Abstract
The novel coronavirus pneumonia (COVID-19) is a contagious acute respiratory infectious disease whose causative agent has been demonstrated to be a novel virus of the coronavirus family, SARSCoV-2. A recent PRE-print study has showed a heme attack on the 1-beta chain of hemoglobin by COVID19. Beta-thalassemia results of a default in the hemoglobin beta-chain synthesis. 1,5% global population are heterozygotes for this disease. In this study, by a multiple linear regression, we have analyzed the evolution of COVID-19 infection in three Italian regions (Puglia, Sardinia, Sicilia) with different beta-thalassemic prevalences, in order to search a link. The results have showed that betathalassemic heterozygote population prevalence is correlated to immunity against COVID-19, by a regression. This paper is only for academic discussion, the hypotheses and conclusions needs to be confirmed by further research.
Keywords: Novel coronavirus, Respiratory distress, Favipiravir, Statistics, Correlation, Beta thalassemia, Immunization, Italy, Sardinia, Regression, Heme
Background
The novel coronavirus pneumonia (COVID-19) is a contagious acute respiratory infectious disease whose causative agent has been demonstrated to be a novel virus of the coronavirus family, SARSCoV-2. Patients with the coronavirus pneumonia have a fever, and the temperature above 38 degrees with symptoms such as dry cough, fatigue, dyspnea, difficulty breathing, and diarrhea [5], [6], [12]. Thrombotic complications are also commonly reported such as acute pulmonary embolism [14], [23]. This pneumonia was first discovered in December 2019 in the South China Seafood Market Hubei Province, China [11]. There is a high contagiosity for this disease [13], [18]. This pneumonia has now turned into a pandemic: Tens of thousands of people are infected worldwide.
Hypothesis.
A recent pre-print study [8] shows that ORF8 and surface glycoproteins of the novel coronavirus could combine to the porphyrin to form a complex. Meanwhile, orf1ab, ORF10, and ORF3a proteins could coordinate attack the heme (porphyrin), formed into the mitochondria, on the 1-beta chain of hemoglobin to dissociate the iron ions from the heme. Furthermore, it revealed that Favipiravir could inhibit the envelope protein and ORF7a protein bind to porphyrin, thus preventing the virus from entering host cells, and catching free porphyrins. Explain here also quickly potential mechanisms of chloroquine if possible.
In addition, B-thalassemia has an interesting physiopathology in this context: genetic mutations induce decreased or even absent beta chains synthesis whereas alpha chains synthesis remains normal. This results in the excess of free alpha chains and less hemoglobin A. Either free alpha chains associate themselves to form unstable 4-alpha tetramers, which subsequently oxidize and precipitate themselves in erythroblast cytoplasm, (inducing oxygenated radicals liberation and then surface glycoproteins oxidation, and then dyserythropoiesis and hemolysis) or they are proteolyzed [1], [3], [16], [19].
The incidence and distribution of B-thalassemia syndromes in Italy and in Sardinia have been previously and broadly documented [2], [4], [9], [10], [15], [17], [20], [21]. Prevalence of B-thalassemia heterozygotes in Sardinia (12,9%) is higher than in Sicilia, which is itself higher than in Puglia.
In this context, we hypothesized that beta-thalassemic patients, most concentrated in Sardinia, could develop an immunity to SARS-CoV-2 infectious consequences on hemoglobin, as the beta chain, potential target of the virus, could be either absent or less prominent in the blood.
Evaluation of the hypothesis
Study and participants
We conducted a multi-group descriptive observational transversal study in order to define a hypothetical relationship between beta thalassemia and SARS-CoV-2 immunization. The source and targeted populations are the whole humanity in view of the ongoing COVID-19 pandemic. The eligible population is constituted by all Italians.
The study was directed by a consortium of two data analysts, a MD-PhD specialized in radiology and brain research, and a medical student in clinical years. NexGen Analytics had no role in designing the study, or making the decision to submit manuscript to the publication, nor did receive any fee or compensation in the context of this work. The first author vouches for the data and analyses, as well as for the fidelity of this report to the study protocol.
Enrollment
Population included and studied gather people of three southern Italian regions: Puglia, Sardinia and Sicilia. There were no exclusion criteria. This choice was made according to differences upon beta-thalassemia heterozygote prevalence between all Italian regions and upon the geographical localization (which allowed us to eliminate environmental variables interference).
We gathered COVID-19-related data from various public health and social sources [7]. A parallel multiple group analysis was performed, and demographic data for each group was collected with reference date 01/01/2020 [22].
Outcome measures
Three primary variables were taken into account: accrued confirmed cases of COVID-19; accrued deaths causatively associated to COVID-19 infection; and accrued healed to death ratio.
The primary and second study outcomes were respectively the accumulated confirmed cases of COVID-19 and the accumulated deaths due to COVID-19. These were useful in order to assess the evolution of pandemic. The secondary study outcome was the accumulated deaths due to COVID-19 infection. To the immune status of the population, we used the daily ratio healed upon dead by day as the third outcome.
Statistical analysis
We found that the logistic function can be used to modelize various kind of epidemiological data such as the number of confirmed cases or the number of deaths. After performing regressions, we found that the calculated functions surprisingly fit many reported data, with NRMSE (Normalized Root Mean Square Error) usually below 0.1%. This excellent fit can be observed at different geographic scales (we studied country-level data as well as smaller geographical subdivisions such as provinces or cities). When the model is relevant to the reported data, we observe the following as we gather more data over time: 1/decreasing NRMSE, 2/the 3 degrees of freedom of the logistic curve should also converge. Regressions will provide the values of the 3 parameters/degrees of freedom of the logistic curve: its maximum (L), the abscissa of its midpoint (x0), and its growth rate (k). The abscissa of the curve's midpoint indicates when the peak of the epidemics will occur/occurred.
For each cluster, a linear regression was established to show contrasts. To assess these differences, a comparison of the linear regression coefficient (A) was done.
Empirical data
Populations
7,048,535 people were included in this study (population on 01/01/20) (Table 1 ) [2], [4], [9], [10], [15], [20], [21], [22].
Table 1.
Demographic data on 01/01/20.
| Puglia | Sardinia | Sicilia | ||
|---|---|---|---|---|
| Inhabitants (number) | 4,029,053 | 1,639,591 | 4,999,891 | |
| Middle age (years) | 44.7 | 47.2 | 44.3 | |
| Sex ratio (male/female) | 0.95 | 0.97 | 0.95 | |
| Male life esperancy (years) | 81.1 | 80.4 | 79.9 | |
| Female life esperancy (years) | 85.2 | 85.9 | 84 | |
| Birth rate | 7.1 | 5.7 | 7.8 | |
| Death rate | 10 | 10.3 | 10.8 | |
| BMI (on subjects over 18 years) | <18 | 2 | 3,1 | 3 |
| 18–25 | 46,9 | 54,7 | 47,7 | |
| 25–30 | 37,7 | 32,3 | 37,4 | |
| >30 | 13,5 | 9,9 | 11,9 | |
| Diabetes prevalence (%) | 7,1 | 4,7 | 6,5 | |
| Thalassemic heterozygotes prevalence (%) | 6,5 | 12,9 | 7,5 | |
According to MANOVA test with a risk of 5%, all the groups were well balanced except on the BMI, the diabetes and the thalassemia heterozygote prevalence.
Populations of those three regions have been studied during the period between 02/25/2020 and 04/10/2020.
Outcomes
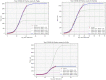
Concerning the primary outcome (accumulated confirmed positive counts), Sicilia is substantially less impacted than Puglia for a population almost similar (respectively 4,999,891 and 4,029,053). Two regions have nearly reached their stage phase, except Sardinia. The confirmed prevalence of COVID-19 on 04/10/2020 is: 0.070% for Puglia, 0.046% for Sicilia, 0.065% for Sardinia (Table 2 ).
Table 2.
Plots with logistic function applied upon accumulated positive confirmed counts for each region (Puglia, Sardinia, Sicilia).
 |
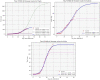
Concerning the secondary outcome (accumulated deceased counts), Sicily is significantly less impacted than Puglia for a population almost similar (respectively 4,999,891 and 4,029,053 inhabitants). Two regions have nearly reached their stage phase, except Sardinia. The death rate due to COVID-19 (number of deaths due to COVID-19 upon number of infected people by COVID-19) on 04/10/2020 is: 8.47% for Puglia, 6.43% for Sicilia, 6.49% for Sardinia (Table 3 ).
Table 3.
Plots with logistic function applied upon deceased counts for each region (Puglia, Sardinia, Sicilia).
 |
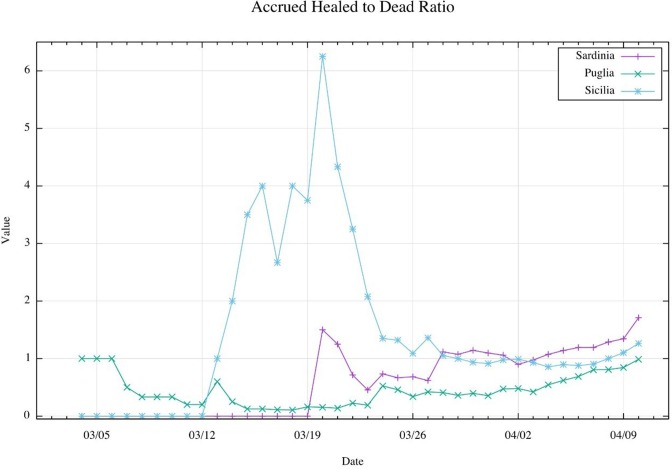
The third outcome is the accrued healed to dead ratio of COVID-19 infected patients (Table 4 ). This tracker has allowed us to define the immunization of population. Different variations could be observed: a Sardinian and Sicilian peak around 03/04/20 and 03/05/20. On 04/10/20, this ratio is 0.987 for Puglia, 1.735 for Sardinia, 1.263 for Sicilia.
Table 4.
| R2 | y= | |
|---|---|---|
| Puglia | −0,344 | 0,0187x |
| Sardinia | −1,266 | 0,0751x |
| Sicilia | −1,025 | 0,0714x |
The relationship of this last plot (Table 4) with the beta-thalassemic prevalence has been drawn through multiple regressions (Table 4, Table 5, Table 6 ). As a first step, we have ordered data with beginning on the first day where the healed to dead ratio is different from 0 (for Puglia on 03/04/20; for Sardinia on 03/20/20; for Sicilia on 03/12/20). Then, a linear regression was done. This one has allowed to show, by the linear coefficient (A), that ratio increased faster in Sardinia (a = 0,0751) than in Sicilia (A = 0,0714) and faster in Sicilia than in Puglia (A = 0,0187).
Table 5.
Matrix for the multiple linear regression.
Table 6.
Multiple least square adjustment.
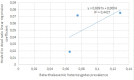 |
Finally, we had to calculate the regression between each regional prevalence and the regional ratio linear regression through a matrix (Table 5).
Discussion
Using a multiple linear regression, we have shown that thalassemia heterozygote population prevalence is significantly correlated to the hypothesized immunity against COVID-19 (Table 6).
This study is of course limited by different important factors. Firstly, the short studying period (1.5 month) didn’t allow to predict perfectly the next evolution of this correlation. That’s a crucial point in this pandemic context. The second limit of our study concerned the population. Indeed, Italy has an important prevalence of B-thalassemic heterozygotes (1/20) [15], but still, they are part of an aphelion disease (world heterozygote prevalence: 1.5%) [4]. The third limit is the immunity tracker; as we didn’t have data concerning serology tests on the Italian population, we have simulated this immunity tracker (accrued healed to dead ratio). Last but not least, there could be a major concern with the influence of well-known but hereby undocumented co-factors that are associated with bad prognosis in Intensive Care Unit population infected by COVID-19, such as BMI or diabetes (Table 1), and other unknown population co-factors, ranging from genetics to political management (PCR tests, face masks, containment of population, social distancing, treatment at early stages with antiviral drugs).
Nevertheless, our regression, linked with the hypothesized physiopathology [1], [3], [16], [19], suggests a first order effect at least. In this global context of medical research concerning the COVID-19 pandemic, our study and its results must be taken into account in order to open new therapeutical and diagnostic perspectives.
Our hypothesis could be confirmed by screening the prevalence of COVID-19 infected among beta-thalassemic patients. In addition, in vitro cell studies and animal models, as the thalassemic erythrocyte or the beta-thalassemic mice [16], could be of interest to test our statistical correlation. Further studies could be conducted to identify other correlations between COVID-19 and other blood pathologies such as drepanocytosis, G6PD deficiency or malaria.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109827.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Arbres décisionnels pour le diagnostic et la caractérisation moléculaire des hémoglobinopathies. ABC 2020;(68):455–64. [DOI] [PubMed]
- 2.Carcassi U. Atti Giornate Studio Microcitemia. 1963. Aspetti medico sociali della microcitemia in Sardegna; pp. 34–48. [Google Scholar]
- 3.Bonello-Palot N., Badens C. Bases moléculaires des syndromes thalassémiques et facteurs génétiques modulateurs de sévérité de la beta-thalassémie. RMGH. 2011 [Google Scholar]
- 4.Galanello R., Origa R. Beta-thlassemia. Orphan J Rare Dis. 2011;5:11. doi: 10.1186/1750-1172-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020:24. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csuthi E, Montuori M. Coronavirus, la situazione in Italia [Internet]. Gedi Visual. Available from: lab.gedidigital.itgedi-visual/2020/coronavrius-i-contagi-in-italia/?refresh_ce.
- 8.Wenzhong L., Hualan L. ChemRxiv; 2020. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. [Google Scholar]
- 9.Silvestroni E., Bianco I. Dati statistici sulla frequenza della microceitemia e del morbo di Cooley nella Sardegna meridonale. Nuovi Annali d’Igiene e Microbilogia. 1960;11:339–348. [Google Scholar]
- 10.Silvestroni E., Bianco I. Atti delle Giornate di Studio. Istituto Italiano di Medicina Sociale; Roma: 1962. Diffusione e frequenza della microcitemia e della anemi microcitemiche nell’Italia continentale e in Sicila. [Google Scholar]
- 11.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020:29. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang D., Minggui L., Lai W. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diao K., Han P., Pang T., Li Y., Yang Z. HCTR Imaging features in representative imported Cases of 2019 novel coronavirus pneumonia. Precis Clin Med. 2020 doi: 10.1093/pcmedi/pbaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La talassemia in cifre [Internet]. Associazione Piera Cutino Guarire dalla Talassemia. Available from: http://www.pieracutino.it/la-talassemia-in-cifre/.
- 16.Leroy-Viard K., Rouyer-Fessard P., Sauvage C., Scoot Mark, Beuzard Y. Modèles expérimentaux de la beta-thalassémie. Synthèse. 1992;8:784–789. [Google Scholar]
- 17.Siniscalco M., Bernini L., Filippi G., Latte B., Meera Khan P., Piomelli S. Population genetics of haemoglobin variants, thalassemia, and glucose-6-phosphate dehydrogenase deficiency with particular reference to the malaria hypothesis. Bull World Health Org. 1966;34:379–394. [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Zai J., Wang X., Li Y. Potential of large ‘first generation’human to human transmission of - 2019 nCoV. J Med Virol. 2020 doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syndromes thalassémiques majeurs et intermédiaires. Protocole national de diagnostic et de soins pour une maladie rare. HAS 2008.
- 20.Cao A., Galanello R., Furbetta M., Muroni P.P., Garbato L., Rosatelli C. Thalassemia types and their incidences in Sardinia. J Med Genet. 1979;15:443–447. doi: 10.1136/jmg.15.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceci A., Mangiarini L., Felisi M., Bartoloni F., Cianco A., Capra M. The management of iron chelation therapy: preliminary data from a national registry of thalassaemic patients. Anemia. 2011;11:2011. doi: 10.1155/2011/435683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Population and household data [Internet]. Instituto Nazionale di Statistica. Available from: istat.it.
- 23.Rotzinger D.C., Beigelman-Aubry C., von Garnier C., Qanadli S.D. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res. 2020 Apr;8:2020. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.