Abstract
Infective endocarditis (IE) caused by Pseudomonas aeruginosa is extremely uncommon. Reported cases have usually been associated with intravenous drug use, prosthetic heart valves, and/or implanted cardiac devices. Traditionally, successful treatment has necessitated a combination of antimicrobial(s) and valve replacement. Yet, P. aeruginosa IE remains difficult to manage, especially in cases where valve replacement may not be an immediate option. We present such a case of P. aeruginosa IE, highlighting that medical management with 2 antipseudomonal synergistic agents may be an alternative to surgery in particularly complicated cases.
Keywords: Pseudomonas, Endocarditis, Aortic, Bacteremia, Nosocomial, Native
Introduction
Infective endocarditis (IE) caused by Pseudomonas aeruginosa is rarely seen in clinical practice [1]. Noted to have a high mortality rate, early diagnosis and intervention with both antibiotics and surgery are crucial in treating P. aeruginosa IE [[1], [2], [3]]. Although there is a rise in infection in patients with prosthetic heart valves or with implanted cardiac devices such as pacemakers or defibrillators, many of the reported cases in literature of P. aeruginosa IE involve right sided disease in intravenous drug users (IVDU) [[2], [3], [4], [5], [6], [7], [8], [9]]. Here, we present the rare case of native-valve left-sided P. aeruginosa endocarditis in a patient with no history of IVDU.
Case presentation
A 72-year-old male with a past medical history of Parkinson’s disease and benign prostatic hypertrophy (BPH) requiring intermittent urethral self-catheterization presented to our facility as a transfer from an outside hospital (OSH) for cardiothoracic surgery (CTS) evaluation given persistent P. aeruginosa bacteremia and findings on transesophageal echocardiogram (TEE) of a large vegetation involving the aortic valve, consistent with a diagnosis of IE.
The patient had originally presented to the OSH approximately two months prior complaining of fever and right sided flank pain. Urinalysis was grossly positive on admission. A subsequent computerized tomography (CT) of the abdomen/pelvis revealed a 1 cm right sided calculus at the ureter-pelvic junction for which a nephrostomy tube was placed followed by percutaneous nephrolithotomy (PCNL). The patient initially received 2 days of piperacillin/tazobactam, however, once the urine culture resulted in P. aeruginosa with no significant associated resistance, the patient was discharged home on a 5-day course of ciprofloxacin 750 mg twice daily (BID) per os (P.O.). Both sets of blood cultures obtained on arrival to the emergency department at the OSH were negative for growth. Although the patient did have an indwelling urinary bladder catheter placed during hospitalization, it was removed prior to discharge and he was told to resume self-catheterization at home.
Two months later, the patient presented back to the OSH with fever. CT abdomen/pelvis revealed passage of the right sided calculus, however, this time, urine and blood cultures were positive for P. aeruginosa with no significant associated resistance. He was started on cefepime and a transthoracic echocardiogram (TTE) obtained on admission was negative for any valvular abnormalities, including vegetation. However, despite treatment with cefepime, the patient’s blood cultures continued to be positive for P. aeruginosa that had no significant associated resistance. One week into hospitalization, a CT chest with contrast was obtained and revealed a paratracheal mass, possibly suggestive of lymphadenopathy. On day 10 at the OSH, a TEE revealed a large vegetation involving the right coronary cusp of the aortic valve with severe aortic insufficiency; it was noted that an abscess was unable to be ruled out. Based on the persistent bacteremia and TEE findings, a diagnosis of infective endocarditis was reached. Tobramycin was added to the cefepime on day 10 to provide a synergistic antipseudomonal effect and the patient was then transferred to our facility for CTS evaluation.
A CT head and magnetic resonance imaging (MRI) of the brain done upon arrival per CTS request revealed multiple punctate hemorrhages in the parietal lobe, the largest of which was a 1.8 cm focus in the left parietal lobe. The patient had no apparent neurological deficits; however, was transferred to the Neuro-Intensive care unit for closer monitoring for approximately 24 h. Additionally, due to the risk of hemorrhage from the heparin bolus needed during valve replacement, CTS felt it was appropriate to defer valve replacement surgery and upon their own review of the TEE, did not see an abscess. Of note, the patient was also experiencing depressive symptoms at this time and did not want surgery or any invasive measures to be taken (Fig. 1, Fig. 2).
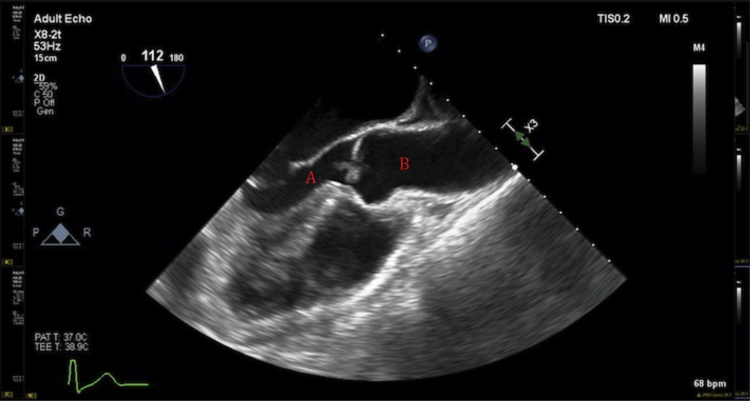
Fig. 1.
Long-axis view on transesophageal echocardiogram depicting the left ventricle (A) and aortic valve (B) with vegetation.
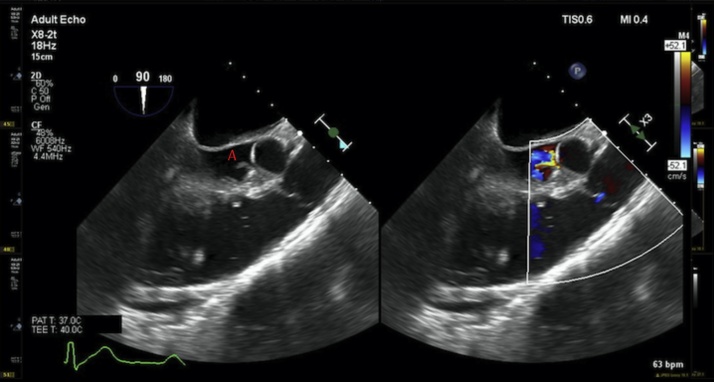
Fig. 2.
Left: Long axis view on transesophageal echocardiogram showing aortic valve with vegetation labeled A. Right: Flow velocimetry through aortic valve showing a severely regurgitant valve.
Blood cultures remained positive with P. aeruginosa with no significant associated resistance despite continual treatment with cefepime and tobramycin. Due to worsening renal function, tobramycin was discontinued on day 8 at our facility and the patient was started on ciprofloxacin 750 mg BID P.O. along with continued cefepime. On day 10, a susceptibility profile for cultures obtained on day 7 resulted in P. aeruginosa with no significant associated resistance again, however with a minimal inhibitory concentration (MIC) of 8 for cefepime compared to an MIC of 2 on the blood cultures obtained on arrival. The cefepime was then changed from 2 g every 8 h administered over 3−5 min to an extended infusion administered over 3 h. Additionally, a TEE done at our facility on day 10 revealed the presence of an enlarged vegetation, 1.26 × 0.51 cm compared to the prior TEE done at the OSH 10 days prior which measured 1.11 × 0.30 cm.
On day 15 at our hospital, two sets of blood cultures drawn on day 10 were found to be negative for growth for 5 days. A PICC line was placed and the patient was discharged to a rehabilitation facility with the same antibiotic regimen for 6 weeks. He was seen in the Infectious Disease outpatient clinic approximately 2 weeks after discharge and stated that he felt well. The plan was to have him follow up 4 weeks later and re-draw blood cultures and discontinue antibiotics upon the first negative culture results and to repeat blood cultures again 2 weeks after that. This plan was discussed in detail with the patient. However, due to the onset of the COVID-19 pandemic, the follow up appointment has been deferred indefinitely. Additionally, the patient decided to follow up with CTS upon discharge regarding possible valve replacement. He was advised to have pre-operative screening measures done first. This has also been deferred indefinitely due to COVID-19.
Discussion
We present a rare case of left-sided P. aeruginosa endocarditis in a patient with no history of IVDU, cardiac device implantation, or the presence of a prosthetic valve. Although in the past P. aeruginosa IE has been associated with intravenous drug users, recently, a shift towards nosocomial, or healthcare associated P. aeruginosa, is being observed [[2], [3], [4], [5], [6], [7], [8], [9]]. A large cohort study done by Morpeth et al. found that of non-HACEK gram negative IE (species other than Haemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species), only 4% of the cases were associated to active IVDU [6]. Additionally, a recent Italian cohort study found that the genitourinary tract was the most frequent source of infection in IE due to gram negative bacteremia (GNB) [7]. In the case of this patient, who was found to have right sided nephrolithiasis requiring PCNL as well as a P. aeruginosa cystitis upon initial presentation to the OSH months prior, it may be argued that the PCNL, the indwelling urinary bladder catheter placed during that admission, and/or the intermittent urethral self-catheterization the patient resumed at discharge, may have introduced bacteria into the bloodstream, possibly in the case of the latter two by damaging the urethral mucosa. No blood cultures, other than the two obtained in the emergency room on arrival that admission, were obtained until the patient presented back to the OSH 2 months later and was found to be bacteremic.
Given the high overall mortality of P. aeruginosa IE, found to be approximately 64 % in left sided disease in non-IVDU according to one literature review, early diagnosis and treatment are essential for optimal outcome [2,8]. As with all cases of endocarditis, blood cultures repeatedly positive for an organism should warrant an early TEE for evidence of valvular vegetations despite an initial negative TTE [2]. Our patient was persistently bacteremic while on adequate antibiotic treatment and had a history of a recent urological procedure and complicated cystitis with the same organism. Therefore, despite a negative TTE, a TEE should not have been deferred until day 12 at the OSH. An earlier TEE may have allowed for an expeditious diagnosis and intervention from CTS prior to the intracranial hemorrhage that delayed the patients care.
Traditionally, successful treatment of left-sided IE has necessitated a combination of antibiotics and source control in the form of valve replacement. Antibiotic therapy for P. aeruginosa IE consists of six weeks of two intravenous antipseudomonal agents, each from a different antimicrobial class to which that strain is susceptible to [2]. This combination draws on the concept of antimicrobial synergy, which allows for two different mechanisms of bacterial killing. In gram-negative infections, synergy has been traditionally seen with beta-lactam and aminoglycoside combinations; the beta-lactam mediated disturbance of the cell walls of gram negative bacteria allows aminoglycosides to pass into the periplasmic space [10]. Recently however, similar data has been emerging with beta-lactam and fluoroquinolone combinations [10].
Although initially our patient was continued on the cefepime and tobramycin that was started at the OSH, the tobramycin was discontinued and ciprofloxacin was started in its place on day 8 due to worsening renal function. Additionally, due to a higher MIC for cefepime that resulted on day 10 for cultures that were obtained on day 7, (MIC of 8 compared to that of 2 from blood cultures obtained on arrival) the cefepime was switched to an extended infusion rate, 2 g every 8 h administered over 3 h, instead of over 3−5 min. This change is based on the pharmacodynamics principle that beta lactam antibiotics have a time dependent effect on bacterial eradication [11,12]. Therefore, increasing the duration of exposure of the antibiotic to the bacteria by extending the infusion rate may be more effective at bacteria eradication especially at higher MICs [11,12].
This case highlights that medical management with 2 antipseudomonal synergistic agents may be an effective alternative to surgery in particularly complicated situations. In the case of our patient surgery was delayed at the time due to a guarded clinical and functional status as well as the presence of hemorrhagic foci in the parietal lobes as evident on imaging. More recently, the COVID-19 pandemic has diminished the patient’s ability to adhere to follow up measures or consult with CTS. In the meantime, the patient has been advised to continue with the regimen of ciprofloxacin 750 mg BID P.O. and extended infusion cefepime. We have good reason to remain hopeful that updated blood cultures will be negative when the patient is in fact able to follow up.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Swetha Ramireddy: Writing - original draft, Writing - review & editing. Smitha Gudipati: Writing - review & editing. Marcus Zervos: Supervision.
Acknowledgements
We would like to thank and acknowledge all the healthcare workers that helped take care of this patient.
Contributor Information
Swetha Ramireddy, Email: Sramire5@hfhs.org.
Smitha Gudipati, Email: Sgudipa2@hfhs.org.
Marcus Zervos, Email: Mzervos1@hfhs.org.
References
- 1.Mahajan A., Amer M., Awan A., Tiruneh F., Gandotra C., Curry B. An invasive case of left-sided endocarditis caused by Pseudomonas aeruginosa in a patient with history of intravenous drug abuse. Cureus. 2017;9(9) doi: 10.7759/cureus.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson N.L., Brumble L.M., Pritt B.S., Yao J.D., Echols J.D., Alvarez S. Left-sided Pseudomonas aeruginosa endocarditis in patients without injection drug use. Medicine (Baltimore) 2011;90(4):250–255. doi: 10.1097/MD.0b013e3182252133. [DOI] [PubMed] [Google Scholar]
- 3.Lin T.I., Huang Y.F., Liu P.Y. Pseudomonas aeruginosa infective endocarditis in patients who do not use intravenous drugs: analysis of risk factors and treatment outcomes. J Microbiol Immunol Infect. 2016;49(4):516–522. doi: 10.1016/j.jmii.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Reyes M.P., Palutke W.A., Wylin R.F. Pseudomonas endocarditis in the Detroit medical center. Medicine (Baltimore) 1973;52(3):173–194. doi: 10.1097/00005792-197305000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Cohen P.S., Maguire J.H., Weinstein L. Infective endocarditis caused by gram-negative bacteria: a review of the literature, 1945–1977. Prog Cardiovasc Dis. 1980;22(4):205–242. doi: 10.1016/0033-0620(80)90010-9. [DOI] [PubMed] [Google Scholar]
- 6.Morpeth S., Murdoch D., Cabell C.H. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147(12):829–835. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 7.Falcone M., Tiseo G., Durante-Mangoni E. Risk factors and outcomes of endocarditis due to non-HACEK gram-negative bacilli: data from the prospective multicenter Italian endocarditis study cohort. Antimicrob Agents Chemother. 2018;62(4) doi: 10.1128/AAC.02208-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagiya H., Tanaka T., Takimoto K. Non-nosocomial healthcare-associated left-sided Pseudomonas aeruginosa endocarditis: a case report and literature review. BMC Infect Dis. 2016;16(1):431. doi: 10.1186/s12879-016-1757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gürtler N., Osthoff M., Rueter F. Prosthetic valve endocarditis caused by Pseudomonas aeruginosa with variable antibacterial resistance profiles: a diagnostic challenge. BMC Infect Dis. 2019;19(1):530. doi: 10.1186/s12879-019-4164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamma P.D., Cosgrove S.E., Maragakis L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25(3):450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer K.A., West J.E., O’Brien J.M., Goff D.A. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2013;57(7):2907–2912. doi: 10.1128/AAC.02365-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacVane S.H., Kuti J.L., Nicolau D.P. Prolonging β-lactam infusion: a review of the rationale and evidence, and guidance for implementation. Int J Antimicrob Agents. 2014;43(2):105–113. doi: 10.1016/j.ijantimicag.2013.10.021. [DOI] [PubMed] [Google Scholar]