rodents.164 However, a meta‐analysis revealed that the anorexigenic effect of oxytocin administration is not statistically significant in humans.161 SNPs of the gene encoding the oxytocin receptor have been shown to be associated with eating disorders including anorexia nervosa and bulimia nervosa.165
On the other hand, although oxytocin receptor‐deficient male mice show late‐onset mild obesity,166 it has been reported that oxytocin receptor deficiency,166 chemogenetic inhibition of hypothalamic paraventricular oxytocin neurones,167 genetically targeted ablation of oxytocin neurones152 and destruction of hypothalamic paraventricular oxytocin neurones150 do not significantly change the total amounts of food intake in mice. Photogenic or chemogenetic activation of hypothalamic paraventricular oxytocin neurones has also be shown to have no significant effect on food intake (re‐feeding) in mice,168, 169 although food intake induced by activation of agouti‐related peptide (AgRP) neurones is reduced by activation of oxytocin neurones.168 On the other hand, an oxytocin receptor antagonist170 or oxytocin receptor deficiency131 increases meal size, suggesting that oxytocin released by food intake plays an essential role for termination of each meal.
Endogenous oxytocin appears to suppress intake of a specific macronutrient, namely carbohydrate. Oxytocin has been shown to reduce preference for carbohydrates but not fat.135 Oxytocin deficiency,171 ablation of oxytocin neurones150 and an oxytocin receptor antagonist172 have been shown in mice to enhance intake of sucrose solutions but not intake of palatable liquid emulsions173 or a high‐fat diet, suggesting that oxytocin preferentially suppresses carbohydrate intake, although no significant preference has been reported in oxytocin receptor‐deficient mice.174
The discrepant findings regarding the actions of oxytocin in food intake remain to be explained. However, effects of oxytocin are dependent on social or metabolic contexts. Oxytocin has been shown to reduce sucrose intake in isolated male rats but not in rats that were allowed social contact with conspecifics.135 Oxytocin has been shown to increase food intake in certain conditions. Oxytocin increases rather than decreases food intake in pregnant rats.175 Oxytocin may increase food intake under stressful conditions by attenuating stress‐induced anorexia. In female prairie voles, social isolation‐induced reduction of sucrose intake has been shown to be recovered by oxytocin, although oxytocin has no significant effect on sucrose intake in control voles.176 Oxytocin has also been shown in mice to reduce novel environment‐induced suppression of food intake177 and to mediate repeated stress‐induced attenuation of delayed gastric emptying,178 thus possibly leading to recovery of food intake.
As we have seen, it is likely that endogenous oxytocin contributes to the termination of each meal. However, oxytocin neurones are not located in the main stream for food intake control. Two distinct neuronal populations in the arcuate nucleus, AgRP/neuropeptide Y (NPY) neurones, which induce food intake, and pro‐opiomelanocortin (POMC) neurones, which induce satiety, play pivotal roles in food intake. These two populations project to melanocortin‐4 receptor‐expressing neurones in the hypothalamic paraventricular nucleus. The melanocortin‐4 receptor‐expressing neurones regulating feeding have been shown in mice to be glutamatergic, and none167 or only a few (2%)179 of them express oxytocin, although magnocellular oxytocin neurones have been shown to express the melanocortin‐4 receptor in rats and appear to receive regulation by α‐MSH.26 The relationship between oxytocin neurones and the main target melanocortin‐4 receptor‐expressing neurones remains to be clarified.
Oxytocin not only terminates food intake, but also affects metabolisms of the body. Activation of hypothalamic paraventricular oxytocin neurones has been reported to increase oxygen consumption in mice.169 Destruction of oxytocin neurones and oxytocin receptor deficiency have been shown in mice to reduce energy consumption and reduce thermogenesis in cold environments.150, 180 These findings suggest that oxytocin facilitates energy consumption and it is likely that decreased energy consumption is a cause of late‐onset obesity in oxytocin receptor‐deficient mice.
Oxytocin has also been shown to act peripherally to control metabolic homeostasis.146, 181 Oxytocin has been reported to increase glucose uptake from muscle and adipose tissue, hepatic gluconeogenesis, insulin secretion from the pancreas, and lipolysis, leading to improved insulin sensitivity and lipid homeostasis. These metabolic actions of oxytocin indicate the possible therapeutic usage of oxytocin for treatment of obese or diabetic patients.
Food intake‐induced activation of oxytocin neurones may also have functions other than control of food intake and energy consumption. Oxytocin has been reported in rats and mice to contribute to post‐prandial sexual appetite,182 natriuresis183 and the inhibition of sodium intake184 and alcohol ingestion.185
In summary, oxytocin has been shown in mice and rats to suppress food intake, especially carbohydrate intake, terminate each meal and increase energy consumption. On the other hand, oxytocin has been reported in laboratory rodents to increase food intake in certain conditions including pregnancy and stressful conditions. Oxytocin neurones themselves are not the main direct target neurones of orexigenic AgRP/NPY neurones or anorexic POMC neurones and oxytocin does not appear to directly control activity of AgRP/NPY or POMC neurones (see the next section). The precise mechanisms by which oxytocin modifies food intake remain to be clarified.
3.3. Sites of action of oxytocin with respect to modulating food intake
Food intake is controlled largely by two anatomically and functionally overlapping systems: a homeostatic or metabolic process and a reward‐related process.186 The homeostatic process is controlled by signals concerning nutritional status or energy reserve status such as signals from the catabolic peptides leptin and insulin and from the anabolic hormone ghrelin. The reward‐related process is associated with hedonia (pleasure, “liking” the food) and incentive or motivational salience (reinforcement or “wanting” the food).187 Reward‐related intake is the consumption of palatable food in the absence of nutrient deficits. Food intake is also modulated by stress‐related emotional processes and by cognitive processes.188 Oxytocin‐oxytocin receptor systems have been suggested to be involved in all of these systems. The oxytocin receptor is located in the homeostatic system including the arcuate nucleus, hypothalamic paraventricular nucleus, dorsomedial hypothalamus, ventromedial hypothalamus, lateral hypothalamus, dorsal vagal complex and parabrachial nucleus, in the reward‐processing system including the ventral tegmental area, nucleus accumbens and ventral pallidum, in emotion processing systems including the amygdala, lateral septum, bed nucleus of the stria terminalis, ventromedial hypothalamus, periaqueductal gray and raphe nucleus, as well as in cognitive systems including the prefrontal cortex, anterior cingulate and insular cortex.13, 114
3.3.1. Prefrontal cortex
Oxytocin has been suggested to reduce the reward value of food by facilitating cognitive processes of shifting attention from short‐term merits to long‐term health consequences. Oxytocin administration with instruction to consider long‐term effects of food intake has been shown in female humans to induce changes in activity of the prefrontal cortex and reduce food craving.189 Oxytocin has also been shown to enhance prefrontal cortex activity and reduce food intake in fasted males.190
3.3.2. Nucleus accumbens and ventral tegmental area
Oxytocin neurones of the hypothalamic paraventricular nucleus have been shown to project to the ventral tegmental area and nucleus accumbens and to regulate activity of dopamine neurones and dopamine release, resulting in control of reward‐related behaviours.191, 192, 193, 194, 195
Oxytocin administration into the ventral tegmental area192 and in the nucleus accumbens core196 has been shown to decrease deprivation‐induced food intake and palatable sucrose intake in rats.197
Oxytocin has also been shown to inhibit addiction by acting on the nucleus accumbens.198 It has been shown that i.c.v. administration of oxytocin blocks methamphetamine‐induced turnover of dopamine in the striatum or nucleus accumbens of mice199 and that administration of oxytocin into the nucleus accumbens reduces methamphetamine‐induced conditioned place preference, drug‐seeking behaviour in rats200 and ethanol‐induced conditioned place preference in mice.201 All of these findings suggest that oxytocin impairs the reward value of addictive drugs and ethanol.
On the other hand, oxytocin has been shown in laboratory rodents to facilitate social reward by acting on the ventral tegmental area or the nucleus accumbens via facilitation of dopamine release.193, 194, 202, 203
Oxytocin has been shown to enhance tonic activity of dopamine neurones of the ventral tegmental area in mice.191, 193 The differential actions of oxytocin to control the activity of dopamine neurones in response to addictive drugs and palatable food and to control the activity of dopamine neurones in response to social behaviour remain to be clarified. However, the effects of oxytocin on activity of dopamine neurones might be different depending on subtypes of dopamine neurones. Oxytocin has been shown in mice to activate dopamine neurones in the ventral tegmental nucleus but to inhibit dopamine neurones in the substantia nigra pars compacta.204 Oxytocin has also been shown in mice to filter certain inputs to dopamine neurones in the ventral tegmental area by inhibiting excitatory synaptic transmission through retrograde endocannabinoid signalling. Thus, oxytocin selectively suppresses inputs that are controlled by the presynaptic cannabinoid receptor and, as a result, modulates the excitability of dopamine neurones in an input‐selective manner.205
Differential activation of dopamine receptors after intake of addictive substances and social behaviour may also be involved in the differential actions of oxytocin. Drug reward appears to be mediated by the dopamine D1 receptor and social reward appears to be mediated by the dopamine D2 receptor, although both pair bonding and drug addiction have been suggested to increase the dopamine D1 receptor in the nucleus accumbens, resulting in selective interest in bonded partners or in drugs.206 Partner preference induced by mating has been shown to be impaired by dopamine D2 receptor antagonists in the nucleus accumbens shell of female prairie voles207 and amphetamine‐induced conditioned place preference has been shown to be blocked by a dopamine D1 receptor antagonist in the nucleus accumbens of male prairie voles.208 Increased activation of the D2 receptor versus D1 receptor has been suggested to induce social rewards over drug rewards.207, 208
In humans, intranasal oxytocin administration has been shown to decrease activation of the ventral tegmental area in response to foods and to increase activities of the dorsal anterior cingulate cortex, frontopolar cortex,165 ventromedial prefrontal cortex, supplementary motor area, anterior cingulate, and ventrolateral prefrontal cortices,190 which are involved in cognitive functions.
In summary, oxytocin reduces the reward value of palatable food intake possibly by inhibiting dopamine release in the nucleus accumbens.
3.3.3. Hypothalamus
The oxytocin receptor is located in various hypothalamic nuclei, including the dorsomedial hypothalamus, arcuate nucleus, ventromedial hypothalamus and lateral hypothalamus,114 which have been shown to be involved in the control of food intake and energy expenditure.209 Administration of oxytocin into the ventromedial hypothalamus has been reported to reduce food intake in rats.210 Chemogenetic activation of oxytocin receptor‐expressing glutamatergic neurones in the arcuate nucleus that project to the hypothalamic paraventricular nucleus has also been reported in mice to rapidly induce satiety, indicating the importance of oxytocin receptor‐expressing arcuate neurones in the control of food intake.211
3.3.4. Amygdala
Oxytocin administration in the basolateral and central amygdala has been shown to suppress food intake moderately in rats.212 On the other hand, an oxytocin receptor antagonist has been reported in mice to impair acquisition of conditioned taste aversion, suggesting that oxytocin facilitates acquisition of conditioned taste aversion.213
3.3.5. Dorsal vagal complex
Oxytocin neurones in the hypothalamic paraventricular nucleus project to the dorsal vagal complex that consists of the nucleus tractus solitarius and dorsal motor nucleus of the vagus (DMV). Oxytocin has been shown in rodents to act on DMV neurones to inhibit gastric motility, resulting in termination of a meal.135 On the other hand, under stressful conditions, it has been suggested that oxytocin has opposite functions in rats and increases gastric tone via suppression of GABAergic inhibitory inputs to DMV neurones, which has facilitative actions on gastric motility.214
In summary, oxytocin has been shown to act on homeostatic systems including the arcuate nucleus and dorsal vagal complex to terminate food intake, on reward systems including the ventral tegmental area and nucleus accumbens to reduce food reward, and on cognition systems including the prefrontal cortex to facilitate cognitive control of food intake.
3.4. Interactions between stress and food intake
Stress responses are influenced by bodily metabolic status.215, 216 Metabolic information is transmitted via neurones or hormones to the hypothalamus, which controls bodily metabolisms and stress responses. Fasting reduces anxiety‐ or fear‐related behaviours. Reduction of anxiety or fear contributes to induction of proactive and risky food‐seeking foraging behaviours during a negative energy balance. On the other hand, perceived stress and glucocorticoid release have been shown to be reduced after acute intake of high carbohydrate or palatable food in humans.188, 217 Stress‐vulnerable individuals may thus learn to self‐medicate comfort food,218 resulting in increased intake of palatable food under stressful conditions.
Stressful stimuli have been shown to modulate food intake and energy expenditure.215, 219 Under stressful conditions such as fight or flight, food intake is suppressed and energy expenditure is increased as a result of elevated sympathetic tones, leading to a negative energy balance. However, in some stressful conditions, hyperphagia220, 221 and/or hypothermia,222 which result in a positive energy balance, are induced. In humans, an increase in perceived stress has been shown to be associated with an increase in high‐fat/carbohydrate palatable food intake188, 218, 223 and visceral obesity.224
The detailed mechanisms of interactions between stress and energy metabolisms remain unclear. However, the involvement of AgRP/NPY neurones has been suggested. Fasting activates AgRP/NPY neurones in the arcuate nucleus, which not only induce food intake, but also are suggested to reduce fear‐related behaviour.225, 226, 227 Arcuate AgRP neurones project to the medial amygdala neurones that innervate the posterior bed nucleus of the stria terminalis, and this AgRP‐medial amygdala‐bed nucleus of the stria terminalis pathway has been shown to be involved in fasting‐induced behavioural changes in mice.226 AgRP/NPY neurones also project to the periaqueductal gray, which is involved in fear and anxiety‐related behaviour. Furthermore, NPY has anxiolytic actions.228 Not only AgRP/NPY, but also the peripheral anorexic hormone leptin and the orexigenic hormone ghrelin have been suggested to be involved in modulation of neuroendocrine stress responses during fasting or satiated conditions. Leptin has been shown to inhibit noradrenaline release in the hypothalamic paraventricular nucleus and adrenocorticotrophic hormone release in response to stressful stimuli, whereas ghrelin facilitated these stress responses in rats.229 On the other hand, the stress‐related hormones CRH and glucocorticoids, and ghrelin have been suggested to be involved in the effects of stress on food intake. A CRH1 receptor antagonist has been reported to suppress stress‐induced eating in humans,230 and CRH neurones have been shown to induce preference for a high‐carbohydrate diet in mice.231 Glucocorticoid administration has also been shown to increase food intake, especially intake of carbohydrates and proteins, in humans232 possibly via activation of brain reward systems.187 Social defeat stress has been shown to facilitate ghrelin release and stress‐induced hyperphagia is impaired in ghrelin receptor‐deficient mice.233
Oxytocin has also been suggested to play a role in interactions between stress and metabolic homeostasis.
As we have seen, stressful stimuli and food intake activate oxytocin‐synthesising neurones, which modulate stress responses and food intake160 by acting on common brain systems including homeostasis, reward, emotion and cognition systems. It is possible that oxytocin contributes to stress‐induced food intake changes and food intake‐induced attenuation of stress responses. Patients with eating disorders frequently show emotional and social dysfunctions, which induce stressful situations and exacerbate symptoms of eating disorders. Genetic predispositions of oxytocin systems have been suggested to induce vulnerability to stressful stimuli and liability to overeating or eating disorders in humans.165, 234, 235, 236 A negative relationship between socioeconomic status and childhood obesity has been shown in A allele carriers of the oxytocin receptor gene SNP rs53576.237 A allele carriers with a low socioeconomic status have been shown to have a high body mass index. On the other hand, no significant association between socioeconomic status and body mass index was found in GG genotyped children. The differential susceptibility of childhood obesity to socioeconomic status is consistent with the view that the oxytocin receptor is involved in effects of adverse environments on energy metabolisms. The sites of action of oxytocin with respect to controlling aberrant eating during stressful stimuli remain to be determined. Oxytocin has been shown to modulate the activity of cognition‐related brain regions in humans.98 Oxytocin may act on the prefrontal cortex to modulate executive functions, to reduce cognitive rigidity and to suppress impulsivity, as been associated with the intake of palatable foods and obesity in humans.238 Oxytocin administration has also been shown to reduce eating‐related concern or attentional bias,239 to attenuate cognitive rigidity, and to decrease salivary cortisol240 in anorexia nervosa, suggesting that oxytocin reduces stress responsiveness related to food intake via improvement of cognitive functions.
4. ROLES OF OXYTOCIN IN THE CONTROL OF ADAPTIVE BEHAVIOURS
Oxytocin has been shown to control not only stress responses and food intake, but also social behaviours. Several hypotheses have been proposed to explain the actions of oxytocin in an integrative way.241 Studies on the role of oxytocin in reproduction‐related behaviours, including pair bonding,206, 242 mating and maternal behaviour of laboratory animals,243 as well as in human behaviours requiring higher cognitive functions, including trust and altruism, have led to the prosocial hypothesis that oxytocin facilitates prosocial behaviours in general.244 Oxytocin paired with charitable social cues has been shown in humans to reduce out‐group rejection even in xenophobic individuals, although the effects of oxytocin appear to be dependent on the experimental conditions.245
On the other hand, oxytocin has also been shown in humans to evoke antisocial behaviours in competitive situations246 and to induce defensive aggression toward out‐group members.247 Intranasal oxytocin application has also been shown to facilitate startle response to threatening stimuli in humans, consistent with the view that oxytocin increases the salience of threatening stimuli.248 In rodents, oxytocin mediates maternal aggression.249 Oxytocin has been shown to facilitate perceptual processing of olfactory signals250 by increasing the signal‐to‐noise ratio via the excitatory drive of inhibitory neurones and that of auditory signals251 and, as a result, enhance social behaviours in rodents. Oxytocin has been reported in rodents to be indispensable for induction of odour‐driven social avoidance from parasitised individuals252 or odour‐dependent learning, although not that of non‐social learning,253 which is either appetitive or aversive, via acting on the medial amygdala, piriform cortex, and anterior olfactory cortex, where olfactory sensory information is processed. Thus, the social salience hypothesis that oxytocin increases the salience of either positive or negative social stimuli by possibly facilitating sensory processing has been proposed.69, 246, 254
Consistent with the idea that oxytocin facilitates sensory processing of socially relevant stimuli, the oxytocin receptor is located densely in the olfactory system in rodents,255 whereas the oxytocin receptor is abundantly located in the visual system or visual attention‐related systems including the superior colliculus and nucleus basalis of Meynert in primates.256 Oxytocin may modulate most relevant sensory systems to regulate social behaviour dependent on species.257 Oxytocin has been shown to enhance eye gaze between humans69 and between dogs and their owners.258 Oxytocin administration in humans has also been shown to enhance recognition of emotion expressed in faces that are either fearful or happy.254
The oxytocin receptor is also located in somatic sensory systems including the trigeminal ganglion, dorsal root ganglion and dorsal horn of the spinal cord. Oxytocin is released after pleasant tactile stimuli in rats259 and it has been proposed to contribute to the formation of social bonding between mothers and their offspring via pleasant tactile stimuli.260 Subjective feelings induced by tactile stimuli are dependent on the social relationship between receivers and givers of touch stimuli. Oxytocin released by intimate social interactions may modulate sensory processing of tactile stimuli to induce a comfort sensation.
The social salience hypothesis, however, cannot fully explain the finding that oxytocin appears to preferentially increase the salience of positive social cues rather than negative social cues.261 Oxytocin has been shown to enhance activity of the amygdala in response to happy facial expressions but to attenuate the activity toward fear faces262 and to improve recognition of happy facial expression selectively.263
Activation of the social reward system may be involved in preferential facilitation of positive social information processing. Oxytocin facilitates dopamine release in response to socially relevant cues in order to evoke positive social behaviours.193, 204, 264, 265 Interactions with serotoninergic projections have been shown to play an important role. Within the nucleus accumbens, the oxytocin receptor is located on presynaptic axon terminals of dorsal raphe serotoninergic neurones, and serotonin released by oxytocin has been shown in mice to promote long‐term depression of glutamatergic transmission via the 5HT1b receptor in the nucleus accumbens to induce social reward.266
Thus, oxytocin increases the reward value of social behaviour (social reward hypothesis) and, as a result, preferentially enhances salience of positive emotional stimuli. The facilitative action of oxytocin on dopamine release appears to be selective to social situations. Oxytocin reduces dopamine release in response to addictive drugs and possibly in response to food, and oxytocin administration has been examined as a therapeutic tool for addiction or obesity.198 The detailed mechanisms for this selective action of oxytocin remain to be clarified.
Oxytocin has also been suggested to facilitate approach behaviour to positive stimuli and to suppress withdrawal behaviour to negative stimuli, regardless of whether they are social or non‐social stimuli (the approach/withdrawal hypothesis).267 Oxytocin has been proposed to act on the prefrontal cortex‐amygdala circuit controlling fear or threat responses to attenuate behavioural avoidance or autonomic stress responses in response to negatively valenced stimuli, whereas, at the same time, oxytocin acts on the dopaminergic reward system to increase approach behaviour in response to emotionally salient stimuli including not only stimuli inducing positive emotion, but also stimuli inducing negative emotion such as anger, envy, and gloating,268 although the role of the dopaminergic system in the control of approach responses to negative emotion‐related stimuli remains to be established.
On the other hand, oxytocin has been suggested to facilitate active coping behaviour in certain conditions and to switch from passive stress‐coping behaviour to active stress‐coping behaviour.24 Recently, oxytocin has been shown to reduce passive coping behaviour such as freezing behaviour by acting on neurones in the lateral part of the central amygdala, which inhibits the activity of periaqueductal gray‐projecting central amygdala neurones, and, as a result, induces active escape behaviour106 or active defensive behaviour.269 Consistent with this idea, oxytocin has been shown to suppress social loss‐induced passive coping behaviour in male prairie voles84 and to induce social defeat‐induced risk assessment behaviour (head orientation) in female mice by acting on the bed nucleus of the stria terminalis.12, 270 Furthermore, oxytocin facilitates expression of social defeat posture, which is an active coping behaviour showing subordination toward dominant conspecifics, by possibly acting on the ventromedial hypothalamus or periaqueductal gray in male mice.13 In rats, the oxytocin receptor in the insular cortex has been shown to be indispensable for showing approach behaviour to distressed juvenile conspecifics and for showing avoidance behaviour to distressed adult conspecifics, suggesting the importance of the oxytocin receptor in appropriate active coping behaviours.271 All of these findings suggest that oxytocin facilitates active stress‐coping behaviour by acting on multiple regions.
These hypotheses are not mutually exclusive. The oxytocin receptor is located in various brain sites, and oxytocin has multiple actions depending on the situation or the individual by acting on various brain sites for an appropriate decision to be made depending on the social environment.272
5. CONCLUSIONS
It is essential for group‐living mammals to find appropriate partners, to bond with and mate with partners, to create and nurture their offspring, to build up friendship, to make an in‐group society, and to deal with difficulties cooperatively under stressful conditions. Oxytocin appears to be involved in these steps to some degree by acting on various brain regions. Oxytocin facilitates sensory processing to preferentially receive socially salient signals and facilitates reward values of prosocial behaviour, especially toward in‐group members and, at the same time, reduces anxiety and induces satiety, resulting in appropriate active coping behaviours being taken to adapt to social environments (Figure 1). Reproduction, food intake, stress responses and social behaviours are associated with each other, and it is interesting to speculate that oxytocin modulates these functions in an integrative way to induce active and adaptive coping behaviours.
Figure 1.
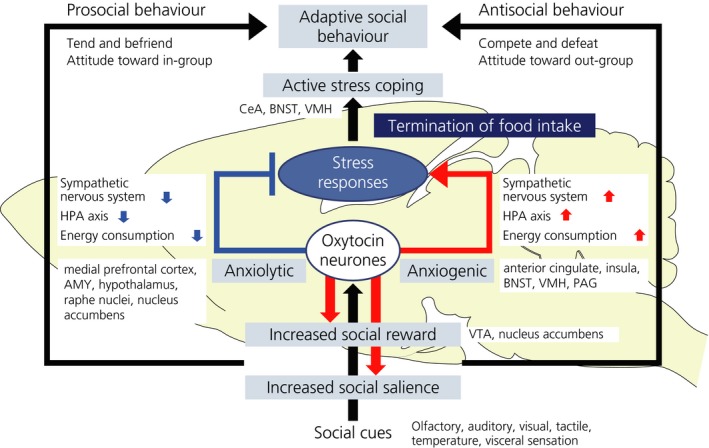
Oxytocin facilitates sensory processing of socially salient stimuli, facilitates reward value of social behaviours, modulates stress responses depending on the situation, induces active coping behaviour and terminates food intake. Oxytocin acts on various brain regions to play multifaceted roles leading to adaptive behaviours. AMY, amygdala; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; HPA, hypothalamic‐pituitary‐adrenal; PAG, periaqueductal gray; VMH, ventromedial hypothalamus; VTA, ventral tegmental area
In stressful conditions, there are at least two aspects of behavioural strategies. One is the “tend‐and‐befriend” or “fight or fright” aspect273 and the other is passive coping behaviour (freezing, withdrawal) or active coping behaviour (active escape, flight, or fight). Oxytocin appears to facilitate the tend‐and‐befriend strategy in non‐competitive situations, whereas it facilitates fight or flight behaviours in competitive situations. Oxytocin also appears to facilitate active coping behaviour rather than passive behaviour. Because behavioural or neuroendocrine stress responses are final common outputs after integrating various processes of internal and external information, oxytocin may induce augmentative or attenuated stress responses depending on experimental situations by acting on multiple brain areas.
However, the detailed mechanisms of the actions of oxytocin remain unclear. It is not known how oxytocin induces apparently opposite behaviours depending on the situation. The detailed functional and anatomical heterogeneity of oxytocin neurones remains to be elucidated. The mechanisms of gender difference274, 275 should also be clarified. Oxytocin has also been reported to act not only on the oxytocin receptor, but also on vasopressin receptors, on δ subunit‐containing GABAA receptors276 and on transient receptor potential vanilloid (TRPV1).277 Although pharmacological studies have shown the importance of vasopressin receptors in the actions of oxytocin,278 molecular and genetic evidence directly showing the roles of vasopressin receptors is not sufficient.279, 280 The physiological roles of these receptors in the actions of oxytocin remain to be clarified. Further studies are also necessary to facilitate therapeutic potentials of oxytocin281, 282 or oxytocin‐related treatments including diet ingredients283 and intestinal microbial symbionts.284
Onaka T, Takayanagi Y. Role of oxytocin in the control of stress and food intake. J Neuroendocrinol. 2019;31:e12700 10.1111/jne.12700
Funding information
Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT)/Japan Society for the Promotion of Science KAKENHI Grants‐in‐Aid for Scientific Research: 17K19636, and 17H04026 (to T.O.) and 17K08573 (to Y.T.). Grant‐in‐Aid for Scientific Research on Innovative Areas “The Evolutionary Origin and Neural Basis of the Empathetic Systems”: 25118008 (to T.O.), Takeda Science Foundation (to T.O.).
[The copyright line for this article was changed on 22 April 2020 after original online publication.]
REFERENCES
- 1. Althammer F, Grinevich V. Diversity of oxytocin neurons: beyond magno‐ and parvocellular cell types? J Neuroendocrinol. 2018;30:e12549. [DOI] [PubMed] [Google Scholar]
- 2. Dolen G. Oxytocin: parallel processing in the social brain? J Neuroendocrinol. 2015;27:516‐535. [DOI] [PubMed] [Google Scholar]
- 3. Knobloch HS, Charlet A, Hoffmann LC, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553‐566. [DOI] [PubMed] [Google Scholar]
- 4. Ludwig M, Leng G. Dendritic peptide release and peptide‐dependent behaviours. Nat Rev Neurosci. 2006;7:126‐136. [DOI] [PubMed] [Google Scholar]
- 5. Takayanagi Y, Yoshida M, Takashima A, et al. Activation of supraoptic oxytocin neurons by secretin facilitates social recognition. Biol Psychiatry. 2017;81:243‐251. [DOI] [PubMed] [Google Scholar]
- 6. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629‐683. [DOI] [PubMed] [Google Scholar]
- 7. Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 2018;98:1805‐1908. [DOI] [PubMed] [Google Scholar]
- 8. Song Z, Albers HE. Cross‐talk among oxytocin and arginine‐vasopressin receptors: relevance for basic and clinical studies of the brain and periphery. Front Neuroendocrinol. 2018;51:14‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337‐354. [DOI] [PubMed] [Google Scholar]
- 10. Onaka T, Okabe S, Takayanagi Y, Yoshida M. Noxious or non‐noxious inputs to oxytocin neurons: possible roles in the control of behaviors. Interdisciplin Info Sci. 2015;21:189‐195. [Google Scholar]
- 11. Onaka T, Yagi K. Differential effects of naloxone on neuroendocrine responses to fear‐related emotional stress. Exp Brain Res. 1990;81:53‐58. [DOI] [PubMed] [Google Scholar]
- 12. Duque‐Wilckens N, Steinman MQ, Busnelli M, et al. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress‐induced social avoidance in female California mice. Biol Psychiatry. 2018;83:203‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nasanbuyan N, Yoshida M, Takayanagi Y, et al. Oxytocin‐oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology. 2018;159:763‐775. [DOI] [PubMed] [Google Scholar]
- 14. Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439‐448. [DOI] [PubMed] [Google Scholar]
- 15. Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11:867‐872. [DOI] [PubMed] [Google Scholar]
- 16. Wotjak CT, Kubota M, Liebsch G, et al. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci. 1996;16:7725‐7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314‐316. [DOI] [PubMed] [Google Scholar]
- 18. Nishioka T, Anselmo‐Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:57‐61. [DOI] [PubMed] [Google Scholar]
- 19. Douglas AJ, Johnstone HA, Wigger A, Landgraf R, Russell JA, Neumann ID. The role of endogenous opioids in neurohypophysial and hypothalamo‐pituitary‐adrenal axis hormone secretory responses to stress in pregnant rats. J Endocrinol. 1998;158:285‐293. [DOI] [PubMed] [Google Scholar]
- 20. Torner L, Plotsky PM, Neumann ID, de Jong TR. Forced swimming‐induced oxytocin release into blood and brain: effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology. 2017;77:165‐174. [DOI] [PubMed] [Google Scholar]
- 21. Rivest S, Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune‐challenged rats. J Neuroendocrinol. 1995;7:501‐525. [DOI] [PubMed] [Google Scholar]
- 22. Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin‐1 on stress‐related neuroendocrine neurons. J Neurosci. 1994;14:897‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onaka T, Takayanagi Y, Yoshida M. Roles of oxytocin neurones in the control of stress, energy metabolism, and social behaviour. J Neuroendocrinol. 2012;24:587‐598. [DOI] [PubMed] [Google Scholar]
- 24. Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649‐659. [DOI] [PubMed] [Google Scholar]
- 25. Hada T, Onaka T, Takahashi T, Hiraga A, Yagi K. Effects of novelty stress on neuroendocrine activities and running performance in thoroughbred horses. J Neuroendocrinol. 2003;15:638‐648. [DOI] [PubMed] [Google Scholar]
- 26. Sabatier N. α‐Melanocyte‐stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol. 2006;18:703‐710. [DOI] [PubMed] [Google Scholar]
- 27. Landgraf R, Hacker R, Buhl H. Plasma vasopressin and oxytocin in response to exercise and during a day‐night cycle in man. Endokrinologie. 1982;79:281‐291. [PubMed] [Google Scholar]
- 28. Jong TR, Menon R, Bludau A, et al. Salivary oxytocin concentrations in response to running, sexual self‐stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381‐388. [DOI] [PubMed] [Google Scholar]
- 29. Hew‐Butler T, Noakes TD, Soldin SJ, Verbalis JG. Acute changes in endocrine and fluid balance markers during high‐intensity, steady‐state, and prolonged endurance running: unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur J Endocrinol. 2008;159:729‐737. [DOI] [PubMed] [Google Scholar]
- 30. Hew‐Butler T, Jordaan E, Stuempfle KJ, et al. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab. 2008;93:2072‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernhard A, van der Merwe C, Ackermann K, Martinelli A, Neumann ID, Freitag CM. Adolescent oxytocin response to stress and its behavioral and endocrine correlates. Horm Behav. 2018;105:157‐165. [DOI] [PubMed] [Google Scholar]
- 32. Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. 2011;36:115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277:2661‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McQuaid RJ, McInnis OA, Paric A, Al‐Yawer F, Matheson K, Anisman H. Relations between plasma oxytocin and cortisol: the stress buffering role of social support. Neurobiol Stress. 2016;3:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu LL, Onaka T. Facilitative role of prolactin‐releasing peptide neurons in oxytocin cell activation after conditioned‐fear stimuli. Neuroscience. 2003;118:1045‐1053. [DOI] [PubMed] [Google Scholar]
- 36. Yoshida M, Takayanagi Y, Onaka T. The medial amygdala‐medullary PrRP‐synthesizing neuron pathway mediates neuroendocrine responses to contextual conditioned fear in male rodents. Endocrinology. 2014;155:2996‐3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hatton GI. Emerging concepts of structure‐function dynamics in adult brain: the hypothalamo‐neurohypophysial system. Prog Neurobiol. 1990;34:437‐504. [DOI] [PubMed] [Google Scholar]
- 38. Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress‐induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829‐2834. [DOI] [PubMed] [Google Scholar]
- 39. Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress‐induced c‐fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo‐pituitary‐adrenal activity. J Neurosci. 2004;24:2974‐2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress‐induced activity of the hypothalamo‐pituitary‐adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235‐243. [DOI] [PubMed] [Google Scholar]
- 41. Uvnäs‐Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819‐835. [DOI] [PubMed] [Google Scholar]
- 42. Cavanaugh J, Carp SB, Rock CM, French JA. Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology. 2016;66:22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersson M, Wiberg U, Lundeberg T, Uvnäs‐Moberg K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides. 2001;22:1479‐1484. [DOI] [PubMed] [Google Scholar]
- 44. Szeto A, Sun‐Suslow N, Mendez AJ, Hernandez RI, Wagner KV, McCabe PM. Regulation of the macrophage oxytocin receptor in response to inflammation. Am J Physiol Endocrinol Metab. 2017;312:E183‐E189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee HJ, Macbeth AH, Pagani JH, Young WS 3rd. Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61:392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ring RH, Malberg JE, Potestio L, et al. Anxiolytic‐like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218‐225. [DOI] [PubMed] [Google Scholar]
- 48. Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858‐865. [DOI] [PubMed] [Google Scholar]
- 49. Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16:319‐324. [DOI] [PubMed] [Google Scholar]
- 50. Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin‐deficient mice display enhanced anxiety‐related behavior. Endocrinology. 2003;144:2291‐2296. [DOI] [PubMed] [Google Scholar]
- 51. Neumann ID, Slattery DA. Oxytocin in general anxiety and social fear: a translational approach. Biol Psychiatry. 2016;79:213‐221. [DOI] [PubMed] [Google Scholar]
- 52. Meyer‐Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663‐668. [DOI] [PubMed] [Google Scholar]
- 53. Holt‐Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta‐analytic review. PLoS Med. 2010;7:e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361:2215‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kiyokawa Y, Hennessy MB. Comparative studies of social buffering: a consideration of approaches, terminology, and pitfalls. Neurosci Biobehav Rev. 2018;86:131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Waldherr M, Neumann ID. Centrally released oxytocin mediates mating‐induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681‐16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nyuyki KD, Waldherr M, Baeuml S, Neumann ID. Yes, I am ready now: differential effects of paced versus unpaced mating on anxiety and central oxytocin release in female rats. PLoS ONE. 2011;6:e23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728‐731. [DOI] [PubMed] [Google Scholar]
- 59. Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389‐1398. [DOI] [PubMed] [Google Scholar]
- 60. Preis A, Samuni L, Mielke A, Deschner T, Crockford C, Wittig RM. Urinary oxytocin levels in relation to post‐conflict affiliations in wild male chimpanzees (Pan troglodytes verus). Horm Behav. 2018;105:28‐40. [DOI] [PubMed] [Google Scholar]
- 61. Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin‐dependent consolation behavior in rodents. Science. 2016;351:375‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li LF, Yuan W, He ZX, et al. Involvement of oxytocin and GABA in consolation behavior elicited by socially defeated individuals in mandarin voles. Psychoneuroendocrinology. 2019;103:14‐24. [DOI] [PubMed] [Google Scholar]
- 64. Paloyelis Y, Krahe C, Maltezos S, Williams SC, Howard MA, Fotopoulou A. The analgesic effect of oxytocin in humans: a double‐blind, placebo‐controlled cross‐over study using laser‐evoked potentials. J Neuroendocrinol. 2016;28: 10.1111/jne.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boll S, Almeida de Minas AC, Raftogianni A, Herpertz SC, Grinevich V. Oxytocin and pain perception: from animal models to human research. Neuroscience. 2018;387:149‐161. [DOI] [PubMed] [Google Scholar]
- 66. Gonzalez‐Hernandez A, Rojas‐Piloni G, Condes‐Lara M. Oxytocin and analgesia: future trends. Trends Pharmacol Sci. 2014;35:549‐551. [DOI] [PubMed] [Google Scholar]
- 67. Gonzalez‐Hernandez A, Manzano‐Garcia A, Martinez‐Lorenzana G, et al. Peripheral oxytocin receptors inhibit the nociceptive input signal to spinal dorsal horn wide‐dynamic‐range neurons. Pain. 2017;158:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 68. Eliava M, Melchior M, Knobloch‐Bollmann HS, et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89:1291‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301‐309. [DOI] [PubMed] [Google Scholar]
- 70. MacDonald K, Feifel D. Oxytocin's role in anxiety: a critical appraisal. Brain Res. 2014;1580:22‐56. [DOI] [PubMed] [Google Scholar]
- 71. Eckstein M, Scheele D, Weber K, Stoffel‐Wagner B, Maier W, Hurlemann R. Oxytocin facilitates the sensation of social stress. Hum Brain Mapp. 2014;35:4741‐4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eckstein M, Scheele D, Patin A, et al. Oxytocin facilitates pavlovian fear learning in males. Neuropsychopharmacology. 2016;41:932‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18:958‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Striepens N, Scheele D, Kendrick KM, et al. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA. 2012;109:18144‐18149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC. Oxytocin enhances observational fear in mice. Nat Commun. 2017;8:2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Crockford C, Deschner T, Wittig RM. The role of oxytocin in social buffering: what do primate studies add? Curr Top Behav Neurosci. 2018;35:155‐173. [DOI] [PubMed] [Google Scholar]
- 77. McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015;27:446‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sabihi S, Dong SM, Maurer SD, Post C, Leuner B. Oxytocin in the medial prefrontal cortex attenuates anxiety: anatomical and receptor specificity and mechanism of action. Neuropharmacology. 2017;125:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lahoud N, Maroun M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology. 2013;38:2184‐2195. [DOI] [PubMed] [Google Scholar]
- 80. Li K, Nakajima M, Ibanez‐Tallon I, Heintz N. A cortical circuit for sexually dimorphic oxytocin‐dependent anxiety behaviors. Cell. 2016;167:60‐72 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nakajima M, Gorlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159:295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brill‐Maoz N, Maroun M. Extinction of fear is facilitated by social presence: synergism with prefrontal oxytocin. Psychoneuroendocrinology. 2016;66:75‐81. [DOI] [PubMed] [Google Scholar]
- 83. Eckstein M, Becker B, Scheele D, et al. Oxytocin facilitates the extinction of conditioned fear in humans. Biol Psychiatry. 2015;78:194‐202. [DOI] [PubMed] [Google Scholar]
- 84. Bosch OJ, Dabrowska J, Modi ME, et al. Oxytocin in the nucleus accumbens shell reverses CRFR2‐evoked passive stress‐coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang H, Duclot F, Liu Y, Wang Z, Kabbaj M. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat Neurosci. 2013;16:919‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Duclot F, Wang H, Youssef C, Liu Y, Wang Z, Kabbaj M. Trichostatin A (TSA) facilitates formation of partner preference in male prairie voles (Microtus ochrogaster). Horm Behav. 2016;81:68‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Donovan M, Liu Y, Wang Z. Anxiety‐like behavior and neuropeptide receptor expression in male and female prairie voles: the effects of stress and social buffering. Behav Brain Res. 2018;342:70‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Blume A, Bosch OJ, Miklos S, et al. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neuorsci. 2008;27:1947‐1956. [DOI] [PubMed] [Google Scholar]
- 89. Smith AS, Tabbaa M, Lei K, et al. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016;63:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF‐containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci. 2013;7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Winter J, Jurek B. The interplay between oxytocin and the CRF system: regulation of the stress response. Cell Tissue Res. 2018;375:85‐91. [DOI] [PubMed] [Google Scholar]
- 92. Chen R, Wu X, Jiang L, Zhang Y. Single‐cell RNA‐Seq reveals hypothalamic cell diversity. Cell Rep. 2017;18:3227‐3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jamieson BB, Nair BB, Iremonger KJ. Regulation of hypothalamic corticotropin‐releasing hormone neurone excitability by oxytocin. J Neuroendocrinol. 2017;29:e12532. [DOI] [PubMed] [Google Scholar]
- 94. Guzman YF, Tronson NC, Sato K, et al. Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology. 2014;231:2097‐2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology. 2014;39:3027‐3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Menon R, Grund T, Zoicas I, et al. Oxytocin signaling in the lateral septum prevents social fear during lactation. Curr Biol. 2018;28:1066‐1078 e1066. [DOI] [PubMed] [Google Scholar]
- 97. Radke S, Volman I, Kokal I, Roelofs K, de Bruijn ERA, Toni I. Oxytocin reduces amygdala responses during threat approach. Psychoneuroendocrinology. 2017;79:160‐166. [DOI] [PubMed] [Google Scholar]
- 98. Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I. Oxytocin and brain activity in humans: a systematic review and coordinate‐based meta‐analysis of functional MRI studies. Psychoneuroendocrinology. 2018;96:6‐24. [DOI] [PubMed] [Google Scholar]
- 99. Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83‐93. [DOI] [PubMed] [Google Scholar]
- 100. Campbell‐Smith EJ, Holmes NM, Lingawi NW, Panayi MC, Westbrook RF. Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context‐conditioned fear in rats. Learn Mem. 2015;22:247‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fam J, Holmes N, Delaney A, Crane J, Westbrook RF. Oxytocin receptor activation in the basolateral complex of the amygdala enhances discrimination between discrete cues and promotes configural processing of cues. Psychoneuroendocrinology. 2018;96:84‐92. [DOI] [PubMed] [Google Scholar]
- 102. Han RT, Kim YB, Park EH, et al. Long‐term isolation elicits depression and anxiety‐related behaviors by reducing oxytocin‐induced GABAergic transmission in central amygdala. Front Mol Neurosci. 2018;11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region‐specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546‐2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Viviani D, Charlet A, van den Burg E, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104‐107. [DOI] [PubMed] [Google Scholar]
- 105. Kritman M, Lahoud N, Maroun M. Oxytocin in the amygdala and not the prefrontal cortex enhances fear and impairs extinction in the juvenile rat. Neurobiol Learn Mem. 2017;141:179‐188. [DOI] [PubMed] [Google Scholar]
- 106. Terburg D, Scheggia D, Triana Del Rio R, et al. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell. 2018;175:723‐735 e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Goode TD, Maren S. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn Mem. 2017;24:480‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shackman AJ, Fox AS. Contributions of the central extended amygdala to fear and anxiety. J Neurosci. 2016;36:8050‐8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gungor NZ, Pare D. Functional heterogeneity in the bed nucleus of the stria terminalis. J Neurosci. 2016;36:8038‐8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Moaddab M, Dabrowska J. Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear‐potentiated startle paradigm in rats. Neuropharmacology. 2017;121:130‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ayers LW, Missig G, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear‐potentiated startle paradigm: peripheral vs central administration. Neuropsychopharmacology. 2011;36:2488‐2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Janecek M, Dabrowska J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies‐potential interaction with the corticotropin‐releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res. 2018;375:143‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yoshida M, Takayanagi Y, Inoue K, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pagani JH, Williams Avram SK, Cui Z, et al. Raphe serotonin neuron‐specific oxytocin receptor knockout reduces aggression without affecting anxiety‐like behavior in male mice only. Genes Brain Behav. 2015;14:167‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Prounis GS, Thomas K, Ophir AG. Developmental trajectories and influences of environmental complexity on oxytocin receptor and vasopressin 1A receptor expression in male and female prairie voles. J Comp Neurol. 2018;526:1820‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Baker M, Lindell SG, Driscoll CA, et al. Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proc Natl Acad Sci USA. 2017;114:11769‐11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Melchior M, Juif PE, Gazzo G, et al. Pharmacological rescue of nociceptive hypersensitivity and oxytocin analgesia impairment in a rat model of neonatal maternal separation. Pain. 2018;159:2630‐2640. [DOI] [PubMed] [Google Scholar]
- 119. Zheng JJ, Li SJ, Zhang XD, et al. Oxytocin mediates early experience‐dependent cross‐modal plasticity in the sensory cortices. Nat Neurosci. 2014;17:391‐399. [DOI] [PubMed] [Google Scholar]
- 120. Sannino S, Chini B, Grinevich V. Lifespan oxytocin signaling: maturation, flexibility, and stability in newborn, adolescent, and aged brain. Dev Neurobiol. 2017;77:158‐168. [DOI] [PubMed] [Google Scholar]
- 121. Takayanagi Y, Yoshida M, Bielsky IF, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor‐deficient mice. Proc Natl Acad Sci USA. 2005;102:16096‐16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiat. 2015;5:e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Opacka‐Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress. 2012;15:1‐10. [DOI] [PubMed] [Google Scholar]
- 124. Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14:954‐958. [DOI] [PubMed] [Google Scholar]
- 125. Gong P, Fan H, Liu J, Yang X, Zhang K, Zhou X. Revisiting the impact of OXTR rs53576 on empathy: a population‐based study and a meta‐analysis. Psychoneuroendocrinology. 2017;80:131‐136. [DOI] [PubMed] [Google Scholar]
- 126. Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Curr Opin Neurobiol. 2013;23:11‐16. [DOI] [PubMed] [Google Scholar]
- 127. Flasbeck V, Moser D, Kumsta R, Brune M. The OXTR Single‐nucleotide polymorphism rs53576 moderates the impact of childhood maltreatment on empathy for social pain in female participants: evidence for differential susceptibility. Front Psychiatry. 2018;9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cicchetti D, Rogosch FA. Gene x Environment interaction and resilience: effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Dev Psychopathol. 2012;24:411‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. McQuaid RJ, McInnis OA, Stead JD, Matheson K, Anisman H. A paradoxical association of an oxytocin receptor gene polymorphism: early‐life adversity and vulnerability to depression. Front Neurosci. 2013;7:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Leng G, Onaka T, Caquineau C, Sabatier N, Tobin V, Takayanagi Y. Oxytocin and appetite. Prog Brain Res. 2008;170:137‐151. [DOI] [PubMed] [Google Scholar]
- 131. Yamashita M, Takayanagi Y, Yoshida M, Nishimori K, Kusama M, Onaka T. Involvement of prolactin‐releasing peptide in the activation of oxytocin neurones in response to food intake. J Neuroendocrinol. 2013;25:455‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313‐321. [DOI] [PubMed] [Google Scholar]
- 133. Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417‐1419. [DOI] [PubMed] [Google Scholar]
- 134. Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Olszewski PK, Klockars A, Levine AS. Oxytocin: a conditional anorexigen whose effects on appetite depend on the physiological, behavioural and social contexts. J Neuroendocrinol. 2016;28: 10.1111/jne.12376. [DOI] [PubMed] [Google Scholar]
- 136. Hume C, Sabatier N, Menzies J. High‐sugar, but not high‐fat, food activates supraoptic nucleus neurons in the male rat. Endocrinology. 2017;158:2200‐2211. [DOI] [PubMed] [Google Scholar]
- 137. von Holstein‐Rathlou S, BonDurant Lucas D, Peltekian L, et al. FGF21 Mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016;23:335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Soberg S, Sandholt CH, Jespersen NZ, et al. FGF21 Is a sugar‐induced hormone associated with sweet intake and preference in humans. Cell Metab. 2017;25:1045‐1053 e1046. [DOI] [PubMed] [Google Scholar]
- 139. Talukdar S, Owen BM, Song P, et al. FGF21 Regulates sweet and alcohol preference. Cell Metab. 2016;23:344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Matsui S, Sasaki T, Kohno D, et al. Neuronal SIRT1 regulates macronutrient‐based diet selection through FGF21 and oxytocin signalling in mice. Nat Commun. 2018;9:4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sladek CD, Stevens W, Song Z, Johnson GC, MacLean PS. The “metabolic sensor” function of rat supraoptic oxytocin and vasopressin neurons is attenuated during lactation but not in diet‐induced obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310:R337‐R345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Velmurugan S, Russell JA, Leng G. Systemic leptin increases the electrical activity of supraoptic nucleus oxytocin neurones in virgin and late pregnant rats. J Neuroendocrinol. 2013;25:383‐390. [DOI] [PubMed] [Google Scholar]
- 143. Tung YC, Ma M, Piper S, Coll A, O'Rahilly S, Yeo GS. Novel leptin‐regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus. J Neurosci. 2008;28:12419‐12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Saito R, So M, Motojima Y, et al. Activation of nesfatin‐1‐containing neurones in the hypothalamus and brainstem by peripheral administration of anorectic hormones and suppression of feeding via central nesfatin‐1 in rats. J Neuroendocrinol. 2016;28: 10.1111/jne.12400. [DOI] [PubMed] [Google Scholar]
- 145. Larsen PJ, Tang‐Christensen M, Jessop DS. Central administration of glucagon‐like peptide‐1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445‐4455. [DOI] [PubMed] [Google Scholar]
- 146. Leng G, Sabatier N. Oxytocin – the sweet hormone? Trends Endocrinol Metab. 2017;28:365‐376. [DOI] [PubMed] [Google Scholar]
- 147. Sloan DK, Spencer DS, Curtis KS. Estrogen effects on oxytocinergic pathways that regulate food intake. Horm Behav. 2018;105:128‐137. [DOI] [PubMed] [Google Scholar]
- 148. Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991;129:785‐791. [DOI] [PubMed] [Google Scholar]
- 149. Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30‐41. [DOI] [PubMed] [Google Scholar]
- 150. Xi D, Long C, Lai M, et al. Ablation of oxytocin neurons causes a deficit in cold stress response. J Endocr Soc. 2017;1:1041‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87‐R96. [DOI] [PubMed] [Google Scholar]
- 152. Wu Z, Xu Y, Zhu Y, et al. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE. 2012;7:e45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Maejima Y, Sedbazar U, Suyama S, et al. Nesfatin‐1‐regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin‐independent melanocortin pathway. Cell Metab. 2009;10:355‐365. [DOI] [PubMed] [Google Scholar]
- 154. Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin‐1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642‐R1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dos‐Santos RC, Grover HM, Reis LC, Ferguson AV, Mecawi AS. Electrophysiological effects of ghrelin in the hypothalamic paraventricular nucleus neurons. Front Cell Neurosci. 2018;12:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Olszewski PK, Bomberg EM, Martell A, Grace MK, Levine AS. Intraventricular ghrelin activates oxytocin neurons: implications in feeding behavior. NeuroReport. 2007;18:499‐503. [DOI] [PubMed] [Google Scholar]
- 157. Calvez J, de Avila C, Timofeeva E. Sex‐specific effects of relaxin‐3 on food intake and body weight gain. Br J Pharmacol. 2017;174:1049‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kania A, Gugula A, Grabowiecka A, et al. Inhibition of oxytocin and vasopressin neuron activity in rat hypothalamic paraventricular nucleus by relaxin‐3‐RXFP3 signalling. J Physiol. 2017;595:3425‐3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Olszewski PK, Klockars A, Levine AS. Oxytocin and potential benefits for obesity treatment. Curr Opin Endocrinol Diabetes Obes. 2017;24:320‐325. [DOI] [PubMed] [Google Scholar]
- 160. Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017;13:700‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Leslie M, Silva P, Paloyelis Y, Blevins J, Treasure J. A systematic review and quantitative meta‐analysis of oxytocin's effects on feeding. J Neuroendocrinol. 2018;30:e12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Skinner JA, Campbell EJ, Dayas CV, Garg ML, Burrows TL. The relationship between oxytocin, dietary intake and feeding: a systematic review and meta‐analysis of studies in mice and rats. Front Neuroendocrinol. 2019;52:65‐78. [DOI] [PubMed] [Google Scholar]
- 163. Maejima Y, Yokota S, Nishimori K, Shimomura K. The anorexigenic neural pathways of oxytocin and its clinical implication. Neuroendocrinology. 2018;107:91‐104. [DOI] [PubMed] [Google Scholar]
- 164. Roberts ZS, Wolden‐Hanson T, Matsen ME, et al. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet‐induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2017;313:R357‐R371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Plessow F, Eddy KT, Lawson EA. The neuropeptide hormone oxytocin in eating disorders. Curr Psychiatry Rep. 2018;20:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor‐deficient mice developed late‐onset obesity. NeuroReport. 2008;19:951‐955. [DOI] [PubMed] [Google Scholar]
- 167. Garfield AS, Li C, Madara JC, et al. A neural basis for melanocortin‐4 receptor‐regulated appetite. Nat Neurosci. 2015;18:863‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sutton AK, Pei H, Burnett KH, Myers MG Jr, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci. 2014;34:15306‐15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus‐brainstem circuit. J Neurosci. 2009;29:8302‐8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828‐R1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Herisson FM, Brooks LL, Waas JR, Levine AS, Olszewski PK. Functional relationship between oxytocin and appetite for carbohydrates versus saccharin. NeuroReport. 2014;25:909‐914. [DOI] [PubMed] [Google Scholar]
- 173. Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1063‐R1068. [DOI] [PubMed] [Google Scholar]
- 174. Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS 3rd. A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256‐3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352‐365. [DOI] [PubMed] [Google Scholar]
- 176. Grippo AJ, Trahanas DM, Zimmerman RR 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long‐term social isolation. Psychoneuroendocrinology. 2009;34:1542‐1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Olszewski PK, Ulrich C, Ling N, Allen K, Levine AS. A non‐peptide oxytocin receptor agonist, WAY‐267,464, alleviates novelty‐induced hypophagia in mice: insights into changes in c‐Fos immunoreactivity. Pharmacol Biochem Behav. 2014;124:367‐372. [DOI] [PubMed] [Google Scholar]
- 178. Babygirija R, Zheng J, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Sustained delayed gastric emptying during repeated restraint stress in oxytocin knockout mice. J Neuroendocrinol. 2010;22:1181‐1186. [DOI] [PubMed] [Google Scholar]
- 179. Li C, Navarrete J, Liang‐Guallpa J, et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 2019;29:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kasahara Y, Sato K, Takayanagi Y, et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology. 2013;154:4305‐4315. [DOI] [PubMed] [Google Scholar]
- 181. Ding C, Leow MK, Magkos F. Oxytocin in metabolic homeostasis: implications for obesity and diabetes management. Obes Rev. 2018;20:22‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Caquineau C, Leng G, Douglas AJ. Sexual behaviour and neuronal activation in the vomeronasal pathway and hypothalamus of food‐deprived male rats. J Neuroendocrinol. 2012;24:712‐723. [DOI] [PubMed] [Google Scholar]
- 183. Verbalis JG, Mangione MP, Stricker EM. Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology. 1991;128:1317‐1322. [DOI] [PubMed] [Google Scholar]
- 184. Stricker EM, Verbalis JG. Central inhibition of salt appetite by oxytocin in rats. Regul Pept. 1996;66:83‐85. [DOI] [PubMed] [Google Scholar]
- 185. Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict Biol. 2017;22:702–711. [DOI] [PubMed] [Google Scholar]
- 186. Caron A, Richard D. Neuronal systems and circuits involved in the control of food intake and adaptive thermogenesis. Ann N Y Acad Sci. 2017;1391:35‐53. [DOI] [PubMed] [Google Scholar]
- 187. Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2015;58:36‐45. [DOI] [PubMed] [Google Scholar]
- 189. Striepens N, Schroter F, Stoffel‐Wagner B, Maier W, Hurlemann R, Scheele D. Oxytocin enhances cognitive control of food craving in women. Hum Brain Mapp. 2016;37:4276‐4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Spetter MS, Feld GB, Thienel M, Preissl H, Hege MA, Hallschmid M. Oxytocin curbs calorie intake via food‐specific increases in the activity of brain areas that process reward and establish cognitive control. Sci Rep. 2018;8:2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Tang Y, Chen Z, Tao H, et al. Oxytocin activation of neurons in ventral tegmental area and interfascicular nucleus of mouse midbrain. Neuropharmacology. 2014;77:277‐284. [DOI] [PubMed] [Google Scholar]
- 192. Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013;1513:85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hung LW, Neuner S, Polepalli JS, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Song Z, Borland JM, Larkin TE, O'Malley M, Albers HE. Activation of oxytocin receptors, but not arginine‐vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward‐like properties of social interactions. Psychoneuroendocrinology. 2016;74:164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol. 2017;525:1094‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Herisson FM, Waas JR, Fredriksson R, Schioth HB, Levine AS, Olszewski PK. Oxytocin acting in the nucleus accumbens core decreases food intake. J Neuroendocrinol. 2016;28:e12381. [DOI] [PubMed] [Google Scholar]
- 197. Klockars A, Levine AS, Olszewski PK. Central oxytocin and food intake: focus on macronutrient‐driven reward. Front Endocrinol. 2015;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Bowen MT, Neumann ID. Rebalancing the addicted brain: oxytocin interference with the neural substrates of addiction. Trends Neurosci. 2017;40:691‐708. [DOI] [PubMed] [Google Scholar]
- 199. Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine‐induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn‐Schmiedeberg's Arch Pharmacol. 2008;376:441‐448. [DOI] [PubMed] [Google Scholar]
- 200. Baracz SJ, Everett NA, McGregor IS, Cornish JL. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine‐seeking behaviour in rats. Addict Biol. 2016;21:316‐325. [DOI] [PubMed] [Google Scholar]
- 201. Bahi A. The oxytocin receptor impairs ethanol reward in mice. Physiol Behav. 2015;139:321‐327. [DOI] [PubMed] [Google Scholar]
- 202. Borland JM, Grantham KN, Aiani LM, Frantz KJ, Albers HE. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology. 2018;95:128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin‐dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276‐2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y. Biased oxytocinergic modulation of midbrain dopamine systems. Neuron. 2017;95:368‐384 e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Xiao L, Priest MF, Kozorovitskiy Y. Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons. eLife. 2018;7:e33892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Walum H, Young LJ. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci. 2018;19:643‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Lieberwirth C, Wang Z. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr Opin Neurobiol. 2016;40:8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor‐mediated mechanism. J Neurosci. 2011;31:7960‐7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683‐701. [DOI] [PubMed] [Google Scholar]
- 210. Noble EE, Billington CJ, Kotz CM, Wang C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol. 2014;307:R737‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Fenselau H, Campbell JN, Verstegen AM, et al. A rapidly acting glutamatergic ARC–>PVH satiety circuit postsynaptically regulated by alpha‐MSH. Nat Neurosci. 2017;20:42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Klockars OA, Klockars A, Levine AS, Olszewski PK. Oxytocin administration in the basolateral and central nuclei of amygdala moderately suppresses food intake. NeuroReport. 2018;29:504‐510. [DOI] [PubMed] [Google Scholar]
- 213. Olszewski PK, Waas JR, Brooks LL, Herisson F, Levine AS. Oxytocin receptor blockade reduces acquisition but not retrieval of taste aversion and blunts responsiveness of amygdala neurons to an aversive stimulus. Peptides. 2013;50:36‐41. [DOI] [PubMed] [Google Scholar]
- 214. Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13:389‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ulrich‐Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure, food intake and emotional state. Stress. 2015;18:381‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Harrell CS, Gillespie CF, Neigh GN. Energetic stress: the reciprocal relationship between energy availability and the stress response. Physiol Behav. 2016;166:43‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Tryon MS, Stanhope KL, Epel ES, et al. Excessive sugar consumption may be a difficult habit to break: a view from the brain and body. J Clin Endocrinol Metab. 2015;100:2239‐2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853‐865. [DOI] [PubMed] [Google Scholar]
- 219. O'Connor DB, Conner M. Effects of stress on eating behavior In: Baum A, Contrada R, eds. The Handbook of Stress Science: Biology, Psychology, and Health. New York, NY: Springer; 2011:275‐286. [Google Scholar]
- 220. Razzoli M, Pearson C, Crow S, Bartolomucci A. Stress, overeating, and obesity: insights from human studies and preclinical models. Neurosci Biobehav Rev. 2017;76:154‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Rowland NE, Antelman SM. Stress‐induced hyperphagia and obesity in rats: a possible model for understanding human obesity. Science. 1976;191:310‐312. [DOI] [PubMed] [Google Scholar]
- 222. Chen X, Herbert J. Regional changes in c‐fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675‐685. [DOI] [PubMed] [Google Scholar]
- 223. Mikolajczyk RT, El Ansari W, Maxwell AE. Food consumption frequency and perceived stress and depressive symptoms among students in three European countries. Nutr J. 2009;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Tenk J, Matrai P, Hegyi P, et al. Perceived stress correlates with visceral obesity and lipid parameters of the metabolic syndrome: a systematic review and meta‐analysis. Psychoneuroendocrinology. 2018;95:63‐73. [DOI] [PubMed] [Google Scholar]
- 225. Burnett CJ, Li C, Webber E, et al. Hunger‐driven motivational state competition. Neuron. 2016;92:187‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Padilla SL, Qiu J, Soden ME, et al. Agouti‐related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci. 2016;19:734‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Jikomes N, Ramesh RN, Mandelblat‐Cerf Y, Andermann ML. Preemptive stimulation of AgRP neurons in fed mice enables conditioned food seeking under threat. Curr Biol. 2016;26:2500‐2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kawakami A, Okada N, Rokkaku K, Honda K, Ishibashi S, Onaka T. Leptin inhibits and ghrelin augments hypothalamic noradrenaline release after stress. Stress. 2008;11:363‐369. [DOI] [PubMed] [Google Scholar]
- 230. Epstein DH, Kennedy AP, Furnari M, et al. Effect of the CRF1‐receptor antagonist pexacerfont on stress‐induced eating and food craving. Psychopharmacology. 2016;233:3921‐3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Okamoto S, Sato T, Tateyama M, et al. Activation of AMPK‐regulated CRH neurons in the PVH is sufficient and necessary to induce dietary preference for carbohydrate over fat. Cell Rep. 2018;22:706‐721. [DOI] [PubMed] [Google Scholar]
- 232. Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317‐E325. [DOI] [PubMed] [Google Scholar]
- 233. Abizaid A. Stress and obesity: The ghrelin connection. J Neuroendocrinol. 2019;44:e12693. [DOI] [PubMed] [Google Scholar]
- 234. Micali N, Crous‐Bou M, Treasure J, Lawson EA. Association between oxytocin receptor genotype, maternal care, and eating disorder behaviours in a community sample of women. Eur Eat Disord Rev. 2017;25:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Acevedo SF, Valencia C, Lutter M, McAdams CJ. Severity of eating disorder symptoms related to oxytocin receptor polymorphisms in anorexia nervosa. Psychiatry Res. 2015;228:641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Davis C, Patte K, Zai C, Kennedy JL. Polymorphisms of the oxytocin receptor gene and overeating: the intermediary role of endophenotypic risk factors. Nutr Diabetes. 2017;7:e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Bush NR, Allison AL, Miller AL, Deardorff J, Adler NE, Boyce WT. Socioeconomic disparities in childhood obesity risk: association with an oxytocin receptor polymorphism. JAMA Pediatr. 2017;171:61‐67. [DOI] [PubMed] [Google Scholar]
- 238. Bongers P, van de Giessen E, Roefs A, et al. Being impulsive and obese increases susceptibility to speeded detection of high‐calorie foods. Health Psychol. 2015;34:677‐685. [DOI] [PubMed] [Google Scholar]
- 239. Kim YR, Kim CH, Cardi V, Eom JS, Seong Y, Treasure J. Intranasal oxytocin attenuates attentional bias for eating and fat shape stimuli in patients with anorexia nervosa. Psychoneuroendocrinology. 2014;44:133‐142. [DOI] [PubMed] [Google Scholar]
- 240. Russell J, Maguire S, Hunt GE, et al. Intranasal oxytocin in the treatment of anorexia nervosa: randomized controlled trial during re‐feeding. Psychoneuroendocrinology. 2018;87:83‐92. [DOI] [PubMed] [Google Scholar]
- 241. Piva M, Chang SWC. An integrated framework for the role of oxytocin in multistage social decision‐making. Am J Primatol. 2018;80:e22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Carter CS. The oxytocin‐vasopressin pathway in the context of love and fear. Front Endocrinol. 2017;8:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Numan M, Young LJ. Neural mechanisms of mother‐infant bonding and pair bonding: similarities, differences, and broader implications. Horm Behav. 2016;77:98‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Meyer‐Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524‐538. [DOI] [PubMed] [Google Scholar]
- 245. Marsh N, Scheele D, Feinstein JS, et al. Oxytocin‐enforced norm compliance reduces xenophobic outgroup rejection. Proc Natl Acad Sci USA. 2017;114:9314‐9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Shamay‐Tsoory SG, Abu‐Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. 2016;79:194‐202. [DOI] [PubMed] [Google Scholar]
- 247. De Dreu CK, Greer LL, Handgraaf MJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408‐1411. [DOI] [PubMed] [Google Scholar]
- 248. Leppanen J, Ng KW, Kim YR, Tchanturia K, Treasure J. Meta‐analytic review of the effects of a single dose of intranasal oxytocin on threat processing in humans. J Affect Disord. 2018;225:167‐179. [DOI] [PubMed] [Google Scholar]
- 249. Bosch OJ. Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Oettl LL, Ravi N, Schneider M, et al. Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron. 2016;90:609‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Marlin BJ, Mitre M, D'Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kavaliers M, Ossenkopp KP, Choleris E. Social neuroscience of disgust. Genes Brain Behav. 2018;18:e12508. [DOI] [PubMed] [Google Scholar]
- 253. Choe HK, Reed MD, Benavidez N, et al. Oxytocin mediates entrainment of sensory stimuli to social cues of opposing valence. Neuron. 2015;87:152‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Shahrestani S, Kemp AH, Guastella AJ. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta‐analysis. Neuropsychopharmacology. 2013;38:1929‐1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Althammer F, Jirikowski G, Grinevich V. The oxytocin system of mice and men‐Similarities and discrepancies of oxytocinergic modulation in rodents and primates. Peptides. 2018;109:1‐8. [DOI] [PubMed] [Google Scholar]
- 256. Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J Neuroendocrinol. 2016;28: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Grinevich V, Stoop R. Interplay between oxytocin and sensory systems in the orchestration of socio‐emotional behaviors. Neuron. 2018;99:887‐904. [DOI] [PubMed] [Google Scholar]
- 258. Nagasawa M, Mitsui S, En S, et al. Social evolution. Oxytocin‐gaze positive loop and the coevolution of human‐dog bonds. Science. 2015;348:333‐336. [DOI] [PubMed] [Google Scholar]
- 259. Okabe S, Yoshida M, Takayanagi Y, Onaka T. Activation of hypothalamic oxytocin neurons following tactile stimuli in rats. Neurosci Lett. 2015;600:22‐27. [DOI] [PubMed] [Google Scholar]
- 260. Uvnas‐Moberg K, Handlin L, Petersson M. Self‐soothing behaviors with particular reference to oxytocin release induced by non‐noxious sensory stimulation. Front Psychol. 2014;5:1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Averbeck BB. Oxytocin and the salience of social cues. Proc Natl Acad Sci USA. 2010;107:9033‐9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence‐related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400‐9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology. 2010;209:225‐232. [DOI] [PubMed] [Google Scholar]
- 264. Groppe SE, Gossen A, Rademacher L, et al. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74:172‐179. [DOI] [PubMed] [Google Scholar]
- 265. Scheele D, Wille A, Kendrick KM, et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA. 2013;110:20308‐20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Kemp AH, Guastella AJ. Oxytocin: prosocial behavior, social salience, or approach‐related behavior? Biol Psychiatry. 2010;67:e33‐e34; author reply e35. [DOI] [PubMed] [Google Scholar]
- 268. Harari‐Dahan O, Bernstein A. A general approach‐avoidance hypothesis of oxytocin: accounting for social and non‐social effects of oxytocin. Neurosci Biobehav Rev. 2014;47:506‐519. [DOI] [PubMed] [Google Scholar]
- 269. Rickenbacher E, Perry RE, Sullivan RM, Moita MA. Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother‐pup interactions. eLife. 2017;6:PMC5469614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Steinman MQ, Duque‐Wilckens N, Trainor BC. Complementary neural circuits for divergent effects of oxytocin: social approach versus social anxiety. Biol Psychiatry. 2018; 10.1016/j.biopsych.2018.1010.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Rogers‐Carter MM, Varela JA, Gribbons KB, et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. 2018;21:404‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Ma Y, Shamay‐Tsoory S, Han S, Zink CF. Oxytocin and social adaptation: insights from neuroimaging studies of healthy and clinical populations. Trends Cogn Sci. 2016;20:133‐145. [DOI] [PubMed] [Google Scholar]
- 273. Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend‐and‐befriend, not fight‐or‐flight. Psychol Rev. 2000;107:411‐429. [DOI] [PubMed] [Google Scholar]
- 274. Gao S, Becker B, Luo L, et al. Oxytocin, the peptide that bonds the sexes also divides them. Proc Natl Acad Sci USA. 2016;113:7650‐7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Bredewold R, Veenema AH. Sex differences in the regulation of social and anxiety‐related behaviors: insights from vasopressin and oxytocin brain systems. Curr Opin Neurobiol. 2018;49:132‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Bowen MT, Peters ST, Absalom N, Chebib M, Neumann ID, McGregor IS. Oxytocin prevents ethanol actions at delta subunit‐containing GABAA receptors and attenuates ethanol‐induced motor impairment in rats. Proc Natl Acad Sci USA. 2015;112:3104‐3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Nersesyan Y, Demirkhanyan L, Cabezas‐Bratesco D, et al. Oxytocin modulates nociception as an agonist of pain‐sensing TRPV1. Cell Rep. 2017;21:1681‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Qiu F, Qiu CY, Cai H, et al. Oxytocin inhibits the activity of acid‐sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171:3065‐3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Sala M, Braida D, Lentini D, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875‐882. [DOI] [PubMed] [Google Scholar]
- 280. Schorscher‐Petcu A, Sotocinal S, Ciura S, et al. Oxytocin‐induced analgesia and scratching are mediated by the vasopressin‐1A receptor in the mouse. J Neurosci. 2010;30:8274‐8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Yamasue H, Okada T, Munesue T, et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol Psychiatry. 2019; 10.1038/s41380-41018-40097-41382 [DOI] [PubMed] [Google Scholar]
- 282. Tachibana M, Kagitani‐Shimono K, Mohri I, et al. Long‐term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013;23:123‐127. [DOI] [PubMed] [Google Scholar]
- 283. Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa‐Mattioli M. Microbial reconstitution reverses maternal diet‐induced social and synaptic deficits in offspring. Cell. 2016;165:1762‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Poutahidis T, Kearney SM, Levkovich T, et al. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE. 2013;8:e78898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Dong N, Du P, Hao X, et al. Involvement of GABAA receptors in the regulation of social preference and emotional behaviors by oxytocin in the central amygdala of female mandarin voles. Neuropeptides. 2017;66:8‐17. [DOI] [PubMed] [Google Scholar]


