Abstract
Objective
The aim of this study was to explore the dose response of licogliflozin, a dual inhibitor of sodium/glucose cotransporter 1 (SGLT1) and 2 (SGLT2), by evaluating change in body weight in adults with overweight or obesity.
Methods
This dose‐response analysis evaluated change in body weight following 24 weeks with four once‐daily and twice‐daily licogliflozin doses (2.5‐150 mg) versus placebo (primary end point). A further 24‐week analysis evaluated the efficacy and safety of two once‐daily licogliflozin doses in maintaining initial weight reduction.
Results
Licogliflozin once daily or twice daily produced a significant dose‐response signal for weight loss versus placebo (P < 0.0001). However, mean adjusted percent changes in body weight after 24 weeks were modest, ranging from −0.45% to −3.83% (in the 50 mg twice daily group [95% CI: −5.26% to −2.48%]; n = 75). Responder analysis of ≥ 5% weight loss at week 24 revealed significant differences versus placebo, which were most pronounced with highest doses of 50 mg twice daily (45.3%) and 150 mg once daily (42.9%) (both P < 0.01). While weight loss was greater at higher doses, gastrointestinal adverse events were also more frequent. The 50‐mg once‐daily dose had perhaps the best balance between efficacy and tolerability.
Conclusions
Licogliflozin produced significant reductions in body weight versus placebo. However, the magnitude of weight reduction was modest.
Study Importance.
What is already known?
-
►
Sodium/glucose cotransporter 2 (SGLT2) inhibitors are antidiabetes agents that reduce body weight by about 3%.
-
►
Licogliflozin, a novel, potent dual SGLT1 and 2 inhibitor, significantly reduced body weight by 5.7% on average versus placebo in a 12‐week proof‐of‐concept study in patients with obesity.
What does this study add?
-
►
This was a dose‐ranging study of eight doses of licogliflozin (once daily or twice daily) over 24 weeks in adults with overweight and obesity. Treatment for a further 24 weeks with lower daily maintenance doses resulted in weight loss up to 5.4% versus placebo for the full study period.
-
►
High licogliflozin doses were associated with improved waist circumference, hemoglobin A1c, and systolic blood pressure.
-
►
The most frequently reported adverse events were gastrointestinal related (particularly at high doses), which improved when doses were lowered.
How might these results change the focus of clinical practice?
-
►
These results provide the first evidence for a dose‐related effect of licogliflozin treatment on body weight in adults with overweight or obesity.
-
►
This study provides a foundation for studying licogliflozin in other conditions, such as nonalcoholic steatohepatitis (NCT04065841).
Introduction
Obesity can be defined as a “chronic, progressive, relapsing, multi‐factorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences” 1. Despite public health nutritional initiatives and physical activity recommendations, the emergence of overweight and obesity and their complications is described as an epidemic, with a limited number of safe and effective antiobesity agents available 1, 2, 3. Furthermore, the amount of weight loss mediated by existing antiobesity agents is often less than the degree desired by patients and some clinicians. For example, the proportion of patients achieving clinically meaningful (at least 5%) weight loss was 75% for phentermine‐topiramate, 63% for liraglutide, 55% for naltrexone‐bupropion, 49% with lorcaserin, and 44% for orlistat compared with 23% for placebo 4.
Sodium/glucose cotransporter 2 (SGLT2) mediates the renal tubular reabsorption of filtered glucose. SGLT2 inhibitors are approved as antidiabetes agents. SGLT2 inhibitors decrease body weight among patients with or without diabetes mellitus by 2% to 3%, largely because of a negative caloric balance as a result of glycosuria 5, 6, 7, 8, 9. SGLT1 mediates the intestinal absorption of simple sugars, such as glucose and galactose. SGLT1 inhibition therefore leads to calorie wasting and it also may enhance incretin hormone secretion, which may contribute to blood glucose and body weight control 10, 11, 12.
Licogliflozin is a dual inhibitor of both SGLT1 and SGLT2 with more than 30‐fold selectivity for SGLT2 (in vitro IC50 [half‐maximal inhibitory concentration] of 20.6 and 0.58nM, respectively). In a previous 12‐week proof‐of‐concept (PoC) study, licogliflozin demonstrated significant weight loss (~6%) versus placebo, with favorable changes in metabolic parameters and incretin hormones 13. A high incidence of diarrhea was observed at higher doses, which was predominantly mild and which mostly declined in frequency over time.
This current study evaluated the efficacy, tolerability, and safety of a dose range of licogliflozin in adults with overweight and obesity and explored weight maintenance doses after initial weight reduction. Taking into account the results from the previous PoC study, 150 mg of licogliflozin was selected as the highest once‐daily (qd) dose in the 24‐week dose‐finding study. Twice‐daily (bid) dosing evaluated whether increased frequency of intestinal licogliflozin exposure could result in greater weight loss and improved tolerability compared with once‐daily dosing. The additional 24‐week study was designed to determine whether the licogliflozin effects could be maintained over a longer period (48 weeks). Two different low‐level doses were tested in the maintenance phase to identify whether a specific dose could contribute to a more beneficial effect.
Methods
Study design and oversight
This was a multicenter, placebo‐controlled, randomized, double‐blind, dose‐finding study in adults with overweight or obesity, which was carried out between May 6, 2017, and August 2, 2018, at 91 centers across seven countries in Europe and North America. Following an initial 2‐week screening period, eligible participants entered a 4‐week placebo run‐in period (to ensure future compliance with the study drug). Participants were then randomized into an initial dose‐finding period (part 1) to evaluate the change in body weight after 24 weeks of treatment with eight different doses and regimens of licogliflozin (2.5 mg, 10 mg, 50 mg, and 150 mg qd; 2.5 mg, 5 mg, 25 mg, and 50 mg bid) compared with placebo. This was followed by 24 weeks of treatment with two doses of licogliflozin (25 mg or 35 mg) or placebo. Participants were instructed on study‐required lifestyle intervention (hypocaloric nutritional intervention and increased physical activity) throughout the study. Participants with weight < 114 kg (250 lb) were advised to follow a 1,200‐ to 1,500‐kcal/d diet, while those with weight ≥ 114 kg (250 lb) were advised to follow a 1,500‐ to 1,800‐kcal/d diet 14. Compliance was reviewed and reinforced at every study visit. Further information on lifestyle information can be found in online Supporting Information.
This study was designed and implemented in accordance with International Conference on Harmonisation Harmonized Tripartite Guidelines for Good Clinical Practice 15 and according to the ethical principles of the Declaration of Helsinki 16. Ethical approval was obtained from the institutional review boards or independent ethics committees of each participating center. All participants provided written informed consent for participation prior to randomization. The trial was overseen by a Ketoacidosis Adjudication Committee. Site monitoring was carried out by Novartis. The study investigator (or a designated staff member) was responsible for data collection and reporting. The study sponsor had access to the trial database and performed all analyses.
Participants
This study included adults between 18 and 75 years old with BMI ≥ 30 or BMI ≥ 27 combined with at least one obesity complication (e.g., history of cardiovascular disease, hypertension, dyslipidemia, dysglycemia, including prediabetes or type 2 diabetes mellitus [T2DM], sleep apnea). Exclusion criteria included participants using pharmacologically active weight loss medications, glucagon‐like peptide agonists, or SGLT2 inhibitors within 3 months of screening or between screening and randomization, as well as bariatric surgery. Other key exclusion criteria comprised a history of ketoacidosis, lactic acidosis, or hyperosmolar coma; symptomatic genital infection or urinary tract infection (UTI); gastrointestinal (GI) disorders associated with chronic diarrhea; congestive heart failure (New York Heart Association class III or IV); and a lack of compliance with lifestyle intervention or study medication (defined as < 80% blinded study drug intake assessed at randomization).
Study procedures
Following the 4‐week run‐in period, eligible participants were randomized via interactive response technology in the ratio of 1:1:1:2:1:1:1:2:2 to licogliflozin (2.5 mg qd, 10 mg qd, 50 mg qd, 150 mg qd, 2.5 mg bid, 5 mg bid, 25 mg bid, and 50 mg bid) or placebo (both as tablets) for 24 weeks. Following this, participants treated with licogliflozin in the once‐daily regimen were switched to 25 mg once daily, while those treated with licogliflozin in the twice‐daily regimen were switched to 35 mg once daily for a further 24 weeks. Participants receiving placebo were switched in a 1:1 ratio to 25 mg of licogliflozin once a day or placebo (Figure 1). All participants received three tablets in the morning and two tablets in the evening regardless of the treatment regimen.
Figure 1.
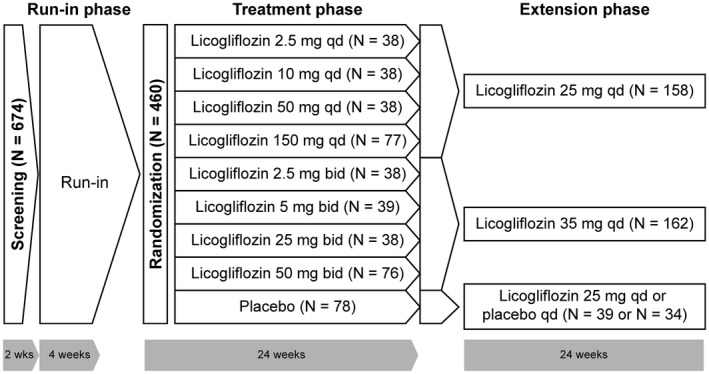
Study design. bid, twice a day; qd, once a day.
Participants were stratified according to their glycemic status at screening. Normoglycemic participants included those with no prior clinical diagnosis of T2DM, fasting plasma glucose (FPG) < 5.6 mmol/L (100 mg/dL), and hemoglobin A1c (HbA1c) < 5.7% at screening. Dysglycemic participants included all those not meeting the criteria for normoglycemic or T2DM at screening. The T2DM group included participants with a prior diagnosis of T2DM or those without prior diagnosis of T2DM but with HbA1c ≥ 6.5 % and FPG ≥ 7.0 mmol/L (126 mg/dL) at screening.
Patients with T2DM taking sulfonylurea had their dose reduced by 50% at randomization because of the risk of hypoglycemia. In the case of persistent deterioration in glycemic control during the study, concomitant background oral antidiabetes treatment was initially escalated to the maximal approved dose, followed by the addition of rescue medication (a dipeptidyl peptidase 4 inhibitor or insulin) as required. For participants treated with insulin, an initial reduction of the daily dose by 10% or more was considered at the investigator’s discretion. Insulin could be up‐titrated following a deterioration in glycemic control. During the initial 24‐week active placebo‐controlled treatment period, doses of T2DM and high blood pressure (BP) medications were adjusted in participants who were considered to be at a safety risk (e.g., risk of repetitive or severe hypoglycemia, symptoms and signs of hypotension, volume depletion).
Efficacy assessments included measurement of body weight, BMI, waist circumference, HbA1c, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting lipid profile (triglycerides, total cholesterol, high‐density lipoprotein [HDL] cholesterol, low‐density lipoprotein [LDL] cholesterol), high‐sensitivity C‐reactive protein (hsCRP), and 24‐hour urinary glucose excretion (UGE24). Safety assessments included assessments of vital signs, clinical signs and symptoms, and changes in physical exam, with recording of all adverse events (AEs) and serious AEs, along with their severity and relationship to study drug, and pregnancies. Pharmacokinetics assessments are described in online Supporting Information. Suspected cases of ketoacidosis were to be reviewed by a Ketoacidosis Adjudication Committee.
Study end points
The primary end point of this study was percent change from baseline in body weight following treatment with four once‐daily doses of licogliflozin (2.5 mg, 10 mg, 50 mg, and 150 mg qd) or four twice‐daily doses of licogliflozin (2.5 mg, 5 mg, 25 mg, and 50 mg bid) versus placebo after 24 weeks.
Secondary end points included response rates according to percent decrease in body weight (≥ 5% or ≥ 10%) from baseline at week 24 for the overall population and by glycemic status (normoglycemic, dysglycemic, and T2DM); weight loss following 24 weeks of treatment according to glycemic status; effect of 24 weeks of licogliflozin versus placebo on waist circumference, FPG, HbA1c, BP, fasting lipid profile, hsCRP, and UGE24; change in weight and secondary efficacy parameters by licogliflozin treatment versus placebo between week 24 and week 48; safety and tolerability of licogliflozin; and pharmacokinetics. Exploratory end points included the percent change in weight from baseline after 48 weeks as well as the effects of licogliflozin on selected efficacy variables over 48 weeks in the individual treatment groups (waist circumference, BP, fasting lipid profile, hsCRP) and the effects of licogliflozin on selected AEs of interest over 48 weeks.
Statistical analysis
The primary analysis for the percent change from baseline in body weight at week 24 was performed using a Multiple Comparison Procedure‐Modeling (MCP‐Mod) methodology 17. To preserve the familywise error rate at a one‐sided significance level of 2.5%, the optimal contrasts derived from the prespecified model candidate set (Emax [maximal effect at high drug concentrations], sigmoid Emax, linear and exponential models) for each dosing regimen were individually compared with the critical value derived using a multiplicity adjustment to account for all tests comparing licogliflozin doses to placebo across all regimens simultaneously. The analysis to derive the test statistics is based on an ANCOVA model, with the percent change in body weight from baseline to week 24 as a response variable, with treatment, baseline glycemic status, and region as factors and baseline weight as a covariate. A model‐averaging approach was used to estimate the dose response in each dosing regimen separately. For further details, see online Supporting Information.
A planned randomized cohort of 432 participants would provide at least 95% power to detect a statistically significant dose‐response signal at one‐sided α of 2.5%, assuming the underlying maximum weight loss effect was 5% or 6% and the SD was 5.5% or 6.5% in either regimen, following adjustment for 15% dropout rate.
Results
Patient disposition
In total, 674 participants were screened for this study. Of the 460 patients who were randomized to the study, the majority (85.7%) completed the initial 24‐week dose‐ranging treatment period (Figure 2). The most common reasons for discontinuation during this time were AEs (6.3%) and participant/guardian decision (4.8%). Study discontinuations were generally greater for higher licogliflozin doses versus lower licogliflozin doses or placebo, particularly at ≥ 10 mg qd or ˃ 5 mg bid.
Figure 2.
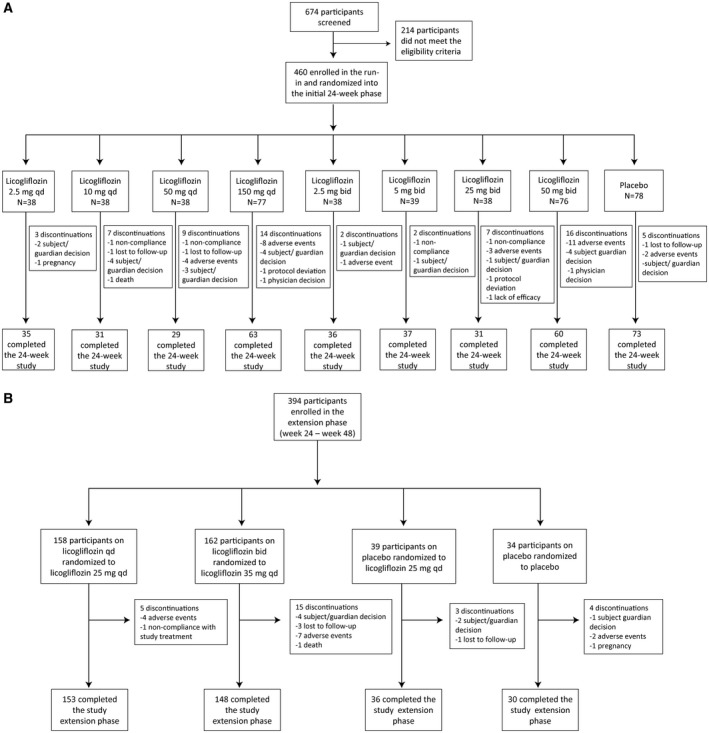
Study profile. (A) The 24‐week study. (B) The 24‐week study extension phase.
Most of the 394 participants who completed the 24‐week treatment period and entered the extension phase (weeks 24‐48) completed the study (93.1%), with discontinuations mainly because of participant/guardian decision (1.8%), AEs (3.3%), and participants lost to follow‐up (1.0%) (Figure 2).
Patient characteristics
Randomized participants were generally comparable across treatment groups (Supporting Information Table S1). Participants had a median age of 53 years (19‐74 years), and they were primarily Caucasian (85.2%) and women (61.5%). Mean BMI was 37.9, with almost all patients (92.0%) having BMI ≥ 30 and 58.3% with BMI ≥ 35. Mean waist circumference was 118.3 cm. The largest group of patients had dysglycemia (40.4%), followed by T2DM (35.2%) and then normoglycemia (24.3%).
Effect of licogliflozin on body weight
The primary end point of the study was the dose‐response percent change in body weight from baseline versus placebo after 24 weeks of one of four once‐daily doses of licogliflozin (2.5 mg, 10 mg, 50 mg, and 150 mg qd) or four twice‐daily doses of licogliflozin (2.5 mg, 5 mg, 25 mg, and 50 mg bid) versus placebo. All candidate models for testing the dose‐response signal in both once‐daily and twice‐daily regimens were statistically significant (adjusted P < 0.0001), among which the best fitted model was the sigmoid Emax model (ED50 [50% effective dose] = 25 mg; hours = 0.7) for the once‐daily regimen (test statistics: 5.606) and the log‐linear model for the twice‐daily regimen (test statistics: 5.884) (Supporting Information Table S2).
A statistically significant (MCP‐Mod–based estimate) dose‐response weight loss signal was demonstrated for both once‐daily and twice‐daily licogliflozin regimens versus placebo (P < 0.0001; Figure 3). The greatest placebo‐subtracted change from baseline in body weight at week 24 was −3.73% (95% CI: −5.04% to −2.49%; n = 77) in the once‐daily regimen (licogliflozin 150 mg) and –3.83% (95% CI: −5.26% to −2.48%; n = 75) in the twice‐daily regimen (licogliflozin 50 mg) (Table 1).
Figure 3.
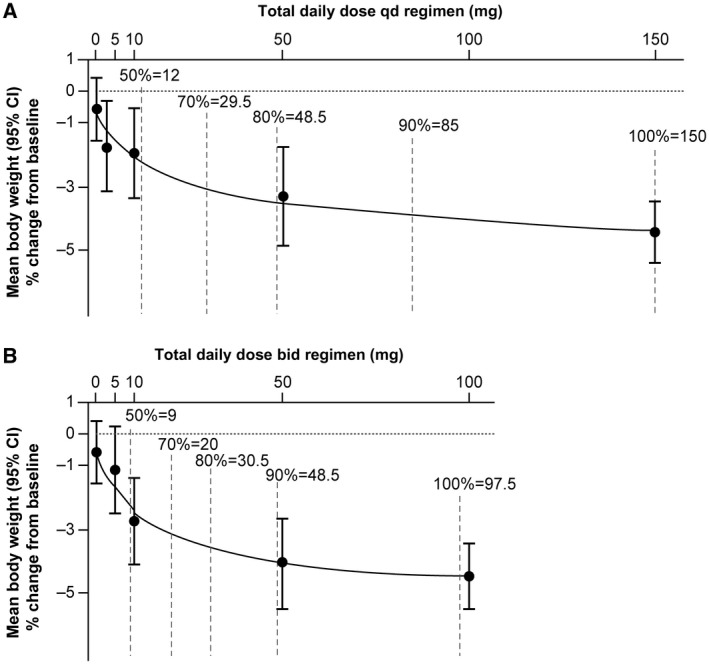
Dose‐response curves based on model averaging over optional models in the candidate sets by regimen. (A) The once‐daily (qd) regimen. (B) The twice‐daily (bid) regimen. Vertical dashed lines represent number of patients achieving a given percent of the maximum weight loss in each regimen. Values are expressed as mean percent change from baseline ± 95% CI.
Table 1.
Dose‐response analysis of percent change in body weight following 24 weeks of treatment with licogliflozin vs. placebo based on model‐averaging method over all models in candidate set (i.e., full analysis set)
| Treatment | Placebo‐subtracted change from baseline (kg) | Dose response | ||
|---|---|---|---|---|
| Model‐based | 95% CI | Model‐based | 95% CI | |
| Placebo | −0.63 | −1.56 to 0.37 | ||
| Licogliflozin 2.5 mg qd (n = 78) | −0.45 | −2.26 to 0.00 | −1.24 | −2.50 to −0.45 |
| Licogliflozin 10 mg qd (n = 38) | −1.38 | −3.21 to −0.06 | −2.04 | −3.36 to −0.88 |
| Licogliflozin 50 mg qd (n = 38) | −2.93 | −4.39 to −0.78 | −3.52 | −4.62 to −1.87 |
| Licogliflozin 150 mg qd (n = 77) | −3.73 | −5.04 to −2.49 | −4.37 | −5.36 to −3.37 |
| Licogliflozin 2.5 mg bid (n = 38) | −0.87 | −2.16 to 0.00 | −1.67 | −2.61 to −0.27 |
| Licogliflozin 5 mg bid (n = 39) | −1.96 | −3.54 to −0.33 | −2.51 | −3.94 to −1.32 |
| Licogliflozin 25 mg bid (n = 37) | −3.52 | −5.06 to −1.63 | −4.06 | −5.52 to −2.87 |
| Licogliflozin 50 mg bid (n = 75) | −3.83 | −5.26 to −2.48 | −4.47 | −5.49 to −3.48 |
Dose‐response analysis based on Multiple Comparison Procedure‐Modeling model‐averaging method over all candidate models used to test dose response.
bid, twice a day; qd, once a day.
Dose‐response curves are presented according to regimen and glycemic status (normoglycemic, dysglycemic, T2DM) (Supporting Information Figure S1). The greatest placebo‐subtracted reduction in weight in the licogliflozin once‐daily regimen was seen with licogliflozin 150 mg in the dysglycemic group (−4.51% [95% CI: −6.58% to −2.62%]; n = 31), compared with normoglycemic (−4.16% [95% CI: −6.71% to −1.55%]; n = 19) or T2DM (−3.0% [95% CI: −4.96% to −1.06%]; n = 27). In the twice‐daily regimen, the greatest reduction was seen in the licogliflozin 50 mg normoglycemic group (−5.29% [95% CI: −8.20% to −2.25%]; n = 17), compared with the dysglycemic (−3.98 [95% CI: −6.17% to −1.69%]; n = 31) or the T2DM (−3.55% [95% CI: −5.60% to −1.56%]; n = 27) groups.
Secondary end point analysis of responders achieving 5% or 10% weight loss revealed statistically significant differences among the individual treatment groups versus placebo for responders with ≥ 5% decrease in body weight from baseline at the higher licogliflozin doses only (licogliflozin 50 mg qd, licogliflozin 150 mg qd, licogliflozin 25 mg bid, and licogliflozin 50 mg bid). The greatest effect was observed with the two highest doses in each dose regimen (45.3% [95% CI: 2.72% to 14.93%], P < 0.001, licoglifozin 50 mg bid; 42.9% [95% CI: 2.41% to 12.88%], P < 0.001, licogliflozin 150 mg qd versus 12.8%, placebo). Very few responders met the criterion of ≥ 10% decrease in body weight from baseline (maximum of 10.8% in the licogliflozin 25 mg bid group compared with 3.8% in the placebo group), and no significant differences were noted across active treatments versus placebo (Table 2).
Table 2.
Responder analysis of body weight (kilograms, percent reduction from baseline) following 24 weeks of treatment with licogliflozin vs. placebo
| End point/time point | Treatment group | n/M (%) | Odds ratio | 95% CI | P |
|---|---|---|---|---|---|
| Percent decrease from baseline ≥ 5% | Licogliflozin 2.5 mg qd | 10/38 (26.3) | 2.38 | 0.85‐6.67 | 0.099 |
| Licogliflozin 10 mg qd | 6/38 (15.8) | 1.18 | 0.36‐3.84 | 0.779 | |
| Licogliflozin 50 mg qd | 13/38 (34.2) | 3.69 | 1.35‐10.11 | 0.011* | |
| Licogliflozin 150 mg qd | 33/77 (42.9) | 5.57 | 2.41‐12.88 | < 0.001* | |
| Licogliflozin 2.5 mg bid | 6/38 (15.8) | 1.15 | 0.36‐3.74 | 0.812 | |
| Licogliflozin 5 mg bid | 8/39 (20.5) | 1.94 | 0.66‐5.69 | 0.229 | |
| Licogliflozin 25 mg bid | 14/37 (37.8) | 4.29 | 1.61‐11.46 | 0.004* | |
| Licogliflozin 50mg bid | 34/75 (45.3) | 6.37 | 2.72‐14.93 | < 0.001* | |
| Placebo | 10/78 (12.8) | ||||
| Percent decrease from baseline ≥ 10% | Licogliflozin 2.5mg qd | 2/38 (5.3) | 1.09 | 0.10‐12.41 | 0.943 |
| Licogliflozin 10 mg qd | 2/38 (5.3) | 2.11 | 0.28‐15.77 | 0.465 | |
| Licogliflozin 50 mg qd | 2/38 (5.3) | 1.60 | 0.15‐17.15 | 0.696 | |
| Licogliflozin 150 mg qd | 5/77 (6.5) | 2.14 | 0.38‐12.10 | 0.389 | |
| Licogliflozin 2.5 mg bid | 2/38 (5.3) | 1.04 | 0.09‐11.87 | 0.976 | |
| Licogliflozin 5 mg bid | 1/39 (2.6) | 1.02 | 0.09‐11.70 | 0.986 | |
| Licogliflozin 25 mg bid | 4/37 (10.8) | 3.51 | 0.56‐22.18 | 0.181 | |
| Licogliflozin 50 mg bid | 7/75 (9.3) | 3.97 | 0.77‐20.54 | 0.100 | |
| Placebo | 3/78 (3.8) |
Statistical model used logistic regression, adjusting for treatment, glycemic status, and region with baseline body weight as covariate. Baseline defined as value at randomization visit.
Statistically significant.
n, number of participants who responded; M, total number of participants in treatment group with response variable defined; bid, twice a day; qd, once a day.
Waterfall plots of individual participants’ percent change in body weight (kilograms) from baseline at week 24 are provided in Supporting Information Figure S2. These plots show that as the dose increased, a greater proportion of patients lost weight, and the extent of weight loss was also greater. Raw mean percent change in body weight (kilograms) from baseline by treatment at week 24 is shown in Figure 4. While an apparent dose‐response effect was observed with both treatment regimens, absolute changes in body weight were small in all treatment arms (−3.7 kg in the licogliflozin 50 mg qd group, −5.4 kg in the licogliflozin 150 mg qd group, and −4.7 kg and −4.8 kg in the licogliflozin 25 mg and 50 mg bid groups, respectively, compared with −0.6 kg in the placebo group).
Figure 4.
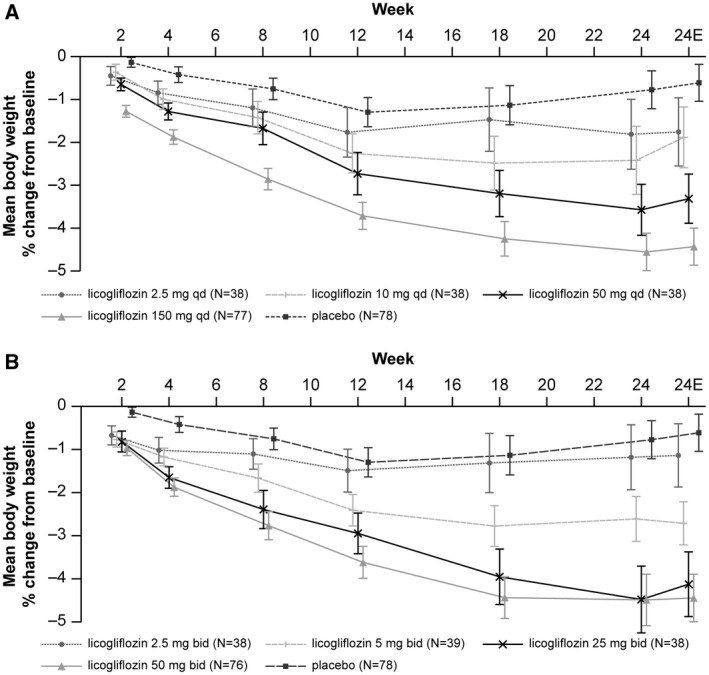
Raw mean percent change in body weight (kilograms) from baseline after 24 weeks by treatment. (A) The qd treatment. (B) The bid treatment. Values are expressed as percent mean change ± SEM. 24E, end of study measurement at week 24; bid, twice a day; qd, once a day.
Percent changes in body weight from baseline between week 24 and week 48 were small (~−0.6%) in patients who switched from the once‐daily and twice‐daily treatment regimens in the first 24 weeks to 25 mg once daily and 35 mg once daily, respectively (Supporting Information Table S3). The weight loss achieved at the higher doses was maintained and even increased slightly by week 48, and it was statistically significant for all treatment groups. The biggest reduction over 48 weeks was 5.4% (P < 0.001) in the licogliflozin 50 mg qd/25 mg qd group versus placebo (Supporting Information Table S4).
Waist circumference, HbA1c, BP, and lipids
Small but statistically significant differences in waist circumference were observed following 24 weeks of treatment with licogliflozin (50 mg qd, 150 mg qd, 5 mg bid, 25 mg bid, and 50 mg bid) versus placebo (Table 3). The greatest reduction in waist circumference was seen in the 150 mg licogliflozin group versus placebo (adjusted mean difference, −4.3 cm [95% CI: −6.06 to −2.54]; P < 0.001). A dose‐related effect was observed with the once‐daily treatment regimen at week 24, which was not seen for each of the three highest twice‐daily treatment arms (Table 3). Raw mean changes in waist circumference over 24 weeks and between‐treatment changes from week 24 at week 48 are shown in Supporting Information Figure S3 and Supporting Information Table S5 respectively.
Table 3.
Change from baseline in waist circumference (centimeters) following 24 weeks of treatment with licogliflozin vs. placebo
| Licogliflozin vs. placebo* | Adjusted mean change (95% CI) | Comparison of adjusted mean changes: licogliflozin vs. placebo | |||
|---|---|---|---|---|---|
| Licogliflozin | Placebo | Difference (licogliflozin‐placebo) | 95% CI | Two‐sided P | |
| Licogliflozin 2.5 mg qd (n = 38) vs. placebo | −2.1 (−3.87 to −0.31) | −1.3 (−2.54 to −0.05) | −0.8 | −2.96 to 1.37 | 0.470 |
| Licogliflozin 10 mg qd (n = 38) vs. placebo | −2.7 (−4.55 to −0.92) | −1.3 (−2.54 to −0.05) | −1.4 | −3.64 to 0.76 | 0.199 |
| Licogliflozin 50 mg qd (n = 38) vs. placebo | −3.7 (−5.55 to −1.82) | −1.3 (−2.54 to −0.05) | −2.4 | −4.63 to −0.15 | 0.037 |
| Licogliflozin 150 mg qd (n = 77) vs. placebo | −5.6 (−6.84 to −4.35) | −1.3 (−2.54 to −0.05) | −4.3 | −6.06 to −2.54 | < 0.001 |
| Licogliflozin 2.5 mg bid (n = 38) vs. placebo | −2.7 (−4.47 to −0.91) | −1.3 (−2.54 to −0.05) | −1.4 | −3.56 to 0.77 | 0.206 |
| Licogliflozin 5 mg bid (n = 39) vs. placebo | −4.3 (−6.07 to −2.57) | −1.3 (−2.54 to −0.05) | −3.0 | −5.18 to −0.88 | 0.006 |
| Licogliflozin 25 mg bid (n = 38) vs. placebo | −4.8 (−6.61 to −2.98) | −1.3 (−2.54 to −0.05) | −3.5 | −5.70 to −1.30 | 0.002 |
| Licogliflozin 50 mg bid (n = 76) vs. placebo | −4.6 (−5.89 to −3.31) | −1.3 (−2.54 to −0.05) | −3.3 | −5.10 to −1.51 | < 0.001 |
Statistical model used repeated‐measure ANCOVA adjusting for treatment, visit, glycemic status, region, and treatment‐by‐visit interaction, with baseline and baseline‐by‐visit interaction as covariates and common unstructured covariance matrix among visits between treatments.
Placebo, n = 78.
n, number of patients with nonmissing value at corresponding time point of interest; bid, twice a day; qd, once a day.
Clinically relevant and statistically significant raw mean changes in HbA1c over 24 weeks (−0.4% to −0.6%) were seen only with the two highest once‐daily doses and the highest twice‐daily dose of licogliflozin versus placebo in patients with T2DM (Table 4). Raw mean HbA1c changes from week 24 at week 48 are shown in Supporting Information Table S6.
Table 4.
Between‐treatment analysis of change from baseline in HbA1c (%) at week 24 in patients with T2DM
| Licogliflozin vs. placebo* | Adjusted mean change (95% CI) | Comparison of adjusted mean changes: licogliflozin vs. placebo | |||
|---|---|---|---|---|---|
| Licogliflozin | Placebo | Difference (licogliflozin‐placebo) | 95% CI | Two‐sided P | |
| Licogliflozin 2.5 mg qd (n = 13) vs. placebo | 0.1 (−0.23 to 0.45) | −0.3 (−0.50 to −0.02) | 0.4 | −0.05 to 0.79 | 0.082 |
| Licogliflozin 10 mg qd (n = 13) vs. placebo | −0.5 (−0.82 to −0.13) | −0.3 (−0.50 to −0.02) | −0.2 | −0.63 to 0.21 | 0.319 |
| Licogliflozin 50 mg qd (n = 12) vs. placebo | −0.9 (−1.24 to −0.50) | −0.3 (−0.50 to −0.02) | −0.6 | −1.05 to −0.16 | 0.008 |
| Licogliflozin 150 mg qd (n = 27) vs. placebo | −0.7 (−0.92 to −0.44) | −0.3 (−0.50 to −0.02) | −0.4 | −0.76 to −0.08 | 0.015 |
| Licogliflozin 2.5 mg bid (n = 13) vs. placebo | −0.3 (−0.68 to 0.00) | −0.3 (−0.50 to −0.02) | −0.1 | −0.50 to 0.34 | 0.707 |
| Licogliflozin 5 mg bid (n = 13) vs. placebo | −0.5 (−0.83 to −0.15) | −0.3 (−0.50 to −0.02) | −0.2 | −0.65 to 0.19 | 0.280 |
| Licogliflozin 25 mg bid (n = 13) vs. placebo | −0.6 (−0.98 to −0.28) | −0.3 (−0.50 to −0.02) | −0.4 | −0.80 to 0.05 | 0.086 |
| Licogliflozin 50 mg bid (n = 27) vs. placebo | −0.6 (−0.87 to −0.40) | −0.3 (−0.50 to −0.02) | −0.4 | −0.72 to −0.04 | 0.030 |
Statistical model used repeated‐measure ANCOVA adjusting for treatment, visit, glycemic status, region, and treatment‐by‐visit interaction, with baseline and baseline‐by‐visit interaction as covariates and common unstructured covariance matrix among visits between treatments.
Placebo, n = 27.
n, number of patients with nonmissing value at corresponding time point of interest; bid, twice a day; qd, once a day.
Overall, SBP tended to be reduced from baseline in all licogliflozin treatment groups versus placebo following 24 weeks of treatment. Statistically significant adjusted mean changes were observed for licogliflozin 50 mg qd (−6.5 mmHg [95% CI: −10.52 to −2.42], n = 38; P = 0.002), licogliflozin 25 mg bid (−4.1 mmHg [95% CI: −8.07 to −0.19], n = 37; P = 0.04), and licogliflozin 50 mg bid (−3.4 mmHg [95% CI: −6.61 to −0.19], n = 75; P = 0.038) versus placebo (n = 78). Changes in DBP versus placebo following 24 weeks of licogliflozin and BP changes between week 24 and week 48 are shown in online Supporting Information.
Significant (P < 0.05) reductions in triglycerides were seen with 50 mg qd (−15.6% [95% CI: −30.30% to −0.86%], n = 32 licogliflozin, n =75 placebo; P = 0.038) and 50 mg bid (−11.4% [95% CI: −22.71% to −0.07%], n = 71 licogliflozin, n =75 placebo; P < 0.05). The greatest increase in total cholesterol was observed with 150 mg qd (+9.9% [95% CI: 2.85% to 16.87%], n = 77 licogliflozin, n =75 placebo; P = 0.006) and was associated with significantly increased HDL cholesterol (+7.3% [95% CI: 1.23% to 13.27%], n = 77 licogliflozin, n =75 placebo; P = 0.02). Changes in LDL cholesterol at week 24 versus placebo were generally small and not statistically significant (online Supporting Information).
Safety
The safety profile of licogliflozin in this study is in line with that previously reported, with the exception that the rate of diarrhea was lower than that previously observed 13. Licogliflozin was generally well tolerated, with just a small percentage of patients discontinued during the first 24 weeks because of AEs (~6%; Figure 2). AEs were reported in ~80% of participants during the first 24 weeks, and they were generally balanced across all treatment groups; at least one AE was also reported in ~80% of participants from the placebo group. The most frequent AEs reported were in the GI disorders (33%‐68%), infections and infestations (21%‐45%), and musculoskeletal and connective tissue disorders (8%‐24%) system organ classes (Supporting Information Table S9). The most frequent GI AEs included diarrhea (16%‐69%) and flatulence (13%‐37%), which appeared to be dose related across both regimens, occurring more frequently at higher doses (Table 5). The highest incidence of diarrhea was reported in the licogliflozin 150 mg qd group (69%). Diarrhea was generally mild in nature, and it tended to decrease over time. Serious AEs occurred in very few patients, with an incidence comparable across treatment groups. One death was reported during the study (caused by multiple drug intoxication), which was not suspected to be related to the study medication.
Table 5.
Most commonly occurring AEs (≥ 10% in any group) up to week 24 by preferred term and treatment
| Preferred term | Licogliflozin 2.5 mg qd (n = 38) | Licogliflozin 10 mg qd (n = 38) | Licogliflozin 50 mg qd (n = 38) | Licogliflozin 150 mg qd (n = 77) | Licogliflozin 2.5 mg bid (n = 38) | Licogliflozin 5 mg bid (n = 39) | Licogliflozin 25 mg bid (n = 38) | Licogliflozin 50 mg bid (n = 76) | Placebo (n = 78) |
|---|---|---|---|---|---|---|---|---|---|
| Number of participants with at least one AE | 30 (78.9) | 25 (65.8) | 30 (78.9) | 66 (85.7) | 30 (78.9) | 27 (69.2) | 31 (81.6) | 60 (78.9) | 62 (79.5) |
| Diarrhea | 7 (18.4) | 6 (15.8) | 21 (55.3) | 53 (68.8) | 10 (26.3) | 8 (20.5) | 15 (39.5) | 43 (56.6) | 15 (19.2) |
| Flatulence | 5 (13.2) | 8 (21.1) | 10 (26.3) | 23 (29.9) | 11 (28.9) | 3 (7.7) | 11 (28.9) | 28 (36.8) | 10 (12.8) |
| Abdominal distension | 2 (5.3) | 2 (5.3) | 5 (13.2) | 10 (13.0) | 7 (18.4) | 3 (7.7) | 4 (10.5) | 11 (14.5) | 11 (14.1) |
| Constipation | 6 (15.8) | 4 (10.5) | 7 (18.4) | 10 (13.0) | 12 (31.6) | 5 (12.8) | 6 (15.8) | 9 (11.8) | 16 (20.5) |
| Defecation urgency | 0 | 0 | 4 (10.5) | 8 (10.4) | 3 (7.9) | 0 | 4 (10.5) | 3 (3.9) | 3 (3.8) |
| Abdominal discomfort | 1 (2.6) | 1 (2.6) | 3 (7.9) | 7 (9.1) | 2 (5.3) | 2 (5.1) | 0 | 0 | 5 (6.4) |
| Abdominal pain upper | 2 (5.3) | 2 (5.3) | 3 (7.9) | 7 (9.1) | 3 (7.9) | 3 (7.7) | 4 (10.5) | 9 (11.8) | 6 (7.7) |
| Nasopharyngitis | 2 (5.3) | 3 (7.9) | 2 (5.3) | 5 (6.5) | 3 (7.9) | 3 (7.7) | 4 (10.5) | 4 (5.3) | 1 (1.3) |
| Gastrointestinal motility disorder | 2 (5.3) | 1 (2.6) | 2 (5.3) | 4 (5.2) | 6 (15.8) | 0 | 2 (5.3) | 3 (3.9) | 3 (3.8) |
| Nausea | 1 (2.6) | 3 (7.9) | 4 (10.5) | 4 (5.2) | 6 (15.8) | 2 (5.1) | 2 (5.3) | 6 (7.9) | 6 (7.7) |
| Back pain | 2 (5.3) | 4 (10.5) | 2 (5.3) | 3 (3.9) | 2 (5.3) | 3 (7.7) | 1 (2.6) | 4 (5.3) | 3 (3.8) |
| Feces hard | 2 (5.3) | 1 (2.6) | 2 (5.3) | 3 (3.9) | 4 (10.5) | 0 | 2 (5.3) | 1 (1.3) | 4 (5.1) |
| Dyspepsia | 2 (5.3) | 1 (2.6) | 2 (5.3) | 2 (2.6) | 9 (23.7) | 5 (12.8) | 3 (7.9) | 8 (10.5) | 9 (11.5) |
Data given as n (%). Preferred terms sorted in descending frequency of AEs in licogliflozin 150 mg qd group. Patient with multiple AEs with same preferred term is counted only once for that preferred term.
AE, adverse event; bid, twice a day; qd, once a day.
Between 24 and 48 weeks, prolonged treatment with a maintenance dose (after a higher licogliflozin dose up to week 24) was well tolerated. Fewer patients (~65%) reported treatment‐emergent AEs during the extension phase, and these were of a similar nature to those reported during the first 24 weeks (Supporting Information Table S10). The average incidence of diarrhea between weeks 24 and 48 (~10%) was similar to placebo (12%) in patients switched from 10 mg, 50 mg, or 150 mg of licogliflozin qd to 25 mg of licogliflozin qd. A higher incidence of diarrhea was reported in patients who received a higher dose of licogliflozin during the extension phase than in the first 24 weeks versus those who received a lower dose during the initial 24‐week treatment period, an effect that was seen with both once‐daily and twice‐daily treatment regimens.
The overall incidence of AEs of special interest was low in all treatment groups. No cases of ketoacidosis occurred, and hypoglycemia occurred in very few patients (0%‐7.9%). The incidence of impaired renal function (0%‐7.9%) and UTI (1.3%‐10.3%) was low across all treatment groups. A low frequency of cardiac events was also reported (0%‐5.9%), although no dose response was observed. No lower‐limb amputations were reported following treatment with licogliflozin (Table 5).
Discussion
In this study, licogliflozin once daily or twice daily produced statistically significant weight loss versus placebo, including a dose‐response signal (P < 0.0001) over 24 weeks. However, mean percent decreases in body weight were mild (< 5% from baseline). This is less robust than the weight loss of ~6% reported in the prior 12‐week PoC study 13. Most of the weight loss associated with licogliflozin treatment appeared to occur in the first 12 weeks and then plateaued. In the 12‐week PoC study with licogliflozin, the reduction in body weight with dual SGLT1 and 2 inhibition was greater in patients with dysglycemia 13. However, this trend was not observed in this study.
The greatest weight loss observed with licogliflozin treatment versus placebo over 48 weeks was 5.4% in the licogliflozin 50 mg qd/25 mg group (5.5‐kg placebo‐subtracted weight loss), which was greater than that previously reported with approved SGLT2 inhibitors that typically lead to ~2% to 3.0% weight loss 7, 8, 9. This additional weight loss was likely due to the SGLT1 effect of licogliflozin 13. Significant ≥ 5% weight reduction from baseline among the licogliflozin treatment groups (34.2% to 45.3% for licogliflozin versus 12.8% for placebo) occurred only at higher doses (150 mg qd, 50 mg qd, 25 mg bid, 50 mg bid), with very few responders meeting the criterion of ≥ 10% weight reduction from baseline (5.3%−10.8% for licogliflozin vs. 3.8% for placebo).
Regarding waist circumference, the 12‐week PoC study revealed that this was reduced by 3.74 cm with licogliflozin treatment 13. In the current study, a similar reduction of 4.3 cm was seen with the same dose of licogliflozin (150 mg qd) over 24 weeks. Some significant reductions in FPG were observed among the licogliflozin treatment groups versus placebo, which were generally greatest in patients with T2DM (Supporting Information Table S7). Significant increases in UGE24 were seen in almost all licogliflozin groups versus placebo at week 24, with even greater increases observed from week 24 at week 48 (Supporting Information Table S8). The clinically meaningful significant reductions in HbA1c at higher doses of licogliflozin (50 mg qd, 150 mg qd, and 50 mg bid) versus placebo in patients with T2DM (ranging from a 0.4% to 0.6% reduction) are consistent with findings with currently approved SGLT2 inhibitors 18, 19.
Higher doses of licogliflozin also significantly reduced SBP following 24 weeks of treatment (−6.5 mmHg, 50 mg qd; −4.1 mmHg, 25 mg bid; −3.4 mmHg, 50 mg bid), with a numerical reduction in DBP observed (up to −2.8 mmHg). These findings did not appear to be dose related. Such BP reductions are reported with both SGLT2 inhibitors 20, 21 and SGLT1 and 2 inhibitors 22, 23, caused by a reduction in plasma volume associated with SGLT inhibition 24, and they are consistent with increases in renal glucose and sodium elimination as well as reduced body weight. Indeed, UGE24 was significantly increased in all treatment groups at week 24, as expected, in line with previous reports in the 12‐week PoC study 13. For further information, see online Supporting Information. Triglyceride levels were significantly reduced with high doses of licogliflozin (50 mg qd and bid). Furthermore, the greatest increase in total cholesterol was with licogliflozin 150 mg qd, driven by a significant increase in HDL cholesterol and LDL cholesterol. These findings are consistent with previous reports following SGLT2 inhibition 25, 26.
Licogliflozin was generally well tolerated and was more frequently discontinued at higher doses. AEs during the first 24 weeks were generally balanced across all treatment groups with no unexpected findings. GI symptoms were increased in the highest once‐daily and twice‐daily doses and were most frequent for the first 3 months of treatment, reaching a maximum at 8 weeks post treatment before diminishing. The most frequent AEs reported for GI disorders included diarrhea and flatulence, which appeared to be dose related across both regimens, supporting the findings of the PoC study with licogliflozin 13. A lower incidence of diarrhea is reported with sotagliflozin 27, 28, suggesting that SGLT1 activity may not be completely inhibited by sotagliflozin in the clinical setting. The diarrhea associated with licogliflozin treatment is likely to be mechanism based and associated with SGLT1 inhibition in the gut in the presence of high carbohydrate intake. Diarrhea is reported to be mitigated by reducing carbohydrate (glucose/galactose) intake during the one meal each day (the breakfast meal) that accompanies licogliflozin dosing 29. A reduction in GI carbohydrate absorption via SGLT1 inhibition, coupled with impaired renal tubular reabsorption of glucose via SGLT2 inhibition, could theoretically increase the risk of euglycemic diabetic ketoacidosis, which is most often described with SGLT2 inhibitors.
SGLT2 inhibitors are also associated with an increased incidence of UTI and genital mycotic infection in patients with T2DM 30, 31, 32, with similar events reported with licogliflozin. The incidence of bone fracture, UTI, and impaired renal function was similar between placebo and licogliflozin‐treated groups. An increase in lower‐limb amputation was observed in long‐term studies with the SGLT2 inhibitor canagliflozin 33. No lower‐limb amputations were reported in adults treated with licogliflozin in this study or in the recent PoC study with licogliflozin 13. Rare cases of ketoacidosis are reported with SGLT2 inhibitors 34, 35, 36, 37 and also with the SGLT1 and 2 inhibitor sotagliflozin 23, 27, 28. In this study, one case of ketosis was reported, with no confirmed cases of ketoacidosis.
Limitations of this study include the homogeneous patient population (85% of patients were Caucasian), the relatively small sample size, and the absence of a direct comparator, which precludes any direct comparisons from being made with other SGLT inhibitors. Longer‐duration studies would also be required to determine the long‐term safety associated with licogliflozin treatment.
In conclusion, licogliflozin treatment produced a statistically significant dose‐responsive reduction in body weight from baseline versus placebo after 24 weeks. However, the percent decreases were numerically small (< 5% for the 24‐week period) and less than expected based on prior PoC data 13. Higher doses of licogliflozin also reduced waist circumference, FPG, HbA1c, SBP, and triglycerides and increased HDL cholesterol and UGE24. Licogliflozin was generally safe, with no new safety signals. The most frequently reported AEs were diarrhea related (particularly at high doses), which diminished when patients switched to lower doses. Overall, we consider 50 mg per day of licogliflozin to be the dose with the most acceptable efficacy and tolerability profile.
Funding agencies
This study was funded by Novartis.
Disclosure
PP, PK, QS, and DK are employed by and own stock in Novartis. HEB has received research grants from Amgen, Arena, Eisai, Eli Lilly, Evadera, Janssen, Johnson and Johnson, Novartis, and Novo Nordisk.
Author contributions
DK, PK, and PP designed the study; HEB, PP, and PK were responsible for conducting the study and collecting data; and QS carried out the statistical analysis of the data. All authors contributed to the drafting of the manuscript and had final approval of the submitted and published versions.
Clinical trial registration
ClinicalTrials.gov identifier NCT03100058.
Supporting information
Acknowledgments
Medical writing and editorial support in the development of this manuscript was provided by Mary‐Clare Cathcart (Novartis Ireland Ltd).
Novartis is committed to sharing with qualified external researchers access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of participants who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on http://www.clinicalstudydatarequest.com.
[Copyright corrected on 2 April 2020 after initial online publication.]
References
- 1. Bays HE, McCarthy W, Christensen S, et al. Obesity Algorithm. Denver: Obesity Medicine Association; 2019. [Google Scholar]
- 2. Fryar CD, Kruszon‐Moran D, Gu Q, Ogden CL.Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999‐2000 through 2015‐2016. National Health Statistics Reports, no. 122. Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 3. World Health Organization . Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Published February 16, 2018. Accessed January 22, 2020.
- 4. Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta‐analysis. JAMA 2016;315:2424‐2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bays H. From victim to ally: the kidney as an emerging target for the treatment of diabetes mellitus. Curr Med Res Opin 2009;25:671‐681. [DOI] [PubMed] [Google Scholar]
- 6. Bays HE, Weinstein R, Law G, Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) 2014;22:1042‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Prato S, Nauck M, Duran‐Garcia S, et al. Long‐term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add‐on therapy to metformin in patients with type 2 diabetes: 4‐year data. Diabetes Obes Metab 2015;17:581‐590. [DOI] [PubMed] [Google Scholar]
- 8. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double‐blind, phase 3 study. Diabetes Care 2015;38:355‐364. [DOI] [PubMed] [Google Scholar]
- 9. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 2016;13:119‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut‐brain axis. Neuropeptides 2012;46:261‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon‐like peptide‐1 receptor agonists on weight loss: systematic review and meta‐analyses of randomised controlled trials. BMJ 2012;344:d7771. doi: 10.1136/bmj.d7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo‐controlled trial. Clin Pharmacol Ther 2012;92:158‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He YL, Haynes W, Meyers CD, et al. The effects of licogliflozin, a dual SGLT1/2 inhibitor, on body weight in obese patients with or without diabetes. Diabetes Obes Metab 2019;21:1311‐1321. [DOI] [PubMed] [Google Scholar]
- 14. Look AHEAD Research Group ; Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med 2001;47:45‐50. [PubMed] [Google Scholar]
- 16. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 17. Pinheiro J, Bornkamp B, Glimm E, Bretz F. Model‐based dose finding under model uncertainty using general parametric models. Stat Med 2014;33:1646‐1661. [DOI] [PubMed] [Google Scholar]
- 18. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care 2018;41:1543‐1556. [DOI] [PubMed] [Google Scholar]
- 19. Wilding J, Fernando K, Milne N, et al. SGLT2 inhibitors in type 2 diabetes management: key evidence and implications for clinical practice. Diabetes Ther 2018;9:1757‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium‐glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015;75:33‐59. [DOI] [PubMed] [Google Scholar]
- 21. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med 2013;159:262‐274. [DOI] [PubMed] [Google Scholar]
- 22. Rosenstock J, Cefalu WT, Lapuerta P, et al. Greater dose‐ranging effects on A1C levels than on glucosuria with LX4211, a dual inhibitor of SGLT1 and SGLT2, in patients with type 2 diabetes on metformin monotherapy. Diabetes Care 2015;38:431‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017;377:2337‐2348. [DOI] [PubMed] [Google Scholar]
- 24. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013;15:853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bode B, Stenlof K, Harris S, et al. Long‐term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab 2015;17:294‐303. [DOI] [PubMed] [Google Scholar]
- 26. Stenlof K, Cefalu WT, Kim KA, et al. Long‐term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52‐week CANTATA‐M study. Curr Med Res Opin 2014;30:163‐175. [DOI] [PubMed] [Google Scholar]
- 27. Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: The North American inTandem1 Study. Diabetes Care 2018;41:1970‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 Study. Diabetes Care 2018;41:1981‐1990. [DOI] [PubMed] [Google Scholar]
- 29. Novartis Pharmaceuticals . Effects of carbohydrate in diet and supplements on the gastrointestinal tolerability of LIK066. https://www.clinicaltrials.gov/ct2/show/NCT03198767?term=LIK066%26draw=2%26rank=11. Accessed August 15, 2019. Updated June 11, 2019.
- 30. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;375:2223‐2233. [DOI] [PubMed] [Google Scholar]
- 31. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care 2011;34:2015‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nyirjesy P, Zhao Y, Ways K, Usiskin K. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co‐transporter 2 inhibitor. Curr Med Res Opin 2012;28:1173‐1178. [DOI] [PubMed] [Google Scholar]
- 33. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 34. Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care 2015;38:1680‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258‐2265. [DOI] [PubMed] [Google Scholar]
- 36. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium‐glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care 2016;39:532‐538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


