Abstract
This review focuses on best practices and recommendations for hygiene and disinfection to limit exposure and transmission of infection in outpatient glaucoma clinics during the current COVID-19 pandemic.
Key Words: coronavirus, disinfection, hygiene, glaucoma, tonometry, visual field testing, optical coherence tomography, lenses
The first physician to sound the alarm about the novel coronavirus causing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was ophthalmologist Lin Wenliang, MD in Wuhan, China. Not long after he first raised awareness for SARS-CoV-2, he was infected by a presymptomatic patient with glaucoma and died.1
Just a few months after Dr Wenliang’s death, several studies have confirmed both presymptomatic and asymptomatic transmission of SARS-CoV-22–5 and the director of the Centers for Disease Control and Prevention (CDC) Dr Robert Redfield estimates that as many as 25% of individuals who test positive for COVID-19 disease are asymptomatic.6 Hence, the fear of transmission of SARS-CoV-2 during an ophthalmology office visit is well warranted and requires ophthalmologists, optometrists, and ancillary personnel to be aware of the signs and symptoms of COVID-19; and to minimize transmission to personnel and other patients.
Reports have suggested that COVID-19 may present with findings of conjunctivitis that are indistinguishable from viral conjunctivitis because of other pathogens. A study of 30 patients in China found that 1 patient with conjunctivitis had SARS-CoV-2 in tear film and conjunctival discharge, suggesting that the virus may cause infection through the conjunctiva or ocular surfaces.7 In a study of 38 patients with clinically confirmed COVID-19, ocular findings such as conjunctival hyperemia and epiphora were associated with more severe clinical disease, higher white blood cell, and neutrophil counts, and greater C-reactive protein elevation compared with patients without ocular symptoms.8 Though the risk of ocular transmission of SARS-CoV-2 through the tear film is likely low,9 the use of ophthalmic diagnostic equipment and procedures that requires close proximity to patients—such as the slit lamp, ophthalmoscope, and ocular manipulation—increases the risk of viral transmission from patient to physician through respiratory droplets and contact with surfaces. In addition, several ophthalmic instruments are commonly used during a single-patient visit and, if not disinfected properly, these tools can become potential sources of transmission.
There were nearly 46.3 million office visits to ophthalmologists in the United States in 2016 according to the most recent CDC National Ambulatory Medical Care Survey.10,11 Glaucoma, 1 of the top 5 reasons for office visits, accounted for 7.19 million visits in the United States in 2016.11 The large volume of annual office visits to outpatient glaucoma clinics combined with the use of ophthalmic tools and the necessary proximity of physicians to patients during these visits puts both patients and health care workers at considerable risk for exposure to pathogens. Moreover, patients visiting a glaucoma clinic are often older adults with comorbidities,12,13 the demographic at highest risk of severe illness from COVID-19.14
How can glaucoma specialists adjust their practice to best protect themselves from exposure to SARS-CoV-2 and avoid becoming a vector for transmission to patients and staff? Through an internet search and communication with ophthalmic equipment manufacturers, we have compiled recommendations for hygiene and disinfection in outpatient glaucoma clinics as a reference guide. The Ophthalmology Department at Columbia University implemented many of these practices before the American Academy of Ophthalmology (AAO) or the CDC-published guidelines for outpatient care. Following the development of an operational task force at Columbia University, the Ophthalmology Department shared several commonsense measures for practice operations starting March 2. The department has continued to be proactive in adjusting best practices for ophthalmology clinics, and these updated protocols and policies, including a Patient Visit Stratification, are publicly available on the department’s COVID-19 Information webpage.15
PREVENTING PERSON TO PERSON TRANSMISSION IN GLAUCOMA OUTPATIENT SETTINGS
The primary mode of transmission of SARS-CoV-2 is thought to be person to person spread in respiratory droplets when an infected person coughs, sneezes, or talks.16 To limit respiratory spread during office visits, ophthalmologists must take precautions to regulate the number of patients entering clinic, to preserve social (physical) distancing, and to minimize direct encounters between staff and patients.17–20
The CDC has published guidelines for health care facilities to protect patients and staff during the current SARS-CoV-2 outbreak.21 These recommendations include screening patients for symptoms of acute respiratory illness—including cough or shortness of breath, sore throat, or systemic symptoms like myalgias or chills—and advising patients to check their temperature before their arrival at the health care facility, asking all patients about history of travel to areas with high prevalence of COVID-19 or contact with possible COVID-19 positive individuals, separating patients with respiratory symptoms from other patients in the waiting area, optimizing the use of telemedicine, limiting the number of staff providing care, installing barriers to limit contact with patients during triage, and emphasizing hand hygiene for patients and staff.22,23
On March 18, 2020, the AAO published “New Recommendations for Urgent and Nonurgent Patient Care,”24 urging ophthalmologists to halt providing any treatment that is nonurgent or nonemergent. The AAO is continually updating their guidelines for practice management specific to ophthalmology. In addition to the aforementioned CDC recommendations, AAO recommendations include the following24:
Reschedule appointments for patients with nonurgent ophthalmic problems and see only patients with emergent needs or those requiring frequent management.
Only allow patients inside the practice; if necessary, a patient may have only 1 person to accompany them.25
Encourage online payment of copays to minimize interaction between patients and health care staff.
Use slit lamp breath shields.
Both patient and physicians should speak as little as possible during slit lamp examination.
For patients with documented COVID-19, persons under investigation, or patients who are “potentially infected with COVID-19,” ophthalmologists should wear personal protective equipment (PPE) including an N95 mask, gown, gloves, and eye protection.
Although the CDC suggests implementing PPE optimization strategies to extend supplies,26 at the moment this review was performed, the AAO has not yet offered an official stance on the use and reuse of PPE. The recommendations and expectations for the use of PPE in the outpatient glaucoma clinics must be considered in the context of the United States’ PPE shortage. For example, N95 masks are not currently readily available for outpatient health care settings, so the AAO has not yet recommended the use of N95 masks in outpatient clinics. CDC guidance should be followed and accessed frequently given our constantly changing knowledge of disease transmission. The purpose of this review is to provide a resource for the disinfection of instruments and devices of particular interest to the glaucoma specialist.
DISINFECTION OF THE CLINIC ENVIRONMENT: WAITING ROOM AND PATIENT ROOMS
Another important mode of transmission of COVID-19 may be spread from contact with contaminated surfaces or objects.16 To minimize disease transmission through surfaces in the outpatient health care setting, both the CDC and AAO recommend routine cleaning and disinfection of waiting rooms, restrooms, examination lanes, office furniture, and instruments.23,27 For example, within the Ophthalmology Department at Columbia University, cleaning of check-in and check-out kiosks, front desk workstations, door entries, and waiting room furniture is performed hourly.28
The AAO suggests following CDC recommendations for appropriate disinfectant agents, which include: diluted household bleach (>1000 ppm sodium hypochlorite), alcohol solutions with at least 70% alcohol, and common EPA-registered household disinfectants (eg, surface wipes with dimethyl benzyl ammonium chloride, such as Clorox and Lysol branded products).27 The EPA published an official list of products that meet criteria for use against SARS-CoV-2 and includes the required amount of time that a surface should be treated with each product to effectively kill the virus.29
DISINFECTION OF EQUIPMENT AND OTHER INSTRUMENTS IN GLAUCOMA CLINICS
Another potential vector of transmission in glaucoma clinics is any device used during patient visits. Slit lamps are commonly discussed in the context of viral transmission given the close proximity of patient to provider and patient-instrument contact during the physical examination. The AAO has encouraged the use of slit lamp breath shields, and several manufacturers including Zeiss and Topcon are offering free slit lamp breath shields. There are also many videos and picture demonstrations on the internet for how to make functional slit lamp breath shields using materials that are easily accessible, such as an A4 plastic folder or a plastic quilting template. Other ophthalmic devices such as handheld tonometers have been implicated in the transmission of viral pathogens as well.30
On April 2, 2020, the AAO recommended using the “same disinfection practices already used” to disinfect ophthalmic instruments and office furniture.27 In order to confirm manufacturers’ disinfection instructions and to provide a reference tool for glaucoma specialists as they continue to see patients in the outpatient setting, customer support lines of several manufacturers were contacted, and we conducted a comprehensive review of manufacturer webpages and equipment user manuals.
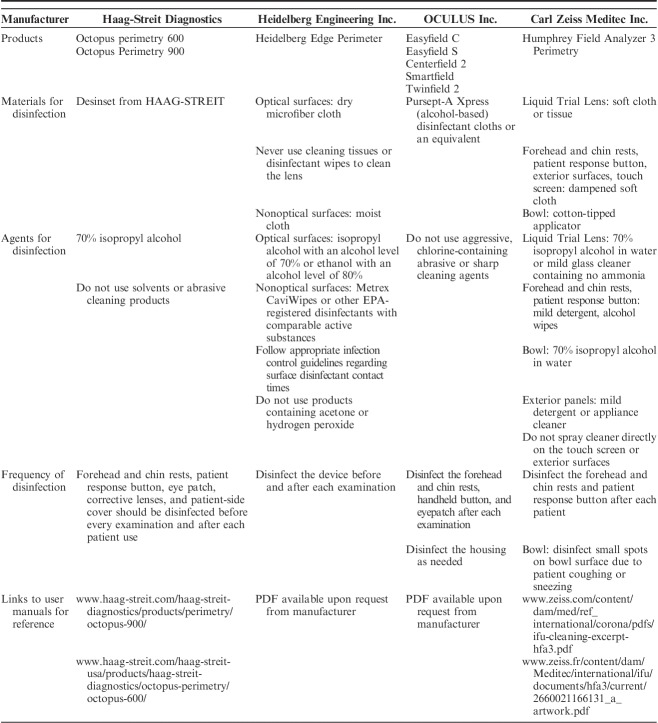
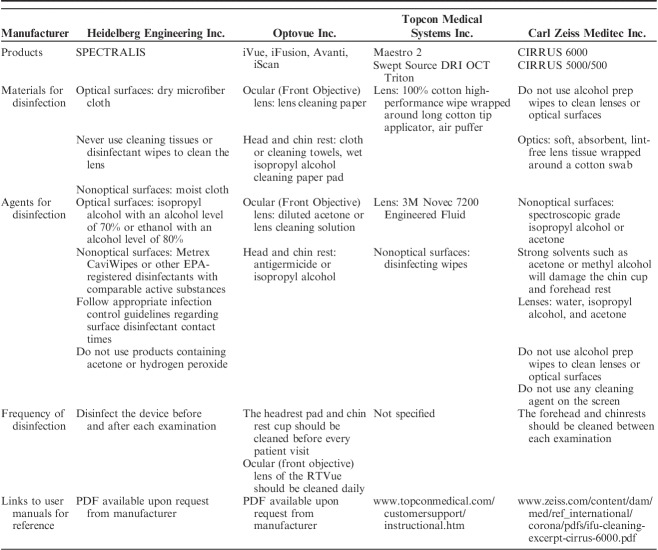
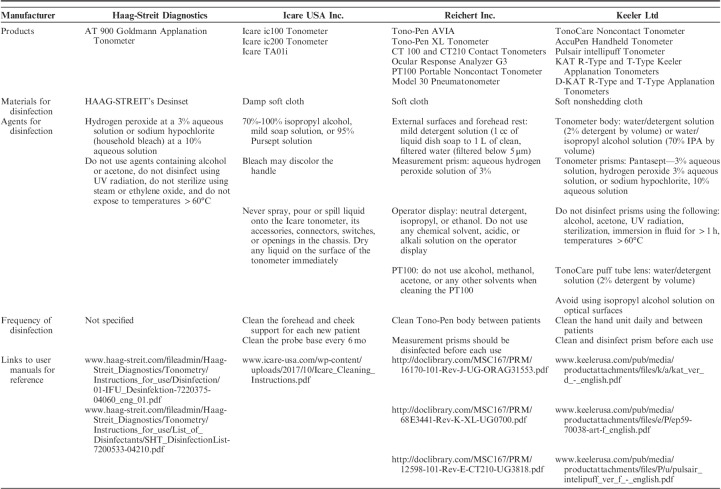
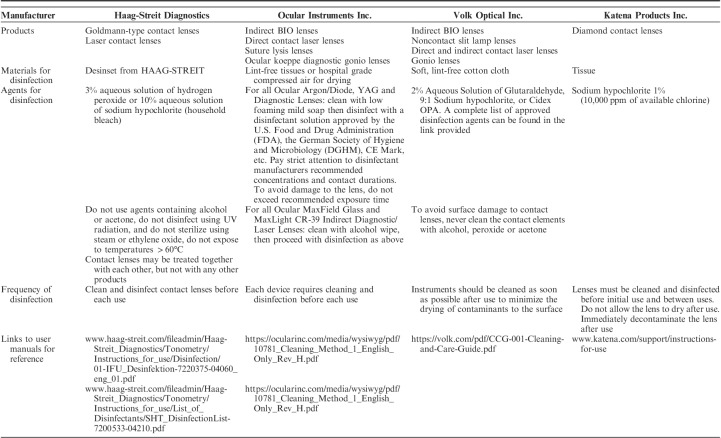
We have compiled the cleaning and disinfection instructions from manufacturers for the following ophthalmic tools that are commonly used in outpatient glaucoma clinics: visual field analyzers (Table 1), optical coherence tomography (OCT) machinery (Table 2), tonometers (Table 3), and lenses (Table 4). Each table is inclusive of commonly used products from a sample of manufacturers but is not meant to be an exhaustive list nor an endorsement of the listed products. The information provided herein is publicly available as of April 5, 2020 on manufacturer websites or as downloadable PDF documents.
TABLE 1.
Summary of Recommendations for the Cleaning and Disinfection of Common Visual Field Analyzers, by Manufacturer
TABLE 2.
Summary of Recommendations for the Cleaning and Disinfection of Common Optical Coherence Tomography Devices, by Manufacturer
TABLE 3.
Summary of Recommendations for the Cleaning and Disinfection of Common Contact Tonometers, by Manufacturer
TABLE 4.
Summary of Recommendations for the Cleaning and Disinfection of Common Lenses, by Manufacturer
VISUAL FIELD TESTING
Visual Field Analyzers of all types require close contact of the patient to the equipment and close proximity of the operator to the patient and equipment (including data entry and patient positioning). Aspects of the equipment that come into direct contact with the patient include the forehead and chin rests, the patient response button, the chair, and the eye occluder. The corrective lens may also come into contact with the patient’s eyelashes or nose. Respiratory droplets may accumulate on these surfaces and importantly within the perimetry bowl itself. The duration of viral particle presence on the bowl surface, how long the particles remain suspended within the bowl, and whether the viral particles can be dislodged or resuspended by normal breathing or talking by the patient undergoing testing is unknown. The eye occluder may be replaced with disposable material such as taped gauze or an amblyopia patch to decrease the amount of time and material used for disinfection between each patient. Disinfection of visual field analyzers including the bowl surface may be difficult, and the AAO previously suggested avoiding the use of “equipment that cannot be safely disinfected, such as some visual field analyzers.”24 Routine perimetry should therefore be performed judiciously and taking into account the aforementioned precautions. A summary of the recommendations for cleaning and disinfection of visual field analyzers is provided in Table 1.
IMAGING DEVICES (OCT)
Imaging devices’ (eg, OCT or cameras) forehead and chin rests come into direct contact with the patient. The exterior surface of the machine may be contaminated by a patient’s respiratory droplets from breathing or speaking during the examination. A summary of the recommendations for cleaning and disinfection of OCT units is provided in Table 2.
TONOMETRY
Contact Tonometry
Previous studies have reported that applanation tonometry has the potential to transmit a variety of infectious agents, including adenovirus 8 and herpes simplex virus 1, and reusable tonometer prisms could, in theory, be a mode of transmission for hepatitis B, hepatitis C, human immunodeficiency virus, and Creutzfeldt-Jakob disease.31–37 A systematic review of 11 studies assessing the disinfection of Goldmann tonometers concluded that there is no definitive optimal technique or agent for the disinfection of Goldmann applanation tonometer prisms.30,33 The AAO supports the use of diluted bleach for the disinfection of reusable tonometer tips for SARS-CoV-2 and adenovirus. If available, single use, disposable tonometer prisms provide an alternative to reusable prisms to prevent cross-contamination.38 A summary of the recommendations for cleaning and disinfection of common contact tonometers is provided in Table 3.
Noncontact Tonometry
In its Infection Prevention and Control Recommendations, the CDC suggests taking precautions when performing aerosol-generating procedures, which could generate the aerosolization of viral particles.23 A 1991 study using florescence photography using specific types of tonometer (Keeler Pulsair and AO NCT II) depicted visible tear film splatter from the air puff in eyes receiving fluorescein drops; however the investigators were unable to demonstrate aerosolization of the tear film in eyes not receiving topical drops.39 Unless aerosolization of the tear film with the puff energy elicited by the current devices is evidenced, non-contact tonometry during the pandemic could be considered after weighing risks and benefits. One potential benefit, for instance, is that these tonometers permit more staff-patient distancing than other methods. When used, care should be taken to properly disinfect contact surfaces between patients.
LENSES
Given their frequent handling, proximity to respiratory droplets and/or direct contact to the eye, diagnostic, or therapeutic lenses [eg, handheld indirect lenses (20D, 28D, 60D, 78D, 90D, etc.) Goldmann-type contact lenses, laser surgery lenses, gonioscopy lenses, and others] are vectors for nosocomial infection during an ophthalmologic examination.40,41 In order to limit the number of patients coming into contact with a single lens, many ophthalmology departments, including our own, have suspended the use of trial lenses and trial framing, and are only performing refractions in rare situations.28 Single-use diagnostic or therapeutic lenses, such as those offered by some manufacturers (eg, Volk Optical and Katena), can reduce the potential for transmission of infection and eliminate the time required for disinfection without affecting image quality.42,43 Alternatively, Haag-Streit Goldmann lenses can be used with an optically neutral disposable shield call the Stery Cup.44 A summary of the recommendations for cleaning and disinfection of lenses commonly used in an office visit for a glaucoma patient is provided in Table 4.
CONCLUSIONS
There are many opportunities for the transmission of pathogens, particularly the coronavirus now causing COVID-19, during a patient visit to an outpatient glaucoma clinic. Proper infection control practices are especially important given the volume of patients in these clinics—even during times of restricted patient flow—and the number of instruments that may be used during a single-patient examination. Careful attention to personal hygiene, social (physical) distancing, and instrument disinfection will decrease the chance of inadvertent transmission among ourselves, office personnel, and patients in the outpatient setting.
By implementing many of these practices in the first week of March, before national organizations gave recommendations for outpatient clinical practices, our department has kept the COVID-19 infection rate among staff and faculty very low. This proactive approach serves as an example for how Ophthalmology Departments can effectively address the next epidemic or pandemic to decrease transmission between patients and providers.
Footnotes
Supported by Bernard and Shirlee Brown Glaucoma Research Laboratory, Edward S. Harkness Eye Institute, Columbia University Irving Medical Center, New York, NY and an unrestricted grant from Research to Prevent Blindness, New York, NY (Columbia University Department of Ophthalmology).
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Parrish RK, II, Stewart MW, Duncan Powers SL. Ophthalmologists are more than eye doctors— In Memoriam Li Wenliang. Am J Ophthalmol. 2020. Doi:10.1016/j.ajo.2020.02.014. [Google Scholar]
- 2.Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 — Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New Engl J Med. 2020;382:970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020 MMWR Morb Mortal Wkly Rep. 2020;69:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead S, Fiebel C. CDC Director on models for the months to come: this virus is going to be with us. National Public Radio. 2020. Available at: https://www.npr.org/sections/health-shots/2020/03/31/824155179/cdc-director-on-models-for-the-months-to-come-this-virus-is-going-to-be-with-us. Accessed April 2, 2020.
- 7.Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020. Doi:10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Jun IS, Anderson DE, Zheng Kang AE, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020. Doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ophthalmology Fact Sheet from the National Ambulatory Medical Care Survey. National Center for Health Statistics. 2015. Available at: https://www.cdc.gov/nchs/data/namcs/factsheets/NAMCS_2014_15_Opthalmology-508.pdf. Accessed April 1, 2020.
- 11.Rui P, Okeyode T. National Ambulatory Medical Care Survey: National summary tables. 2016. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_ web_tables.pdf. Accessed April 2, 2020.
- 12.Bennion JR, Wise ME, Carver JA, et al. Analysis of glaucoma-related mortality in the United States using death certificate data. J Glaucoma. 2008;17:474–479. [DOI] [PubMed] [Google Scholar]
- 13.Sharma T, Salmon JF. Ten-year outcomes in newly diagnosed glaucoma patients: mortality and visual function. Br J Ophthalmol. 2007;91:1282–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention People who are at higher risk for severe illness. Coronavirus disease 2019 (COVID-19). 2020. Available at: www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html Accessed April 3, 2020. [PubMed]
- 15.Columbia University Department of Ophthalmology Columbia ophthalmology COVID-19 information. 2020. Available at: https://www.columbiaeye.org/content/columbia-ophthalmology-covid-19-information. Accessed March 31, 2020.
- 16.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19): how it spreads. 2020. Available at: www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html. Accessed April 3, 2020.
- 17.Liebmann JM, Barton K, Weinreb RN, et al. Evolving guidelines for intracameral injection. J Glaucoma. 2020;29 (suppl 1):S1–S7. [DOI] [PubMed] [Google Scholar]
- 18.Doshi RR, Leng T, Fung AE. Reducing oral flora contamination of intravitreal injections with face mask or silence. Retina. 2012;32:473–476. [DOI] [PubMed] [Google Scholar]
- 19.Garg SJ, Dollin M, Hsu J, et al. Effect of a strict ‘no-talking’ policy during intravitreal injection on post-injection endophthalmitis. Ophthalmic Surg Lasers Imaging Retina. 2015;46:1028–1034. [DOI] [PubMed] [Google Scholar]
- 20.McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31:654–661. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention For healthcare professionals. Coronavirus disease 2019 (COVID-19). 2020. Available at: www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Accessed April 3, 2020.
- 22.Centers for Disease Control and Prevention Steps healthcare facilities can take. Coronavirus Disease 2019 (COVID-19). 2020. Available at: www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/steps-to-prepare.html. Accessed March 31, 2020.
- 23.Centers for Disease Control and Prevention Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. Coronavirus disease 2019 (COVID-19). 2020. Available at: www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. Accessed April 1, 2020.
- 24.American Academy of Ophthalmology New recommendations for urgent and nonurgent patient care. 2020. Available at: www.aao.org/headline/new-recommendations-urgent-nonurgent-patient-care. Accessed March 31, 2020.
- 25.American Academy of Ophthalmology Coronavirus impact: practice operations and safety considerations. Available at: www.aao.org/practice-management/article/coronavirus-practice-operations-safety-advice. Accessed April 3, 2020.
- 26.Centers for Disease Control and Prevention Strategies to optimize the supply of PPE and equipment. 2020. Available at: www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Accessed April 11, 2020.
- 27.Chodosh J, Holland GN, Yeh S. Important coronavirus updates for ophthalmologists. The American Academy of Ophthalmology. 2020. Available at: www.aao.org/headline/alert-important-coronavirus-context. Accessed April 4, 2020.
- 28.Columbia University Department of Ophthalmology COVID-19 recommendations for practice operations. Available at: columbiaeye.org. Accessed March 8, 2020.
- 29.List N: Disinfectants for use against SARS-CoV-2. United States Environmental Protection Agency. 2020. Available at: www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Accessed April 6, 2020.
- 30.Ragan A, Cote SL, Huang JT. Disinfection of the Goldman applanation tonometer: a systematic review. Can J Ophthalmol. 2018;53:252–259. [DOI] [PubMed] [Google Scholar]
- 31.Junk AK, Chen PP, Lin SC, et al. Disinfection of tonometers: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:1867–1875. [DOI] [PubMed] [Google Scholar]
- 32.Nagington J, Sutehall GM, Whipp P. Tonometer disinfection and viruses. Br J Ophthalmol. 1983;67:674–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkins N, Hodge W, Li B. A systematic review regarding tonometry and the transmission of infectious diseases. J Clin Med Res. 2018;10:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepose JS, Linette G, Lee SF, et al. Disinfection of Goldmann tonometers against human immunodeficiency virus type 1. Arch Ophthalmol. 1989;107:983–985. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu H, Inui A, Sogo T, et al. Tears from children with chronic hepatitis B virus (HBV) infection are infectious vehicles of HBV transmission: experimental transmission of HBV by tears, using mice with chimeric human livers. J Infect Dis. 2012;206:478–485. [DOI] [PubMed] [Google Scholar]
- 36.Davanipour Z, Sobel E, Ziogas A, et al. Ocular tonometry and sporadic Creutzfeldt-Jakob disease (sCJD): a confirmatory case-control study. Br J Med Med Res. 2014;4:2322–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal WA, Pirnazar JR, Arens M, et al. Disinfection of Goldmann tonometers after contamination with hepatitis C virus. Am J Ophthalmol. 2001;131:184–187. [DOI] [PubMed] [Google Scholar]
- 38.Salvi SM, Sivakumar S, Sidiki SS. Use of disposable prism tonometry in routine clinical practice. Eye (Lond). 2005;19:743–746. [DOI] [PubMed] [Google Scholar]
- 39.Britt JM, Clifton BC, Barnebey HS, et al. Microaerosol formation in noncontact “Air-Puff” tonometry. Arch Ophthalmol. 1991;109:225–228. [DOI] [PubMed] [Google Scholar]
- 40.Sobolewska B, Buhl M, Liese J, et al. Slit lamps and lenses: a potential source of nosocomial infections? Eye (Lond). 2018;32:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sammons JS, Graf EH, Townsend S, et al. Outbreak of adenovirus in a neonatal intensive care unit: critical importance of equipment cleaning during inpatient ophthalmologic examinations. Ophthalmology. 2019;126:137–143. [DOI] [PubMed] [Google Scholar]
- 42.Culham L. Single-use contact lenses aid imaging, diagnosis: multiple mirror, fundus, and retina lenses provide quality images. Optometry Times. 2015. Available at: https://www.optometrytimes.com/modern-medicine-cases/single-use-contact-lenses-aid-imaging-diagnosis. Accessed April 2, 2020.
- 43.Lee B, Szirth BC, Fechtner RD, et al. Are disposable and standard gonioscopy lenses comparable? J Glaucoma. 2017;26:e157–e159. [DOI] [PubMed] [Google Scholar]
- 44.HAAG-STREIT AG. Stery Cup; 2020. Available at: www.haag-streit.com/fileadmin/Haag-Streit_Diagnostics/Disposables/Brochures_Flyers/Sterycup/FLY_SteryCup_7200437-02030_Web_.pdf. Accessed April 2, 2020.