Abstract
Circulating ensembles of tumor‐associated cells (C‐ETACs) which comprise tumor emboli, immune cells and fibroblasts pose well‐recognized risks of thrombosis and aggressive metastasis. However, the detection, prevalence and characterization of C‐ETACs have been impaired due to methodological difficulties. Our findings show extensive pan‐cancer prevalence of C‐ETACs on a hitherto unreported scale in cancer patients and virtual undetectability in asymptomatic individuals. Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples of 16,134 subjects including 5,509 patients with epithelial malignancies in various organs and 10,625 asymptomatic individuals with age related higher cancer risk. PBMCs were treated with stabilizing reagents to protect and harvest apoptosis‐resistant C‐ETACs, which are defined as cell clusters comprising at least three EpCAM+ and CK+ cells irrespective of leucocyte common antigen (CD45) status. All asymptomatic individuals underwent screening investigations for malignancy including PAP smear, mammography, low‐dose computed tomography, evaluation of cancer antigen 125, cancer antigen 19‐9, alpha fetoprotein, carcinoembryonic antigen, prostate specific antigen (PSA) levels and clinical examination to identify healthy individuals with no indication of cancer. C‐ETACs were detected in 4,944 (89.8%, 95% CI: 89.0–90.7%) out of 5,509 cases of cancer. C‐ETACs were detected in 255 (3%, 95% CI: 2.7–3.4%) of the 8,493 individuals with no abnormal findings in screening. C‐ETACs were detected in 137 (6.4%, 95% CI: 5.4–7.4%) of the 2,132 asymptomatic individuals with abnormal results in one or more screening tests. Our study shows that heterotypic C‐ETACs are ubiquitous in epithelial cancers irrespective of radiological, metastatic or therapy status. C‐ETACs thus qualify to be a systemic hallmark of cancer.
Keywords: circulating tumor cells, circulating tumor‐associated cells, circulating tumor emboli, circulating tumor cell clusters, cancer related thrombosis, circulating metastatic disease
Short abstract
What's new?
Circulating Ensembles of Tumor Associated Cells (C‐ETACs) comprised of tumor emboli, immune cells, and fibroblasts pose well‐recognized risks of thrombosis and aggressive metastasis. However, the detection and characterization of C‐ETACs have been impaired by methodological difficulties. Here, the authors have developed a label‐free non‐mechanical process that permits enrichment of viable apoptosis‐resistant C‐ETACs from peripheral blood. They show that heterotypic C‐ETACs are not merely incidental findings in cancer but rather a systemic manifestation of malignancy. C‐ETACs are present in a significant proportion of all solid organ malignancies and are rare in asymptomatic individuals. Monitoring of C‐ETACs could help inform cancer management.
Abbreviations
- AFP
alpha fetoprotein
- CA125
cancer antigen 125
- CA19‐9
cancer antigen 19‐9
- CAP
College of American Pathologists
- CD45
leucocyte common antigen
- CEA
carcinoembryonic antigen
- C‐ETACs
circulating ensembles of tumor‐associated cells
- CMD
circulating metastatic disease
- CSC
cancer stem cells
- CTCs
circulating tumor cells
- EpCAM
epithelial cell adhesion molecule
- HPE
histopathological evaluation
- IHC
immunohistochemistry
- LDCT
low‐dose computed tomography
- MRD
minimum residual disease
- NED
no evidence of disease
- pan‐CK
pan‐cytokeratins
- PBMCs
peripheral blood mononuclear cells
- PSA
prostate‐specific antigen
- SoC
standard of care
- TAL
tumor‐associated T‐lymphocytes
- TAM
tumor‐associated macrophages
- VTE
venous thromboembolism
Introduction
The focus of prior research efforts in regard to the release of viable cells from the tumor has been to capture and characterize single cells rather than clusters. However, there is growing evidence that has led researchers to hypothesize that in addition to (or rather than) circulating tumor cells (CTCs), metastasis is facilitated more aggressively by dissemination of cell clusters containing CTCs.1, 2 Though there have been prior attempts at identification and characterization of CTC clusters, these efforts have employed methods and devices primarily designed for isolation of single CTCs, such as epitope (epithelial cell adhesion molecule [EpCAM]) capture or microfluidic devices.3, 4, 5 There appear to be no reports on definitive methods for harvesting tumor derived emboli or CTC clusters. We hypothesized that prior attempts may have been suboptimal in recovering intact viable clusters due to methodological limitations, and may have inadvertently underrepresented the prevalence of CTC aggregates.2 We have developed a label free nonmechanical process that permits enrichment of viable apoptosis resistant circulating tumor‐associated cells (C‐TACs) and their assemblages (circulating ensembles of tumor‐associated cells [C‐ETACs]) from peripheral blood. This process detects and yields C‐ETACs for qualitative and quantitative analysis. Samples from a large cohort of cancer patients (n = 5,509) as well as asymptomatic individuals (n = 10,625) were processed to identify and harvest C‐ETACs. We show that heterotypic C‐ETACs comprising tumor cells and diverse immune cells are commonly detected in patients with epithelial solid organ malignancies at higher prevalence rates than previously thought and are virtually undetectable in the asymptomatic population. Our study findings qualify C‐ETACs as a systemic hallmark of cancer with potential implications in cancer detection and management.
Methods
Study design
We present data from two separate prospective observational studies. The first observational study is titled, “Realtime Enrichment Screen for Outright detection of Latent Undiagnosed malignant Tumors in asymptomatic individuals Efficiently—RESOLUTE” (WHO ICTRP ID CTRI/2019/01/017219). The second observational study is titled “Tissue biopsy Replacement with Unique Evaluation of circulating bio‐markers for morphological evaluation and clinically relevant molecular typing of malignancies from BLOOD sample—TrueBlood” (WHO ICTRP ID CTRI/2019/03/017918). Both studies have been approved by the respective Institutional Ethics Committees of participating centers. Evaluation of participant samples was carried out at a facility which offers College of American Pathologists (CAP) accredited services.
Study participants and samples
The present study screened 16,134 individuals including 5,509 cancer patients (TrueBlood) and 10,625 asymptomatic individuals (RESOLUTE). The TrueBlood Study recruited adult (≥18) male and female patients with confirmed diagnosis of solid organ cancers irrespective of stage, grade or therapy status (>21 days since most recent systemic therapy or radiology for pretreated patients). Details of the True Blood study are available at http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/03/017918. The RESOLUTE Study recruited adult males (49–75 years) and females (40–75 years) with no known diagnosis or clinical suspicion of cancer. Details of the RESOLUTE study are available at http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/01/017219. All screened individuals were counseled regarding the study objectives and procedures and those who provided written informed consent were enrolled. Venous blood was collected in EDTA containers from all recruited participants. Cancer patients in the TruBlood Study did not undergo any further evaluations and their most recent clinical records including histopathology, treatment summary and radiological evaluations were referred for disease status. All asymptomatic individuals in the RESOLUTE study underwent prescribed gender‐relevant cancer screening procedures including mammography, low‐dose computed tomography (LDCT) scan and PAP smear, as well as evaluation of cancer antigen 125 (CA125), cancer antigen 19‐9 (CA19‐9), carcinoembryonic antigen (CEA), alpha fetoprotein (AFP) and prostate‐specific antigen (PSA) levels. Asymptomatic individuals with abnormal findings in any of the screening procedures (e.g., elevated CA marker or suspicious findings on imaging) were identified and considered as “at risk” population, while those with normal findings were considered as “healthy” population in all further evaluations. Demographic and clinical stratification details of cancer patients and asymptomatic individuals are provided in Supporting Information Tables S1 and S2, respectively.
Enrichment and harvesting of C‐ETACs
Peripheral blood mononuclear cells (PBMCs) were obtained from 15 ml whole blood using RBC lysis buffer (Thermo Fisher Scientific, Waltham, MA) and finally resuspended in buffer as per manufacturer's instructions. Resuspended PBMCs were divided into several aliquots, which were transferred into multiwell plates and treated with epigenetically activated media for up to 100 hr at 37°C under hypoxic (5% O2) conditions. The epigenetically activated media comprises of DMEM (Thermo Fisher) containing FBS (Thermo Fisher) which is enriched with Tumor Necrosis Factor Receptors (TNFR), Nuclear Factor kappa B (NF‐kB) and the Janus kinase/signal transducer and activator of transcription (Jak/Stat) pathway related transcripts and factors. Additional cell growth factors (CGF) such as F12 nutrient mixture (Thermo Fisher), epidermal growth factor (EGF, Thermo Fisher), fibroblast growth factor (FGF, Thermo Fisher) and N‐2 supplements (Thermo Fisher) are also blended. Since epithelial cells and hematolymphoid cells have significantly different apoptotic pathways, the media provokes differential apoptosis in cells of these lineages. This approach selectively kills hematolymphoid cells with proficient apoptotic mechanisms in response to intense progrowth stimuli. The cells which survive are “apoptosis resistant” and are therefore direct tumor cells or those who are recruited by the tumors such as, but not limited to, tumor‐associated macrophages (TAMs) and tumor‐associated fibroblasts (TAFs). The procedure being label‐free and singularly premised upon the exploitation of apoptosis proficient/resistant characteristic of normal versus tumor cells affords the benefit of harvesting clusters without dependence on antigen epitopes or the mechanical hobbling or stresses typical of microfluidic devices. Processed samples were thereafter observed by phase contrast microscopy on the fifth day and cell clusters if any were harvested by aspiration for further characterization. Harvested clusters were immunostained with fluorophore conjugated antibodies against EpCAM (phycoerythrin [PE]), pan‐cytokeratins (pan‐CK; fluorescein isothiocyanate [FITC]) and leucocyte common antigen (CD45; CY5), and finally stained with the nucleic acid dye (4,6‐diaminodino‐2‐phenylindole [DAPI]). Fluorescence imaging was performed on Cell Insight CX7 High‐Content Screening Platform (ThermoFisher Scientific). For the purpose of our study, C‐ETACs were defined as clusters of at least three cells that were positive by immunostaining for EpCAM and pan‐CK, irrespective of CD45 status. The C‐ETAC enrichment media formulation and isolation protocol is the subject matter of Patent applications (United States Patent Office Provisional Application Numbers 62849840 and 62796098).
Immunostaining for identification and characterization of C‐ETACs
Harvested cell clusters were used for preparation of cytospin slides by using standard procedures. One slide was used for identification of C‐ETACs by immunofluorescent staining using anti‐EpCAM, anti‐panCK and anti‐CD45 antibodies, as well as DAPI to confirm intact (nucleated) cells. Additional slides were used for immunostaining with markers such as CD44 (cancer stem cells [CSCs]) and CD8 (tumor‐associated leucocytes). In a set of samples from known cases of breast, lung, prostate, cervix and gastric cancers, general, organ‐specific and nonorgan specific markers were evaluated by immunostaining of C‐ETACs. All slides were scanned using a multiwavelength fluorescent scanner (CellInsight, Thermofisher). A sample was treated as positive if at least one C‐ETAC was detected in 1 ml PBMC equivalent of peripheral blood. All primary and secondary antibodies used in immunostaining, their manufacturers as well as cell lines used as positive controls for each antibody are listed in Supporting Information Table S3. The immunostaining workflow is provided in Supporting Information Table S6. All antibodies were used at manufacturer recommended dilutions with dilutions being prepared in manufacturer provided or recommended dilution buffers. All human cell lines were procured within the last 3 years. All experiments were performed with mycoplasma‐free cells.
Tumorigenic origin of C‐ETACs
In order to establish a direct causative link with the existence of a tumor, we obtained samples from a subcohort of 223 cancer patients prior to undergoing surgical resection of the tumor as well as 8 hr after the surgical procedure. C‐ETACs were harvested and enumerated to discern their presurgery and postsurgery numbers. Details of this subcohort are provided in Supporting Information Table S4.
C‐ETACs and radiological status
Another subcohort of the study population included 589 patients who had previously (>21 days ago) received treatments for cancer and where recent radiological evaluation indicated no evidence of disease (NED). Details of this subcohort are provided in Supporting Information Table S5. Samples from this subcohort were compared to those of patients with radiological evidence of disease to determine differences in prevalence of C‐ETACs.
Data availability
Data may be made available from the authors upon reasonable request.
Results
Study cohort
The present study included 5,509 patients with a confirmed diagnosis of cancer (Supporting Information Table S1) with a median age of 55 years including 2,482 (45.1%) males and 3,027 (54.9%) females. Then, 4,920 patients had radiological evidence of active cancer at the time of blood sampling (irrespective of prior treatment status) and 589 had no radiological evidence of disease post prior treatment(s). Then, 3,098 patients (56.2%) had metastatic disease and 1,138 (20.7%) cases were nonmetastatic; metastatic status was unavailable in 1,273 (23.1%) cases. Then, 3,413 patients (62.0%) had received prior treatment whereas 1,828 (33.2%) were treatment naïve. Therapy status of 268 patients (4.8%) was unknown. The asymptomatic cohort (Supporting Information Table S2) screened 10,625 individuals with a median age of 54 years including 3,898 (36.7%) males and 6,727 (63.3%) females, of whom 3,475 were postmenopausal. Among these 10,625 individuals, 2,132 (898 males +1,234 females) had either significant findings in LDCT, Mammography or PAP smear or elevated level(s) of CA125, CA19‐9, CEA, AFP or PSA and were hence considered as “at risk” population. The remaining 8,493 individuals were considered as healthy population and consisted of 3,000 (35.3%) males and 5,493 (64.7% females) with a median age of 53 years (range: 40–75 years).
C‐ETACs are heterotypic
Figure 1 shows representative phase contrast microscope images of cell assemblages from various cancers as observed on the fifth day. Figures 2 a–2 i show representative images of clusters (3–50 cells) staining positively for EpCAM, CK or CD45. C‐ETACs included cells that were negative for EpCAM and CK but positive for CD8a (tumor‐associated leucocytes; Figs. 3 a–3 e) as well as cells that stained positively for CD44 (CSCs, Figs. 3 f–3 j). The varied immunomorphology indicated that these C‐ETACs are not merely aggregates of CTCs but represent a snapshot of heterotypic multicellular associations. C‐ETACs were immunostained with organ of origin specific markers in samples from Ca Breast (Figs. 4 a–4 e), Ca Lung (Figs. 4 f–4 j), Ca Ovary (Figs. 4 k–4 o) and Ca Prostate (Figs. 4 p–4 t) indicating that the C‐ETAC can be used to identify the organ of origin. All tested samples were found to be concordant for the organ‐specific markers tested and had undetectable reactivity with nonspecific markers.
Figure 1.
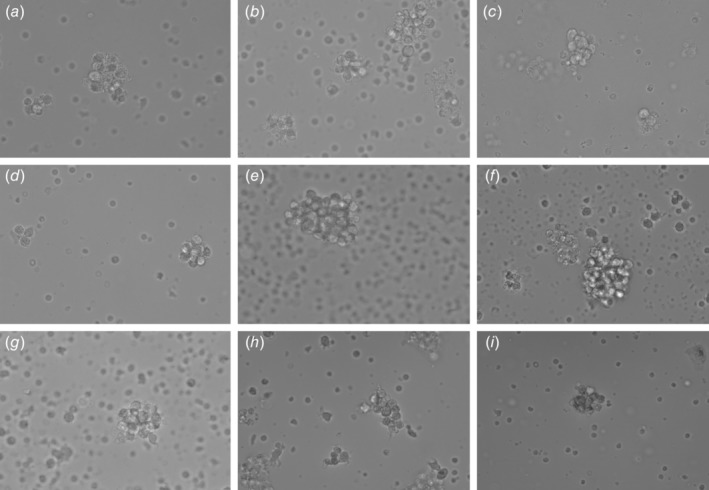
Cell assemblages on Day 5. Viable intact cell assemblages (white arrow) were imaged under a phase contrast microscope at 40× magnification. Samples from various cancer types are depicted (a) breast, (b) lung, (c) prostate, (d) stomach, (e) gallbladder, (f) kidney, (g) bladder, (h) buccal mucosa and (i) pancreas. Field width is ~160 μm.
Figure 2.
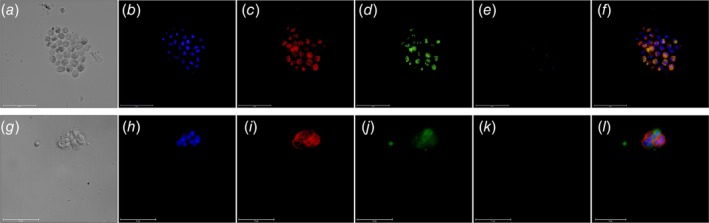
Immunostaining of C‐ETACs. Cytospin smears prepared from cell‐assemblages obtained on Day 5 from a case of Ca lung (a–f) and Ca endometrium (g–l) were stained with DAPI, anti‐EpCAM, anti‐CK and anti‐CD45. (a, g) Bright field; (b, h) DAPI; (c, i) EpCAM; (d, j) panCK; (e k) CD45; (f, l) composite overlay (without bright field).
Figure 3.
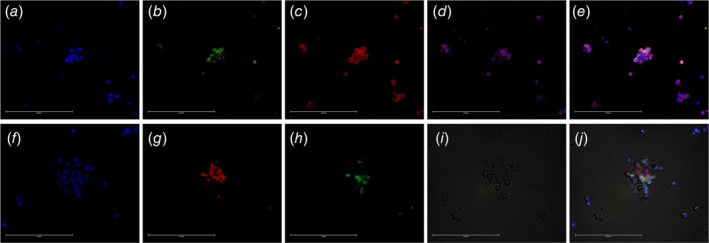
C‐ETACs are heterotypic. Cytospin smears of confirmed C‐ETAC samples were immunostained for CD44 in a known case of Ca buccal mucosa (a–e) and CD8a in a case of Ca Breast (f–j). C‐ETACs in a–d were stained for DAPI, panCK, CD44 and CD45, respectively, while e is the composite overlay. C‐ETACs in f–h were stained for DAPI, EpCAM and CD8a, while i is the bright field image and j is the composite overlay.
Figure 4.
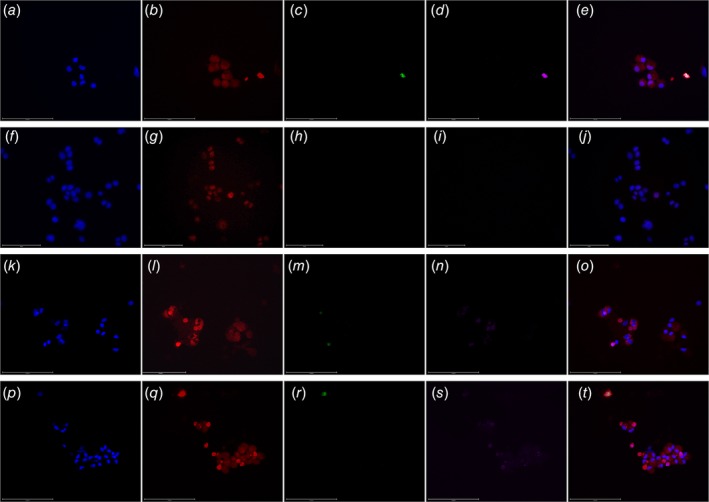
Organ specificity of C‐ETACs. Cytospin smears of confirmed C‐ETAC samples were immunostained for organ‐specific and organ nonspecific markers in a case of Ca Breast (a–e), Ca Colon (f–j), Ca Ovary (k–o) and Ca Prostate (p–t). C‐ETACs from Ca Breast were stained for DAPI, specific marker GCDFP15 (unconjugated primary and PE‐conjugated secondary), negative marker CDX‐2 (FITC) and CD45 (Cy5.5). C‐ETACs from Ca Colon were stained for DAPI, specific marker CDX‐2 (unconjugated primary and PE‐conjugated secondary), negative marker GCDFP‐15 (FITC) and CD45 (Cy5.5). C‐ETACs from Ca Ovary were stained for DAPI, specific marker CA125 (unconjugated primary and PE‐conjugated secondary), negative marker GFAP (FITC) and CD45 (Cy5.5). C‐ETACs from Ca Prostate were stained for DAPI, AMACR (unconjugated primary and PE‐conjugated secondary), negative marker GFAP (FITC) and CD45 (Cy5.5).
C‐ETACs are ubiquitous in epithelial malignancies
Blood samples from 5,509 cancer patients were processed for stabilization and isolation of C‐ETACs. Viable C‐ETACs were discernible in 4,944 cases (89.7%) across all epithelial solid organ malignancies. Figure 5 a depicts the cancer‐wise proportion of samples where C‐ETACs were detectable. Histopathological evaluation (HPE) and tumor grade data was available for a subset of samples; however, no significant differences were observed based on differences in HPE subtype or tumor grade (data not shown). C‐ETACs were detected in 1,006 (88.4%) patients out of 1,138 with local disease and 2,750 (88.8%) patients of 3,098 with metastatic disease (Fig. 5 b). C‐ETACs were detected in 1,645 (90.0%) of 1,828 recently diagnosed (radiologically evident) therapy naïve patients as well as in 3,062 (89.7%) of 3,413 pretreated patients irrespective of present radiological status. C‐ETACs were also detectable in 4,403 (89.5%) of 4,920 patients with radiologically evident disease, irrespective of treatment status. A subset (n = 589) of the pretreated cancer population included patients with NED in the most recent radiological scan; C‐ETACs were detected in 541 (91.9%) of these patients (Fig. 5 c). In another subcohort of 223 cancer patients (Supporting Information Table S4) who underwent surgical resection of tumor, C‐ETACs were enumerated in samples collected prior to surgery and 8 hr postsurgery. It was observed that while presurgery samples had a median density of 10 C‐ETACs/field, postsurgery samples had a median density of 5 C‐ETACs/field (Fig. 5 d). In postsurgery samples, majority (77%) of samples showed decrease in C‐ETACs, while 15% of samples showed increased C‐ETACs and 8% of samples showed no change.
Figure 5.
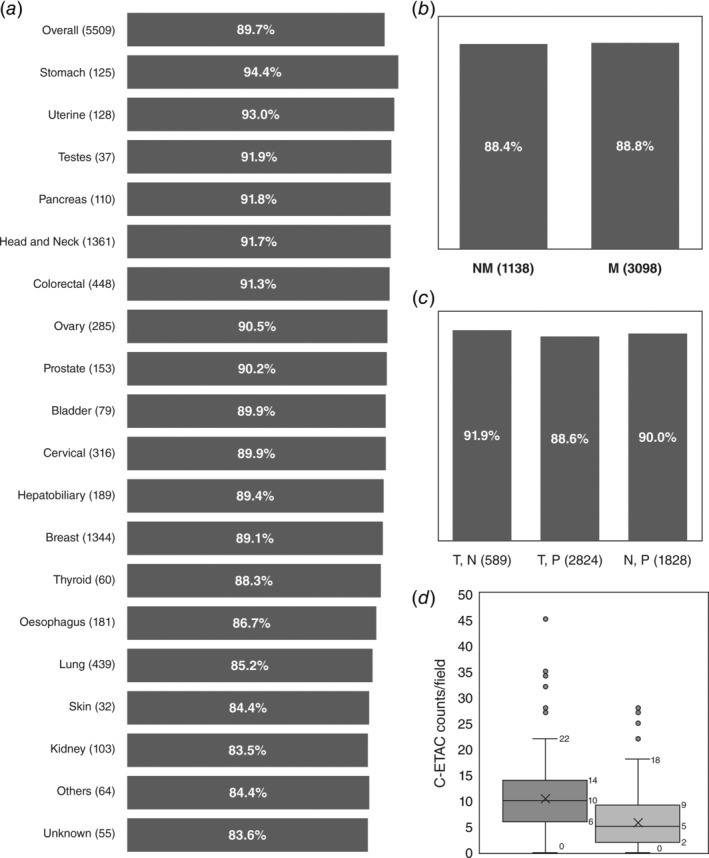
Ubiquity of C‐ETACs. (a) C‐ETACs were evaluated in 5,509 previously diagnosed cases of cancers. Dark bars represent percentage of total samples in each cancer type (and overall) where C‐ETACs could be detected. (b) C‐ETACs were detected with comparable frequency in metastatic (M) as well as nonmetastatic (NM) cancer samples (UA: metastatic status unavailable). (c) C‐ETACs detection was irrespective of treatment and radiological status. T, N: treated with presently no radiological evidence of disease; T, P: treated with radiologically evident disease; N, P: therapy naïve with radiologically evident disease. (d) C‐ETAC counts in presurgery (dark bar) and postsurgery (light bar) sample.
C‐ETACs in asymptomatic population
Among the 10,625 asymptomatic individuals, 8,493 had no abnormal findings in screening for cancer and were deemed as healthy population. C‐ETACs were detected in 255 (3.0%) of these 8,493 individuals’ samples. Among the 2,132 individuals with deranged findings on any of the screening investigations, C‐ETACs were detected in 137 (6.4%) cases. The occurrence of C‐ETACs in patients with normal (negative) and abnormal (elevated/significant) findings in various screening investigations are provided in Supporting Information Table S7. In males, higher probability of C‐ETACs detection was associated with abnormal findings in CA‐19‐9 (10.3%) and total PSA (8.9%). In females, higher probability of C‐ETACs detection was associated with abnormal findings in CEA (8.5%) and PAP smear (10.3%). Among the 487 (out of 10,625) asymptomatic individuals with a known family history (first‐/second‐degree blood relatives) of cancer, C‐ETACs were detected in 14 (2.9%) of these cases which was comparable to the 3% detection rate in asymptomatic individuals with no aberrant findings. Among the 985 (out of 10,625) asymptomatic individuals who reported habits such as tobacco addiction as well as individuals with risk of exposure to carcinogens due to occupational hazard, C‐ETACs were detected in 47 (4.8%) cases. At the time of submission of this article, none of the 392 individuals where C‐ETACs were detected had presented with clinical or radiological manifestations of cancer. However, they have been advised follow up to identify any early clinical presentation.
Discussion
The onset of any sustained neoplastic expansion that disturbs the cellular equilibrium in the human body is a major disruptive event with possibly fatal consequences. Such uncontrolled cell growth coupled with resistance to apoptosis is part of a cascade of survival and proliferative events that form the cellular and molecular hallmarks of malignancy.6, 7 What remains largely unknown is the existence of systemic hallmarks of cancer, that is, extracellular features or events that are ubiquitous to cancers and actively involved in oncological processes. Although the significance of CTCs in cancer has been extensively studied,8 the prevalence of CTC assemblages has been largely underestimated2 and not been studied from the perspective of being definitive attributes of malignant neoplasia. Here, we present evidence which indicates that C‐ETACs qualify as a systemic hallmark of cancer.
Mechanistically, an important feature of the disorganized process of uncontrolled proliferation of cells in solid organ tumors is the outflow of loosely attached epithelial cells and their emboli into the vasculature.1, 9, 10 Normal parenchymal cells which have torn away from their cellular scaffolds due to either injury or infections but are not part of the malignant or premalignant population succumb to anoikis.11, 12 However, cells that have acquired apoptosis‐resistant phenotypes adapt to the hematolymphoid habitat and survive for extended periods of time or remain senescent in safe niches.13, 14 Recent studies have shown that the lineage and ensemble of such cells is quite diverse including tumor‐associated macrophages (TAM), tumor‐associated lymphocytes, CSCs and TAM‐cancer cell hybrids,15, 16, 17, 18 and that they perhaps obtain immune privilege using multiple camouflages and even get layered protection from treatment agents19 as well as any other extrinsic antitumor factors. Indeed, the active recruitment and reprogramming of normal cells, including immune cells, by tumor cells has been previously described20 as one of the means by which tumor cells subvert immune machinery to achieve tumor survival and proliferation. This agrees with our own observations of CD8a positive cells in the C‐ETACs. Owing to the selective cytotoxicity of our process, all immune cells (being nontumorigenic) are eliminated. Accordingly, neither single immune cells nor homotypic clusters of immune cells were detectable and the only detectable immune cells (CD8a+) were found in C‐ETACs. We speculate that the CD8a+ cells may have undergone some reprogramming after recruitment by the tumor‐associated cells.
C‐ETACs are a further potent danger because it has been shown that they have a very high metastatic potential1, 9, 10, 21 besides posing the imminent threat of thromboembolic complications.22, 23 Though C‐ETACs have received due attention in recent years, their composite detection, harvest and culture has remained difficult and sporadic, with only few anecdotal successes.3 The limited successes of prior efforts may be attributable to the processes relying on devices and methods originally designed for detection/capture of single CTC. Microfluidic devices4, 5 used for single cell capture may be associated with shear forces which could lead to destruction of cells or disruption of cell clusters and result in lower detection rates.4 Label (e.g., EpCAM) based detection/isolation methods have been used extensively; the FDA‐approved CellSearch24 is a more contemporary example where CTCs are defined as EpCAM+, CK+ and CD45−. However, EpCAM‐based approaches are not suitable for identification of CTCs that have undergone epithelial to mesenchymal transition.25 EpCAM‐based approaches also have limited efficacy in isolation of heterotypic C‐ETACs for the same reason: EpCAM+ cells in viable C‐ETACs can be obscured from detection due to sequestration with a plethora of cells such as post‐EMT CTCs, tumor‐associated T‐lymphocytes (TAL), TAM and CSCs.15, 16, 17, 18 The C‐ETAC isolation process used in our approach is neither microfluidic nor epitope‐based and is hence unaffected by the limitations of the respective approaches. In vitro processing of viable cells may introduce artifacts due to inherent complexities in tumor biology as well as interactions with media or reagents. Such artifacts may include passive cell aggregation due to metabolic intermediates,26 as well as active chemotaxis and cellular‐adherence in viable cells induced by media or reagents. Supporting Information Video S1 is a time lapse video (Day 0–Day 5) of a representative sample showing persistence of existing clusters (stabilization), elimination of most single cells and absence of new cluster formation. The in vitro C‐ETAC isolation process was also used with cell lines (SiHa Cervical Cancer, SKBR3 Breast Cancer) and TDCs from freshly biopsied tumor (Liver, Ovarian) tissue and PBMC samples from healthy individuals and no cell assemblages were observed in any of these samples (Supporting Information Fig. S1) indicating the fidelity of the process.
We evaluated the prevalence of C‐ETACs across a range of cancer types in 5,509 samples. Prior investigations2, 27, 28, 29, 30, 31, 32 reported significant variations in detection of cell clusters ranging between 14.5% (n = 55) to 100% (n = 7) in lung cancers, 17.4% (n = 115) to 61.9% (n = 21) in breast cancers, 50% (n = 8) to 68.8% (n = 32), 4.5% (n = 44) in hepatocellular carcinoma, 33.3% (n = 42) in renal cell carcinoma, 2.8% (n = 36) to 80% (n = 10) in prostate cancers and 22% (n = 18) to 96.2% (n = 53) in pancreatic cancers. In contrast, we report a pan‐cancer (epithelial malignancies) C‐ETAC prevalence of 89.7%.
It may be intuitive to expect a higher incidence of CTCs and tumor emboli in metastatic cancers. However, C‐ETACs were detected at comparable frequencies in metastatic as well as nonmetastatic patients in our study. Though nonmetastatic solid organ cancers may be viable for surgical resection with curative intent, presence of C‐ETACs in patients with nonmetastatic disease emphasizes the need for proactive disease surveillance postsurgery. Some reports have suggested that tumor cell clusters may increase after surgical resection.33, 34 In the subcohort of patients with paired presurgery and postsurgery samples, a decreasing trend of C‐ETACs was observed postsurgery. These observations also reaffirm the tumorigenic origin of C‐ETACs.
No significant differences were observed between detection rates of C‐ETACs in therapy naïve and pretreated individuals. In a subset of the study cohort where patients had received prior treatment and recent radiological scan indicated NED, C‐ETACs were detected in 91.9% of these patients. The findings suggest that C‐ETACs tend to remain in circulation for extended periods even though the disease is radiologically undetectable posttherapy, and may also be indicative of potential predisposition toward recurrence or metastasis, subject to availability of supportive niches. Although it is well accepted that absence of radiological evidence does not infer absence of malignancy, NED is often a significant yardstick for critical treatment‐related decisions including drug and dose modifications or a shift to metronomic regimens. Akin to the concept of minimum residual disease35 (MRD) in hematological malignancies, viable remnant CTCs in radiologically undetectable cancers are linked to risks of recurrence due to drug resistant clonal subtypes as well as resurgent populations in light of therapy inadequacy. Hence, we propose the term circulating metastatic disease (CMD) in solid organ malignancies, which can be accurately determined by evaluation of C‐ETACs to better guide disease management especially treatment related decisions.
Treatment decisions in standard of care (SoC) are based on organ of origin and often use information on antigen markers determined by immunohistochemistry (IHC) analysis on tumor tissue after biopsy. Prior efforts36, 37 at determination of organ‐specificity and replication of IHC markers using CTCs favor the development of these noninvasive assays. Accordingly, we evaluated C‐ETACs from various cancer types and observed that they reported organ‐specificity with high fidelity, with little or no interference from other organ‐specific markers. Based on these findings, we have initiated a larger study on utility of C‐ETACs for diagnosis and treatment decisions in cancers. The study data will be published separately.
C‐ETACs were detected in 3% of the 8,493 healthy individuals as well as 6.4% of the 2,132 asymptomatic individuals with aberrant findings on screening investigations. The C‐ETAC detection rates among the screened negative (healthy) as well as at risk populations have to be viewed primarily in the context of age‐associated higher risk of cancer. Elevated levels of CA19‐9 and PSA appeared to be most highly associated with increased incidences of C‐ETAC detection among males whereas the findings of LDCT (higher lung‐RADS) inversely correlated with C‐ETACs presence. Similarly, elevated levels of CEA as well as suspicious findings in PAP smear appeared to be most highly associated with increased incidences of C‐ETAC detection among females while lung‐RADS appeared to have lowest association. We did not investigate association of C‐ETACs with quantitative differences in any of the screening investigations since it was beyond the scope of the present study. Individuals with risks of carcinogen exposure due to tobacco addiction as well as occupational hazards appeared to be at a higher risk of C‐ETAC positivity as compared to the asymptomatic population. Surprisingly, individuals with a known family history (first‐/second‐degree blood relatives) of cancer did not appear to be at a higher risk of C‐ETAC positivity. The extremely high incidence of C‐ETACs in the cancer cohort indicates that C‐ETACs represent the biological prevalence of malignancy, irrespective of clinical or radiological status. However, the probability of a future clinical presentation of cancer in these 392 asymptomatic individuals (with C‐ETAC positivity) cannot be presently predicted, nor can the “clinical false‐positive” fraction, that is, those individuals among the 392 in whom cancer will not manifest clinically in their lifetimes. Hypothesizing that the cancer will not clinically manifest in any of the 392 individuals among the total 10,625 yields a hypothetical‐maximum false‐positive rate of 3.7% which is yet significantly and unambiguously lower than the false positives observed for LDCT (12.9–25.9%),38 mammography (7–12% at first mammogram39 and 50–60% after 10 yearly mammograms40) and CA markers (e.g., 66% for PSA,41 29% for CA‐125,42 10–60% for CA19‐943) which are routinely used in early detection screening. Radiological scans such as LDCT and mammography not only have high false positive rates, but are also nonconfirmatory, that is, necessitate an invasive biopsy for histopathological confirmation of suspected malignancy, as well as being associated with radiation exposure risks.44, 45 Though PAP smears offer direct evidence of malignancy, false‐negative findings due to suboptimal samples are not uncommon.46 Coupled with the high specificity for cancers as well as the noninvasiveness of the procedure, C‐ETACs appear to be a superior analyte for detection of malignancy in asymptomatic individuals. C‐ETAC based cancer screening of populations is also expected to significantly reduce instances of confirmatory biopsies as well as radiological scans, both of which may be unnecessarily necessitated in suspected cases due to false positives.
The scope of the present study extended to establish the ubiquity of C‐ETACs in epithelial malignancies and rarity in asymptomatic populations, which has been demonstrated. Enumeration of C‐ETACs is presently a cumbersome and laborious manual process and hence has been attempted only in a single subcohort. Further development and refinements of methods will enable quantitative correlation of C‐ETACs with treatment status, radiological findings and extent of disease. These findings will be published at fruition. Though the present study was based on a South Asian population, we do not anticipate variations based on ethnicity or geographical location. We conclude that C‐ETACs are not merely incidental findings in malignancy but rather its systemic manifestation, the monitoring of which would better inform cancer management. Ubiquitous C‐ETACs qualify as a systemic hallmark of cancer and their presence in an individual's blood is the colloquial “smoking gun”—the absolute and direct evidence of viable neoplastic disease.
Conflict of interest
D.A., D.P., V.D., C.S., R.P., P.F., P.F., N.S., P.D., S.A., S.P., S.P., A.A., S.P., A.A., R.C., M.A. and A.S. are in full time employment of the Study Sponsor. R.D. is the Founder and CMD of the Study Sponsor. T.C., S.L., R.P. and A.R. have no competing interests.
Supporting information
Appendix S1. Supporting Information.
Video S1 Time lapse (Day 0–Day 5) of a representative sample showing persistence of existing clusters (stabilization), elimination of single cells and absence of new cluster formation.
Acknowledgements
The authors acknowledge all the patients and asymptomatic individuals who consented to participate in the study and provide blood samples. Samples from asymptomatic individuals were obtained from Medall Healthcare Pvt. Ltd. (multiple pan‐India locations) while cancer patients’ samples were obtained from HCG Manavata Cancer Centre (Nasik, India), NueClear Healthcare (Mumbai, India), Chandak Cancer Hospital (Jalgaon, India) and HCG Cancer Centre (Bengaluru, India). Contributions of Ms Swati Deshpande, Mr Pankaj Porje, Mr Milind Agnihotri, Dr Jitendra Karlekar, Dr Shalom Syed, Ms Rukhsar Patel, Ms Ashwini Pawar, Mr Ashish Rojekar, Ms Rimple Shah, Ms Disha Mathew, Ms Shweta Shinde and Ms Kanchan Tidke in managing various aspects of the study are acknowledged. The entire study was self‐funded by Datar Cancer Genetics Limited and no external funding was received for our study.
References
- 1. Giuliano M, Shaikh A, Lo HC, et al. Perspective on circulating tumor cell clusters: why it takes a village to metastasize. Cancer Res 2018;78:845–52. [DOI] [PubMed] [Google Scholar]
- 2. Hong Y, Fang F, Zhang Q. Circulating tumor cell clusters: what we know and what we expect (review). Int J Oncol 2016;49:2206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Au SH, Storey BD, Moore JC, et al. Clusters of circulating tumor cells traverse capillary‐sized vessels. Proc Natl Acad Sci USA 2016;113:4947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Au SH, Edd J, Stoddard AE, et al. Microfluidic isolation of circulating tumor cell clusters by size and asymmetry. Sci Rep 2017;7:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarioglu AF, Aceto N, Kojic N, et al. A microfluidic device for label‐free, physical capture of circulating tumor cell clusters. Nat Methods 2015;12:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 8. Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science 2013;341:1186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol 2017;11:40–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aceto N, Toner M, Maheswaran S, et al. En route to metastasis: circulating tumor cell clusters and epithelial‐to‐mesenchymal transition. Trends Cancer 2015;1:44–52. [DOI] [PubMed] [Google Scholar]
- 11. Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 2001;13:555–62. [DOI] [PubMed] [Google Scholar]
- 12. Taddei ML, Giannoni E, Fiaschi T, et al. Anoikis: an emerging hallmark in health and diseases. J Pathol 2012;226:380–93. [DOI] [PubMed] [Google Scholar]
- 13. Cao Z, Livas T, Kyprianou N. Anoikis and EMT: lethal "liaisons" during cancer progression. Crit Rev Oncog 2016;21:155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 2013;1833:3481–98. [DOI] [PubMed] [Google Scholar]
- 15. Song W, Mazzieri R, Yang T, et al. Translational significance for tumor metastasis of tumor‐associated macrophages and epithelial‐mesenchymal transition. Front Immunol 2017;8:1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamilton G, Rath B. Circulating tumor cell interactions with macrophages: implications for biology and treatment. Transl Lung Cancer Res 2017;6:418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding J, Jin W, Chen C, et al. Tumor associated macrophage × cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS One 2012;7:e41942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agnoletto C, Corrà F, Minotti L, et al. Heterogeneity in circulating tumor cells: the relevance of the stem‐cell subset. Cancers (Basel) 2019;11:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Q, Liao Q, Zhao Y. Myeloid‐derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med Hypotheses 2016;87:34–9. [DOI] [PubMed] [Google Scholar]
- 20. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–22. [DOI] [PubMed] [Google Scholar]
- 21. Divella R, Daniele A, Abbate I, et al. The presence of clustered circulating tumor cells (CTCs) and circulating cytokines define an aggressive phenotype in metastatic colorectal cancer. Cancer Causes Control 2014;25:1531–41. [DOI] [PubMed] [Google Scholar]
- 22. Ünlü B, Versteeg HH. Cancer‐associated thrombosis: the search for the holy grail continues. Res Pract Thromb Haemost 2018;2:622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tormoen GW, Haley KM, Levine RL, et al. Do circulating tumor cells play a role in coagulation and thrombosis? Front Oncol 2012;2:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cellsearch . Available from https://www.cellsearchctc.com/. Accessed July 18, 2019.
- 25. Gorges TM, Tinhofer I, Drosch M, et al. Circulating tumour cells escape from EpCAM‐based detection due to epithelial‐to‐mesenchymal transition. BMC Cancer 2012;12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sigma‐Aldrich . Available from https://www.sigmaaldrich.com/technical-documents/articles/biology/cell-culture-troubleshooting-cell-clumping.html. Accessed July 18, 2019.
- 27. Molnar B, Ladanyi A, Tanko L, et al. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res 2001;7:4080–5. [PubMed] [Google Scholar]
- 28. Mu Z, Wang C, Ye Z, et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced‐stage breast cancer. Breast Cancer Res Treat 2015;154:563–71. [DOI] [PubMed] [Google Scholar]
- 29. Wendel M, Bazhenova L, Boshuizen R, et al. Fluid biopsy for circulating tumor cell identification in patients with early‐and late‐stage non‐small cell lung cancer: a glimpse into lung cancer biology. Phys Biol 2012;9:016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kats‐Ugurlu G, Roodink I, de Weijert M, et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol 2009;219:287–93. [DOI] [PubMed] [Google Scholar]
- 31. Loh J, Jovanovic L, Lehman M, et al. Circulating tumor cell detection in high‐risk non‐metastatic prostate cancer. J Cancer Res Clin Oncol 2014;140:2157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang MC, Chang YT, Chen JY, et al. Clinical significance of circulating tumor microemboli as a prognostic marker in patients with pancreatic ductal adenocarcinoma. Clin Chem 2016;62:505–13. [DOI] [PubMed] [Google Scholar]
- 33. Martin OA, Anderson RL, Narayan K, et al. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol 2017;14:32–44. [DOI] [PubMed] [Google Scholar]
- 34. Katharina P. Tumor cell seeding during surgery‐possible contribution to metastasis formations. Cancers (Basel) 2011;3:2540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luskin MR, Murakami MA, Manalis SR, et al. Targeting minimal residual disease: a path to cure? Nat Rev Cancer 2018;18:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang YT, Kim YJ, Lee TH, et al. Cytopathological study of the circulating tumor cells filtered from the cancer patients' blood using hydrogel‐based cell block formation. Sci Rep 2018;8:15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cummings J, Sloane R, Morris K, et al. Optimisation of an immunohistochemistry method for the determination of androgen receptor expression levels in circulating tumour cells. BMC Cancer 2014;14:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pinsky PF, Bellinger CR, Miller DP Jr. False‐positive screens and lung cancer risk in the National Lung Screening Trial: implications for shared decision‐making. J Med Screen 2018;25:110–2. [DOI] [PubMed] [Google Scholar]
- 39. Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta‐analysis to update the 2009 U.S. preventive services task force recommendation. Ann Intern Med 2016;164:244–55. [DOI] [PubMed] [Google Scholar]
- 40. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false‐positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med 2011;155:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kilpeläinen TP, Tammela TL, Roobol M, et al. False‐positive screening results in the European randomized study of screening for prostate cancer. Eur J Cancer 2011;47:2698–705. [DOI] [PubMed] [Google Scholar]
- 42. Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. J Clin Pathol 2005;58:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19‐9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence‐based appraisal. J Gastrointest Oncol 2012;3:105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fabrikant MS, Wisnivesky JP, Marron T, et al. Benefits and challenges of lung cancer screening in older adults. Clin Ther 2018;40:526–34. [DOI] [PubMed] [Google Scholar]
- 45. Heywang‐Köbrunner SH, Hacker A, Sedlacek S. Advantages and disadvantages of mammography screening. Breast Care (Basel) 2011;6:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lieu D. The Papanicolaou smear: its value and limitations. J Fam Pract 1996;42:391–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Video S1 Time lapse (Day 0–Day 5) of a representative sample showing persistence of existing clusters (stabilization), elimination of single cells and absence of new cluster formation.
Data Availability Statement
Data may be made available from the authors upon reasonable request.


