Abstract
Some patients undergoing routine SPECT/CT and PET/CT examinations during the COVID-19 pandemic may incidentally reveal findings of COVID-19–associated pneumonia (C-19AP) on localizing CT. It is critical for nuclear medicine physicians to develop diagnostic skills for timely recognition of typical findings of C-19AP on a localizing CT. Furthermore, it is our responsibility to know the optimal practices for safely isolating and managing such patients while protecting the staff, other patients at the facility, family and/or friend accompanying the patients, and the public in general from risky exposure to COVID-19 sources. We offer several steps following an encounter suspicious of C-19AP.
Key Words: coronavirus, coronavirus identified in 2019, COVID-19, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, pneumonia, viral, COVID-19–associated pneumonia, betacoronavirus, single photon emission computed tomography computed tomography, nuclear medicine, tomography, x-ray computed
A novel coronavirus 2 was identified and provisionally named SARS-CoV-2 by the World Health Organization (WHO), whereas the respiratory illness it causes was named COVID-19.1 When the disease progresses to cause inflammation in the lungs, it is called COVID-19–associated pneumonia (C-19AP) and a chest CT usually displays characteristic evolving opacities.2 C-19AP may lead to its most feared complication, a severe acute respiratory syndrome (SARS). Consequently, the novel coronavirus 2 was officially named SARS-CoV-2.3
The COVID-19 epidemic in China and beyond was declared a Public Health Emergency of International Concern on January 30, 2020, and a pandemic on March 11, 2020 by the WHO.1 As it continues to spread, virtually every healthcare personnel is likely to encounter a patient with COVID-19. Nuclear medicine physicians (NMPs) reported on cases of C-19AP identified on PET/CT examinations.4,5 It is the localizing CT that may suggest this diagnosis, which should place a patient under investigation (PUI) for COVID-19. Therefore, SPECT/CT or PET/CT could lead to incidental findings of C-19AP necessitating management of a PUI.
As an example, discussed here forth is a 60-year-old woman with extensive orthopedic history who was referred for the management of chronic cervical and shoulder pain. A bone scan with SPECT/CT of the cervical spine was ordered by the orthopedic surgeon; however, the patient developed an acute respiratory illness. The examination was postponed, whereas her primary care physician evaluated the influenza-like viral illness and prescribed oseltamivir. However, her viral testing panel returned negative the following day. After approximately 9 days of febrile respiratory illness, she gradually began to improve and decided to proceed with the bone scan.
On March 5, 2020, the patient underwent a bone scan with SPECT/CT of the cervical spine, which includes images of the upper chest (Fig. 1). Unexpectedly, review of the low-dose localizing CT images revealed multiple, bilateral, ground glass opacities (GGOs) with areas of nodular consolidation (Fig. 2A). The localizing CT augmented by the edge enhancement revealed additional features that are typical of C-19AP, including vascular enlargement in peripheral distribution, air-bronchograms, and the reverse halo sign (Fig. 2). Conspicuous interlobular and intralobular septal thickening was also present, especially prominent peripherally (Fig. 2A). These findings were typical of C-19AP. Less than an hour after the study completion, an NMP recognized the incidental C-19AP findings and immediately informed the facility's administrator who activated the infection control measures (Fig. 3). The NMP next telephoned the patient but had to leave a message, asking the patient to immediately return the call. After the decontamination of the imaging room, it was closed for 2 hours. Anyone who had contact with the PUI was decontaminated according to the institutional policy.
FIGURE 1.
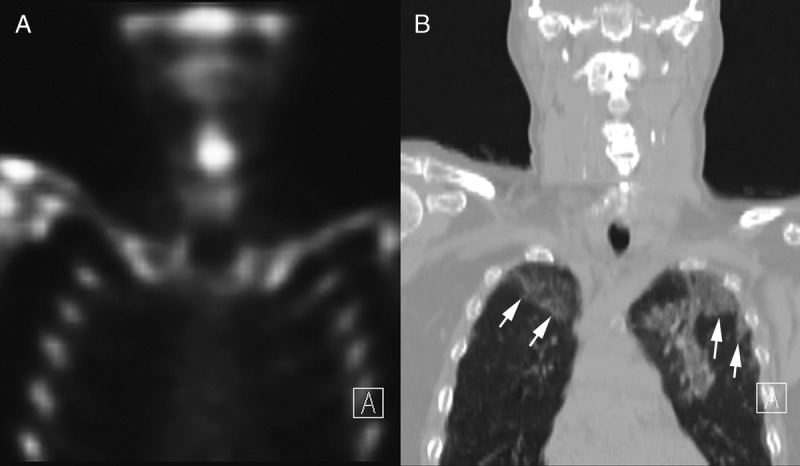
Skeletal SPECT/CT through the mid chest, showing coronal skeletal SPECT (A) and the matching slice of the low-dose localizing CT (B) that is displayed in a lung window. There are large, peripheral GGOs (arrows) in both lungs.
FIGURE 2.
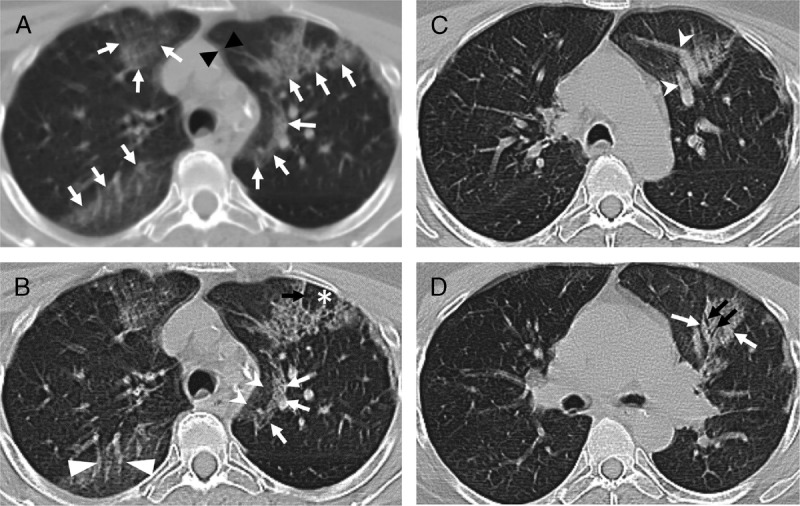
Shown are axial slices of localizing low-dose CT obtained with a non-breathholding technique as part of SPECT/CT study that revealed findings commonly described in patients with C-19AP. A, The localizing, unenhanced, axial CT image is shown. Respiratory motion artifact is creating double shadows (black arrowheads) and blurring the fine detail. Nevertheless, multiple scattered areas of GGOs are noted in the peripheral distribution (white arrows), a few of which in the left lung demonstrate nodular consolidation. B, The same image was processed using the edge enhancement filter, which improved visualization of associated interlobular and intralobular septal thickening (black arrow) with central lobular sparing (asterisk). A semilunar-shaped reticular-nodular infiltrate (white arrows) is surrounding a GGO (white indented-base arrowheads), called a reversed halo sign. There is a noticeable vascular enlargement coursing through a GGO (white flat-base arrowheads). C, Another slice showing peripheral GGO containing vascular enlargement. D, A slice showing reticular-nodular opacity (white arrow) with an air-bronchogram (black arrows).
FIGURE 3.
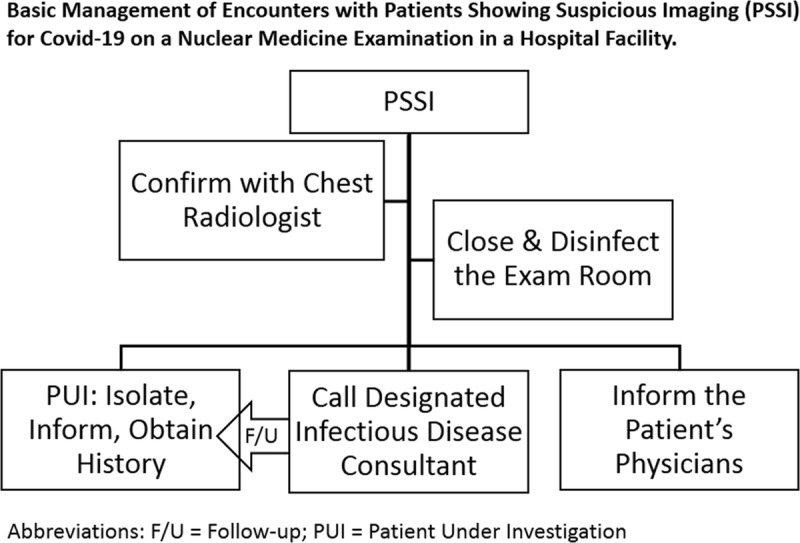
Schematic of basic steps in managing an encounter with a patient showing suspicious imaging for C-19AP.
When the patient called back, she was informed by the NMP about the CT findings, asked to stay quarantined at home, and expect a call from our designated infectious disease consultant. The PUI reported no recent travel or known exposure to individuals at high risk of SARS-CoV-2 exposure. She reported that her symptoms started about 10 days prior, including severe fatigue, body aches, sore throat, dry cough, and a fever of up to 103°F (39.4°C). She had dyspnea of several days duration that started to improve a few days before the bone scan. When she arrived at our registration desk, she continued to experience an occasional dry cough and generalized mild weakness. The medical office associate at the registration desk asked the screening questions provided by our hospital administration, which at that time included (1) travel to specific countries with a high prevalence of COVID-19, (2) upper respiratory illness in the past 14 days, and (3) a fever. Contemporaneous institutional policy required rescheduling and isolation only if a patient answered affirmatively to all three questions. Hence, she was allowed to proceed into the imaging area for the test.
At our institution, any PUI must be reported to a designated infectious disease consultant for further management and reporting to the Pennsylvania Department of Health. The NMP also informed the primary care physician and the referring orthopedic surgeon about the incidental CT findings.
At the time of our encounter with the patient, there were no confirmed cases of COVID-19 in the state of Pennsylvania. The testing kits for SARS-CoV-2 were rationed at the time because of severe shortage, allowing testing of only symptomatic patients with a positive history of travel to high-risk countries or a contact with someone who did. Hence, our patient did not qualify for SARS-CoV-2 testing but was managed as a PUI based on incidental CT findings. Six days later, the WHO declared COVID-19 a pandemic.1 The following day, the director of the National Institute of Allergy and Infectious Diseases stated at the House of Representatives hearing that “The system is not really geared to what we need right now,” when asked about SARS-CoV-2 test kits availability in the United States.6
The test used for identification of SARS-CoV-2 is a real-time reverse polymerase chain reaction. The sensitivity for such testing improved in the following order of sampling: bronchoalveolar lavage fluid (93%), sputum (72%), nasal swabs (63%), fibrobronchoscope brush biopsy (46%), pharyngeal swabs (32%), feces (29%), and blood (1%).7 The chest CT has a high diagnostic sensitivity for COVID-19 infection, but it is not as specific as the molecular testing. All viral pneumonias have overlapping findings on a chest CT.8 In one study, C-19AP was more likely to have a peripheral distribution (80% vs 57%, P < 0.001), GGO (91% vs 68%, P < 0.001), fine reticular opacity (56% vs 22%, P < 0.001), and vascular thickening (59% vs 22%, P < 0.001), but less likely to have a central and peripheral distribution (14.% vs 35%, P < 0.001), pleural effusion (4.1 vs 39%, P < 0.001), and lymphadenopathy (2.7% vs 10.2%, P < 0.001).2
It is critical to be familiar with up-to-date institutional and/or your facility's policy and procedure for handling a PUI encounter. Our index patient was handled according to the contemporaneous institutional policy. It is important to be up-to-date on the policies issued by the Centers for Disease Control and Prevention.9 It is also important to monitor useful resources emerging from related specialty organizations.10
The localizing low-dose CT performed as part of a SPECT/CT examination is suboptimal but diagnostic. Its shortcomings include tidal breathing that creates motion artifact and hypoinflation. However, quality of localizing low-dose CT (Fig. 2A) can be improved by edge enhancement, which is standard with most radiology image display workstations. It is common at our facility to apply edge enhancement that significantly improved image quality (Figs. 2B–D). Knowledge of typical CT features of C-19AP2,11,12 by NMPs is essential in timely recognition of PUIs. In most states, PUIs must be reported to the Departments of Health. Therefore, every institution has to clearly chart the pathway for completing this requirement and associated responsibilities (Fig. 3).
SARS-CoV-2 can spread when aerosolized, such as during aerosol ventilation scintigraphy.13 It is prudent to suspend the use of aerosol ventilation imaging, performing only perfusion scintigraphy for pulmonary thromboembolism. There is a validated approach for the interpretation of perfusion scintigraphy in combination with a chest x-ray.14
It is important for nuclear medicine physicians to become familiar with diagnostic characteristics of C-19AP on CT, enabling prompt recognition on a SPECT/CT or a PET/CT examination. Identified PUIs must be systematically managed and reported to a state's health department, which should be part of a standard operating procedure.
Footnotes
Conflicts of interest and sources of funding: none declared.
REFERENCES
- 1.Ghebreyesus TA. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. WHO Speaches. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed March 15, 2020.
- 2.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020;200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin C, Liu F, Yen TC, et al. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou S, Zhu X. FDG PET/CT of COVID-19. Radiology. 2020;200770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuck E. ‘It is a failing. Let's admit it,’ Fauci says of coronavirus testing C-19APacity. NBC News [NBC News website]. 2020. Available at: https://www.nbcnews.com/health/health-news/it-failing-let-s-admit-it-fauci-says-coronavirus-testing-n1157036. Accessed March 15, 2020. [Google Scholar]
- 7.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260:18–39. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19). National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Accessed March 15, 2020. [Google Scholar]
- 10.Kooraki S, Hosseiny M, Myers L, et al. Coronavirus (COVID-19) outbreak: what the department of radiology should know. J Am Coll Radiol. 2020;17:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X, Gong Z, Xiao Z, et al. Novel coronavirus pneumonia outbreak in 2019: computed tomographic findings in two cases. Korean J Radiol. 2020;21:365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020:1–7. [DOI] [PubMed] [Google Scholar]
- 13.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sostman HD, Miniati M, Gottschalk A, et al. Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. J Nucl Med. 2008;49:1741–1748. [DOI] [PubMed] [Google Scholar]


