Abstract
5-HT1B receptors modulate synaptic serotonin (5-HT) levels and play a significant role in the regulation of emotional behaviors. These receptors are Gαi/o-coupled and inhibit adenylyl cyclase but have also been reported to activate MAP kinases; however, the details of signaling cascades downstream of 5-HT1B receptor activation remain unclear, particularly in neuronal cells. We generated a stable 5-HT1B receptor-expressing Neuro2A (N2A-1B) neuronal cell line and demonstrate that activation of these receptors by the selective 5-HT1B agonist CP-94253 results in activation of ERK1/2 but not of other closely related MAP kinases. Phosphoproteomics revealed four novel phosphorylation sites on the third intracellular loop of the 5-HT1B receptor, and mutations of serine-256 and serine-291 to alanine led to reduced levels of ERK1/2 phosphorylation following receptor activation. Inhibition of Gαi/o signaling with pertussis toxin, as well as MEK1/2 inhibition with U0126, also reduced 5-HT1B-mediated ERK1/2 phosphorylation. Finally, we found that knockout of either β-arrestin 1 or β-arrestin 2 prevented 5-HT1B -mediated phosphorylation of ERK1/2. Taken together, these results show that 5-HT1B receptor activation selectively induces ERK1/2 activation through both the Gαi subunit and β-arrestin proteins. This work elucidates the signal transduction pathway of 5-HT1B receptors, as well as key phosphorylation sites within the receptor that modulate ERK1/2 activation, and further characterizes the intracellular mechanisms that underlie 5-HT1B receptor function.
Keywords: serotonin, 5-HT1B receptor, GPCR, G protein, β-arrestin, signal transduction
INTRODUCTION
The serotonergic system is important in modulating a range of emotional behaviors. Disturbances of this system are implicated in psychiatric conditions such as posttraumatic stress disorder, depression, and anxiety. 5-HT1B receptors are primarily localized adjacent to axon terminals and exist both as autoreceptors on serotonergic neurons and as terminal heteroreceptors on nonserotonergic cells.1–4 They serve to regulate 5-HT synthesis, 5-HT release, and 5-HT reuptake5–9 and modulate a wide variety of behavioral effects.10, 11
Despite its importance in neuromodulation throughout the brain, 5-HT1B receptor function and its underlying biochemical mechanisms are not well understood. 5-HT1B receptors are Gαi/o-coupled receptors that are well known for inhibiting adenylyl cyclase and opening inward rectifying potassium channels.12–15 5-HT1B receptors have been shown to couple to ERK2 and stimulate p70 S6 kinase in a pertussis- and phosphoinositide 3-kinase-sensitive manner in transfected non-neuronal cells.16, 17 5-HT1B receptor stimulation also leads to indirect activation of Akt1 in a MEK1/2-dependent manner.18 Furthermore, these receptors directly interact with glycogen synthase kinase-3β (GSK3β), which is important for agonist-induced 5-HT1B receptor activation and internalization, and with p11, which increases 5-HT1B surface expression and enhances receptor function.19–22
While all G-protein coupled receptors (GPCRs) couple to G proteins to engage canonical signaling pathways such as activation or inhibition of adenylyl cyclase, other signaling pathways are mediated primarily by β-arrestins.23, 24 Recent evidence suggests that this β-arrestin signaling is dependent on G proteins and supports the idea of β-arrestin-G protein coupling.25 Arrestins may also trigger desensitization and internalization of GPCRs.26, 27 Of the multitude of possible signaling pathways, mitogen-activated protein kinases (MAPKs) are ubiquitously expressed, evolutionarily conserved, and activated following extracellular stimulation of a variety of different receptors. MAPKs are serine-threonine kinases, and the most well-studied members of this family are extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-Terminal kinase (JNK), p38 MAPK, and ERK5.28 Remarkably, MAPK activation by GPCRs sometimes involve both G protein- and β-arrestin-dependent mechanisms.29, 30 The relative contribution of different second messenger pathways to the cellular effects of 5-HT1B receptors has not been examined, but identifying these pathways may lead to the development of novel ligands with biased signaling effects that might have therapeutic advantages over nonbiased ligands.
Although 5-HT1B receptors have been reported to activate MAPK signaling, our understanding of the molecular mechanisms involved is incomplete. Interestingly, fear behaviors are affected by ERK phosphorylation in the brain.31–35 Since we have observed that 5-HT1B autoreceptors are critical in modulating fear responses,10, 36, 37 the relationship between 5-HT1B receptors and ERK signaling warrants further investigation. The purpose of this study is to elucidate the signal transduction pathway of 5-HT1B receptors in a neuronal cell line and to identify phosphorylation sites in the 5-HT1B receptor that are involved in activating this downstream cascade.
RESULTS
Neuro2A cells express a variety of neuronal and serotonin-related genes endogenously, and stably transfected N2A-1B cells express 5-HT1B receptors on the cell surface
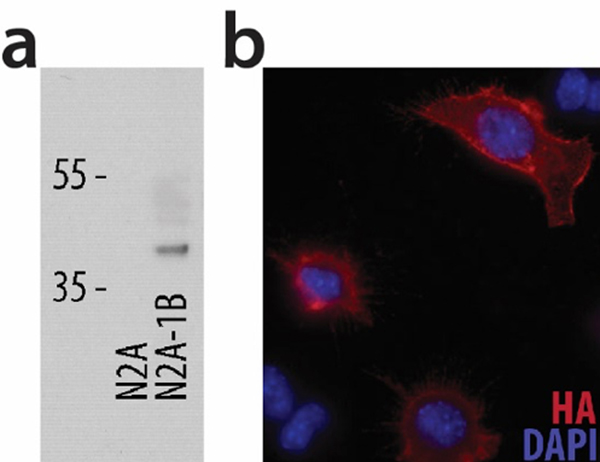
To model endogenous neuronal signaling pathways, we chose the mouse neuroblastoma Neuro2A cell line and investigated the presence of serotonergic and neuronal markers. RNA was purified from untransfected N2A and stably transfected N2A-1B cells, and ten genes were probed for endogenous expression. N2A cells expressed a variety of serotonin-related genes, including 5-HT1A, SERT, and Tph2 (Table 1). 5-HT1B mRNA was essentially absent in untransfected N2A cells but abundant in N2A-1B cells. To verify 5-HT1B protein expression, N2A and N2A-1B cells were lysed and western blotting was performed for the HA-tagged 5-HT1B receptor (HA-5-HT1B). HA is detected in N2A-1B cells but not in N2A cells (Figure 1a). Immunocytochemistry for HA was performed on non-permeabilized N2A-1B cells and verified that a subset of the receptors was present at the cell surface (Figure 1b).
Table 1.
Quantitative RT-PCR measurements of serotonergic and neuronal marker genes in untransfected N2A and stably transfected N2A-1B cells.
| Gene | Normalized Ct Values | Fold Enrichment | |
|---|---|---|---|
| N2A cells | N2A-1B cells | ||
| 5-HT1B | 34.9 | 24.0 | 2012.39 |
| 5-HT1A | 27.1 | 26.9 | 1.28 |
| SERT | 31.7 | 32.4 | 0.79 |
| Pet-1 | 33.4 | 34.9 | 0.47 |
| Tph2 | 30.8 | 31.2 | 0.90 |
| Tph1 | 27.0 | 26.9 | 1.24 |
| AADC | 17.8 | 17.6 | 1.17 |
| GCH1 | 23.0 | 23.1 | 1.02 |
| VGLUT3 | 34.0 | 34.1 | 1.16 |
| VMAT2 | 24.5 | 24.8 | 0.94 |
Figure 1. HA-tagged 5-HT1B receptors are present in stably transfected N2A-1B cells on the cell surface.
(a) Western blot for HA shows the presence of the HA-5-HT1B receptors in N2A-1B cells but not in untransfected N2A cells. (b) Immunocytochemical staining of non-permeabilized N2A-1B cells demonstrates cell surface expression of HA-5-HT1B receptors.
5-HT1B receptor activation increases phosphorylation of ERK1/2 in a dose-dependent manner in N2A-1B cells but not in untransfected N2A cells, with no effect on total levels of ERK1/2 or other closely related MAP kinases
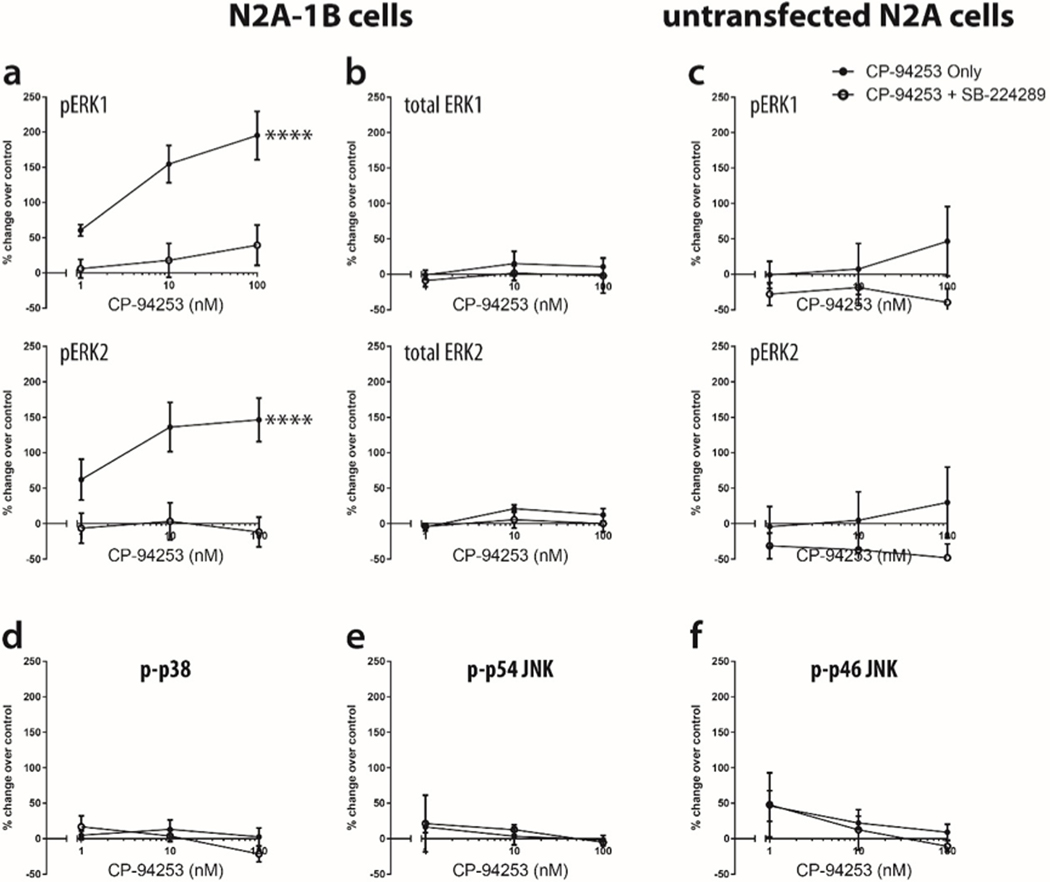
Untransfected N2A and stably transfected N2A-1B cells were treated with the selective 5-HT1B agonist, CP-94253, at 1–100 nM for ten minutes. Levels of both ERK1 and ERK2 phosphorylation increased in N2A-1B cells, and this effect was blocked by preincubation with the 5-HT1B antagonist SB-224289 (1 μM) for one hour prior to agonist treatment; at 100 nM CP-94253, the highest concentration used, phospho-ERK1 levels increased by 195%, while phospho-ERK2 levels increased by 146% (Figure 2a, Supplemental Figure 1a). Total levels of ERK1/2 remained constant in N2A-1B cells, regardless of agonist and antagonist treatments (Figure 2b, Supplemental Figure 1b). Agonist-induced increases in phospho-ERK1/2 were absent in untransfected N2A cells (Figure 2c, Supplemental Figure 1c). Additionally, the 5-HT1B agonist CP-93129 produced a similar increase in ERK1/2 phosphorylation (Supplemental Figure 2). Treatment with CP-94253 did not increase phosphorylation of p38, p54 JNK, or p46 JNK (Figures 2d, 2e, 2f, Supplemental Figure 1d, 1e).
Figure 2. CP-94253 increases levels of phospho-ERK1/2, but not phospho-p38 or phospho-SAPK/JNK, in N2A-1B cells, but not in untransfected N2A cells.
(a) Treatment with CP-94253 (1–100 nM) for ten minutes increased phosphorylation of ERK1 and ERK2 in N2A-1B cells compared to unstimulated N2A-1B control cells treated with vehicle (PBS), but this was blocked by pretreatment with the antagonist SB-224289 (pERK1 F1,24 = 34.07, p < 0.0001; pERK2 F1,24 = 28.38, p < 0.0001). (b) No change was observed in total ERK in N2A-1B cells (total ERK1 F1,18 = 0.89, p = 0.36; total ERK2 F1,18 = 1.79, p = 0.20). (c) No change was observed in phospho-ERK1/2 with agonist treatment in untransfected wild-type N2A cells (pERK1 F1,24 = 3.62, p = 0.07; pERK2 F1,24 = 3.52, p = 0.07). Agonist treatment did not change levels of (d) phospho-p38 (F1,18 = 0.58, p = 0.46), (e) phospho-p54 JNK (F1,18 = 0.06, p = 0.81), and (f) phospho-p46 JNK (F1,18 = 0.21, p = 0.65). Data are expressed as the percent change in pERK signal compared to the no agonist control from each independent biological replicate. Error bars represent SEM and data are averages of 4–5 independent biological replicates (two-way ANOVA; ****p < 0.00001).
Phosphoproteomics identifies putative 5-HT1B receptor phosphorylation sites
We used immobilized metal affinity chromatography (IMAC) beads to enrich phosphopeptides for LC-MS analyses and to identify novel phosphorylation sites on the 5-HT1B receptor using the N2A-1B cell line with or without stimulation with the selective 5-HT1B agonist CP-94253. We identified four novel phosphorylation sites in the 5-HT1B sequence on three distinct phosphopeptides, all within the C3 loop; one of these phosphopeptides was found to be doubly phosphorylated (Table 2). All four novel phosphorylation sites were found to be phosphorylated both under basal conditions and after agonist treatment.
Table 2.
Novel C3 loop phosphorylation sites detected through phosphoproteomics.
| Residue # | 5-HT1B phosphopeptides |
| 256 | AQLITDSPGSTSSVTSINSR |
| 277, 279 | VPEVPSESGSPVYVNQVK |
| 291 | VRVSDALLEK |
Mutations of putative 5-HT1B receptor phosphorylation sites alter agonist-induced increases in phospho-ERK1
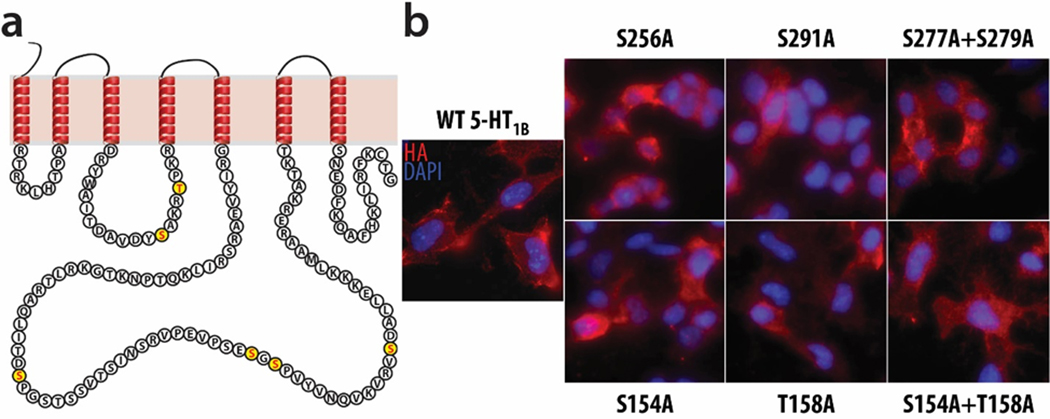
Phosphorylation-null 5-HT1B mutant receptors were designed based on the three phosphopeptides discovered with phosphoproteomics, which included four novel putative phosphorylation sites on 5-HT1B receptor. Serine residues were mutated to alanine using site-directed mutagenesis to generate three mutant receptors that are unable to be phosphorylated at these sites: 1) S256A, 2) S291A, and 3) S277A+S279A. Three additional mutants were generated to mutate sites of the 5-HT1B receptor known to be crucial for phosphorylation by GSK3β: 1) S154A, 2) T158A, and 3) S154A+T158A.20 The novel putative phosphorylation sites are all located in the third intracellular loop, while the GSK3β phosphorylation sites are in the second intracellular loop (Figure 3a).
Figure 3. Four novel phosphorylation sites were identified by phosphoproteomics, and six phosphorylation-null mutant receptors were generated.
(a) Phosphorylation at four previously unreported sites in the 5-HT1B receptor C3 loop was detected and is illustrated graphically, along with two sites in the 5-HT1B receptor C2 loop known to be phosphorylated and important for GSK3β signaling. These six sites were used to generate six phosphorylation-null mutant receptors, in which individual or dual site-directed mutations replaced serine or threonine with alanine, thereby preventing phosphorylation. Three mutants target two sites in the second intracellular loop known to be phosphorylated by GSK3β: S154A, T158A, and S154A+T158A. Three other mutants target the four novel putative sites in the third intracellular loop: S256A, S291A, and S277A+S279A. (b) N2A cells were stably transfected with each of these mutant receptors, and immunocytochemical staining on non-permeabilized cells demonstrates that the clones selected for further analysis successfully expressed their respective mutant receptor at the cell surface.
The wild-type 5-HT1B receptor and the six mutant receptors were transfected into N2A cells to generate seven stable cell lines. Immunocytochemical staining for HA on non-permeabilized cells confirmed the presence of the receptor at the cell surface in all stable cell lines (Figure 3b). Stable cell lines were selected based on similar expression levels of the various 5-HT1B mutant receptors observed with qPCR and immunohistochemistry (Figure 3b).
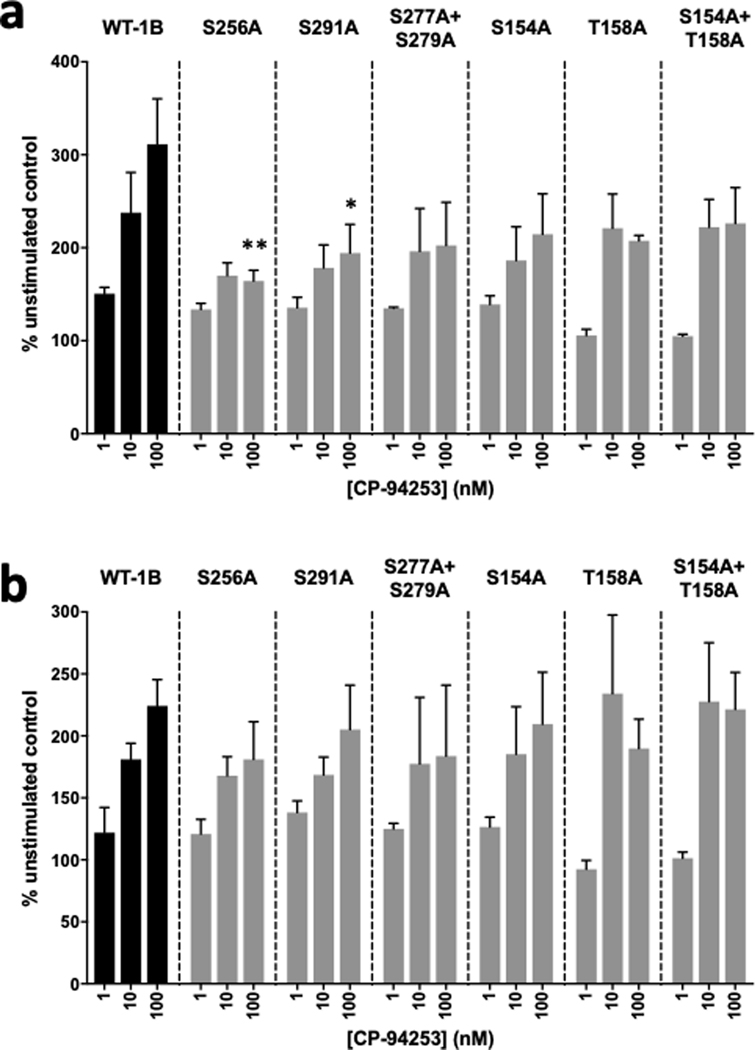
Cells were treated with 1–100 nM CP-94253 for ten minutes, with or without 1 μM SB-224289, then probed for phospho-ERK1/2 expression. All six mutant receptors showed varying levels of phospho-ERK1/2 induction following CP-94253 treatment (Figure 4); this response was sensitive to blockade by SB-224289 (data not shown). Of the three novel mutants, mutations of both S256A and S291A resulted in significantly lower levels of agonist-induced ERK1 phosphorylation; compared to the WT receptor, which showed a 211% increase in phospho-ERK1 levels in response to CP-94253, the S256A and S291A mutant receptors showed phospho-ERK1 levels of 64% and 94% increases in phospho-ERK1 levels, respectively, relative to unstimulated controls (Figure 4a). None of the mutant receptors showed significantly different levels of ERK2 phosphorylation compared with the WT receptor (Figure 4b).
Figure 4. Mutations of 5-HT1B phosphorylation sites alter phospho-ERK1/2 responses to CP-94253.
CP-94253 (1–100 nM) produced dose-dependent increases in (a) ERK1 phosphorylation and (b) ERK2 phosphorylation in N2A cells expressing wild-type HA-5-HT1B receptors (WT-1B) compared to unstimulated N2A-1B control cells treated with vehicle (PBS). (a) At 100 nM of CP-94263, phospho-ERK1 levels were significantly decreased in cells expressing the S256A and S291A mutant receptors (S256A mutant p = 0.005; S291A mutant p = 0.033; S277A+S279A mutant p = 0.054; S154A mutant p = 0.104; T158A mutant p = 0.071; S154A+T158A mutant p = 0.184) compared to WT-1B cells. (b) Phospho-ERK2 levels were not significantly different between WT-1B and mutant cell lines with 100 nM of CP-94263 (S256A mutant p = 0.845; S291A mutant p = 0.996; S277A+S279A mutant p = 0.876; S154A mutant p = 0.999; T158A mutant p = 0.935; S154A+T158A mutant p > 0.999). Data are expressed as the percent change in pERK signal compared to the no agonist control from each independent biological replicate. Error bars represent SEM and data are averages of 3 independent biological replicates for each receptor (two-way ANOVA with Dunnett’s post-hoc tests; **p < 0.01, *p < 0.05).
5-HT1B-mediated phosphorylation of ERK1/2 is a Gαi-dependent process that is reduced with MEK1/2 inhibition
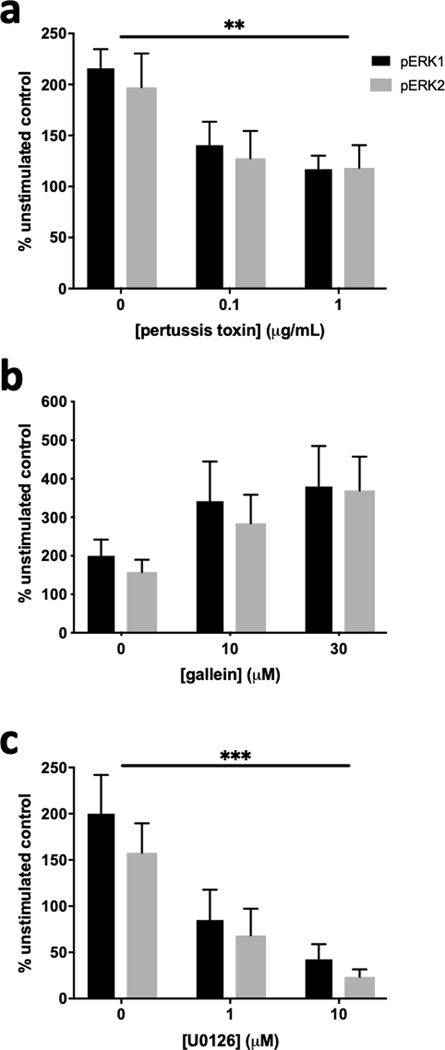
To investigate the signaling intermediates between 5-HT1B receptor activation and phosphorylation of ERK1/2, N2A-1B cells were pretreated with pharmacological inhibitors one hour prior to CP-94253 treatment. Pertussis toxin was used to inhibit Gαi/o signaling, gallein was used to inhibit Gβγ signaling, and U0126 was used to inhibit MEK1/2 signaling. Increased phosphorylation of both ERK1 and ERK2 was blocked when pertussis toxin was present (Figure 5a) but was not blocked by gallein (Figure 5b). Pretreatment with U0126 also prevented the phosphorylation of ERK1/2 (Figure 5c).
Figure 5. Phosphorylation of ERK1/2 by 5-HT1B receptors is sensitive to pertussis toxin and U0126 but not gallein.
N2A-1B cells were pretreated for one hour with inhibitors prior to treatment with 100 nM CP-94253 for ten minutes and compared to unstimulated N2A-1B control cells treated with vehicle (PBS). (a) 5-HT1B-mediated phosphorylation of ERK1/2 was blocked by pertussis toxin (inhibitor effect: F2,18 = 7.98, p = 0.0033). (b) 5-HT1B-mediated phosphorylation of ERK1/2 was not sensitive gallein (inhibitor effect: F2,12 = 3.198, p = 0.077). (c) 5-HT1B-mediated phosphorylation of ERK1/2 was blocked by the MEK1/2 inhibitor U0126 (inhibitor effect: F2,12 = 13.34, p = 0.0009). Data are expressed as the percent change in pERK signal compared to the no agonist control from each independent biological replicate. Error bars represent SEM and data are averages of 3–4 independent biological replicates for each experiment (two-way ANOVA; ***p < 0.001, **p < 0.01).
5-HT1B-mediated phosphorylation of ERK1/2 is dependent on β-arrestins
We performed a preliminary investigation of the role of β-arrestins using mouse embryonic fibroblasts (MEFs) and found that knockout of β-arrestin 2 alone or double knockout of β-arrestin 1 and 2 resulted in blockade of 5-HT1B-mediated activation of ERK1, and knockout of either or both β-arrestin 1 and 2 led to blockade of 5-HT1B-mediated activation of ERK2 (Supplemental Figure 3).
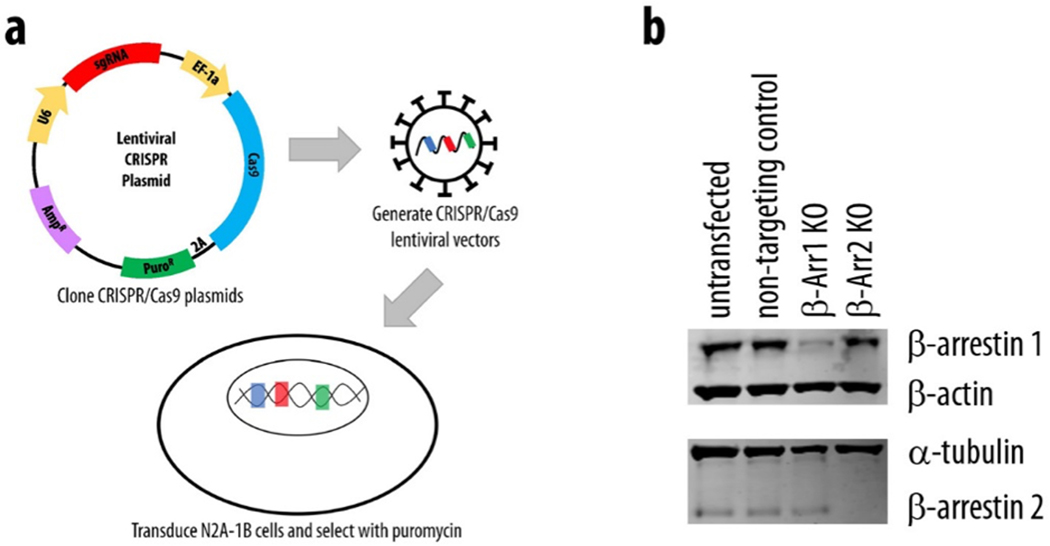
Because of these intriguing results, we next developed stable β-arrestin knockout N2A-1B cell lines using CRISPR/Cas9 gene editing to further investigate the role of β-arrestins in 5-HT1B-mediated ERK1/2 activation in neuronal cells (Figure 6a). β-arrestin knockout lines were generated by transduction of CRISPR/Cas9 lentiviral vectors with sgRNAs specific for β-arrestin 1 (β-Arr1 KO) or β-arrestin 2 (β-Arr2 KO), while N2A-1B control cells were transduced with a non-targeting control sgRNA sequence. Transduction of N2A-1B cells with targeted lentiviral CRISPR/Cas9 vectors induced specific and efficient reduction of β-arrestin 1 and β-arrestin 2 expression, while transduction with a non-targeting control vector (“control” cells) did not alter β-arrestin expression compared with untransfected N2A-1B cells (Figure 6b).
Figure 6. Transduction of N2A-1B cells with a lentiviral CRISPR/Cas9 vector induces specific and efficient knockout (KO) of β-arrestin 1 and β-arrestin 2.
(a) Lentiviral CRISPR/Cas9 vectors were used to generate stable β-arrestin KO N2A-1B cell lines. (b) Transduction with a non-targeting control vector does not alter the expression of β-arrestin 1 or β-arrestin 2. Transduction with CRISPR/Cas9 vectors specific for β-arrestin 1 does not alter the expression of β-arrestin 2, and transduction with vectors specific for β-arrestin 2 does not alter the expression of β-arrestin 1.
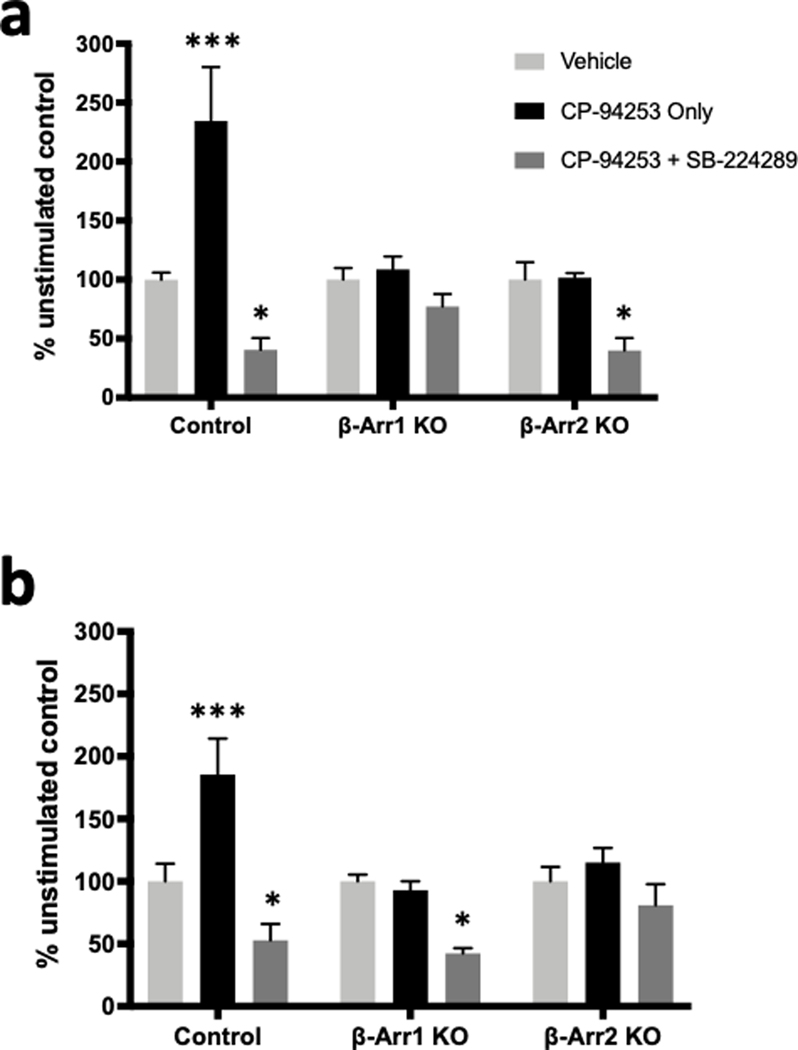
Control, β-Arr1 KO, and β-Arr2 KO cells were incubated with or without 100 nM CP-94253 for ten minutes. Agonist treatment significantly increased phospho-ERK1 and phospho-ERK2 levels in control cells, but not in β-Arr1 KO or β-Arr2 KO cells (Figure 7). Preincubation with 1 μM SB-224289 blocked both ERK1 and ERK2 activation in control cells, as well as significantly reduced ERK1 phosphorylation in β-Arr2 KO cells and ERK2 phosphorylation in β-Arr1 KO cells.
Figure 7. 5-HT1B-mediated phosphorylation of ERK1/2 is dependent on β-arrestins in N2A-1B cells.
N2A-1B control cells, β-arrestin 1 KO cells (β-Arr1 KO), and β-arrestin 2 KO cells (β-Arr2 KO) received treatment with the 5-HT1B agonist CP-94253 (100 nM) alone or pretreatment with the 5-HT1B antagonist SB-224289 (1 μM) 1 hour prior to agonist treatment and compared to unstimulated control cells treated with vehicle (PBS). (a) Levels of phospho-ERK1 significantly differed by treatment (F2,36 = 21.4, p < 0.0001) and cell type (F2,36 = 4.79, p = 0.014), with a significant interaction between treatment and cell type (F4,36 = 7.017, p < 0.001). 5-HT1B receptor stimulation with agonist significantly increased levels of phospho-ERK1 in control cells (p < 0.0001) but not in β-Arr1 KO (p = 0.918) or β-Arr2 KO cells (p = 0.997). (b) Levels of phospho-ERK2 also significantly differed by treatment (F2,36 = 19.3, p < 0.0001) and cell type (F2,36 = 4.362, p = 0.020), with a significant interaction between treatment and cell type (F4,36 = 4.469, p = 0.049). 5-HT1B receptor stimulation significantly increased levels of phospho-ERK2 in control cells (p = 0.0003) but not in β-Arr1 KO (p = 0.917) or β-Arr2 KO cells (p = 0.680). Furthermore, SB-224289 blocks agonist-induced ERK1/2 activation, reducing phospho-ERK1 levels in control (p = 0.045) and β-Arr2 KO cells (p = 0.043) (a) and reducing phospho-ERK2 levels in control (p = 0.048) and β-Arr1 KO cells (p = 0.014) (b). Data are expressed as the percent change in pERK signal compared to the no agonist control from each independent biological replicate. Error bars represent SEM and data are averages of 5 independent biological replicates for all groups (two-way ANOVA with Dunnett’s post hoc tests; ***p < 0.001, *p < 0.05).
DISCUSSION
Disturbances of the serotonergic system are implicated in disorders including addiction, fear, anxiety, stress, and depression. Presynaptic 5-HT1B autoreceptors located at serotonergic nerve terminals provide feedback that allows for precise spatial and temporal regulation of synaptic levels of serotonin, thereby affecting serotonergic neurotransmission throughout the entire brain. Postsynaptic 5-HT1B receptors are also localized on axon terminals of diverse but important neuron populations and these may also contribute to potential therapeutic effects of these receptors.10, 11 While canonical signaling via inhibition of adenylyl cyclase is well established for this receptor,38, 39 we focused on noncanonical signaling mechanisms, as they may also be important for mediating the impact of these receptors. Understanding the mechanisms by which 5-HT1B receptors signal may provide new insights into therapeutic opportunities for targeting 5-HT1B receptors in neuropsychiatric disorders.
Previous studies of 5-HT1B receptor signaling utilized non-neuronal cell lines.16, 17, 40–43 To best mimic endogenous signaling pathways in neurons, we chose the mouse neuroblastoma Neuro2A cell line. Compared with non-neuronal lines, the use of N2A cells has advantages because of their endogenous expression of several key serotonergic and neuronal genes, so these may be more physiologically similar to 5-HT1B-expressing neurons in vivo. Although N2A cells do not endogenously express 5-HT1B receptors, transfection and generation of stable cell lines allowed for not only the expression of wild-type and mutant 5-HT1B proteins but also reliable trafficking to the cell surface. It is important to note that while 5-HT1B receptors are usually localized at axon terminals, N2A cells do not demonstrate this polarized receptor trafficking1–4; thus, overall levels of receptor expression in N2A cells do not necessarily correspond to localized levels of receptor expression in neuronal axon terminals. Activation of these 5-HT1B receptors in N2A-1B cells with CP-94253 induced robust phosphorylation of ERK1/2 but not of other MAP kinases in the same family, demonstrating the specificity of the phospho-ERK1/2 response. We used a combination of a highly selective 5-HT1B agonist and antagonist in this report to increase our confidence that the 5-HT1B receptor was responsible for inducing ERK1/2 phosphorylation, especially since these cells express low levels of 5-HT1A receptors. Although the degree of biased agonism of CP-94253 for canonical versus noncanonical signaling has not been reported, the structure of the 5-HT1B receptor has been reported to be less likely to lead to biased agonism than other serotonin receptors such as the 5-HT2B receptor, which was shown to display β-arrestin-biased signaling for numerous agonists.44
Interrogation of the 5-HT1B protein with phosphoproteomics revealed four novel putative phosphorylation sites on the receptor, which are highly conserved across multiple species including humans, rats, and mice. Site-directed mutagenesis of these residues to an alanine residue, which cannot be phosphorylated, revealed that serine-256 and serine-291 may be necessary for the maximal ERK1/2 response. Interestingly, previous studies of GPCRs have shown that movement of transmembrane helices V and VI after ligand binding is necessary for activation of downstream signaling cascades.44, 45 These phosphorylated 5-HT1B receptor residues are within the third intracellular loop, which likely displaces upon receptor activation to facilitate signal transduction intracellularly. In our analyses, the response to agonist in the mutant receptors was quantitatively different for phospho-ERK1 versus phospho-ERK2; it is unclear what, if any, physiological significance this has, as the two ERK isoforms have been suggested in the literature to be functionally redundant.46 It is possible that higher agonist concentrations are required for maximal activation of ERK2 in order to detect differences; however, low doses of CP-94253 resulted in similar levels of phosphorylation of ERK1 in both wild-type and mutant receptors. Because the mutant 5-HT1B receptors showed a reduced level, rather than complete absence, of phospho-ERK signaling, it appears that blocking the phosphorylation of these residues on the 5-HT1B receptor reduces the efficiency of, but does not abolish, activation of ERK1/2. Future studies might examine the effects of mutation of these critical serine/threonine residues to aspartic acid, which would mimic the phosphorylated state, to determine whether such a change increases agonist potency or even renders the receptor constitutively active.
We found that pertussis toxin, but not gallein, was able to block ERK1/2 phosphorylation by 5-HT1B receptors completely. This suggests that 5-HT1B receptors must couple to Gαi/o in order to activate ERK1/2 signaling, and that this ERK1/2 activation does not depend on Gβγ signaling. Further, CRISPR/Cas9-mediated knockout of either isoform of β-arrestin in N2A-1B cells resulted in blockade of agonist-induced ERK1/2 phosphorylation, suggesting that 5-HT1B receptor-mediated phosphorylation of ERK1/2 is dependent on both β-arrestin 1 and β-arrestin 2 in neuronal cells. β-arrestin 1 and β-arrestin 2 are known to form heterodimers, which influences their localization and signaling properties.47 This finding is consistent with several other studies of GPCRs that require both β-arrestin isoforms for signaling, including 5-HT2C and PTH1 receptors,48, 49 and may explain why both isoforms appear to be necessary for 5-HT1B receptor-mediated ERK1/2 activation. Additionally, GPCRs that exhibit equivalent affinities for both β-arrestin isoforms can form long-lasting receptor-arrestin complexes that subsequently bind ERK1/2.50 Thus, it is possible that 5-HT1B receptors form complexes with β-arrestin to induce phosphorylation of ERK1/2. We were surprised to find that 5-HT1B-mediated ERK1/2 phosphorylation required interactions both with Gαi/o and with β-arrestins. However, an intriguing report recently found that when all Gαi/o proteins were blocked or deleted by CRISPR/Cas9, β-arrestins still interacted with GPCRs but no longer activated ERK1/2 phosphorylation.25 β-arrestins may still play important roles in regulating cell surface GPCR density independent of G proteins, but our results lend further support to the complementary involvement of both G proteins and β-arrestins in forming complexes that can mediate noncanonical MAPK signaling by 5-HT1B receptors.
We also found that inhibition of MEK1/2 with U0216 prevented phosphorylation of ERK1/2 following 5-HT1B stimulation. While MEK1/2 inhibition reduced levels of phospho-ERK1/2 even in the absence of 5-HT1B agonist (data not shown), this is not surprising given that MEK1/2 is the major upstream activator of ERK1/2,30, 51, 52 and our results demonstrate that 5-HT1B-mediated ERK1/2 phosphorylation does not occur in the absence of MEK1/2 signaling.
Interestingly, preincubation with the 5-HT1B antagonist SB-224289 not only prevented agonist-induced phosphorylation of ERK1/2, but also significantly reduced phosphorylation of ERK1 in control and β-Arr2 KO N2A-1B cells, and ERK2 in control and β-Arr1 KO N2A-1B cells. SB-224289 is a potent inverse agonist and reduces the constitutive activity of 5-HT1B receptors.53, 54 Our results raise the possibility that the constitutive activity of the 5-HT1B receptor influences ERK1 and ERK2 differentially via the two β-arrestin isoforms, with β-arrestin 1 being necessary for basal activation of ERK2 and β-arrestin 2 being necessary for ERK1 activation. Different GPCR ligands affect not only the affinity of receptors for the β-arrestins but also the affinity of the receptor-arrestin complexes for their downstream effectors.50 Future studies should investigate whether there is an interaction between 5-HT1B receptors and ERK1 and ERK2 that is mediated preferentially by β-arrestin 2 and β-arrestin 1, respectively. Furthermore, the possibility that there is constitutive activity of 5-HT1B receptors for β-arrestin-mediated signaling in vivo should be examined in future studies.
We observed a different pattern of β-arrestin isoform involvement in non-neuronal MEF cells. In those cells, ERK1 phosphorylation depended only on β-arrestin 2, while either β-arrestin 1 or β-arrestin 2 deletion blocked the phosphorylation of ERK2. Previous studies show that the mechanism by which GPCRs engage ERK1/2 is highly cell type-specific and dependent on the expression of various isoforms of upstream molecules.51 Additionally, levels of ERK1/2 expression are known to vary depending on cell type,55 and the MEF cells were transiently transfected with HA-5-HT1B, which produces varying levels of receptor expression in each individual experiment. Thus, interactions with binding partners may vary with the receptor expression level. It appears that the main conclusion from these experiments is that β-arrestins are necessary for ERK1/2 activation in MEF cells, but the participation of each β-arrestin isoform in these experiments is less clear than in our N2A-1B experiments.
Taken together, our data show that agonist-induced 5-HT1B receptor activation leads to selective phosphorylation of ERK1/2, with contributions of G protein-dependent signaling through the Gαi/o subunit, as well as interactions with β-arrestins 1 and 2. This work sheds light on the complexity of signal transduction mechanisms that may underlie the diverse functions of the 5-HT1B receptor in neurons.
METHODS
Cell culture and drug treatments
Neuro2A (N2A) cells were maintained with growth media consisting of Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum (FBS), and 1x Antibiotic-Antimycotic (Gibco) at 37°C in 5% CO2. N2A cells were transfected with a plasmid expressing HA-tagged rat 5-HT1B receptor in a pcDNA3 backbone (N2A-1B) using Lipofectamine LTX (Invitrogen), and selection for the stably transfected cell lines was achieved with 500 μg/mL geneticin (G418). Cells were plated in 60 mm plates 48 hours before treatment with growth media consisting of DMEM, 10% dialyzed serum, and 1x Antibiotic-Antimycotic (Gibco), and fed with fresh dialyzed growth media 24 hours before treatment. One hour before agonist treatment, cells were switched to serum-free Opti-MEM to wash out any residual 5-HT, with or without the presence of antagonists as described. Following agonist treatment, cells were lysed with modified RIPA buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.25% sodium deoxycholate, 1% CHAPS, and 1x protease and phosphatase inhibitors) and briefly vortexed. Cell debris was pelleted by centrifugation at 15,000 x g for five minutes. The protein concentration of the lysate was measured using the 660 nm protein assay (Pierce). Treatment drugs used were: CP-94253,56 SB-224289,53, gallein (Tocris), pertussis toxin (Novex), and U0126 (Cell Signaling). These drugs were applied one hour prior to the addition of agonists.
For β-arrestin experiments, N2A-1B β-arrestin knockout (KO) cells were grown in media additionally supplemented with 2 μg/ml puromycin. Cells were plated in 60 mm plates 24 hours prior to treatment. One hour before agonist treatment, cells were switched to serum-free Opti-MEM, with or without the presence of the selective 5-HT1B antagonist SB-224289 (1 μM), then treated with the selective 5-HT1B agonist CP-94253 (100 nM) for ten minutes. Cell lysates were prepared as described above.
For mouse embryonic fibroblast (MEF) experiments, wild-type, β-arrestin 1 knockout, β-arrestin 2 knockout, and β-arrestin 1 and 2 double knockout MEF cells57 were maintained with growth media consisting of DMEM, 10% FBS, and 1% penicillin/streptomycin at 37°C in 7% CO2. Cells were plated in 60 mm plates 72 hours prior to drug treatment. Using Lipofectamine 2000 (Invitrogen), cells were transiently transfected 48 hours prior to drug treatment with 16.525 μg DNA of a plasmid mix containing 30% HA-tagged rat 5-HT1B receptor, 30% Clover, a bright green-yellow fluorescent protein derived from GFP, and 40% pCAGGS, an empty vector control plasmid.58 Plates were fed with fresh growth media 24 hours prior to drug treatment. Immediately before drug treatment, presence of Clover fluorescence was confirmed; Clover expression was used as a marker of successful plasmid transfection in each experiment. One hour before agonist treatment, cells were switched to serum-free Opti-MEM, with or without the presence of the selective 5-HT1B antagonist SB-224289 (1 μM), then treated with the selective 5-HT1B agonist CP-94253 (100 nM) for ten minutes. Cell lysates were prepared as described above.
Quantitative PCR
Cells were detached from growth flasks and pelleted. RNA was purified with the RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions, and then treated with TURBO DNase (Ambion). 2 μg of total RNA was reverse transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo) with oligo(dT)18 primers. Quantitative PCR was performed using EXPRESS SYBR GreenER (Invitrogen) with 2 μM primers (Supplemental Table 1) and run for 40 cycles on the ViiA 7 Real Time PCR System (Life Technologies). First-strand cDNA synthesis products were diluted 1:20 before adding to the qPCR reaction. Ct values were normalized to that of GAPDH, and fold enrichment was obtained using the ΔΔCt method.
Immunocytochemistry
Cells were plated onto glass coverslips that were pretreated with poly-L-lysine and grown for 24–48 hours before fixation. Cells were then fixed with warm 4% paraformaldehyde-PHEMS buffer for 20 minutes. To achieve cell surface staining, cell membranes were not permeabilized. Cells were washed once with PBS, blocked with 10% BSA for one hour at room temperature, then incubated with rabbit anti-HA antibody (Cell Signaling, 1:4000) overnight at 4°C. After washing cells three times with PBS, cells were incubated with Alexa Fluor 594 goat anti-rabbit IgG secondary antibody (Molecular Probes, 1:4000) for two hours at room temperature. Cells were washed three times with DPBS, then mounted with Prolong Gold with DAPI (Molecular Probes).
Western blot
4x NuPAGE LDS Sample Buffer (Novex) containing 10 mM DTT was mixed with cell lysates and heated to 70°C for ten minutes. Samples were loaded into Bolt 4–12% Bis-Tris gels (Novex) and run for 45 minutes at 140 V. Protein was transferred to a 0.2 μm nitrocellulose membrane with the Mini Trans-Blot Cell (Bio-Rad) for one hour at 80 V or with the Bolt Mini Blot Module (Novex, Life Technologies) for one hour at 30 V. Membranes were blocked for one hour at room temperature in 1x Tris-buffered saline (TBS) with 5% nonfat milk. Primary and secondary antibodies were diluted in 1x TBS with 1% Triton X-100 (TBST), with either 5% bovine serum albumin (BSA) for detection of phosphorylated proteins, or with 5% nonfat milk for all other proteins. For the β-arrestin experiments, Aqua Block buffer (Abcam) was used instead of BSA and nonfat milk. Primary antibodies were incubated for two hours at room temperature with gentle shaking or overnight at −4°C. Secondary antibodies (Dylight 680 and Dylight 800) were incubated for two hours at room temperature with gentle shaking. Protein bands were detected on the Odyssey CLx and analyzed with Image Studio (LI-COR Biosciences). Signal intensity of protein bands were normalized to that of α-tubulin, β-actin, or GAPDH. Antibodies from Cell Signaling Technology were diluted as follows: rabbit anti-phospho-p44/p42 MAPK (pERK1/2), 1:2500; rabbit anti-p44/p42 MAPK (ERK1/2), 1:2500; rabbit anti-HA, 1:1000; rabbit anti-phospho-p38, 1:1000; rabbit anti-phospho-p54 and p46 JNK, 1:1000; rabbit anti-β-arrestin 2, 1:1000; mouse anti-α-tubulin, 1:2500; mouse anti-β-actin, 1:2000; mouse anti-GAPDH, 1:2000; goat anti-rabbit IgG DyLight 800, 1:8000; goat anti-mouse IgG DyLight 680, 1:8000. Antibodies from Abcam were diluted as follows: rabbit anti-β-arrestin 1, 1:1000.
Immobilized metal affinity column (IMAC) enrichment of phosphopeptides
Cells were treated with or without the selective 5-HT1B agonist CP-94253 (100 nM) for ten minutes, rinsed twice with cold PBS and lysed in 8M urea containing 50 mM Tris pH 8, and 1x protease and phosphatase inhibitors. DNA was sheared by passing lysates 15 times through a 28-gauge needle. The lysates were further sonicated in a cup-horn sonicator (30 sec on, 90 sec off, for ten minutes at full power). Lysates were centrifuged at 20,000 x g for 15 minutes to remove cell debris. Protein concentrations of clarified lysates were measured using the 660 nm protein assay (Pierce). Endoproteinase Lys-C (Wako, 1:100 enzyme:substrate ratio) was added to digest proteins at room temperature for three hours. The digest was then diluted to 1.5 M urea with addition of 50 mM Tris pH 8 buffer, and trypsin (Pierce, 1:100 enzyme:substrate ratio) was added to digest proteins overnight at 37°C in a shaking incubator. The digest was centrifuged at 14,000 x g for 15 min to remove precipitated protein; the supernatant was acidified to 1% formic acid to halt protein digestion. Peptides were desalted on a 150 mg 6 cc HLB desalting column (Waters), eluted with 2 × 400 μL 80% acetonitrile with 0.1% TFA in water, and dried to remove organic solvent. Peptides were resuspended in 300 μL 80% acetonitrile with 0.1% TFA in water and incubated for one hour with 10 μL Fe3+ and Ga2+ charged Ni-NTA Superflow agarose beads (Qiagen) on an end-over-end rotator at room temperature. The IMAC beads were transferred to equilibrated C18 StageTips, and phosphopeptides were eluted with 500 mM dibasic sodium phosphate pH 7, desalted, evaporated, and resuspended in 5% acetonitrile with 0.1% TFA in water for liquid chromatography-mass spectrometry (LC-MS) analyses.
Liquid chromatography-mass spectrometry
Peptides were injected on a ~10 cm x 75 μm ID column of 3 μm Reprosil C18.aq beads (Dr. Maisch, Germany) with a Dionex Ultimate 3000 RSLCnano for online peptide separation in a gradient of increasing acetonitrile content (3 – 35% B in 90 minutes) and data-dependent acquisition of mass spectrometry MS and tandem MS (MS/MS) by a Thermo Orbitrap Elite. Profile FTMS spectra were collected (R=30,000 at 400 m/z) and CID linear ion trap MS/MS data was collected with a Top15 method. The duty cycle of the instrument operated in this way is ~2.5 seconds. Data was processed by MaxQuant v. 1.3.0.559 and peptide-spectrum matches obtained with the Andromeda search engine60 from a Uniprot Mouse protein database (July 2013). A maximum 1% false discovery rate was applied for protein, peptide, and phosphorylation sites. The minimum Andromeda phosphopeptide score was set at 40.
Site-directed mutagenesis
Point mutations were introduced into the wild-type rat 5-HT1B receptor sequence using the GeneArt Site-Directed Mutagenesis System (Invitrogen) according to manufacturer’s instructions with 20 ng starting DNA per reaction (Supplemental Table 2). Resulting clones were sequenced through their entire coding sequence to verify the successful introduction of the desired mutation and to confirm that no additional mutations had been introduced inadvertently.
Generation and validation of β-arrestin knockout N2A-1B cell lines
Using the CRISPOR program,61 specific guide sequences were designed, four each for β-arrestin 1 and β-arrestin 2, and ordered as DNA cassettes (Invitrogen GeneArt Gene Synthesis). Each guide sequence was subsequently cloned into a pLentiCRISPRv2 plasmid, a retroviral vector that encodes Cas9, a guide sequence, and puromycin resistance.
Retroviral virus-like particles (VLPs) were generated as described elsewhere.62 Briefly, HEK293T cells were cotransfected with one of the pLentiCRISPRv2 plasmids, psPAX2 (HIV-1 Gag/Pol/Rev/Tat packaging plasmid), and pMD2.G (vesicular stomatitis virus glycoprotein [VSV-G] envelope plasmid) at a ratio of 1:1:0.5 using the FuGENE 6 transfection reagent (Promega) according to manufacturer’s instructions. Forty-eight hours after transfection, HEK293T cell supernatants containing the VLPs were collected and concentrated using Amicon Ultracel 100 K filters (Millipore) to yield approximately 200 μl of concentrated VLPs. N2A-1B cells were plated into six well plates at a density of 2×105 cells/well 24 hours prior to transduction with VLPs. Transduction occurred via spinoculation at 1200 x g for 90 minutes in complete medium supplemented with 500 μg/ml of G418. Twenty-four hours after transduction, cells were passaged into new T75 flasks and cultured in G418-containing media for 48 hours. Subsequently, cells were passaged and cultured in complete media supplemented with both 500 μg/ml of G418 and 2 μg/ml of puromycin to select for transgene expression. Each N2A-1B KO cell line expressed Cas9 and a single guide sequence, with four cell lines specific for β-arrestin 1, four cell lines for β-arrestin 2, and one cell line that expressed a non-targeting control sequence.
Sequences that yielded the most efficient sgRNAs were as follows: non-targeting control, 5’-CCGGGAGATTAACGTTAATT-3’; β-arrestin 1, 5’-GCATTGACCTCGTGGACCCCG-3’; and β-arrestin 2, 5’-GAAGTCGAGCCCTAACTGCA-3’. To validate CRISPR/Cas9 knockout of β-arrestin 1 and β-arrestin 2, each cell line was plated on 60 mm plates and lysed 24 hours later with modified RIPA buffer. Samples were briefly vortexed and cell debris was pelleted by centrifugation at 15,000 x g for 15 minutes. Cell lysates were then collected and analyzed via western blot as described above for the presence or absence of β-arrestin 1 and β-arrestin 2. For β-arrestin 1, signal intensity of protein bands was normalized to that of β-actin, while that of β-arrestin 2 was normalized to α-tubulin.
Data analysis
In each experiment, independent biological replicates were analyzed in separate assays; the number of independent biological replicates is indicated in the legend for each figure. Signal intensity of protein bands for phospho-ERK1/2 were first normalized to α-tubulin. Signal intensities for samples treated with agonist and antagonist were then compared to signal intensities for vehicle-treated controls from the same replicate to yield a percent of unstimulated control. All data were analyzed using a two-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc tests when multiple comparisons were made. All statistical analyses were performed using GraphPad Prism 7 or Excel software.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Theodore Gobillot for his assistance with VLP production, the lab of Dr. Patrick Paddison at the Fred Hutchinson Cancer Research Center for their generous gift of the pLentiCRISPRv2 plasmid, and Dr. Amit Sharma and Dr. Julie Overbaugh for their guidance, support, and the generous gift of the non-targeting control vector.
Funding: This work was supported in part by the National Institutes of Health grants F30-DA043941 (A.W.G), P50-MH106428 (J.F.N.), R21-MH099748 (J.F.N.), and T32-NS099578 (M.R.L.).
ABBREVIATIONS
- 5-HT
5-hydroxytryptamine (serotonin)
- ANOVA
analysis of variance
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- HA
hemagglutinin
- IMAC
immobilized metal affinity column
- JNK
c-Jun N-Terminal kinase
- LC-MS
liquid chromatography-mass spectrometry
- MAPK
mitogen-activated protein kinase
- MEK
MAP/ERK kinase
- MEF
mouse embryonic fibroblast
- N2A
Neuro2A neuroblastoma cell line
- sgRNA
single-guide RNA
- VLP
virus-like particle
Footnotes
SUPPORTING INFORMATION
Primers used for qPCR in untransfected N2A and stably transfected N2A-1B cells. Primers used for site-directed mutagenesis of the wild-type 5-HT1B receptor. CP-94253 increases levels of phospho-ERK1/2, but not phospho-SAPK/JNK or phospho-p38, in N2A-1B cells, but not in untransfected N2A cells. CP-93129 increases levels of phospho-ERK1/2 in N2A-1B cells. 5-HT1B-mediated phosphorylation of ERK1/2 is dependent on β-arrestin signaling in mouse embryonic fibroblast (MEF) cells.
REFERENCES
- [1].Hen R. (1992) Of mice and flies: commonalities among 5-HT receptors, Trends Pharmacol Sci 13, 160–165. [DOI] [PubMed] [Google Scholar]
- [2].Boschert U, Amara DA, Segu L, and Hen R. (1994) The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals, Neuroscience 58, 167–182. [DOI] [PubMed] [Google Scholar]
- [3].Ghavami A, Stark KL, Jareb M, Ramboz S, Segu L, and Hen R. (1999) Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons, J Cell Sci 112 ( Pt 6), 967–976. [DOI] [PubMed] [Google Scholar]
- [4].Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, and Descarries L. (2000) Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain, J Comp Neurol 417, 181–194. [PubMed] [Google Scholar]
- [5].Middlemiss DN, and Hutson PH. (1990) The 5-HT1B receptors, Ann N Y Acad Sci 600, 132–147; discussion 347–148. [DOI] [PubMed] [Google Scholar]
- [6].Hjorth S, Suchowski CS, and Galloway MP. (1995) Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain, Synapse 19, 170–176. [DOI] [PubMed] [Google Scholar]
- [7].Daws LC, Gould GG, Teicher SD, Gerhardt GA, and Frazer A. (2000) 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo, J Neurochem 75, 2113–2122. [DOI] [PubMed] [Google Scholar]
- [8].Hagan CE, McDevitt RA, Liu Y, Furay AR, and Neumaier JF. (2012) 5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes, Synapse 66, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Montanez S, Munn JL, Owens WA, Horton RE, and Daws LC. (2014) 5-HT1B receptor modulation of the serotonin transporter in vivo: studies using KO mice, Neurochem Int 73, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McDevitt RA, Hiroi R, Mackenzie SM, Robin NC, Cohn A, Kim JJ, and Neumaier JF. (2011) Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior, Biol Psychiatry 69, 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McDevitt RA, and Neumaier JF. (2011) Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective, J Chem Neuroanat 41, 234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andrade R, Malenka RC, and Nicoll RA. (1986) A G protein couples serotonin and GABAB receptors to the same channels in hippocampus, Science 234, 1261–1265. [DOI] [PubMed] [Google Scholar]
- [13].Bouhelal R, Smounya L, and Bockaert J. (1988) 5-HT1B receptors are negatively coupled with adenylate cyclase in rat substantia nigra, Eur J Pharmacol 151, 189–196. [DOI] [PubMed] [Google Scholar]
- [14].Schoeffter P, and Hoyer D. (1989) 5-Hydroxytryptamine 5-HT1B and 5-HT1D receptors mediating inhibition of adenylate cyclase activity. Pharmacological comparison with special reference to the effects of yohimbine, rauwolscine and some beta-adrenoceptor antagonists, Naunyn Schmiedebergs Arch Pharmacol 340, 285–292. [DOI] [PubMed] [Google Scholar]
- [15].Ghavami A, Baruscotti M, Robinson RB, and Hen R. (1997) Adenovirus-mediated expression of 5-HT1B receptors in cardiac ventricle myocytes; coupling to inwardly rectifying K+ channels, Eur J Pharmacol 340, 259–266. [DOI] [PubMed] [Google Scholar]
- [16].Pullarkat SR, Mysels DJ, Tan M, and Cowen DS. (1998) Coupling of serotonin 5-HT1B receptors to activation of mitogen-activated protein kinase (ERK-2) and p70 S6 kinase signaling systems, J Neurochem 71, 1059–1067. [DOI] [PubMed] [Google Scholar]
- [17].Mendez J, Kadia TM, Somayazula RK, El-Badawi KI, and Cowen DS. (1999) Differential coupling of serotonin 5-HT1A and 5-HT1B receptors to activation of ERK2 and inhibition of adenylyl cyclase in transfected CHO cells, J Neurochem 73, 162–168. [DOI] [PubMed] [Google Scholar]
- [18].Hsu EH, Lochan AC, and Cowen DS. (2001) Activation of Akt1 by human 5-hydroxytryptamine (serotonin)1B receptors is sensitive to inhibitors of MEK, J Pharmacol Exp Ther 298, 825–832. [PubMed] [Google Scholar]
- [19].Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, and Greengard P. (2006) Alterations in 5-HT1B receptor function by p11 in depression-like states, Science 311, 77–80. [DOI] [PubMed] [Google Scholar]
- [20].Chen L, Salinas GD, and Li X. (2009) Regulation of serotonin 1B receptor by glycogen synthase kinase-3, Mol Pharmacol 76, 1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li X, and Jope RS. (2010) Is glycogen synthase kinase-3 a central modulator in mood regulation?, Neuropsychopharmacology 35, 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou W, Chen L, Paul J, Yang S, Li F, Sampson K, Woodgett JR, Beaulieu JM, Gamble KL, and Li X. (2012) The effects of glycogen synthase kinase-3beta in serotonin neurons, PLoS One 7, e43262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liggett SB. (2011) Phosphorylation barcoding as a mechanism of directing GPCR signaling, Sci Signal 4, pe36. [DOI] [PubMed] [Google Scholar]
- [24].Reiter E, Ahn S, Shukla AK, and Lefkowitz RJ. (2012) Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors, Annu Rev Pharmacol Toxicol 52, 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, Ruttiger N, Ziegler N, Benkel T, Schmitt NK, Ishida S, Muller I, Reher R, Kawakami K, Inoue A, Rick U, Kuhl T, Imhof D, Aoki J, Konig GM, Hoffmann C, Gomeza J, Wess J, and Kostenis E. (2018) Lack of beta-arrestin signaling in the absence of active G proteins, Nat Commun 9, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling, Pharmacol Rev 53, 1–24. [PubMed] [Google Scholar]
- [27].Lefkowitz RJ, and Shenoy SK. (2005) Transduction of receptor signals by beta-arrestins, Science 308, 512–517. [DOI] [PubMed] [Google Scholar]
- [28].Keshet Y, and Seger R. (2010) The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions, Methods Mol Biol 661, 3–38. [DOI] [PubMed] [Google Scholar]
- [29].Naor Z, Benard O, and Seger R. (2000) Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor, Trends Endocrinol Metab 11, 91–99. [DOI] [PubMed] [Google Scholar]
- [30].Luttrell LM, and Miller WE. (2013) Arrestins as regulators of kinases and phosphatases, Prog Mol Biol Transl Sci 118, 115–147. [DOI] [PubMed] [Google Scholar]
- [31].Villarreal JS, and Barea-Rodriguez EJ. (2006) ERK phosphorylation is required for retention of trace fear memory, Neurobiol Learn Mem 85, 44–57. [DOI] [PubMed] [Google Scholar]
- [32].Huh KH, Guzman YF, Tronson NC, Guedea AL, Gao C, and Radulovic J. (2009) Hippocampal Erk mechanisms linking prediction error to fear extinction: roles of shock expectancy and contextual aversive valence, Learn Mem 16, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guedea AL, Schrick C, Guzman YF, Leaderbrand K, Jovasevic V, Corcoran KA, Tronson NC, and Radulovic J. (2011) ERK-associated changes of AP-1 proteins during fear extinction, Mol Cell Neurosci 47, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ishikawa S, Saito Y, Yanagawa Y, Otani S, Hiraide S, Shimamura K, Matsumoto M, and Togashi H. (2012) Early postnatal stress alters extracellular signal-regulated kinase signaling in the corticolimbic system modulating emotional circuitry in adult rats, Eur J Neurosci 35, 135–145. [DOI] [PubMed] [Google Scholar]
- [35].Vetere G, Piserchia V, Borreca A, Novembre G, Aceti M, and Ammassari-Teule M. (2013) Reactivating fear memory under propranolol resets pre-trauma levels of dendritic spines in basolateral amygdala but not dorsal hippocampus neurons, Front Behav Neurosci 7, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Clark MS, Vincow ES, Sexton TJ, and Neumaier JF. (2004) Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner, Brain Res 1007, 86–97. [DOI] [PubMed] [Google Scholar]
- [37].Liu Y, Kelly MA, Sexton TJ, and Neumaier JF. (2015) 5-HT1B autoreceptors differentially modulate the expression of conditioned fear in a circuit-specific manner, Neuroscience 298, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hamblin MW, McGuffin RW, Metcalf MA, Dorsa DM, and Merchant KM. (1992) Distinct 5-HT(1B) and 5-HT(1D) serotonin receptors in rat: Structural and pharmacological comparison of the two cloned receptors, Mol Cell Neurosci 3, 578–587. [DOI] [PubMed] [Google Scholar]
- [39].Barnes NM, and Sharp T. (1999) A review of central 5-HT receptors and their function, Neuropharmacology 38, 1083–1152. [DOI] [PubMed] [Google Scholar]
- [40].Xie Z, Lee SP, O’Dowd BF, and George SR. (1999) Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed, FEBS Lett 456, 63–67. [DOI] [PubMed] [Google Scholar]
- [41].Berg KA, and Clarke WP. (2001) Regulation of 5-HT(1A) and 5-HT(1B) receptor systems by phospholipid signaling cascades, Brain Res Bull 56, 471–477. [DOI] [PubMed] [Google Scholar]
- [42].Salim K, Fenton T, Bacha J, Urien-Rodriguez H, Bonnert T, Skynner HA, Watts E, Kerby J, Heald A, Beer M, McAllister G, and Guest PC. (2002) Oligomerization of G-protein-coupled receptors shown by selective co-immunoprecipitation, J Biol Chem 277, 15482–15485. [DOI] [PubMed] [Google Scholar]
- [43].Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, Manivet P, Launay JM, and Maroteaux L. (2007) Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors, Mol Pharmacol 71, 1463–1474. [DOI] [PubMed] [Google Scholar]
- [44].Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu M, Siu FY, Liu W, Xu HE, Cherezov V, Roth BL, and Stevens RC. (2013) Structural features for functional selectivity at serotonin receptors, Science 340, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Katritch V, Cherezov V, and Stevens RC. (2013) Structure-function of the G protein-coupled receptor superfamily, Annu Rev Pharmacol Toxicol 53, 531–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Busca R, Pouyssegur J, and Lenormand P. (2016) ERK1 and ERK2 Map Kinases: Specific Roles or Functional Redundancy?, Front Cell Dev Biol 4, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Storez H, Scott MG, Issafras H, Burtey A, Benmerah A, Muntaner O, Piolot T, Tramier M, Coppey-Moisan M, Bouvier M, Labbe-Jullie C, and Marullo S. (2005) Homo- and hetero-oligomerization of beta-arrestins in living cells, J Biol Chem 280, 40210–40215. [DOI] [PubMed] [Google Scholar]
- [48].Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, and Lefkowitz RJ. (2006) Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation, J Biol Chem 281, 10856–10864. [DOI] [PubMed] [Google Scholar]
- [49].Labasque M, Reiter E, Becamel C, Bockaert J, and Marin P. (2008) Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling, Mol Biol Cell 19, 4640–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peterson YK, and Luttrell LM. (2017) The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling, Pharmacol Rev 69, 256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Luttrell DK, and Luttrell LM. (2003) Signaling in time and space: G protein-coupled receptors and mitogen-activated protein kinases, Assay Drug Dev Technol 1, 327–338. [DOI] [PubMed] [Google Scholar]
- [52].Luttrell LM, and Lefkowitz RJ. (2002) The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals, J Cell Sci 115, 455–465. [DOI] [PubMed] [Google Scholar]
- [53].Gaster LM, Blaney FE, Davies S, Duckworth DM, Ham P, Jenkins S, Jennings AJ, Joiner GF, King FD, Mulholland KR, Wyman PA, Hagan JJ, Hatcher J, Jones BJ, Middlemiss DN, Price GW, Riley G, Roberts C, Routledge C, Selkirk J, and Slade PD. (1998) The selective 5-HT1B receptor inverse agonist 1’-methyl-5-[[2’-methyl-4’-(5-methyl-1,2, 4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydro- spiro[furo[2,3-f]indole-3,4’-piperidine] (SB-224289) potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo, J Med Chem 41, 1218–1235. [DOI] [PubMed] [Google Scholar]
- [54].Selkirk JV, Scott C, Ho M, Burton MJ, Watson J, Gaster LM, Collin L, Jones BJ, Middlemiss DN, and Price GW. (1998) SB-224289--a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity, Br J Pharmacol 125, 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cargnello M, and Roux PP. (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases, Microbiol Mol Biol Rev 75, 50–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Koe BK, Nielsen JA, Macor JE, and Heym J. (1992) Biochemical and behavioral studies of the 5‐HT1B receptor agonist, CP‐94,253, Drug Development Research 26, 241–250. [Google Scholar]
- [57].Kohout TA, Lin FS, Perry SJ, Conner DA, and Lefkowitz RJ. (2001) beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking, Proc Natl Acad Sci U S A 98, 1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lesiak AJ, Brodsky M, Cohenca N, Croicu AG, and Neumaier JF. (2018) Restoration of Physiological Expression of 5-HT6 Receptor into the Primary Cilia of Null Mutant Neurons Lengthens Both Primary Cilia and Dendrites, Mol Pharmacol 94, 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cox J, and Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification, Nat Biotechnol 26, 1367–1372. [DOI] [PubMed] [Google Scholar]
- [60].Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, and Mann M. (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment, J Proteome Res 10, 1794–1805. [DOI] [PubMed] [Google Scholar]
- [61].Haeussler M, Schonig K, Eckert H, Eschstruth A, Mianne J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, Joly JS, and Concordet JP. (2016) Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR, Genome Biol 17, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nahabedian J, Sharma A, Kaczmarek ME, Wilkerson GK, Sawyer SL, and Overbaugh J. (2017) Owl monkey CCR5 reveals synergism between CD4 and CCR5 in HIV-1 entry, Virology 512, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.